Abstract
Percutaneous radiofrequency ablation (RFA), a generally accepted alternative therapy for patients with liver metastases, is a minimally invasive approach with a favorable safety profile and a lower rate of major complications. The use of RFA or combined RFA plus resection can produce total tumor clearance in patients with unresectable liver metastases. However, the relatively high rate of local tumor progression has prevented the widespread use of RFA. Furthermore, its efficacy is controversial because there have been no comparisons for its effect on overall survival compared with standard options such as systemic chemotherapy. Meanwhile, immunotherapy has become a major research focus for oncology based on the recent successes reported for immune checkpoint inhibitors for melanoma, non-small cell lung cancer, gastric cancer, and other cancers. Immune checkpoints negatively regulate T cell function, and inhibition prevents the blockade of the immune system by cancer cells to prevent their destruction. Unfortunately, only some patients (< 25%) respond to immuno-oncology drugs, whereas other patients acquire resistance. However, RFA can induce massive necrotic cell death which might activate immunity and the presentation of cryptic antigens to induce tumor-specific T cell response. Because RFA can induce the rapid release of large amounts of tumor antigens, it can potentially stimulate transient immune responses to much tumor antigens. Combination therapies have induced synergistic enhancement of anticancer immune response in preclinical studies, indicating great promise for the future of oncologic treatment.
Key Points
• Only some patients respond to immuno-oncology drugs.
• RFA causes the release of large amounts of cellular debris, a source of tumor antigens that elicit immune responses against tumors.
• Combination RFA for liver metastases and immune checkpoint inhibitor therapies might synergistically enhance antitumor immunity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Percutaneous radiofrequency ablation (RFA) of liver metastases is a generally accepted alternative therapy for patients who are poor surgical candidates and those with post therapeutic recurrences [1]. RFA is a minimally invasive approach with a better safety profiles, lower complication rates, and a shorter hospital stay compared with hepatectomy [2, 3]. The use of RFA or combined RFA plus resection can produce total tumor clearance in the liver of patients with unresectable colorectal liver metastases [4,5,6,7]. Patients treated with RFA for unresectable colorectal cancer liver metastases were reported to have 5-year overall survival (OS) rates of 20–48.5%, similar to those for surgical resection [2, 3, 8]. However, the local recurrence rates for liver metastases in colorectal cancer patients after RFA range from 8.8 to 40% [8] and have prevented the widespread use of RFA [4, 8]. Furthermore, the efficacy of RFA is controversial because its effect on OS is poor in comparison with the standard care of option, systemic chemotherapy [2, 9, 10].
Immunotherapy is a promising new therapy for some advanced or metastatic cancers. Recent successes with immune checkpoint inhibitors have demonstrated that the immune system can control cancers with various histologies. Furthermore, some inhibitions have induced responses that are more long-lasting compared with many anticancer drugs [11]. Immune checkpoints are initiated primarily through T cell–inhibiting and T cell–stimulating receptors and their negative ligands, including CTLA-4, PD-1, and PD-L1. Checkpoint inhibitors function similar to anti-CTLA-4 and anti-PD1/PD-L1, which negatively regulate T cell function, and their inhibition prevents the blockade of the immune system by cancer cells to prevent their destruction [12,13,14]. Several checkpoint inhibitors have been developed: pembrolizumab, nivolumab, atezolizumab, avelumab, durvalumab, and ipilimumab. Blockade of immune checkpoints has been approved for the treatment of advanced melanoma, renal cell cancer, non-small cell lung cancer, relapsed refractory Hodgkin’s lymphoma, gastric cancer, and urothelial cancer. However, only some patients (< 25%) respond to immuno-oncology drugs, whereas other patients acquire resistance. To date, several resistance mechanisms have been identified including T cell exhaustion, overexpression of caspase-8 and β-catenin, PD-1/PD-L1 gene amplification, and MHC-I/II mutations [15]. Therefore, there is an urgency to improve tumor response and to overcome resistance to immune-oncology drugs.
