Abstract
Objectives
Bing–Neel syndrome (BNS) is a rare neurological complication of Waldenström’s macroglobulinemia. The aim of this study is to describe the spectrum of radiological manifestations of this syndrome and their prevalence in order to facilitate its early diagnosis.
Methods
Twenty-four patients with BNS were diagnosed between 1994 and 2016 in eight centres in France. We retrospectively examined the medical records of these patients as well as the corresponding literature, focusing on imaging studies. Recorded data were statistically analysed and radiological findings described.
Results
The mean age of our patients was 62.4 years (35–80 years). The vast majority of patients were men, with a male to female ratio of 9:1. Findings included parenchymal or meningeal involvement or both. The most common finding was leptomeningeal infiltration, either intracranial or spinal, with a prevalence reaching 70.8%. Dural involvement was present in 37.5% of patients. In 41.7% (10/24) of patients, there was parenchymal involvement with a higher prevalence of brain comparing to medullar involvement (33.3% and 23.1% respectively). High T2 signal of the parenchyma was identified in 41.7% of patients and high signal in diffusion was evident in 25% of them. Intraorbital or periorbital involvement was also detected in four cases. A proposition regarding the appropriate imaging protocol completed our study.
Conclusion
BNS’s diagnosis remains challenging. Central nervous system MRI findings in the setting of known or suspected Waldenström’s macroglobulinemia appear to be highly suggestive of BNS and appropriate imaging protocols should be implemented for their depiction.
Key Points
• Diagnosis of Bing–Neel syndrome (BNS) remains challenging and recent expert recommendations include MRI in the diagnostic criteria for the syndrome.
• The most common radiological manifestations of BNS are leptomeningeal/dural infiltration or parenchymal involvement of brain or spinal cord, but many atypical forms may exist with various presentations.
• Appropriate imaging protocol for BNS should include enhanced MRI studies of both brain and spine.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Waldenström’s macroglobulinemia (WM) is a rare lymphoproliferative disorder classified in the 2008 World Health Organization classification [1] as a lymphoplasmacytic lymphoma and characterised by bone marrow and lymphoid tissue B cell infiltration as well as the presence of IgM monoclonal gammopathy in the serum. It represents approximately 2% of monoclonal gammopathies, with neurological complications in about 25% of cases [2]. The commonest manifestations include anaemia and lymphoplasmacytic infiltration of the bone marrow, lymph nodes, spleen and rarely other organs. Neurological impairment in the setting of WM is most commonly related to IgM gammopathy (hyperviscosity syndrome or demyelinating peripheral neuropathy due to immunoglobulin M antibodies to neural antigens) [3]. Central nervous system (CNS) involvement by direct malignant cell infiltration in WM is a rare and probably underdiagnosed complication of WM referred to as Bing–Neel syndrome (BNS). It was named after two physicians, Jens Bing and Axel Valdemar Neel, who were the first to describe, as early as in 1936, two cases of women presenting with rapid neurodegeneration in the setting of hyperglobulinemia [4].
Lately, there is increasing interest among researchers in this extremely rare entity and an active effort to describe the clinical, biological and radiological criteria in order to improve the diagnosis of BNS as well as to define guidelines for its treatment options. In the literature there are several case reports and some small cohort studies available, as well as studies based on the review of several case reports cited in the literature [5,6,7,8,9,10], describing typical or atypical presentations of BNS. Two multi-institutional studies including 34 [11] and 44 [3] patients respectively, summarizing data on these patients’ clinical and haematological findings but with limited reference to their radiological findings, have also been published recently. An article proposing guidelines for the diagnosis and management of BNS has been the outcome of a task force on BNS, established during the 8th international workshop on WM held in London in 2014 [12]. Finally, there is a recent study by Guenette et al describing CNS magnetic resonance imaging (MRI) findings in patients with leukaemia and positive findings in CSF [13].
To our knowledge, there is to date no cohort study focusing on the radiological findings of BNS. In this article, we summarise the radiological manifestations of BNS and their prevalence, based on the findings of our cohort as well as on the available literature, in order to familiarise radiologists, neurologists and haematologists with this infrequent presentation of WM and thus facilitate its early detection and appropriate management.
