Abstract
Objectives
Our goal was to investigate the correlation between histopathology and diffusion parameters by utilising the most repeatable region-of-interest (ROI) strategy for diffusion parameters in rectal cancer on a 3T scanner.
Methods
113 patients underwent DKI-MR and 66 of these patients received surgery without neoadjuvant chemoradiotherapy. Two readers independently measured the parameters using three slice protocols including single slice, three slices and whole-tumour slice (WTS), combined with one of two ROIs, including outline and round ROI. ANOVA, Kruskal-Wallis, a paired sample t-test, interclass correlation coefficient (ICC), Bland-Altman, Student’s t-tests, receiver operating characteristic curves and z statistic were used for statistical analysis.
Results
There were no significant differences among the three slice protocols in ADC values (p = 0.822, 0.987), K values (p = 0.842, 0.859) and D values (p = 0.917, 0.988) using round and outline ROI, respectively. The ADC and D values derived from outline ROIs were higher than those from round ROIs (all p < 0.001 for ADC, all p < 0.001 for D), while K values derived from outline ROIs were lower than those from round ROIs (p < 0.001, p = 0.001, p < 0.001) using three slice protocols, respectively. The WTS-outline ROI resulted in the best intra- and inter-observer ICC. Utilising the WTS-outline ROI method, the AUC for assessment of well-differentiated tumours was 0.871 by K and 0.809 by ADC; and the AUC for T2 was 0.768 by K.
Conclusions
The most repeatable strategy was the WTS-outline ROI method. In addition to DWI, DKI also have diagnostic value for rectal cancer histopathological characteristics utilising the WTS-outline ROI on a 3T scanner.
Key Points
• DKI using a 3T scanner is feasible for assessing rectal cancer.
• ROI and slice protocol show considerable influence on DKI parameters.
• DKI parameters exhibit excellent repeatability using whole-tumour slice-outline ROI on 3T scanner.
• DKI has considerable diagnostic value for the estimation of rectal cancer characteristics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Several studies have demonstrated that DKI not only performed better than conventional ADC values in the detection and grading of varies lesions, but also identified early microstructural changes in tissues and could assess treatment responses [1,2,3,4,5,6]. Zhu et al. [6] demonstrated that the kurtosis parameter from the DKI model yields a higher correlation with histological grades compared to the diffusivity and ADC of rectal adenocarcinomas using 1.5T scanner. However, it has been established that a single-shot EPI DWI sequence is very sensitive to susceptibility artifacts, which are more severe on 3T versus 1.5T systems, due to the imperfect B0 field homogeneity. The susceptibility artifact might reduce the performance of DKI in characterising rectal lesions. Thus, it is important and necessary to verify that the DKI model could have equal performances on the 1.5T and 3T systems.
The repeatability of DKI measurements in rectal cancer needs to be evaluated on a 3T scanner, because standardising the ROI is the first step in clinical application. However, some studies have shown that the size and the positioning of the region of interest (ROI) influenced the statistical results of DWI parameters and their inter-observer variability [7,8,9,10]. Lambregts et al. [7] reported that the most reproducible results were obtained when measuring the ADC of the whole-tumour volume for rectal cancer based on their study conducted at 1.5T. Compared with conventional DWI, the DKI sequence utilises higher diffusion gradients to implement higher b values (normally a maximum b value of > 2,000 mm2/s is required), which might result in more image distortions due to the eddy current caused by the high magnetic gradient pulse. Therefore, whether the whole-tumour volume strategy could help generate more reproducible results for DKI, especially at 3T, needs to be confirmed. Moreover, DKI-derived parameter Kurtosis and conventional ADC refer to different phenomena of the tumour microstructure [11,12,13]. It is necessary to explore the most repeatable ROI strategy for diffusion kurtosis imaging parameters in rectal cancer.
Thus, the objective of this study was to explore the influence of different ROI strategies on the measurement of diffusion parameters using a 3T scanner. We examined DKI and conventional DWI to access the correlation between these diffusion parameters from the most repeatable ROI strategy for identifying rectal cancer characteristics.
