Abstract
Purpose
To (i) devise a new semi-quantitative scoring system known as Early Rheumatoid Arthritis Magnetic Resonance Score (ERAMRS) to assess inflammation of the wrist on magnetic resonance imaging in early rheumatoid arthritis and to (ii) test ERAMRS and other MR scoring systems against everyday used clinical scorings.
Materials and methods
One hundred six treatment-naïve patients (81 females, 25 males, mean age 53 ± 12 years) with early rheumatoid arthritis (ERA) underwent clinical/serological testing as well as 3-T MRI examination of the most symptomatic wrist. Clinical assessment included Disease Activity Score-28 and Health Assessment Questionnaire; erythrocyte sedimentation rate and C-reactive protein were measured. MR imaging data was scored in all patients using three devised MR semi-quantitative scoring systems, namely, the (a) ERAMRS system, (b) Rheumatoid Arthritis Magnetic Resonance Imaging Score (RAMRIS) system, and the (c) McQueen Score system.
Results
Synovitis was present in 106 (100%), tenosynovitis in 98 (92%), and bone marrow edema in 84 (79%) of 106 ERA wrists. ERAMRS had the highest correlation with clinical disease activity scores (r = 0.476, p < 0.001) and serological parameters (r = 0.562, p < 0.001). RAMRIS system had the lowest correlation (r = 0.369, p < 0.001 for clinical disease activity; r = 0.436, p < 0.001 for serological parameters). RAMRIS synovitis subscore had a lower correlation than ERAMRS for clinical disease activity (r = 0.410, p < 0.001) and for serological parameters (r = 0.456, p < 0.001).
Conclusion
The ERAMRS system, designed to grade inflammation on wrist MRI in ERA, provided the best correlation with all clinical scoring systems and serological parameters, indicating its improved clinical relevance over other MR scoring systems.
Key Points
• We devised a clinically relevant, easy-to-use semi-quantitative scoring system for scoring inflammation on MRI of the wrist in patients with early rheumatoid arthritis.
• ERAMRS system showed better correlation with all clinical and serological assessment of inflammation in patients with early rheumatoid arthritis indicating its improved clinical relevance over other MR scoring systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The term “early rheumatoid arthritis” (ERA) is used when patient symptoms are present for less than 24 months [1, 2]. Synovitis in ERA most commonly involves the small joints of the wrist and hand [3] and, less frequently, the tenosynovium of the wrist [4, 5]. The main symptoms of ERA are joint pain, stiffness, swelling, and tenderness as well as functional impairment and constitutional symptoms [1, 6]. Several non-imaging-based clinical semi-quantitative scoring systems are used in everyday practice and research to incorporate the different clinical features and, in some cases, serological parameters (erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP)) into a single, clinically useable score that reflects patient disease activity.
Earlier diagnosis and treatment of ERA can slow down or prevent disease progression [2]. Other than soft tissue swelling and juxta-articular osteopenia, radiographs in ERA are usually normal with no visible erosions or joint space narrowing [7]. Ultrasound and magnetic resonance imaging (MRI) are the main imaging modalities used to assist diagnosis and management of ERA [7, 8]. Contrast-enhanced MRI has become an established tool for demonstrating inflammation in RA, allowing appreciation of all the inflammatory features of ERA, namely, synovial proliferation and enhancement, tenosynovial proliferation and enhancement, and osteitis, which manifest as bone marrow edema [9,10,11,12,13]. Even within the wrist, inflammation in RA is patchy in location and varied in type. Given this variability, it is helpful to have a scoring system that incorporates the different MR features of inflammation in different wrist areas into a single score that reflects overall disease activity. Two semi-quantitative MR-based scoring systems are currently used with Rheumatoid Arthritis Magnetic Resonance Imaging Score (RAMRIS) being the most widely used. The less frequently used system is the McQueen system. However, due to inclusion of some less relevant MR features and non-inclusion of other more relevant features, these two scoring systems currently employed are not ideally suited for use in ERA. We therefore devised a new, more inclusive, scoring system specifically for use in ERA, known as the Early Rheumatoid Arthritis Magnetic Resonance Imaging Score (ERAMRS). The clinical relevance of this new ERAMRS system and that of other MR scoring systems was gauged by seeing how well they correlated with clinical scoring systems.