Materials and methods
Patient selection
Between 1994 and 2016, 24 patients were identified for diagnosis and treatment of BNS at eight referral hospitals in France. Following approval from the ethics committee of our hospital, we retrospectively examined the medical records of the patients to collect any useful demographic, clinical or radiological information available. Informed written consent was waived by the institutional review board. Twelve of those patients were previously included in a study conducted by Simon et al [3], focusing on clinical, haematological criteria and appropriate treatment of BNS with only little reference to radiological manifestations.
The diagnosis of BNS was based on the criteria defined by the 2nd international workshop on WM [12]. On the basis of these criteria, the patients included in the study were adults who had a non-ambiguous cytological or histopathological documentation of CNS involvement by a lymphoplasmacytic proliferation, concomitant with a systemic WM disease. Exclusion criteria included (a) patients with a diagnosis of aggressive B cell lymphoma resulting from the transformation of WM, (b) patients whose neurological symptoms were not related to a direct infiltration of CNS by lymphoplasmacytic cells, but most commonly related to hyperviscosity syndrome or IgM-related peripheral neuropathy and (c) patients with no MRI available to study (two female patients of 73 and 48 years respectively whose diagnostic work-up was conducted in private institutions, as well as one patient for whom only CT and PET-CT scans were available as a result of MRI safety issues with the patient).
Laboratory analysis
Biochemical and cytological analyses of the CSF, in search of abnormally high protein levels, lymphoplasmacytic cells and monoclonality, were performed in all patients to confirm diagnosis of BNS. Molecular biology findings—and in particular positive L265P mutation in the MYD88 gene of lymphoplasmacytic cells—were also in favour of BNS diagnosis in some cases. If the result of these investigations was inconclusive, a histopathological study of the brain or the spine was performed.
Imaging analysis
Radiological findings were based on the revision of MRI studies available at the moment of diagnosis and during follow-up.
The imaging protocol varied according to the technical availability of each institution, as well as the routine clinical practice of each centre or the neuroradiologist performing the study. Despite the lack of uniformity of the protocol applied, all MRI studies were performed in 1.5- or 3-Tesla devices and included standard sequences (T1, T2, fluid-attenuated inversion recovery (FLAIR) sequences for the brain and T1, T2 for the spine, as well as T1 sequences after gadolinium administration for both brain and spinal studies). For some of the brain studies, diffusion sequences were also available and their findings were also evaluated.
All images were independently analysed by two neuroradiologists, one from the referral institution and one from our department (each with at least 5 years of experience) in search of abnormal signal in T1, T2, FLAIR, diffusion (if available) and enhanced T1 images (looking for abnormal parenchymal or meningeal enhancement). Results were classified as follows: hypoattenuation in T1 sequence, hyperattenuation in T2/FLAIR sequences, abnormal signal in diffusion, localisation in the meninges (dural or leptomeningeal), intracranial, spinal or orbital involvement, as well as the specific localisation in the brain (subcortical, subependymal/periventricular or infiltration of the brainstem). In case of disagreement on the findings, a third neuroradiologist (with 15 years of experience) blinded to both results was used as a referral.
Statistical analysis
Demographic, clinical and radiological data were analysed statistically and continuous variables (quantitative data) were summarised by medians and ranges or standard deviation (SD), while qualitative data were analysed by frequency and percentage. The Open Source Epidemiologic Statistics for Public Health (OpenEpi, Version. www.OpenEpi.com, updated 2013/04/06) was used for this purpose.
Results
Following the application of the exclusion criteria, 24 patients presenting with biologically or histopathologically confirmed BNS were identified for our study. There were only two women (2/24 or 8.3%) among our study’s patients. The mean age was 62.4 years (range 35–80 years, SD = 11.1). Clinical manifestations at presentation were very heterogeneous but we managed to obtain this information for only 17 of the 24 patients. In 6 of 17 patients (35.3%) there was predominantly a sensitive or motor deficit of the limbs, most commonly the lower limbs. Four of the patients (23.5%) presented with an altered mental status (disorientation/confusion, memory loss or cognitive decline) as the main symptom, while in five patients there was gait ataxia among other symptoms (29.4%). In five patients (29.4%) a visual impairment or an optic ataxia was present as a result of leptomeningeal involvement of optic/oculomotor nerves or orbital involvement in the course of the disease, while in three cases seizures were present (17.6%).
