Abstract
Objectives
To correlate the degree of ascites enhancement during hepatobiliary phase after gadobenate dimeglumine (Gd-BOPTA) administration with ascites aetiology.
Methods
IRB-approved retrospective study, need for informed consent was waived. We included 74 consecutive ascitic patients who underwent Gd-BOPTA-enhanced liver MRI including hepatobiliary phase (HBP) images between January 2014 and December 2017. Ascites appearance on unenhanced and HBP images was classified as hypo-, iso- or hyperintense in comparison to paraspinal muscles. Ascites signal intensity on unenhanced and HBP images was measured using round ROIs and was normalised to paraspinal muscles (NSI). Normalised relative enhancement (NRE) between native phase and HBP was calculated. The results were related to ascites aetiology using Wilcoxon and Mann-Whitney tests.
Results
On native images, ascites appeared hypointense in 95.9% of the cases and isointense in 4.1%, whereas on HBP images, it appeared hyperintense in 59.4% of the cases, isointense in 36.5% and hypointense in 4.1%. Mean ascites NSI was 0.52 on unenhanced images and 1.50 on HBP ones (p < 0.0001). Mean ascites NRE was 201 ± 133%. Ascites of non-malignant aetiology showed mean NRE of 210 ± 134%, whereas malignant ascites showed mean NRE of 92 ± 20% (p = 0.001). ROC analysis showed that a NRE < 112.5% correlates with malignant aetiology with 100% sensitivity and 83.4% specificity (LR = 5.667). NRE did not show any significant correlation with ascites thickness, eGFR and time interval between contrast administration and HBP acquisition (p > 0.05).
Conclusions
Ascites NRE in HBP after Gd-BOPTA administration is significantly lower in patients with ascites secondary to peritoneal carcinomatosis than in patients with non-malignant ascites.
Key Points
• Ascites enhancement in the hepatobiliary phase after Gd-BOPTA administration may determine false positive findings when looking for biliary leaks.
• Ascites enhancement in the hepatobiliary phase after Gd-BOPTA administration is lower in patients with peritoneal carcinomatosis than in patients with portal hypertension or congestive heart failure.
• None of the patients with peritoneal carcinomatosis showed an ascites enhancement of more than 112% as compared with unenhanced images.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gadobenate dimeglumine (Gd-BOPTA; Multihance, Bracco Imaging) is an ionic linear gadolinium chelate characterised by mixed elimination: about 95% of the injected dose is excreted through the renal pathway, whereas about 5% is excreted through the biliary pathway, with a compensatory biliary or urinary excretion in cases of impaired renal or hepatic function [1]. Thanks to its hepatocyte uptake and biliary excretion, Gd-BOPTA is particularly indicated for characterisation of liver lesions and for detection of biliary leakages [2,3,4,5,6,7].
Ascites may be the consequence of a wide range of different pathologies including portal hypertension in patients with liver cirrhosis, peritoneal carcinomatosis, inflammation/infection and congestive heart failure [8]. The mechanisms underlying ascites formation are different and still not completely understood. In portal hypertension and congestive heart failure, ascites formation is mainly secondary to an increase in hydrostatic pressure gradient between capillaries and peritoneal space, whereas in peritoneal carcinomatosis, ascites formation represents the consequence of decreased lymphatic absorption, increased capillary membrane surface and increased intraperitoneal protein concentration [9,10,11]. Ascites of unknown cause represents a diagnostic challenge; many laboratory and imaging features have been proposed in order to reach a definite diagnosis [12,13,14]; however, the aetiology of ascites cannot always be determined.
A recent paper by Ciolina and colleagues demonstrated that peritoneal and pleural effusions appear iso-hyperintense on HBP after administration of both the commercially available hepatobiliary contrast agents, namely gadoxetic acid (Gd-EOB-DTPA) and Gd-BOPTA [15]. This paper also found that the relative enhancement of peritoneal free fluid is higher in patients with chronic liver disease than in the others, suggesting that the pathophysiological mechanism underlying that condition may influence the degree of relative enhancement.
The aim of our study was to correlate the degree of relative enhancement of peritoneal effusions during hepatobiliary phase after Gd-BOPTA administration with their aetiology.