Therefore, the aim of this study was to (i) present a new MR scoring system specifically for use in ERA, (ii) determine the usability and reliability of this ERAMRS system, and (iii) determine how well ERAMRS correlated with clinical scoring systems compared with other MR scoring systems.
Materials and methods
We analyzed the clinical, serological, and MRI data from a cross-sectional prospective study of treatment-naïve ERA patients recruited from October 2012 to January 2016. The study protocol was approved by the local Ethics Committee with signed informed consent being obtained from each patient.
Patients
All 106 patients (81 females, mean age 52 ± 13 years; 25 males, mean age 57 ± 9 years, p = 0.053) fulfilled the 2010 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) classification criteria for RA with symptom duration of less than 24 months at the time of recruitment. In addition to clinical assessment, ESR (mm/h) and CRP (mg/dl), both of which relate to the level of systemic inflammation, were routinely measured in all ERA patients [14].
Clinical scoring systems
Clinical assessment on each patient, performed by experienced rheumatologists, included the Disease Activity Score-28 (DAS28) and Health Assessment Questionnaire (HAQ). DAS28 is a clinician-assessed scoring system which takes into account the number of tender and swollen joints, physician- and patient-perceived disease activity (utilizing a visual analogue scale), and ESR/CRP level. HAQ is a patient-assessed perception of functional status (Table 1).
MR imaging protocol
Magnetic resonance imaging (MRI) of the most symptomatic wrist was performed in all patients. Patients were scanned in the prone position on a 3.0-T system (Achieva TX, Philips Healthcare) with a dedicated wrist coil to optimize signal reception. The MR protocol and scanning time for each sequence used are outlined in Table 2. The total scanning time is about 25 min including contrast injection (0.5 mmol/ml Dotarem Guerbet) at a volume of 0.01 mmol/kg using a rapid hand-held technique.
MR imaging analysis
A new MR scoring system known as the Early Rheumatoid Arthritis Magnetic Resonance Score (ERAMRS) was devised. This ERAMRS incorporated features of other systems pertinent to ERA as well as new features. All MR datasets were scored using both this ERAMRS system and the two previously devised MR scoring systems. All MRI scoring was undertaken by a single musculoskeletal radiologist with 7 years’ experience of analyzing wrist MRI examinations. Scoring was undertaken on a standard viewing workstation (PACS), blinded to other MR scores as well as blinded to clinical scores and serological data.
Early Rheumatoid Arthritis Magnetic Resonance Score system (Appendix 1 in Supplementary Material)
Synovial proliferation and enhancement were assessed on post-contrast T1-weighted axial fat–suppressed axial images. Similar to a previously devised MR scoring system, six joint areas were assessed in each wrist [15]. These six joint areas were the distal radioulnar joint, radiocarpal joint, intercarpal joint, first carpometacarpal joint, second to fifth carpometacarpal joints, and the pisotriquetral joint. Each of these joint areas was scored as 0, 1, 2, or 3 depending on whether the degree of synovial proliferation present was 0, mild, moderate, or severe. Mild, moderate, or severe synovial proliferation was defined by the degree (lower third, mid-third, or upper third) of the expected maximum synovial proliferation for that joint area based on experience and a previously devised MR imaging reference atlas [16, 17]. Maximum score for synovitis proliferation was 3 × 6 joints = 18. Synovial enhancement was also scored in all six joint areas as 1, 2, or 3 points according to whether the perceived average enhancement for that joint area was mild, moderate, or severe in degree relative to the expected maximum enhancement (Figs. 1, 2, 3, and 4). Maximum enhancement was similar to the adjacent vessels, i.e., very high signal intensity (Figs. 3 and 4). Minimal enhancement was similar to muscle (Fig. 1). Maximum score for synovial enhancement was 3 × 6 joints = 18.