All 24 patients in our cohort had radiological findings of BNS, either cerebral or spinal. The most common radiological finding was subarachnoid enhancement in T1 images of the brain or spine (or FLAIR images of the brain) after gadolinium enhancement (Figs. 1 and 2), compatible with leptomeningeal infiltration, either cerebral or spinal, with a prevalence reaching 70.8% (17 in 24 patients). Enhancement of spinal nerves, most commonly of the cauda equina (Fig. 2d), was present in 11 of 13 patients (84.6%) for whom there was a spinal MRI study available. Dural involvement was present in 37.5% (9/24) of the patients, demonstrated as a thickening and enhancement of brain or spinal dura, best evaluated in contrast-enhanced T1 or FLAIR images (Fig. 1a, b). In 41.7% (10/24) of the patients, there was parenchymal involvement with a higher prevalence of brain comparing to medullar involvement (33.3% or 8/24 patients with brain parenchymal involvement and 23.1% or 3/13 patients with medullar parenchymal involvement, while in one patient there was simultaneous brain and medullar parenchymal involvement). High T2 signal (Fig. 3a) of the parenchyma was identified in 41.7% of the patients (10/24 patients, among them 3 with medullar lesions). Among the 10 patients with high T2 signal lesions, there were 7 with associated low T1 signal (7/24 or 29.2%) (Fig. 3b), while in the three studies left (3/24 or 12.5%), there was no abnormality in T1 sequence. Nodular or ring-shaped enhancement, with or without surrounding oedema, was present in all cases with brain parenchymal involvement (Fig. 3c–e). Abnormal signal in diffusion (high signal compatible with vasogenic oedema in all cases; Fig. 3f, g) was evident in 25% (4 of 16 MRI studies including a diffusion sequence).
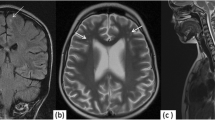
60-year-old male patient suffering from Waldenström’s macroglobulinemia, presenting with rapidly progressive dementia and peripheral neuropathy. a Axial FLAIR brain MR image acquired in a late phase (10–20 min) after contrast media administration at the level of frontal and parietal lobes, demonstrating leptomeningeal (arrows) and dural (asterisks) enhancement, consistent with lymphoplasmacytic cell leptomeningeal and dural infiltration. b Axial FLAIR brain MR image acquired in a late phase (10–20 min) after contrast media administration at the level of the brainstem, demonstrating enhancement of the basal cisterns (asterisk) and the tentoria (arrows), consistent with lymphoplasmacytic cell leptomeningeal and dural infiltration, confirmed in histopathologic studies of the brain (c, haematoxylin and eosin stain, ×100 magnification)
63-year-old male patient with unremarkable medical history presenting with sciatic nerve pain and gait ataxia. Bilateral thickening and enhancement of the Vth cranial nerve (arrows) in axial T1 image after gadolinium administration at the level of the brainstem is shown in a, while in b, a thickening of the cauda equina (arrow) in sagittal T2 unenhanced image is clearly demonstrated. c, d Cauda equina thickening and enhancement (arrows) in sagittal T1 image before and after gadolinium administration respectively at the level of lumbar spine on the same patient
54-year-old male patient with known history of Waldenström’s macroglobulinemia presenting with confusion, aphasia and behavioural changes. MR axial T2 image of the brain at the level of frontal and parietal lobes (a) demonstrates confluent subcortical and deep white matter lesions with high T2 signal in both prefrontal regions (asterisks). In sagittal unenhanced T1 image (b) at the level of the sylvian fissure, corresponding lesions present as low T1 attenuation areas (asterisk). Some of the lesions demonstrate punctuate enhancement (black arrows in subcortical localisation and white arrow in subependymal localisation) in coronal reconstruction of 3D T1 image after gadolinium administration in c. Axial MR images at the level of basal ganglia showing a small ring-shaped enhancing subcortical right occipital lesion (arrow), surrounded by vasogenic oedema, presenting as a low attenuation area in T1 image after gadolinium administration (d) and high attenuation area in FLAIR image (e), with no restriction of diffusion (as shown in diffusion image and corresponding map of average diffusion coefficient in f and g, respectively)
Localisation of brain parenchymal involvement was predominantly periventricular/subependymal (Fig. 3c) with a prevalence of 37.5% (3/8 patients). In 25% (or 2/8 of the patients), lesions were identified in subcortical regions (Fig. 3a–e); in another 25% (2/8) of the patients, lesions were localised in the brainstem, while in one patient (12.5%) there was diffuse infiltration involving all of the above regions. Intra- or periorbital involvement was also demonstrated in four cases (16%) (Fig. 4a–c). Radiological findings of our study are summarised in Table 1.