Materials and methods
Patients
Institutional review board approval was obtained for this retrospective study. 314 patients with rectal cancer who were diagnosed and treated at our hospital between 9 January 2016 and 21 January 2017 were selected for review in our study. Inclusion criteria were: (1) biopsy-proven rectal adenocarcinoma, (2) evaluation MRI with DKI and DWI sequence before treatment, and (3) the absence of any anti-tumour treatment. 149 patients who met the inclusion criteria were selected. Exclusion criteria were: (1) restaging MRI with DKI and DWI sequence after receiving neoadjuvant chemoradiotherapy (NCRT) (n=24), (2) poor signal-to-noise ratio (SNR) and severe bowel motion artifacts (n=8), (3) no identified tumour signal on ADC, K or D map (n=2), and (4) a postoperative pathology-proven mucinous tumour (n = 2). In total, 113 patients (64 male, 49 female) with a median age of 59 years (range: 27–86 years) were enrolled to explore the most repeatable tumour strategy on DKI in the first section. A subgroup of the patients was selected for the second section using the following selection criteria: (1) underwent curative surgery alone, (2) the pathological reports were available, and (3) the time interval between MRI and surgery was less than 2 weeks. Finally, there were 66 patients (41 male, 25 female), with a median age of 61 years (range: 30–83 years) in the second section. Clinical and imaging data were retrieved from a patient database. The workflow is displayed in Fig. 1.
MRI technique
All MRIs were performed on a 3T MR scanner (MAGNETOM Skyra, Siemens Healthineers) with a 16-channel phase-array body coil. The MRI protocol mainly included a sagittal T2-weighted TSE (turbo spin echo), an oblique axial thin-section T2-weighted TSE, and an oblique axial multi-b EPI (single-shot echo-planar imaging) DWI sequence. The multi-b EPI DWI sequence was performed using the following parameters: orientation = oblique axial; b values = 0, 700, 1,400 and 2,100 s/mm2; repetition time (TR)/echo time (TE) = 4,800/79 ms; parallel imaging factor = 2; fat suppression = spectral adiabatic inversion recovery; field of view (FOV) = 240×180 mm2; matrix = 120×120; section thickness = 5.5 mm; number of sections = 20; no. of average = 1, 2, 4 and 6 (for 0, 700, 1,400 and 2,100 s/mm2 respectively); Bandwidth = 1,894 Hz/pixel. Patients did not receive any bowel preparation before the MRI examinations.
The parameter maps of DKI (corrected diffusion coefficient, D; diffusion kurtosis coefficient, K) and conventional DWI (apparent diffusion coefficient, ADC) were calculated from the multi-b DWI data with all measured b values by using prototype post-processing software Body Diffusion Toolbox (Siemens Healthineers). The multi-b DW images were fitted voxel-by-voxel using the DK signal decay equation:
In this equation, S(b) is the signal intensity at a certain b-value, S0 is the baseline signal without diffusion weighting, D is the corrected diffusion coefficient (unit: 10-3mm2/s), and K is the excess diffusion kurtosis coefficient. K describes the degree to which molecular motion deviates from the perfect Gaussian distribution. When K is equal to 0, equation (1) degrades to a conventional monoexponential equation:
The difference between D and ADC is that D is a corrected form of ADC (unit: 10-3mm2/s) for use in non-Gaussian circumstances.
Image analysis
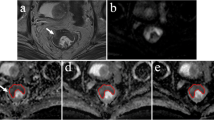
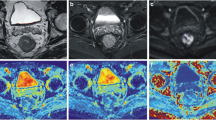
Two radiologists (TT and SYQ, with 10 and 4 years of experience in rectal cancer imaging, respectively), blind to the clinical patient data and pathological report, drew ROIs on axial ADC maps independently. The tumour was defined as a focal mass showing low signal intensity on an ADC map compared with the signal from the adjacent tissue and exhibited high signal intensity on DWI and intermediate signal intensity on the anatomical T2-weighted images (T2WI). Each radiologist performed the measurements twice at 1-month intervals.