Materials and methods
Patient population
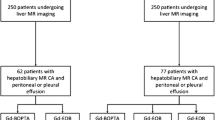
This is a retrospective study approved by our Institutional Review Board; need for informed consent was waived. We considered for inclusion 1478 consecutive adult patients who underwent contrast-enhanced liver MRI using Gd-BOPTA in two centres between January 2013 and December 2018. Inclusion criteria were as follows: presence of ascites on T2-weighted images with maximum perihepatic thickness ≥ 5 mm (174/1478 patients), availability of pre-contrast and hepatobiliary phase (HBP) GRE T1-weighted axial images without any imaging parameter change between the two phases (165/174 patients), and availability of clinical/imaging/laboratory/pathological data sufficient for clearly defining the cause of ascites (151/165 patients). Exclusion criteria were as follows: unknown contrast material administered dose (15/151 patients), recent (< 30 days) abdominal surgery (14/151 patients), suspected biliary leak (23/151 patients) or significant motion artefacts (25/151 patients). Therefore, our patient population included 74 patients, 54 males and 20 females, with a median age of 65 years (range 30–89 years).
Imaging protocol
MR examinations were performed on three different scanners (57/74 on a 1.5-T Ingenia, Philips Healthcare; 13/74 on a 1.5-T Magnetom Avanto, Siemens; and 4/74 on a 3-T Ingenia, Philips Healthcare) using multichannel phased array body coils. Imaging parameters of the evaluated GRE T1weighted sequences are reported in Table 1. A total of 0.05 mmol/kg of body weight (0.1 mL/kg) of Gd-BOPTA followed by a 20-mL saline flush was administered to each patient using a power injector. HBP images were acquired with a median delay of 113′ (range 60–214′) from contrast material administration.
Clinical-radiological data collection
Glomerular filtration rate was estimated from the most recent pre-MRI creatinine value (range 0–7 days before the examination) using the Cockroft-Gault formula. Total bilirubin values (range 1–23 days before the examination) were obtained. Clinical, radiological, laboratory and pathological reports of each patient were retrieved from the Institutional databases in order to define ascites aetiology. We considered ascites malignant if patient’s ascitic fluid cytological analysis detected malignant cells (5/74) and/or if peritoneal implants were recognizable at MRI (6/74), whereas we considered it non-malignant if patient’s ascitic fluid cytological analysis did not detect malignant cells (12/74) and/or if peritoneal implants were not recognizable at MRI (68/74). Moreover, we considered ascites secondary to portal hypertension if the patient showed liver cirrhosis with splenomegaly (bipolar diameter > 12 cm) and porto-systemic collaterals at MRI, whereas we considered it secondary to congestive heart failure if the patient underwent a positive cardiologic evaluation within 3 months from the MR examination (2/74 patients showed biventricular systolic dysfunction and 1/74 right ventricular systolic dysfunction).
Qualitative image analysis
Two radiologists with 15 and 9 years of experience in abdominal MRI, unaware of any clinical information, independently performed qualitative image analysis on dedicated workstations using commercially available PACS. Ascites appearance was assessed as hypointense, isointense or hyperintense in comparison to paraspinal muscles both on unenhanced and HBP T1-weighted images. The same evaluation was performed for pleural effusion, if present. Discrepancies were solved by consensus.
Quantitative image analysis
One radiologist with 3 years of experience in abdominal MRI performed quantitative image analysis on a dedicated workstation using commercially available PACS. Axial T2-weighted images, pre-contrast T1-weighted images and HBP T1-weighted images were manually aligned on the slice in which ascites showed the maximum perihepatic thickness. Maximum perihepatic ascites thickness was measured on axial T2-weighted images. Ascites signal intensity on both unenhanced and HBP axial T1-weighted images was calculated as the mean value of three adjacent round ROIs drawn in the site of maximum perihepatic ascites thickness (Figs. 1a, b and 2a, b). The ROIs were drawn as large as possible in order to include the maximum amount of ascites without including adjacent structures. The same evaluations were performed on pleural effusion, if present; in those cases, maximum fluid thickness was measured in the posterior costophrenic recess. Finally, right paraspinal muscle signal intensity was calculated as the mean value of three 2 cm2 round ROIs on both unenhanced and HBP T1-weighted images. Ascites and pleural effusion signal intensities in both unenhanced and hepatobiliary phases were normalised to the paraspinal muscle according to the formula NSI = SI ascites (or pleural effusion) ∕ SI paraspinal muscle. Paraspinal muscle was used as a reference as it is not expected to significantly change its signal intensity in the HBP. The normalised relative enhancement (NRE) for ascites and pleural effusion was calculated as follows: NRE (%) = (NSIpost − NSIpre) ∕ NSIpre × 100.