57-year-old male patient with ocular ataxia and visual impairment. Coronal T2 MR image showing low attenuation expansive intraorbital lesions surrounding both optic nerves (white arrows in a) and enhancing after contrast media administration in coronal (white arrows in b) and axial (white arrows in c) T1 images after gadolinium administration and fat suppression at the level of the orbits
A detailed review of the medical literature via the PubMed database provided a total of 48 articles, describing 59 case reports, 2 reviews of case reports of 36 and 43 patients and 2 large series of 34 and 44 patients, respectively. In Table 2, there is a detailed comparison of our findings with the findings of all cases and series found in the literature. During this review we observed some unusual presentations of BNS, presented as case reports; these presentations were frequently mimicking other more common conditions, making diagnosis challenging. Rigamonti et al described the case of a patient presenting with symptoms of cervical spinal cord compression by a pseudotumoural epidural infiltration, necessitating urgent surgical decompression [14]; other researchers have described isolated pseudotumoural forms of the cerebrum [15,16,17,18,19] or cerebellum [20,21,22]. Some cases of hydrocephalus [23, 24] or spinal cord hydromelia [25] have also been reported. Franzini et al described the case of a patient whose clinical and CT findings mimicked a bilateral subdural haematoma [26] and Ritzenthaler et al a case of ventriculitis [27]. Moreover, Morabito et al [28] and Pham et al [29] reported an intracranial venous thrombosis as a complication of BNS and Bhatti et al a bilateral sixth nerve transient palsy [30]. During this review we also observed a lack of standardisation in the radiological diagnostic approach of BNS and this motivated us to propose an imaging protocol in cases of suspected BNS. The protocol proposed by our team is summarised in Table 3.
Discussion
Our study demonstrated that clinical and imaging characteristics of the patients suffering from BNS can be very heterogeneous and mimic other more frequent conditions. The most common clinical manifestations were gait ataxia, motor or sensitive limb deficits and altered mental status. All MRI studies of our cohort had abnormal findings, including intra- or extra-axial lesions of the brain or the spine. These radiological findings were not specific, and clinical and laboratory correlation was necessary to establish the BNS diagnosis. The most frequent radiological manifestation was leptomeningeal infiltration (70.8%) depicted as an enhancement of subarachnoid spaces or cranial/spinal nerves, most frequently of the cauda equina, explaining the frequent presentation of limb deficit as the main symptom. This is consistent with previous histopathologic studies, which have revealed lymphoid cell infiltration of the meninges and along vessels with plasma cell accumulation in Virchow–Robin spaces [5, 31]. As a result, other common localisations of BNS infiltration of the brain were subependymal/periventricular regions, whereas subcortical regions or the brainstem were less commonly affected. Those lesions were visualised as high T2 signal in 41.7% of the patients, with or without gadolinium enhancement, which in some cases could coalesce and take the form of tumour-like lesions. Gadolinium uptake could be either nodular or ring-shaped. Another interesting finding was that parenchymal involvement was more commonly intracranial compared to spinal and that the preferential site for spinal involvement was leptomeningeal, affecting the spinal roots. We also noticed that parenchymal involvement could not be depicted in T1 unenhanced images in almost one-third of the cases and in three quarters of the cases in diffusion sequences. These findings are explained by the fact that malignant cell infiltration in the perivascular spaces or IgM toxicity [10] can cause damage to the blood–brain barrier, resulting in extravasation of plasma, which may present as hyperintensities on T2 and FLAIR images and as vasogenic oedema in diffusion, whereas at a later stage, demyelination, petechial haemorrhage and cellular degeneration [31] can appear as high T2/FLAIR signal, low T2 gradient echo (GRE) signal and contrast enhancement, respectively. Dural infiltration was another common manifestation, present at about one-third of our study population (37.5%).