Two different ROIs were positioned on the slice (Fig. 2): (1) round ROI: a round ROI was manually placed within solid tumour parts in the largest possible area and (2) outline ROI: a freehand ROI was drawn along the border of the lower signal of the tumour to cover the entire tumour area on each slice. Both round and outline ROIs excluded visible necrosis, the intestinal lumen and artifacts. The number of slices with ROI placement was determined by three protocols: (1) single slice (SS): one slice with the largest area of tumour, (2) three slices (TS): containing (a) and its adjacent upper and lower slice, and (3) whole-tumour slice (WTS): all slices of the tumour. ROIs were copied to K and D maps automatically by the software. Mean ADC, D and K values were obtained for each reader in each case.
Histopathology evaluation
A gastrointestinal pathologist with 8 years of experience, who was unaware of other clinical or molecular data, reviewed haematoxylin- and eosin-stained tissue sections from all rectal cancer cases. The histopathology factors included histological grade, T category, N category, circumferential resection margin (CRM), perineural invasion and lymphovascular invasion (LVI). The tumour category was determined according to the TNM classification system recommended by the International Union against Cancer and the American Joint Committee on Cancer (AJCC), 7th ed., 2010 [14].
Statistical analysis
All continuous variables as diffusion parameters and ROI are average values among readers. The Kolmogorov–Smirnov test was used to ascertain the distribution of continuous numeric data. Moreover, the distribution of measurement in all methods and based on histopathological factors was normal, except for the measurable data of T1, N2 and CRM (positive) not normally distributed. The data were provided as the mean ± standard deviation (SD) with normal distribution. The median value and interquartile range (IQR) were described with no normal distribution. The ANOVA test was used to compare the diffusion parameters of the three different slice protocols. A paired sample t-test was used to compare round and outline ROI diffusion parameters.
The first and second measurements of radiologist SYQ were used to analyse the intra-observer variability. The first measurements of radiologist SYQ and radiologist TT were used to analyse the inter-observer variability. The intra- and inter-observer variability for the ADC, K and D values were evaluated using the intraclass correlation coefficient (0.00–0.20 poor, 0.21–0.40 fair, 0.41–0.60 moderate, 0.61–0.80 good and 0.81–1.00 excellent correlation). In addition, Bland-Altman plots with limits of intra- and inter-reader agreement (mean difference ± 1.96 × SD) were used for graphical representation of the data. The most repeatable strategy was selected based on intra-observer and inter-observer variability. The averaged ADC, K and D values of two radiologists were used for the next part of this study.
Student’s t-tests (independent-samples t-test) were used to assess the differences of diffusion parameters based on histological grade, PNI and LVI. Kruskal-Wallis tests were used to assess differences in diffusion parameters based on T, N and CRM categories. The sensitivity, specificity and area under the curve (AUC) of the receiver operating characteristic (ROC) for evaluation of histopathological factors were also calculated. The pairwise comparison of ROC curves between the DKI and conventional DWI models was performed using z statistic. Cut-off values were established by calculating the maximal Youden index: Youden index = sensitivity - (1–specificity).
Statistical analysis was performed using SPSS (version 21.0; SPSS) and MedCalc (version 12.7.2; MedCalc Software). P values < 0.05 were considered statistically significance.
Results
Patient characteristics
A total of 113 patients were analysed in the present study. The study population was mainly male (56.6 %) with a median age of 59 years (range 27–86 years). In all, there were 47 (41.6%) patients who received NCRT followed by surgery and 66 (58.4%) patients who received curative surgery alone. The patient characteristics are summarised in Table 1.
The mean and SD of the thickness and the size of the ROIs
The mean thickness of the round and outline ROI was 40.5 ± 12.4 mm and 45.0 ± 12.6 mm using WTS, respectively. The mean size of the round ROI was 302.0 ± 106.1 mm2, 223.2 ± 79.9 mm2 and 177.5 ± 87.9 mm2 in the single slice, three slices and WTS methods, respectively. The mean size of the outline ROI was 591.7 ± 192.1 mm2, 493.4 ± 179.5 mm2 and 371.6 ± 177.5 mm2 for the single slice, three slices and WTS methods, respectively.