RFA destroys tumoral tissue by delivering a high-frequency alternating current with ionic agitation and frictional heating [2, 8, 16]. RFA induces massive necrotic cell death releasing large amount of cellular debris in situ that activates the immune system and promotes the presentation of otherwise cryptic antigens, which induce tumor-specific T cell response [17, 18]. Therefore, the combination treatment with RFA and checkpoint inhibitors might significantly enhance tumor antigen-specific T cell responses and synergistically inhibit the growth of distant tumors. This paper reviews the clinical potential of combined RFA therapy and checkpoint inhibitors and discusses the major issues facing this rapidly evolving field.
The combination of molecular-targeted drug and RFA for liver metastases
A recent impact study reported the use of chemotherapy and bevacizumab with or without RFA for the treatment of unresectable liver metastases in patients with colorectal cancer (clinical trials: NCT00043004) [19]. This primary endpoint of this European intergroup randomized phase II study was the OS. Patients were randomly assigned to systemic treatment plus local treatment by RFA with or without additional resection or systemic treatment alone groups. This study reported a statistically significant difference in OS for the combined modality arm with a median follow-up of 9.7 years (HR = 0.58, 95% CI = 0.38 to 0.88, p = 0.01). The 5-year OS was 43.1% (95% CI = 30.3% to 55.3%) in the combined modality arm and 30.3% (95% CI = 19.0% to 42.4%) in the systemic treatment arm. The median OS was 45.6 months (95% CI = 30.3 to 67.8 months) in the combined modality arm and 40.5 months (95% CI = 27.5 to 47.7 months) in the systemic treatment arm. This was the first randomized study to demonstrate that aggressive local treatment can prolong OS in patients with unresectable colorectal liver metastases. However, we need to find more evidence in RFA for liver metastases.
Unexpected tumor progression after RFA
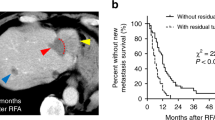
The histomorphology of the metastatic front in liver metastases has been easily evaluable on imaging and growth patterns can provide important insights into the biological mechanisms that support tumor growth in the liver. Several different growth patterns have been described: (1) “desmoplastic” or “encapsulated” pattern, (2) “pushing” or “expansive” pattern, and (3) “replacement” or “invasive” pattern. The tumor border of “desmoplastic” or “pushing” growth pattern is clearly demarcated because of a rim of fibrotic stroma or flattened liver plates. Meanwhile, a “replacement” pattern shows inconspicuous because cancer cells invade diffusely into surrounding tissue. Reported median frequency for the desmoplastic pattern was 41.7% (range, 10.5–80%), for the pushing pattern 37.1% (range, 1.7–91.5%), and for the replacement pattern 43.3% (range, 7.7–65.2%) [20]. Unexpected deeper invasive infiltration in the liver could be frequently demonstrated in the case of replacement pattern; therefore, crescentic or circumferential local progression can be developed after RFA (Fig. 1).
Late local recurrences following RFA of colorectal cancer liver metastases. a Baseline portal phase CT image shows two low-density tumors (arrows) in the right lobe of the liver. b Portal phase CT image 1 day after RFA shows that the tumor and the surrounding area (arrow) were not enhanced. c Follow-up CT image obtained 6 months later shows extrazonal crescentic local progressions of disease (arrow heads)
Ablation-induced immunostimulatory effects and malignant tumor formation
RFA induces inflammatory effects that prevent the cycle of immune evasion by creating a substantial in situ source of acute inflammatory signals and tumor antigens in the form of necrotic tumor cells and cellular debris that generate systemic immunity [21]. In addition, RFA was reported to markedly increase the infiltration of intratumoral CD8+ T lymphocytes and the number of antigen-specific CD8+ T cells within the tumor microenvironment (Fig. 2). Phenotypic analysis demonstrated the increased expression of activation and cytotoxic surface markers on circulating T and natural killer cells [22, 23]. Furthermore, RFA could increase CD8+ effector T cell infiltration at residual tumor sites, which is indicative of innate and/or adaptive immune system activation [24].