T1-weighted axial fat–suppressed post-contrast MR image in a 41-year-old female with ERA showing mild synovial proliferation (score 1) of the intercarpal joint with mild enhancement of this synovium (score 1) (open arrows). The extensor carpi ulnaris tendon shows moderate tenosynovitis (score 2) with mild enhancement (score 1) (arrow)
T1-weighted axial fat–suppressed post-contrast MR image in a 53-year-old female with ERA showing moderate synovial proliferation (score 2) of the intercarpal joint with moderate enhancement of this synovium (score 2) (open arrows). Mild tenosynovitis of the flexor carpi radialis tendon (score 1) is present with moderate enhancement (score 2) (arrow)
T1-weighted axial fat–suppressed post-contrast MR image in a 61-year-old female with ERA showing severe synovial proliferation (score 3) of the intercarpal joint with moderate enhancement of this synovium (score 2) (open arrow). Moderate tenosynovitis of the flexor carpi radialis tendon (score 2) is present with severe enhancement (score 3) (arrow) while mild tenosynovitis of the flexor digitorum tendons (score 1) is present with moderate enhancement (score 2) (arrowhead)
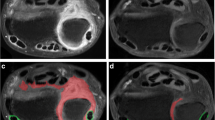
Tenosynovial proliferation of the 6 extensor tendon compartments and 3 flexor tendon areas was scored. These tendons were the extensor tendon compartments 1 to 6, as well as three flexor tendon areas, namely, the (i) flexor carpi radialis tendon, (ii) flexor digitorum superficialis tendons and flexor digitorum profundus tendons and (iii), flexor pollicis longus tendon. The flexor carpi ulnaris tendon was not included as it does not have a tendon sheath. Each tendon was scored as 0, 1, 2, and 3 according to whether tenosynovial proliferation (i.e., tendon sheath thickening) was absent, mild, moderate, or severe in degree. Tendon sheath thickening was measured from the outer border of the tendon to the outer border of the enhancing tendon sheath (Figs. 1, 2, 3, and 4). Tendon sheath thickening was assessed on images transverse and not oblique to the long axis on the tendon and was required to be apparent on more than one image to avoid confusion with a vessel. A tendon sheath thickness < 1 mm was defined as mild, ≥ 1 mm and < 2 mm as moderate, and ≥ 2 mm as severe. Maximum score for tenosynovial proliferation was 3 × 9 tendons = 27. Tenosynovial enhancement for each tendon group was also scored similar to synovial enhancement as described above. Maximum score for tenosynovial enhancement was 3 × 9 tendons = 27. Bone marrow edema was defined as an area within trabecular bone, with ill-defined margins and signal characteristics consistent with increased water content. Bone marrow edema was scored in 15 bone areas (distal 1 cm of radius and ulna, all eight carpal bones and the proximal 1 cm of the five metacarpal bones) on T2-weighted fat–suppressed coronal images as 0, 1, and 2 depending on the perceived percentage fraction of each bone or bone area involved by scrolling the coronal images from volar to dorsal. A bone marrow edema score of “0” for none, “1” for edema involving < 50% of the bone area, and “2” for edema involving > 50% of the bone area (Fig. 5). Maximum score for bone marrow edema was 2 × 15 bones = 30. If bone marrow edema was present in conjunction with erosions or unequivocal bone cysts, only the bone marrow edema component was scored according to the percentage of bone affected, ignoring the eroded or cystic component (Table 1).
Overall maximum ERAMRS score was 120 with 18 for synovial proliferation, 18 for synovial enhancement, 27 for tenosynovial proliferation, 27 for tenosynovial enhancement, and 30 for bone marrow edema. Each ERAMRS evaluation took about 5 min to complete.
Rheumatoid Arthritis Magnetic Resonance Image Score system [16, 17] (Appendix 2 in Supplementary Material)
Synovitis of the distal radioulnar joint, radiocarpal joint, and intercarpal-carpometacarpal joint areas was each scored along with bone erosions and bone marrow edema from 15 bones or bone areas. Meanwhile, tenosynovitis of 9 wrist tendon compartments and joint space narrowing at 17 wrist joints were also scored. Maximum RAMRIS score is 299 with 9 for synovitis, 45 for bone marrow edema, 27 for tenosynovitis, 150 for bone erosion, and 68 for joint space narrowing (Table 1).
McQueen Score [15] (Appendix 2 in Supplementary Material)
Synovitis of seven wrist joint areas, tenosynovitis of nine wrist tendon compartments, and bone marrow edema and bone erosion of 15 wrist bones were scored. Maximum McQueen Score is 124 with 14 for synovial proliferation, 14 for synovial enhancement, 9 for tenosynovitis, 9 for tendon inflammation, 18 for tendon size, 30 for bone marrow edema, and 30 for bone erosion (Table 1).