Seventy-year-old patient with peritoneal carcinomatosis and liver metastases secondary to advanced gastric cancer; eGFR 75 mL/min. Pre-contrast axial T1-weighted VIBE image (a) shows a discrete perihepatic liver effusion (asterisk) that appears hypointense to the paraspinal muscle (arrow). Hepatobiliary phase axial T1-weighted VIBE image (b) acquired 111 min after Gd-BOPTA injection shows an increase in the signal intensity of ascites, which has become isointense to paraspinal muscle. Three round ROIs were drawn in the site of maximum ascites thickness on both images. Normalised relative enhancement was 112%
Sixty-four-year-old patient with portal hypertension secondary to alcoholic liver cirrhosis; eGFR 64 mL/min. Pre-contrast axial T1-weighted THRIVE image (a) shows an abundant perihepatic liver effusion (asterisk) that appears hypointense to the paraspinal muscle (arrow); abundant left pleural effusion (triangle) coexists, also appearing hypointense to the paraspinal muscle. Hepatobiliary phase axial T1-weighted THRIVE image (b) acquired 153 min after Gd-BOPTA injection shows an increase in the signal intensity of both ascites and pleural effusion that have become hyperintense to the paraspinal muscle. Three round ROIs were drawn in the site of maximum ascites thickness on both images. Normalised relative enhancement was 198% for ascites and 121% for pleural effusion
Statistical analysis
Quantitative data are presented as mean (± SD) or median (range), categorical variables as number and percentages.
Mann-Whitney U test, Wilcoxon matched-pairs signed rank test, paired t test, Kruskal-Wallis test and ROC analysis were performed using GraphPad Prism version 6.00 for Mac OS X (GraphPad Software). p values were considered significant when ≤ 0.05.
Inter-observer agreement for qualitative parameters was assessed according to the k-statistics using GraphPad QuickCalcs (GraphPad Software). The strength of agreement was assessed as follows: < 0.20 poor, 0.21–0.40 fair, 0.41–0.60 moderate, 0.61–0.80 good, 0.81–1.0 excellent agreement.
Results
The cause of ascites was portal hypertension in chronic liver disease in 65/74 (87.8%) cases, peritoneal carcinomatosis in 6/74 (8.1%) cases and congestive heart failure in 3/74 (4.1%) cases. Patients showed a median estimated GFR of 91 mL/min (41–130) and a median total bilirubin value of 1.35 mg/dL (0.20–15.90); 37/74 (50%) patients showed impaired liver function (total bilirubin > 1.40 mg/dL). Mean total bilirubin value was significantly higher in patients with ascites secondary to portal hypertension (2.95 ± 4.19) than in patients with ascites secondary to other causes (0.96 ± 0.99) (p < 0.001). The median maximum perihepatic ascites thickness on axial T2-weighted images was 18 mm (range 6–61 mm). The mean time elapsed between contrast administration and HBP was 118 ± 34 min.
On native images, both readers defined ascites as hypointense in 71/74 (95.9%) of the cases (Figs. 1a and 2a) and as isointense in 3/74 (4.1%) (k = 1.000). On HBP images, reader 1 classified ascites as hypointense in 3/74 (4.1%) of the cases, as isointense in 28/74 (37.8%) and as hyperintense in 43/74 (58.1%), whereas reader 2 classified it as hypointense in 3/74 (4.1%) of the cases, as isointense in 26/74 (35.1%) and as hyperintense in 45/74 (60.8%) (k = 0.947). After consensus, ascites was considered hypointense in 3/74 (4.1%) cases, isointense in 27/74 (36.5%) (Fig. 1b) and hyperintense in 44/74 (59.4%) (Fig. 2b).