However, heat-exposed cancer cells underwent enhanced proliferation and there is a concern that RFA may predispose patients to local recurrence with an aggressive phenotype and unfavorable prognosis, suggesting RFA might induce the further malignant transformation of cancer cells [25]. Of note, the implantation of heat-exposed cancer cells into nude mice induced significantly larger, more aggressive tumors compared with implantation of untreated cells [26]. Cancer cell survival after heat ablation can prompt epithelial-mesenchymal transition and transformation to a progenitor-like, highly proliferative cellular phenotype in vitro and in vivo. In addition, the use of suboptimal RFA transiently induced an aggressive cellular phenotype that accelerates cancer cell growth and spread. Clinically, sarcomatoid carcinoma of the liver can be generally induced by necrosis and degeneration caused by the use of repeated non-surgical therapies including transcatheter arterial chemoembolization or RFA [27].
Enhanced tumor immune activity by checkpoint inhibitors
TAAs presented by MHC molecular expressed on tumor cells are recognized by activated T cells, which are then activated to secrete perforin, granzyme, and cytokines including IL-2 and IFN-γ that induce the apoptosis of target cells to prevent tumor progression [28]. However, normal T cells do not efficiently recognize tumors because tumor cells often lack MHC expression and therefore are weak immunogenic [29]. When leukocytes with suppressed immune functions infiltrate the tumor microenvironment, they are used by the neoplasm for their ability to produce growth factors for tumor cell proliferation, angiogenesis, and prolonged survival.
Under normal physiological conditions, the levels of immune checkpoint molecules are balanced, which allows the maintenance of T cell immune response to minimize damage to the surrounding normal tissue and avoid autoimmune reactions. Previous studies reported that immunotherapy with antagonistic antibodies to block checkpoint pathways release cancer inhibition and facilitate antitumor activity [30,31,32] (Fig. 3). T cells, especially Tregs, express CTLA-4, cell membrane protein receptor that binds to CD80 (B7) and CD86 on APCs to prevent binding to CD28 expressed on to T cells and triggering their activation. PD-1, another surface protein expressed on T cells, inhibits T cell activation and promotes apoptosis of antigen-specific T cells upon PD-L1 binding [33, 34]. Finally, the overexpression of immune checkpoint inhibitors on various cells within the tumor microenvironment forms a multi-factorial suppressive signal that prevents strong T cell–mediated responses.
Inhibition of T cell activation and checkpoint inhibition. Within the tumor microenvironment, distinct signaling pathways such as PD-1/PD-L1 or CTLA-4/B7 induce immune tolerance via the induction of T cell apoptosis or T cell dysfunction. Immune checkpoint inhibitors reinvigorate antitumor immune responses by disrupting co-inhibitory T cell signaling
Resistance to checkpoint inhibitors within the tumor microenvironment
It was previously reported that checkpoint inhibitors can become ineffective when tumors evolve to evade innate and adaptive immunity [35]. Tumor-intrinsic mechanisms of immune evasion include genetic and epigenetic alterations that influence neoantigen formation, presentation, and/or processing, as well as alterations in cell signaling pathways that disrupt cytotoxic T cell function [36]. Tumor-extrinsic mechanisms include the effects of non-cancerous stromal or immune cells or other systemic influences (e.g., host microbiota) that combine with cancer cells to promote tumor growth and resistance to checkpoint inhibitors.
Of note, successful checkpoint inhibitor therapy can reactivate T cells, and a lack of suitable neoantigens and alterations in antigen processing and/or presentation is associated with impaired antitumor immune response. The evolution of neoantigen might explain the acquisition of resistance by the outgrowth of tumor cell clones that never expressed the neo-Ag, despite the effective killing of all other clones, or the acquisition of genetic changes that lead to the loss of neo-Ag expression. Evolution of the mutational landscape was reported in patients who developed acquired resistance to checkpoint inhibitor therapy. Furthermore, the efficacy of checkpoint inhibitor therapy might be blocked by factors in the tumor microenvironment that effectively prevent correct T cell function despite successful neoantigen presentation/cross-presentation and T cell priming.