Inter- and intra-reader reliability of ERAMRS system
To reflect clinical and academic practice, three readers with various levels of experience in reading MRI examinations of the wrist tested reliability. The first was a musculoskeletal radiologist with 28 years’ MRI reporting experience (reader 1). The second was a musculoskeletal radiologist with 7 years’ MRI reporting experience (reader 2), and the third was a research assistant with 6 months’ experience of analyzing wrist MRI examinations (reader 3). The third, less experienced reader, underwent one full-day equivalent of direct teaching and testing on how to grade synovial/tenosynovial proliferation and enhancement as well as score bone marrow edema according to ERAMRS prior to analyzing the MR datasets. All readers independently scored 30 randomly selected patient image datasets blinded to the clinical and serological data. One week later, each reader re-scored these 30 cases again blinded to the results of the first reading.
Statistical analysis
All analyses were performed using SPSS. All data were expressed as mean ± standard deviation unless stated otherwise. The Mann-Whitney U test was used to test the means between two groups. Spearman’s correlation was used to test for correlation between MRI scoring systems and clinical or serological parameters. The following criteria were applied to these correlations: r > 0.8 excellent, 0.6–0.8 good, 0.4–0.6 moderate, 0.2–0.4 fair, < 0.2 poor. Multiple comparisons were adjusted by using Bonferroni’s correction. Correlations of different scoring systems with clinical or serological parameters were compared using t tests, with Bonferroni’s correction. Intra-class correlation (ICC) was used to evaluate the inter-reader and intra-reader reliability. The Bland-Altman plots were applied to compare between measurements. For all tests, a probability p level of < 0.05 was regarded as statistically significant.
Results
Prevalence of synovitis, tenosynovitis, and bone marrow edema
Synovitis was present in 106 (100%), tenosynovitis in 98 (92%), and bone marrow edema in 84 (79%) of the 106 ERA wrists at presentation. The extensor carpi ulnaris (57%), extensor carpi radialis longus, and brevis tendons (45%) were the most frequently affected tendons while the abductor pollicis longus and extensor pollicis brevis (14%) were the least affected in the 106 ERA wrists examined. ERA patients with tenosynovitis tended to have higher RAMRIS synovitis score (6.17 ± 2.37) than those without tenosynovitis (3.17 ± 2.57, p = 1.2 × 10−5).
Correlation between MR scoring systems and clinical scoring systems/serological parameters
The correlation between MR scoring systems and clinical scoring systems/serological parameters is shown in Table 3. ERAMRS showed the highest correlation with all clinical scoring systems and serology. The McQueen Score system and RAMRIS system had mild correlation with clinical scoring systems and serological parameters in ERA, lower than that of ERAMRS. When each subscore of RAMRIS was separately analyzed, the RAMRIS synovitis score yielded the highest correlation with clinical and serological parameters though the correlation achieved was still lower than that of ERAMRS (Table 3). The difference in correlation coefficient between ERAMRS and RAMRIS was significant for all clinical and serological parameters (HAQ: p = 0.013; DAS28: p = 0.015; ESR: p = 0.0002; and CRP: p = 0.002). This correlation coefficient difference was still significant for ESR (p = 0.005) and CRP (p = 0.023) when ERAMRS was compared with RAMRIS synovitis subscore.
Reliability of ERAMRS
For inter- and intra-reader reliability of ERAMRS, high single-measure inter- and intra-reader reliability of the ERAMRS system was found (Table 4) [15, 18].
Discussion
Detection and effective treatment during the early “window of opportunity” can ameliorate symptoms, improve quality of life, and prevent damage accrual in patients with RA [19,20,21]. Although ERA is a systemic disease, a single wrist is usually examined by MRI as this is a commonly affected joint and disease activity at the wrist is reflective of systemic disease activity [15, 22, 23].
Clinical parameters are used as a good standard in ERA. However, when considering whether patients respond to treatment, not only clinical remission, but also imaging remission should be considered as it is a more important factor with respect to long-term development of structural damage. Studies have shown the importance of detecting subclinical inflammation and its prediction in further boney structural change using ultrasound and MRI [24,25,26,27]. Given the moderate correlation between MRI features of inflammation and clinical parameters, we can appreciate that patients with the same clinical symptoms can have a different degree of inflammation on MRI.