Mean ascites NSI was 0.52 ± 0.21 on pre-contrast images and 1.50 ± 0.48 on HBP ones (Wilcoxon test, p < 0.0001). Ascites of non-malignant aetiology showed mean NSI of 0.51 ± 0.17 on unenhanced images and of 1.50 ± 0.48 on HBP ones (Wilcoxon test, p < 0.0001), whereas malignant ascites showed mean NSI of 0.77 ± 0.38 on unenhanced images and of 1.43 ± 0.53 on HBP ones (Wilcoxon test, p = 0.0313). Mean ascites NSI on unenhanced images did not vary significantly between the different scanners (Kruskal-Wallis test, p > 0.05).
Mean NRE between unenhanced and HBP images was 201 ± 133%. In ascites of non-malignant aetiology, mean NRE was 210 ± 134%, whereas in malignant ascites, mean NRE was 92 ± 20% (Mann-Whitney test, p = 0.0010). Receiver operator curve (ROC) analysis (Fig. 3) showed that NRE has an area under the curve (AUC) of 0.88 (95% CI 0.80–0.96) for identifying patients with ascites secondary to peritoneal carcinomatosis. A NRE < 112.5% is correlated with malignant ascites with 100% sensitivity (95% CI 54.1–100%), 83.4% specificity (95% CI 71.2–90.5%) and likelihood ratio of 5.667. We did not find any significant correlation between NRE and maximum ascites thickness, eGFR, total bilirubin value and time interval between contrast administration and HBP acquisition (p > 0.05).
Pleural effusion was present in 19/74 (25.7%) patients and showed median maximum thickness of 14 mm (5–55 mm). On unenhanced images, both readers classified pleural effusion as hypointense in 18/19 (94.7%) of the cases (Fig. 2a) and as isointense in 1/19 (5.3%) (k = 1.000), whereas on HBP images, both readers classified it as isointense in 8/19 (42.1%) cases and as hyperintense in 11/19 (57.9%) (Fig. 2b) (k = 1.000).
Pleural effusion had a mean NSI of 0.59 ± 0.16 on pre-contrast images and of 1.36 ± 0.28 on HBP ones; pleural effusion showed a significantly higher NSI on HBP than on unenhanced images (paired t test, p < 0.0001). Mean pleural effusions’ NSI on unenhanced images was not significantly different between the different scanners (Kruskal-Wallis test, p > 0.05).
Mean NRE between unenhanced and HB phases was 143 ± 62%. No differences were found between pleural effusion’s NRE in patients with or without peritoneal carcinomatosis (p > 0.05). There was no correlation between NRE and maximum pleural effusion thickness, eGFR and time between contrast administration and HBP acquisition (p > 0.05).
Discussion
Our study demonstrated that abdominal and pleural effusions significantly increase their signal intensity in the hepatobiliary phase after Gd-BOPTA administration, thus appearing iso/hyperintense. The degree of relative enhancement was not correlated with the time elapsed between contrast administration and HBP acquisition, the estimated glomerular filtration rate or the maximum ascites (or pleural effusion) thickness. Normalised relative enhancement was significantly lower in ascites secondary to peritoneal carcinomatosis than in ascites due to other aetiologies (Fig. 4).
The fact that iodinated contrast agents can diffuse into peritoneal effusions is well known in CT literature since the early 1990s, but the underlying mechanism is not fully understood [16,17,18]. As previously speculated by Cooper, Benedetti and colleagues, contrast diffusion may be related to increased vascular permeability due to peritoneal disease or increased hydrostatic pressure, being the latter very common in portal hypertension and congestive heart failure [16, 17]. The difference in the degree of relative enhancement we found between ascites secondary to peritoneal carcinomatosis and ascites secondary to portal hypertension or congestive heart failure may be explained by the different pathophysiological mechanisms underlying their formation. In peritoneal carcinomatosis, besides an increase in peritoneal microvasculature and permeability, ascites is mainly due to a production of peptides by neoplastic cells, causing fluid exudation, whereas in chronic liver disease and congestive heart failure, ascites is due to a simple increase of hydrostatic pressure within peritoneal capillaries, leading to transudation. Therefore, fluid exchanges between blood and ascitic fluid are slower in peritoneal carcinomatosis than in portal hypertension or congestive heart failure, justifying lower contrast permeability in the first case. The results of our study have two main clinical consequences. First, the detection of ascitic fluid with low relative enhancement on HBP images obtained during liver MRI should raise the suspicion of peritoneal carcinomatosis. It is known that MRI with DWI has high sensitivity