Combination with adaptive immunotherapies
Several immunotherapies that target the adaptive system have also been reported [22, 37, 38]. A study by Shi et al reported that combined anti-PD-1 antibodies and RFA therapy in a murine colon cancer model overcame a major checkpoint to induce a systemic immune response [18]. RFA treatment initially enhanced a strong T cell–mediated immune response in tumors. However, tumors quickly evaded the immune responses by inhibiting CD8+ and CD4+ T cell functions, by increasing the Treg/Teff ratio and upregulating PD-1/PD-L1 expression. The addition of PD-1 inhibition to RFA significantly decreased tumor volume and significantly increase survival (p < 0.001).
Den Brok et al showed blocking CTLA-4 with specific antibodies prior to either RFA or cryoablation enhanced the treatment response [39]. Furthermore, cryoablation or RFA combined with CTLA-4 inhibition significantly increased the survival rate in a B16OVA model of melanoma after rechallenge compared with untreated controls or those receiving combination therapy with sham IgG antibodies (p < 0.05).
Some researchers have shown that the PD-L1 expression on colorectal cancer cells was relatively lower in contrast with other cancer types including non-small cell lung cancer, melanoma, renal cell carcinoma, and other tumors [40]. However, it is reported that RFA for liver metastases could upregulate PD-L1 expression [18]. On day 8 after RFA, the CD8+ T cell to Treg ratio was significantly decreased compared with that on day 3 (p < 0.001). An increase in T cell infiltration in primary colorectal cancer after RFA for liver metastasis could be associated with the upregulation of PD-L1 expression. This can suggest that the anti-PD-1/PD-L1 therapy has potentially benefited from RFA.
If progression on a checkpoint inhibitor after initial response was confined to one or two sites (“oligoprogression”), local ablation therapy to the sites of progression with the continuation of immunotherapy may represent an alternative therapeutic option to salvage immune-oncology therapy. In a retrospective study for acquired resistance to PD-1/PD-L1 axis inhibitor therapy in 26 patients with advanced non-small cell lung cancer, 15 patients (58%) received local therapy to site(s) of oligoprogression [41]. Among them, 11 continued their respective immune checkpoint inhibitor after local therapy. The 2-year survival rate from acquired resistance among these 15 patients was 92% (95% CI, 0.77–1).
Conclusion
The liver is a common site for cancer metastasis, and tumors in the liver allow relatively easy access for percutaneous ablation. Combined RFA and immune checkpoint inhibitor therapies were reported to synergistically enhance antitumor immunity in these early studies. There are some potential advantages of combining RFA and immune checkpoint inhibitors. First, RFA is widely used in patients with primary or secondary hepatic malignancies; therefore, it will be relatively simple to investigate its role in a novel combined modality. Second, RFA promotes the instant release of large amounts of tumor antigens, which can potentially stimulate transient immune responses to a wide of variety of tumor antigens. Third, immune-oncology therapy has shown promising results in many different types of cancer, and a study showed that the microsatellite-unstable subset of liver metastasis might be a good candidate for checkpoint blockade immunotherapy.
There are several limitations associated with this review. The clinical benefit of combination therapy cannot be verified in a large-scale study yet. Much work is needed to elucidate the beneficial effects induced by the combination of ablation with checkpoint inhibitor. Nevertheless, the synergistic enhancement of the anticancer immune response induced by such combination therapies shows great promise for the future of oncologic treatment.