Demographical characteristics of our study population were similar to the findings of the two large series reported by Castillo et al. [11] and Simon et al. [3]: there was clearly a male predominance (although more prominent in our study) and the mean age of patients at the time of diagnosis was similar. Radiological characteristics of our cohort were also consistent with other researchers’ findings [3, 10, 11, 32,33,34,35,36,37,38,39,40,41,42]. Orbital/periorbital involvement was found in four of our cohort patients and has been reported by several other researchers [3, 11, 43,44,45,46,47,48,49,50] as an infiltrative mass or optic neuritis, in addition to more frequent vaso-occlusive conditions related to hyperviscosity syndrome causing visual problems. On the other hand, Arias et al. [51] and Vargas et al. [52] have described multiple brain or medullar infarcts, for which a neoplastic vascular obstruction mechanism similar to that involved in malignant angioendotheliomatosis was proposed; these lesions were characterised by restricted diffusion contrary to the vasogenic oedema usually found in BNS [40, 52] and demonstrated in our cohort. In our study we have not found any atypical presentation similar to those cited in the literature as case reports [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30]. Finally, some researchers [3, 5, 38, 42] distinguish two forms of BNS: the diffuse form presenting as meningeal and perivascular involvement and the tumoural form presenting as expansive lesions. In our study we chose not to follow this classification, because the available literature was confusing on this topic and from our experience some lesions were difficult to classify in either of these forms, with coexistence in many cases of both forms.
Following the recommendations for imaging of CNS primary lymphoma, we believe that appropriate imaging of BNS should include contrast-enhanced brain and spine MRI studies, because of the contrast-enhanced MRI’s increased sensitivity to detect leptomeningeal disease [53]. Contrast-enhanced CT for the CNS evaluation is nowadays reserved only if an MRI study is contraindicated. The role of PET-CT has not yet been established, although some cases have been reported [54,55,56]. MRI should be performed prior to lumbar puncture in order to exclude focal mass effects and/or obstructive hydrocephalus as well as to avoid nonspecific meningeal enhancement that occurs after CSF sampling [53, 57]. In addition to standard MRI sequences (T2 and T1 before and after gadolinium injection for both brain and spine), we also recommend FLAIR for brain imaging following gadolinium injection in a late phase (10–20 min after injection), because of the superiority of enhanced FLAIR images regarding leptomeningeal enhancement compared to T1-enhanced images [58]. Diffusion sequences could be useful to characterise different forms of BNS complications such as vasogenic (most commonly) or cytotoxic (exceptionally) oedema, as well as to help differentiate between them and other conditions such as more aggressive CNS lymphomas or ischemia related to hyperviscosity syndrome; however, this differential diagnosis can be challenging, and correlation with other radiological signs as well as with clinical and biological data is always necessary. In order to detect haemorrhage as a complication of BNS, we also recommend to include T2 susceptibility-weighted imaging (SWI) or GRE sequences in the protocol. Specific sequences focused on the orbits can also be added in case of suspected orbital involvement. Total estimated scan time for both brain and spine is about 60–70 min, depending on the findings and patients’ compliance.
Our study carried certain limitations. A weakness of our cohort was a certain degree of missing data, which is common in this type of multi-institutional retrospective study; however, this was rare and mostly included clinical data of our patients. Neuroimaging for BNS in our cohort was not uniform, which is a reflection of the lack of standardisation in the diagnostic approach for BNS and represents another limitation of our study. All available studies, though, included basic sequences (T1, T2) and post-contrast T1 sequences for both brain and spine, as well as FLAIR images for brain. Finally, an additional limitation was the absence of spinal imaging for the entire cohort.
In conclusion, BNS is an extremely rare syndrome presenting with a wide range of symptoms and imaging findings, including intra-axial and extra-axial brain or spinal lesions, and therefore its diagnosis remains challenging. MRI was abnormal—though nonspecific—in our series. Therefore, it could be considered diagnostic of BNS with a great degree of confidence in the setting of known or suspected WM. Even though more prospective studies with larger cohorts are indicated in order to confirm our findings, we believe that familiarisation of radiologists with the imaging spectrum of this rare presentation of WM’s CNS involvement as well as implementation of appropriate imaging protocols is of great importance for an optimal evaluation of this group of patients, whose prognosis remains poor, although recent advances in the treatment at early stages of the disease seem to improve outcomes.