For the clinician deciding on whether to start or adjust treatment, the degree of inflammation present is of far greater importance than the degree of inherent structural damage, especially in ERA where structural damage is limited. Contrast-enhanced MRI is excellent in revealing all of the known inflammatory features of ERA. Joint space narrowing and erosions are additional features of ERA seen on MRI, though these are a consequence of the inflammatory process rather than a primary marker of inflammation per se. Erosion and joint space narrowing will not change with effective treatment while all the other inflammatory parameters will greatly improve [28]. Of the inflammatory features evident on MRI, synovial and tenosynovial inflammation is the best predictor of functional impairment [22, 23], bone marrow edema the best predictor of erosive change [29, 30], and synovial perfusion the most responsive to treatment change [31]. The rate of synovial enhancement correlates with the degree of synovial vascularity while the degree of enhancement correlates with the severity of synovial inflammation [32].
The variety and range of inflammatory changes seen on MRI in ERA emphasizes the need to have a workable semi-quantitative scoring system that can incorporate all these changes into a single score. Any MR-based scoring system should be easy to use, reliable, and clinically relevant [33]. Clinical relevance of the MR-based scoring system can be optimized by (a) incorporating the most clinically pertinent imaging inflammatory biomarkers into the scoring system and (b) ensuring that these imaging biomarkers are suitably weighted relative to their clinical relevance. Two currently employed semi-quantitative MRI scoring systems are not without limitations. The widely used RAMRIS system was not developed specifically for use in ERA. The overall RAMRIS score places undue emphasis on scoring erosions (maximum score 150) and joint space narrowing (maximum score 68) with relatively little emphasis on synovitis (maximum score 9) and tenosynovitis (maximum score 27). Subscores of RAMRIS can also be applied [17]. The “McQueen Score” scores erosions as well as changes in tendon signal intensity and caliber which are not direct features of active inflammation. To more fully encompass the range of inflammation-specific changes seen on MRI in ERA, we devised a new scoring system, known as ERAMRS, specifically designed for use in ERA.
ERAMRS incorporates all the relevant inflammatory parameters seen on MRI suitably weighted to reflect their perceived importance in ERA. Six different joint areas are evaluated as joint involvement in the wrist is commonly patchy in terms of severity in ERA. Both synovial/tenosynovial proliferation and synovial/tenosynovial enhancement are more heavily weighted than bone marrow edema as synovial inflammation is the hallmark of ERA and the earliest imaging feature seen. A relatively heavy weighting was intentionally afforded to tenosynovial inflammation to reflect the known association between tenosynovial inflammation and functional impairment [22, 23]. In practice, we found the ERAMRS system easy to apply and time efficient taking all three readers only 5 min to complete. Also, the reliability was found to be high comparing well with the reported reliability of other scoring systems [15, 18, 23].
ERAMRS correlated better with all clinical scoring systems and serological tests in ERA than other MR scoring systems. ERAMRS also correlated better with clinical symptoms than any of the RAMRIS subscores. Although ERAMRS fared better than other scoring systems, the level of correlation between clinical and MR scoring systems as well as serological tests was still only moderate to good. This modest correlation is not surprising as clinical scores reflect patient symptoms and general well-being while ERAMRS purely scores inflammatory change in the wrist.
This study has some limitations. First, we used correlation with clinical scoring systems and serology as the sole measure of clinical relevance of MR scoring systems. Other gauges of clinical relevance such as predicting disease progression and response to treatment were not evaluated in this cross-sectional study. Second, this was a single-center study. The value of any scoring can only be fully ascertained though independent testing by other centers. Third, as the number of male patients included in the study was low, we did not fully explore sex differences in MR-related inflammation and clinical activity scores. Lastly, we did not include a control group in this study. The average age of patients in our study was 53 ± 12 years. Low levels of joint synovitis and bone marrow edema are seen in patients without RA as a feature of osteoarthritis [34]. Inclusion of a control group would have allowed us to appreciate the degree of synovitis/bone marrow edema present in patients of comparable age and sex without ERA.
In conclusion, we developed a new system for scoring the inflammatory change seen on MRI in ERA. This new system, known as ERAMRS, is time efficient and reliable and correlated better with clinical scoring systems and serological markers of inflammation than other currently used MR scoring systems.