Abbreviations
- APCs:
-
Antigen-presenting cells
- CI:
-
Confidence interval
- CTLA-4:
-
Cytotoxic T lymphocyte–associated protein 4
- DC:
-
Dendritic cell
- FDA:
-
Food and Drug Administration
- HR:
-
Hazard ratio
- IFN:
-
Interferon
- IL:
-
Interleukin
- MHC:
-
Major histocompatibility complex
- OS:
-
Overall survival
- PD-1:
-
Programmed cell death-1
- PD-L1:
-
Programmed cell death ligand-1
- RFA:
-
Radiofrequency ablation
- TAA:
-
Tumor-associated antigen
- Teff:
-
Effector T cell
- Treg:
-
Regulatory T cell
References
Torzilli G, Adam R, Viganò L et al (2016) Surgery of colorectal liver metastases: pushing the limits. Liver Cancer 6(1):80–89
Shady W, Petre EN, Gonen M et al (2016) Percutaneous radiofrequency ablation of colorectal cancer liver metastases: factors affecting outcomes--a 10-year experience at a single center. Radiology 278(2):601–611
Solbiati L, Ahmed M, Cova L, Ierace T, Brioschi M, Goldberg SN (2012) Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology 265(3):958–968
Wong SL, Mangu PB, Choti MA et al (2010) American Society of Clinical Oncology 2009 clinical evidence review on radiofrequency ablation of hepatic metastases from colorectal cancer. J Clin Oncol 28(3):493–508
Gervais DA, Goldberg SN, Brown DB, Soulen MC, Millward SF, Rajan DK (2009) Society of Interventional Radiology position statement on percutaneous radiofrequency ablation for the treatment of liver tumors. J Vasc Interv Radiol 20(7 Suppl):S342–S347
Crocetti L, de Baere T, Lencioni R (2010) Quality improvement guidelines for radiofrequency ablation of liver tumours. Cardiovasc Intervent Radiol 33(1):11–17
Jones RP, Kokudo N, Folprecht G et al (2016) Colorectal liver metastases: a critical review of state of the art. Liver Cancer 6(1):66–71
Minami Y, Kudo M (2013) Radiofrequency ablation of liver metastases from colorectal cancer: a literature review. Gut Liver 7(1):1–6
Ahmed M, Solbiati L, Brace CL et al (2014) Image-guided tumor ablation: standardization of terminology and reporting criteria—a 10-year update. Radiology 273(1):241–260
Stang A, Fischbach R, Teichmann W, Bokemeyer C, Braumann D (2009) A systematic review on the clinical benefit and role of radiofrequency ablation as treatment of colorectal liver metastases. Eur J Cancer 45(10):1748–1756
Kudo M (2016) Immune checkpoint blockade in hepatocellular carcinoma: 2017 update. Liver Cancer 6(1):1–12
Okazaki T, Honjo T (2006) The PD-1-PD-L pathway in immunological tolerance. Trends Immunol 27(4):195–201
Alsaab HO, Sau S, Alzhrani R et al (2017) PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol 23(8):561
Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12(4):252–264
Dempke WCM, Fenchel K, Uciechowski P, Dale SP (2017) Second- and third-generation drugs for immuno-oncology treatment-the more the better? Eur J Cancer 74:55–72
Lee JW, Choi MH, Lee YJ et al (2017) Radiofrequency ablation for liver metastases in patients with gastric cancer as an alternative to hepatic resection. BMC Cancer 17(1):185
Shen S, Peng H, Wang Y et al (2018) Screening for immune-potentiating antigens from hepatocellular carcinoma patients after radiofrequency ablation by serum proteomic analysis. BMC Cancer 18(1):117
Shi L, Chen L, Wu C et al (2016) PD-1 blockade boosts radiofrequency ablation-elicited adaptive immune responses against tumor. Clin Cancer Res 22(5):1173–1184
Ruers T, Van Coevorden F, Punt CJ et al (2017) Local treatment of unresectable colorectal liver metastases: results of a randomized phase ii trial. J Natl Cancer Inst 109(9)
Fernández Moro C, Bozóky B, Gerling M (2018) Growth patterns of colorectal cancer liver metastases and their impact on prognosis: a systematic review. BMJ Open Gastroenterol 5(1):e000217. https://doi.org/10.1136/bmjgast-2018-000217
Chu KF, Dupuy DE (2014) Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer 14(3):199–208
Slovak R, Ludwig JM, Gettinger SN, Herbst RS, Kim HS (2017) Immuno-thermal ablations - boosting the anticancer immune response. J Immunother Cancer 5(1):78