Abbreviations
- BNS:
-
Bing–Neel syndrome
- CNS:
-
Central nervous system
- CSF:
-
Cerebrospinal fluid
- CT:
-
Computed tomography
- FLAIR:
-
Fluid-attenuated inversion recovery
- GRE:
-
Gradient echo
- MRI:
-
Magnetic resonance imaging
- PET-CT:
-
Positron emission tomography–computed tomography
- SD:
-
Standard deviation
- SE:
-
Spin echo
- SWI:
-
Susceptibility-weighted imaging
- WM:
-
Waldenström’s macroglobulinemia
References
Swerdlow SH (2008) World Health Organization WHO classification of tumours of hematopoietic and lymphoid tissues, 4th edn, vol 2. International Agency for Research on Cancer, Lyon
Grewal JS, Brar PK, Sahijdak WM, Tworek JA, Chottiner EG (2009) Bing–Neel syndrome: a case report and systematic review of clinical manifestations, diagnosis, and treatment options. Clin Lymphoma Myeloma 9:462–466
Simon L, Fitsiori A, Lemal R et al (2015) Bing–Neel syndrome, a rare complication of Waldenström macroglobulinemia: analysis of 44 cases and review of the literature. A study on behalf of the French Innovative Leukemia Organization (FILO). Haematologica 100:1587–1594
Bing J, Neel AV (1936) Two cases of hyperglobulinaemia with affection of the central nervous system on a toxi-infectious basis. Acta Med Scand 88:492–506
Varettoni M, Defrancesco I, Diamanti L, Marchioni E, Farina LM, Pichiecchio A (2017) Bing–Neel syndrome: illustrative cases and comprehensive review of the literature. Mediterr J Hematol Infect Dis 9:e2017061
Vos JM, Kersten MJ, Kraan W et al (2016) Effective treatment of Bing–Neel syndrome with oral fludarabine: a case series of four consecutive patients. Br J Haematol 172:461–464
Boudin L, Romeo E, Mavrovi E, Tsitsi Nding P, Blade JS, de Jaureguiberry JP (2015) Bing–Neel syndrome: report of 4 cases and literature review. Rev Med Interne 36:418–422
Poulain S, Boyle EM, Roumier C et al (2014) MYD88 L265P mutation contributes to the diagnosis of Bing–Neel syndrome. Br J Haematol 167:506–513
Ly KI, Fintelmann F, Forghani R, Schaefer PW, Hochberg EP, Hochberg FH (2011) Novel diagnostic approaches in Bing–Neel syndrome. Clin Lymphoma Myeloma Leuk 11:180–183
Fintelmann F, Forghani R, Schaefer PW, Hochberg EP, Hochberg FH (2009) Bing–Neel syndrome revisited. Clin Lymphoma Myeloma 9:104–106
Castillo JJ, D'Sa S, Lunn MP et al (2016) Central nervous system involvement by Waldenström macroglobulinaemia (Bing–Neel syndrome): a multi-institutional retrospective study. Br J Haematol 172:709–715
Minnema MC, Kimby E, D'Sa S et al (2017) Guideline for the diagnosis, treatment and response criteria for Bing–Neel syndrome. Haematologica 102:43–45
Guenette JP, Tirumani SH, Keraliya AR, Shinagare AB, Ramaiya NH, Jagannathan JP (2016) MRI findings in patients with leukemia and positive CSF cytology: a single-institution 5-year experience. AJR Am J Roentgenol 207:1278–1282
Rigamonti A, Lauria G, Melzi P et al (2014) A case of Bing–Neel syndrome presenting as spinal cord compression. J Neurol Sci 346:345–347 8
Delgado J, Canales MA, Garcia B, Alvarez-Ferreira J, Garcia-Grande A, Hernandez-Navarro F (2002) Radiation therapy and combination of cladribine, cyclophosphamide, and prednisone as treatment of Bing–Neel syndrome: case report and review of the literature. Am J Hematol 69:127–131
Imai F, Fujisawa K, Kiya N et al (1995) Intracerebral infiltration by monoclonal plasmacytoid cells in Waldenström's macroglobulinemia-case report. Neurol Med Chir (Tokyo) 35:575–579
Civit T, Coulbois S, Baylac F, Taillandier L, Aqua J (1997) Waldenström's macroglobulinemia and cerebral lymphoplasmocytic proliferation: Bing and Neel syndrome. Apropos of a new case. Neurochirurgie 43:245–249
Fain O, Wechsler B, Vidailhet M, Raphael M, Schuller E, Godeau P (1992) Involvement of central nervous system disclosing Waldenström's disease: demonstration of intrathecal secretion of immunoglobulin M. Rev Med Interne 13:58–60