Abbreviations
- CRP:
-
C-reactive protein
- DAS28:
-
Disease Activity Score-28 Calculator
- ERA:
-
Early rheumatoid arthritis
- ERAMRS:
-
Early Rheumatoid Arthritis Magnetic Resonance Score
- ESR:
-
Erythrocyte sedimentation rate
- HAQ:
-
Health Assessment Questionnaire
- MRI:
-
Magnetic resonance imaging
- RAMRIS:
-
Rheumatoid Arthritis Magnetic Resonance Imaging Score
References
Scott DL (2007) Early rheumatoid arthritis. Br Med Bull 81-82:97–114
Raza K, Buckley CE, Salmon M, Buckley CD (2006) Treating very early rheumatoid arthritis. Best Pract Res Clin Rheumatol 20:849–863
Fleming A, Crown JM, Corbett M (1976) Incidence of joint involvement in early rheumatoid arthritis. Rheumatol Rehabil 15:92–96
McQueen F, Beckley V, Crabbe J, Robinson E, Yeoman S, Stewart N (2005) Magnetic resonance imaging evidence of tendinopathy in early rheumatoid arthritis predicts tendon rupture at six years. Arthritis Rheum 52:744–751
Navalho M, Resende C, Rodrigues AM et al (2012) Bilateral MR imaging of the hand and wrist in early and very early inflammatory arthritis: tenosynovitis is associated with progression to rheumatoid arthritis. Radiology 264:823–833
Suresh E (2004) Diagnosis of early rheumatoid arthritis: what the non-specialist needs to know. J R Soc Med 97:421–424
Rahmani M, Chegini H, Najafizadeh SR, Azimi M, Habibollahi P, Shakiba M (2010) Detection of bone erosion in early rheumatoid arthritis: ultrasonography and conventional radiography versus non-contrast magnetic resonance imaging. Clin Rheumatol 29:883–891
Sugimoto H, Takeda A, Masuyama J, Furuse M (1996) Early-stage rheumatoid arthritis: diagnostic accuracy of MR imaging. Radiology 198:185–192
Ostergaard M (1997) Different approaches to synovial membrane volume determination by magnetic resonance imaging: manual versus automated segmentation. Rheumatology 36:1166–1177
Chand AS, McHaffie A, Clarke AW et al (2011) Quantifying synovitis in rheumatoid arthritis using computer-assisted manual segmentation with 3 tesla MRI scanning. J Magn Reson Imaging 33:1106–1113
Yang H, Rivoire J, Hoppe M et al (2015) Computer-aided and manual quantifications of MRI synovitis, bone marrow edema-like lesions, erosion and cartilage loss in rheumatoid arthritis of the wrist. Skeletal Radiol 44:539–547
Crowley AR, Dong J, McHaffie A et al (2011) Measuring bone erosion and edema in rheumatoid arthritis: a comparison of manual segmentation and RAMRIS methods. J Magn Reson Imaging 33:364–371
Aizenberg E, Shamonin DP, Reijnierse M, van der Helm-van Mil AHM, Stoel BC (2018) Automatic quantification of tenosynovitis on MRI of the wrist in patients with early arthritis: a feasibility study. Eur Radiol. https://doi.org/10.1007/s00330-018-5807-2
Wolfe F (2009) The many myths of erythrocyte sedimentation rate and C-reactive protein. J Rheumatol 36:1568–1569
McQueen FM, Stewart N, Crabbe J et al (1998) Magnetic resonance imaging of the wrist in early rheumatoid arthritis reveals a high prevalence of erosions at four months after symptom onset. Ann Rheum Dis 57:350–356
Østergaard M, Edmonds J, McQueen F et al (2005) An introduction to the EULAR-OMERACT rheumatoid arthritis MRI reference image atlas. Ann Rheum Dis 64(Suppl 1):i3–i7
Østergaard M, Peterfy CG, Bird P et al (2017) The OMERACT rheumatoid arthritis magnetic resonance imaging (MRI) scoring system: updated recommendations by the OMERACT MRI in Arthritis Working Group. J Rheumatol 44:1706–1712
Haavardsholm EA, Ostergaard M, Ejbjerg BJ et al (2005) Reliability and sensitivity to change of the OMERACT rheumatoid arthritis magnetic resonance imaging score in a multireader, longitudinal setting. Arthritis Rheum 52:3860–3867
Heidari B (2011) Rheumatoid arthritis: early diagnosis and treatment outcomes. Casp J Intern Med 2:161–170
Davis JM, Matteson EL (2012) My treatment approach to rheumatoid arthritis. Mayo Clin Proc 87:659–673