Zerbini A, Pilli M, Penna A et al (2006) Radiofrequency thermal ablation of hepatocellular carcinoma liver nodules can activate and enhance tumor-specific T-cell responses. Cancer Res 66(2):1139–1146
Ito F, Ku AW, Bucsek MJ et al (2015) Immune adjuvant activity of pre-resectional radiofrequency ablation protects against local and systemic recurrence in aggressive murine colorectal cancer. PLoS One 10(11)
Obara K, Matsumoto N, Okamoto M et al (2008) Insufficient radiofrequency ablation therapy may induce further malignant transformation of hepatocellular carcinoma. Hepatol Int 2(1):116–123
Yoshida S, Kornek M, Ikenaga N et al (2013) Sublethal heat treatment promotes epithelial-mesenchymal transition and enhances the malignant potential of hepatocellular carcinoma. Hepatology 58(5):1667–1680
Yoshida N, Midorikawa Y, Kajiwara T et al (2013) Hepatocellular carcinoma with sarcomatoid change without anticancer therapies. Case Rep Gastroenterol 7(1):169–174
Papaioannou NE, Beniata OV, Vitsos P, Tsitsilonis O, Samara P (2016) Harnessing the immune system to improve cancer therapy. Ann Transl Med 4(14):261
Wang Y, Luo F, Yang J, Zhao C, Chu Y (2017) New chimeric antigen receptor design for solid tumors. Front Immunol 22(8):1934
Buchbinder EI, Desai A (2016) CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol 39(1):98–106
Ostrand-Rosenberg S, Horn LA, Haile ST (2014) The programmed death-1 immune-suppressive pathway: barrier to antitumor immunity. J Immunol 193(8):3835–3841
Kreamer KM (2014) Immune checkpoint blockade: a new paradigm in treating advanced cancer. J Adv Pract Oncol 5(6):418–431
Friedman D, Baird JR, Young KH et al (2017) Programmed cell death-1 blockade enhances response to stereotactic radiation in an orthotopic murine model of hepatocellular carcinoma. Hepatol Res 47(7):702–714
Du Y, Jin Y, Sun W, Fang J, Zheng J, Tian J (2018) Advances in molecular imaging of immune checkpoint targets in malignancies: current and future prospect. Eur Radiol. https://doi.org/10.1007/s00330-018-5814-3
Jenkins RW, Barbie DA, Flaherty KT (2018) Mechanisms of resistance to immune checkpoint inhibitors. Br J Cancer 118(1):9–16
Pitt JM, Vétizou M, Daillère R et al (2016) Resistance mechanisms to immune-checkpoint blockade in cancer: tumor-intrinsic and –extrinsic factors. Immunity 44(6):1255–1269
Widenmeyer M, Shebzukhov Y, Haen SP et al (2011) Analysis of tumor antigen-specific T cells and antibodies in cancer patients treated with radiofrequency ablation. Int J Cancer 128(11):2653–2662
Patel SA, Minn AJ (2018) Combination cancer therapy with immune checkpoint blockade: mechanisms and strategies. Immunity 48(3):417–433
den Brok MH, Sutmuller RP, Nierkens S et al (2006) Efficient loading of dendritic cells following cryo and radiofrequency ablation in combination with immune modulation induces anti-tumour immunity. Br J Cancer 95(7):896–905
Dovedi SJ, Adlard AL, Lipowska-Bhalla G et al (2014) Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res 74(19):5458–5468
Gettinger SN, Wurtz A, Goldberg SB et al (2018) Clinical features and management of acquired resistance to PD-1 axis inhibitors in 26 patients with advanced non-small cell lung cancer. J Thorac Oncol 13(6):831–839
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Prof. Masatoshi Kudo.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was not required for this study because it is a review of literature.
Ethical approval
Approval from the institutional animal care committee was not required because it is a review of literature.
Methodology
• Performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Minami, Y., Nishida, N. & Kudo, M. Radiofrequency ablation of liver metastasis: potential impact on immune checkpoint inhibitor therapy. Eur Radiol 29, 5045–5051 (2019). https://doi.org/10.1007/s00330-019-06189-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06189-6