Quilichini R, Benderitter T, Lafeuillade A, Chaffanjon P, Aubert L (1989) Waldenström's macroglobulinemia with cerebral lymphoproliferative involvement of tumor appearance (Bing and Neel syndrome). An anatomo-clinical case. Ann Med Interne (Paris) 140:25–29
Tabouret E, Coso D, Matta M, Barrié M, Bouabdallah R, Chinot O (2014) Bing–Neel syndrome: a cerebral Richter syndrome? Neurol India 62:229–230
Rigual D, Qiu J, Fenstermaker RA, Fabiano AJ (2013) Tumoral Bing–Neel syndrome presenting as a cerebellar mass. Clin Neurol Neurosurg 115:823–826
Kikukawa Y, Yamamura-Fujimoto A, Endo S et al (2015) Successful treatment of Bing–Neel syndrome accompanying Waldenström's macroglobulinemia with R-MPV: a case report. J Clin Exp Hematop 55:113–119
Jammes T, Mann C, Delhom E et al (1990) Bing-Need syndrome revealed by normal pressure hydrocephalus. Unusual clinical/course of Waldenström disease. Presse Med 19:1992
Van Cauwenberge MG, Depreter B, Dumoulin EN, Emmerechts J, Nollet F, Vanopdenbosch LJ (2015) Bing–Neel syndrome: two unexpected cases and a review of the literature. J Neurol Sci 356:19–22
Artemiadis A, Terentiou A, Kolokythopoulos D, Triantafyllou N, Nikolaou G (2017) Bing–Neel syndrome. Arq Neuropsiquiatr 75:262
Franzini A, Gribaudi G, Pirola E et al (2017) Waldenström macroglobulinemia presenting as a bilaterous subdural hematoma. J Clin Neurosci 40:89–91
Ritzenthaler T, Leray V, Bourdin G et al (2013) Ventriculitis revealing Bing–Neel syndrome in a patient without Waldenström's macroglobulinemia. Clin Neurol Neurosurg 115:82–84
Morabito R, Grasso G, Barresi V, La Spina P, Garufi G, Alafaci E (2016) Intracranial venous sinus thrombosis as unusual presentation of Bing–Neel syndrome: case illustration. J Neurosurg 2:1–2
Pham C, Griffiths JD, Kam A, Hunn MK (2017) Bing–Neel syndrome – bilateral cavernous sinus lymphoma causing visual failure. J Clin Neurosci 45:134–135
Bhatti MT, Yuan C, Winter W, McSwain AS, Okun MS (2005) Bilateral sixth nerve paresis in the Bing–Neel syndrome. Neurology 64:576–577 9
Sánchez-Guerrero S, Castillo JJ (2015) Bing–Neel syndrome: a rare complication of Waldenström macroglobulinemia. Blood 126:1390
Abdallah AO, Atrash S, Muzaffar J et al (2013) Successful treatment of Bing–Neel syndrome using intrathecal chemotherapy and systemic combination chemotherapy followed by BEAM auto-transplant: a case report and review of literature. Clin Lymphoma Myeloma Leuk 13:502–506
Drouet T, Behin A, Psimaras D, Choquet S, Guillevin R, Hoang Xuan K (2010) Bing–Neel syndrome revealing Waldenström's macroglobulinemia. Rev Neurol (Paris) 166:66–75
Edgar R, Dutcher TF (1961) Histopathology of the Bing–Neel syndrome. Neurology 11:239–245
Cabannes-Hamy A, Lemal R, Goldwirt L et al (2016) Efficacy of ibrutinib in the treatment of Bing–Neel syndrome. Am J Hematol 91:E17–E19
Malkani RG, Tallman M, Gottardi-Littell N et al (2010) Bing–Neel syndrome: an illustrative case and a comprehensive review of the published literature. J Neurooncol 96:301–312
Drappatz J, Akar S, Fisher DC, Samuels MA, Kesari S (2008) Imaging of Bing–Neel syndrome. Neurology 70:1364
Crombe A, Alberti N, Menegon P, Desblache J, Frulio N, Tourdias T (2014) B type-Bing–Neel syndrome with MRI follow-up: a case report and review of its presentations. J Neuroradiol 41:362–365
Leschziner GD, Roncaroli F, Moss J, Guiloff RJ (2009) Nineteen-year follow-up of Waldenström's-associated neuropathy and Bing–Neel syndrome. Muscle Nerve 39:95–100
Kolbaske S, Grossmann A, Benecke R, Wittstock M (2009) Progressive gait ataxia and intention tremor in a case of Bing-Need syndrome. J Neurol 256:1366–1368
Kim HJ, Suh SI, Kim JH, Kim BJ (2009) Brain magnetic resolution imaging to diagnose Bing–Neel syndrome. J Korean Neurosurg Soc 46:588–591