Irvine S, Munro R, Porter D (1999) Early referral, diagnosis, and treatment of rheumatoid arthritis: evidence for changing medical practice. Ann Rheum Dis 58:510–513
Burgers LE, Nieuwenhuis WP, van Steenbergen HW et al (2016) Magnetic resonance imaging-detected inflammation is associated with functional disability in early arthritis—results of a cross-sectional study. Rheumatology 55:2167–2175
Glinatsi D, Baker JF, Hetland ML et al (2017) Magnetic resonance imaging assessed inflammation in the wrist is associated with patient-reported physical impairment, global assessment of disease activity and pain in early rheumatoid arthritis: longitudinal results from two randomized controlled trials. Ann Rheum Dis 76:1707–1715
Gul HL, Ferreira JF, Emery P (2015) Remission in rheumatoid arthritis: is it all the same? Expert Rev Clin Pharmacol 8:575–586
Gandjbakhch F, Conaghan PG, Ejbjerg B et al (2011) Synovitis and osteitis are very frequent in rheumatoid arthritis clinical remission: results from an MRI study of 294 patients in clinical remission or low disease activity state. J Rheumatol 38:2039–2044
Haavardsholm EA, Lie E, Lillegraven S (2012) Should modern imaging be part of remission criteria in rheumatoid arthritis? Best Pract Res Clin Rheumatol 26:767–785
Anandarajah A, Thiele R, Giampoli E et al (2014) Patients with rheumatoid arthritis in clinical remission manifest persistent joint inflammation on histology and imaging studies. J Rheumatol 41:2153–2160
Møller-Bisgaard S, Ejbjerg BJ, Eshed I et al (2017) Effect of a treat-to-target strategy based on methotrexate and intra-articular betamethasone with or without additional cyclosporin on MRI-assessed synovitis, osteitis, tenosynovitis, bone erosion, and joint space narrowing in early rheumatoid arthritis: results from a 2-year randomized double-blind placebo-controlled trial (CIMESTRA). Scand J Rheumatol 46:335–345
Gandjbakhch F, Foltz V, Mallet A, Bourgeois P, Fautrel B (2011) Bone marrow oedema predicts structural progression in a 1-year follow-up of 85 patients with RA in remission or with low disease activity with low-field MRI. Ann Rheum Dis 70:2159–2162
Nieuwenhuis WP, van Steenbergen HW, Stomp W et al (2016) The course of bone marrow edema in early undifferentiated arthritis and rheumatoid arthritis: a longitudinal magnetic resonance imaging study at bone level. Arthritis Rheumatol 68:1080–1088
Tam LS, Griffith JF, Yu AB, Li TK, Li EK (2007) Rapid improvement in rheumatoid arthritis patients on combination of methotrexate and infliximab: clinical and magnetic resonance imaging evaluation. Clin Rheumatol 26:941–946
Gaffney K, Cookson J, Blades S, Coumbe A, Blake D (1998) Quantitative assessment of the rheumatoid synovial microvascular bed by gadolinium-DTPA enhanced magnetic resonance imaging. Ann Rheum Dis 57:152–157
Griffith JF, Wang YX, Antonio GE et al (2007) Modified Pfirrmann grading system for lumbar intervertebral disc degeneration. Spine 32:E708–E712
Mangnus L, van Steenbergen HW, Reijnierse M, van der Helm-van Mil AH (2016) Magnetic resonance imaging-detected features of inflammation and erosions in symptom-free persons from the general population. Arthritis Rheumatol 68:2593–2602
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is James F. Griffith.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Jason Chi Shun Leung
The Chinese University of Hong Kong Jockey Club Centre for Osteoporosis Care and Control
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• prospective
• cross-sectional study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 41 kb)
Rights and permissions
About this article
Cite this article
Xiao, F., Griffith, J.F., Hilkens, A.L. et al. ERAMRS: a new MR scoring system for early rheumatoid arthritis of the wrist. Eur Radiol 29, 5646–5654 (2019). https://doi.org/10.1007/s00330-019-06060-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06060-8