and specificity for peritoneal metastasis detection, with values ranging from 85 to 90% and from 88 to 91%, respectively, and an overall diagnostic accuracy of 81–85% [19, 20]. In this setting, the degree of relative enhancement can be used as an additional tool for confirming the suspicion of carcinomatosis when peritoneal nodules are visible or for suggesting additional investigations when peritoneal implants are not recognizable. The second main clinical implication lies in the interpretation of MRI scans performed in the suspicion of biliary leakages. In these cases, radiologists should be aware that ascites physiologically becomes iso- to hyperintense in HBP after Gd-BOPTA administration to avoid misinterpreting this phenomenon as a biliary leakage. To our best knowledge, only one paper in the literature focused on the relative enhancement of abdominal effusions after hepatobiliary contrast media administration. Ciolina et al recently found that ascites becomes iso- to hyperintense on HBP images in 88–100% of the cases after both Gd-BOPTA and Gd-EOB-DTPA administration, with a degree of enhancement that is greater in patients with chronic liver disease [15]. Our study confirms their results, as also in our series, ascites became iso/hyperintense in the large majority of the cases (95.9%) with a degree of enhancement that was significantly higher in patients with non-malignant ascites. Moreover, in our series, ROC analysis highlighted that normalised relative enhancement values < 112.5% are able to identify malignant ascites with 100% sensitivity and 83.4% specificity. Despite this, our results showed some differences from the findings of Ciolina and colleagues. First of all, in our series, no correlation was found between degree of ascites relative enhancement and free fluid volume. Differently from their work, in which the amount of ascites was determined qualitatively, we tried to quantify the amount of ascites by measuring its maximum thickness in the perihepatic space. Secondly, we did not observe any correlation between NRE and time elapsed from contrast administration. This may be explained by the fact that we included only patients who received Gd-BOPTA as contrast agent and, therefore, HBP images were acquired with a minimum delay of 60 min. Moreover, in our series, no correlation was observed between ascites NRE and eGFR. However, our series did not include any patient with severe renal insufficiency, being the minimum eGFR 41 mL/min.
Our study has some limitations, mainly due to its retrospective design that limited the number of included patients. In particular, in our series, the number of patients with ascites secondary to peritoneal carcinomatosis was quite low (6/74 patients, 8.1%). This is due to the fact that few patients with proven peritoneal carcinomatosis undergo liver MRI with Gd-BOPTA administration, as in these cases, CT is generally the modality of choice for primary staging and re-staging after chemo/radiotherapy. Moreover, no patients with peritoneal tuberculosis or other rare causes of ascites were included. Therefore, larger prospective studies are needed to confirm our data. Another potential limitation resides in the fact that the evaluated MR examinations were performed on three different scanners; this might lead to different signal intensity values. This limit has been partially overcome by normalising ascites and pleural effusion signal intensities to the paraspinal muscle, trying in this way to limit the effect of noise and field strength differences; moreover, mean ascites NSI on unenhanced images did not vary significantly between the different scanners. Lastly, in our study, a single reader performed quantitative image analysis; a double independent quantitative image analysis might be useful for confirming method reproducibility.
In conclusion, peritoneal and pleural effusions show a significant increase in their signal intensity on HBP after Gd-BOPTA administration. Ascites normalised relative enhancement on hepatobiliary phase images after Gd-BOPTA administration is significantly lower in patients with peritoneal carcinomatosis than in patients with ascites from other causes. According to our results, NRE < 112.5% has high sensitivity and specificity for identifying malignant ascites and might be used as an additional tool for characterising abdominal free fluid of unknown origin. Anyway, it must be kept into account that our patient population included only 6 patients with peritoneal carcinomatosis; therefore, our results must be confirmed on larger patient population, possibly in a prospective manner.