Logothetis J, Silverstein P, Coe J (1960) Neurologic aspects of Waldenström’s macroglobulinemia; report of a case. Arch Neurol 3:564–573
Cuenca Hernández R, Guzman de Villoria Lebiedziejewski JA, Roa Martínez E, Menarguez Diaz J (2015) Bing–Neel syndrome as an initial sign of Waldenström macroglobulinaemia associated with orbital infiltration. Neurologia 30:252–255
Kumar S, Das S, Goyal JL, Chauhan D, Sangit V (2005) Bilateral orbital tumor formation and isolated facial palsy in Waldenström’s macroglobulinemia. Int Ophthalmol 26:235–237
Morita K, Yoshimi A, Masuda A, Ichikawa M, Yatomi Y, Kurokawa M (2013) Unique association of Waldenström macroglobulinemia with optic neuritis and monoclonal T cell expansion. Int J Hematol 98:247–249
Stacy RC, Jakobiec FA, Hochberg FH, Hochberg EP, Cestari DM (2010) Orbital involvement in Bing–Neel syndrome. J Neuroophthalmol 30:255–259
Doshi RR, Silkiss RZ, Imes RK (2011) Orbital involvement in Bing–Neel syndrome. J Neuroophthalmol 31:94–95
Hughes MS, Atkins EJ, Cestari DM, Stacy RC, Hochberg F (2014) Isolated optic nerve, chiasm, and tract involvement in Bing–Neel syndrome. J Neuroophthalmol 34:340–345
Jennane S, Doghmi K, Mahtat EM, Messaoudi N, Varet B, Mikdame M (2012) Bing and Neel syndrome. Case Rep Hematol 2012:845091
Bacquet JL, Weiss N, Meyniel C et al (2016) Neither the patient nor the physician could see anything: atypical Bing–Neel syndrome. Am J Hematol 91:858–859
Arias M, Pereiro Zabala I, Requena Caballero I, Sesar Ignacio A, Arias Rivas S, Villamayor Blanco B (2004) Rapidly progressing dementia as the presenting symptom of Waldenström's macroglobulinemia: findings from magnetic resonance imaging of the brain in Bing–Neel syndrome. Rev Neurol 38:640–642
Vargas A, Dixit KS, Quigley JG, Testai FD (2016) Spinal cord and cranial Bing–Neel syndrome complicated by cerebral ischemia: a case report. J Neurol Sci 366:44–46
Castillo JJ, Garcia-Sanz R, Hatjiharissi E et al (2016) Recommendations for the diagnosis and initial evaluation of patients with Waldenström macroglobulinemia: a task force from the 8th international workshop on Waldenström macroglobulinemia. Br J Haematol 175:77–86
Halperin D, Hallam S, Haroon A, Butler T, Agrawal S (2016) Bing–Neel syndrome case report: a previously undocumented IgG variant with MRI, PET/CT and PET/MRI imaging. Case Rep Hematol 2016:3931709
Donix M, Beuthien-Baumann B, von Kummer R, Gahn G, Thomas F, Holthoff V (2007) Nonfluent aphasia in a patient with Waldenström's macroglobulinemia. J Clin Neurosci 14:601–603
Mason C, Savona S, Rini JN et al (2017) Ibrutinib penetrates the blood brain barrier and shows efficacy in the therapy of Bing Neel syndrome. Br J Haematol 179:339–341
Lancellotti G, Cohen-Bittan J, Makdessi S et al (2014) Late-onset Bing–Neel syndrome associated with delirium and Lewy body dementia. J Am Geriatr Soc 62:2225–2227
Tsuchiya K, Katase S, Yoshino A, Hachiya J (2001) FLAIR MR imaging for diagnosing intracranial meningeal carcinomatosis. AJR Am J Roentgenol 176:1585–1588
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Prof. Stephane Kremer.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the institutional review board.
Ethical approval
Institutional review board approval was obtained.
Study subjects or cohorts overlap
Some study subjects (12 patients) have been previously reported in Haematologica by Simon et al.
Methodology
• retrospective
• multicentre study
Rights and permissions
About this article
Cite this article
Fitsiori, A., Fornecker, LM., Simon, L. et al. Imaging spectrum of Bing–Neel syndrome: how can a radiologist recognise this rare neurological complication of Waldenström’s macroglobulinemia?. Eur Radiol 29, 102–114 (2019). https://doi.org/10.1007/s00330-018-5543-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5543-7