Abbreviations
- HBP:
-
Hepatobiliary phase
- NRE:
-
Normalised relative enhancement
- NSI:
-
Normalised signal intensity
References
Kirchin MA, Lorusso V, Pirovano G (2015) Compensatory biliary and urinary excretion of gadobenate ion after administration of gadobenate dimeglumine (MultiHance(®)) in cases of impaired hepatic or renal function: a mechanism that may aid in the prevention of nephrogenic systemic fibrosis? Br J Radiol 88:20140526
Fontarensky M, Montoriol PF, Buc E, Poincloux L, Petitcolin V, Da Ines D (2013) Advantages of gadobenate dimeglumine-enhanced MR cholangiography in the diagnosis of post-liver transplant bile leakage. Diagn Interv Imaging 94:443–452
Francisco FA, de Araújo AL, Oliveira Neto JA, Parente DB (2014) Hepatobiliary contrast agents: differential diagnosis of focal hepatic lesions, pitfalls and other indications. Radiol Bras 47:301–309
Frydrychowicz A, Lubner MG, Brown JJ et al (2012) Hepatobiliary MR imaging with gadolinium-based contrast agents. J Magn Reson Imaging 35:492–511
Melamud K, LeBedis CA, Anderson SW, Soto JA (2014) Biliary imaging: multimodality approach to imaging of biliary injuries and their complications. Radiographics 34:613–623
Scali EP, Walshe T, Tiwari HA, Harris AC, Chang SD (2017) A pictorial review of hepatobiliary magnetic resonance imaging with hepatocyte-specific contrast agents: uses, findings, and pitfalls of gadoxetate disodium and gadobenate dimeglumine. Can Assoc Radiol J 68:293–307
Seale MK, Catalano OA, Saini S, Hahn PF, Sahani DV (2009) Hepatobiliary-specific MR contrast agents: role in imaging the liver and biliary tree. Radiographics 29:1725–1748
Huang LL, Xia HH, Zhu SL (2014) Ascitic fluid analysis in the differential diagnosis of ascites: focus on cirrhotic ascites. J Clin Transl Hepatol 2:58–64
Cárdenas A, Bataller R, Arroyo V (2000) Mechanisms of ascites formation. Clin Liver Dis 4:447–465
Hou W, Sanyal AJ (2009) Ascites: diagnosis and management. Med Clin North Am 93:801–817
Tamsma JT, Keizer HJ, Meinders AE (2001) Pathogenesis of malignant ascites: Starling’s law of capillary hemodynamics revisited. Ann Oncol 12:1353–1357
Abdel-Razik A, Mousa N, Elalfy H et al (2017) A novel combination of C-reactive protein and vascular endothelial growth factor in differential diagnosis of ascites. J Gastrointest Cancer 48:50–57
Risson JR, Macovei I, Loock M, Paquette B, Martin M, Delabrousse E (2012) Cirrhotic and malignant ascites: differential CT diagnosis. Diagn Interv Imaging 93:365–370
Brogna A, Bucceri AM, Catalano F, Ferrara R, Leocata V (1996) Ultrasound demonstration of gallbladder wall thickening as a method to differentiate cirrhotic ascites from other ascites. Invest Radiol 31:80–83
Ciolina M, Di Martino M, Bruno O, Pommier R, Vilgrain V, Ronot M (2018) Peritoneal and pleural fluids may appear hyperintense on hepatobiliary phase using hepatobiliary MR contrast agents. Eur Radiol 7:3020–3031
Cooper C, Silverman PM, Davros WJ, Zeman RK (1993) Delayed contrast enhancement of ascitic fluid on CT: frequency and significance. AJR Am J Roentgenol 161:787–790
Benedetti N, Aslam R, Wang ZJ et al (2009) Delayed enhancement of ascites after i.v. contrast material administration at CT: time course and clinical correlation. AJR Am J Roentgenol 193:732–737
Wise SW, DeMeo JH, Austin RF (1996) Enhancing ascites: an aid to CT diagnosis. Abdom Imaging 21:67–68
Bozkurt M, Doganay S, Kantarci M et al (2011) Comparison of peritoneal tumor imaging using conventional MR imaging and diffusion-weighted MR imaging with different b values. Eur J Radiol 80:224–228
Low RN, Sebrechts CP, Barone RM, Muller W (2009) Diffusion-weighted MRI of peritoneal tumors: comparison with conventional MRI and surgical and histopathologic findings--a feasibility study. AJR Am J Roentgenol 193:461–470
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Matteo Bonatti.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors (Dr. Fabio Lombardo) has significant statistical expertise. No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• observational
• multicenter study
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bonatti, M., Valletta, R., Zamboni, G.A. et al. Ascites relative enhancement during hepatobiliary phase after Gd-BOPTA administration: a new promising tool for characterising abdominal free fluid of unknown origin. Eur Radiol 29, 2830–2836 (2019). https://doi.org/10.1007/s00330-018-5932-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5932-y