Effect of slice protocols on ADC, K and D values
The statistics were based on diffusion parameters, which were the average of two readers’ measurements. The diffusion parameters using different slice protocols are shown in Table 2. In analysing the whole study population, there were no significant differences in the ADCs derived from different slice protocols (p = 0.822, 0.987 using round and outline ROI, respectively), and the same results were observed for K (p = 0.842, 0.859 using round and outline ROI, respectively) and D (p = 0.917, 0.988 using round and outline ROI, respectively) values. Therefore, the slice protocols were not significantly influenced by ADC, K and D values.
Effect of ROI on ADC, K and D values
Similarly, the statistical diffusion parameters were the average of two readers’ measurements. The diffusion parameters using different ROIs are shown in Table 3 Tumour ADC values from outline ROIs were significantly higher than those from round ROIs (p < 0.001, using single slice, three slices and WTS, respectively). Tumour D values from outline ROIs were also significantly higher than those from round ROIs (p < 0.001 using single slice, three slices and WTS, respectively). K values derived from the round ROIs were significantly higher than those from the outline ROIs (p < 0.001, =0.001, < 0.001 using single slice, three slices and WTS, respectively). Therefore, the ROI had considerable influence on the ADC, K and D values.
The intra- and inter-observer variability of ADC, K and D values for the different strategies
The intra- and inter-observer variability of ADC, K and D values for the different strategies are given in Table 4. On the whole, the intra- and inter-observer ICCs were excellent for the ADC (0.965, 0.825, respectively), K (0.909, 0.945, respectively) and D (0.902, 0.874, respectively) using WTS-outline ROI. For the remanding ROI protocols, the intra-observer and inter-observer variabilities were not better than for the WTS-outline ROI. Overall, the WTS-outline ROI strategy had the lowest limits of agreement (+0.05/-0.05, +0.11/-0.13 for ADC intra- and inter-observer, +0.08/-0.10, +0.12/-0.13 for K intra- and inter-observer and +0.22/-0.16, +0.24/-0.24 for D intra- and inter-observer respectively) among the six strategies. The above results showed the most repeatable strategy is the WTS-outline ROI. Figure 3 shows the Bland-Altman plots for the WTS-outline ROI measurements performed.
The correlation between histopathological characteristics of rectal cancer and diffusion parameters derived from the WTS-outline ROI
The diffusion parameters were used to explore the correlation with the histopathological characteristics of rectal cancer, and the average of the two readers’ measurements utilising the most repeatable strategy, namely the WTS-outline ROI method. The correlation between histopathological characteristics and diffusion parameters is shown in Table 5. The ADC of poorly to moderately differentiated tumours (0.664 ± 0.066) × 10-3mm2/s was lower than in well differentiated tumours (0.721 ± 0.064) × 10-3mm2/s (p = 0.034), but trends for K show the opposite effect (0.993 ± 0.105 vs. 0.905 ± 0.059) (p = 0.037). K is different in the T1-3 category. There were significant differences between T1 and T2 (0.814 (0.773–1.052) vs. 0.998 ± 0.086, p = 0.007) and T1 and T3 (0.814 (0.773–1.052) vs. 0.991 ± 0.106, p = 0.008), while the difference between T2 and T3 (0.998 ± 0.086 vs. 0.991 ± 0.106) was not significant (p = 0.782). For T category, there was no significant difference in the ADC and D values. The ADC, K and D values were different in the subgroups based on N category, CRM, perineural invasion and LVI, but these differences were not statistically significant (p > 0.05).
Performance of ADC, K and D values to distinguish characteristics of rectal cancer
Similarly, the diffusion parameters are the average of the two readers’ measurements utilising the WTS-outline ROI. The ROC curves were used to assess the diagnostic performance of the parameters of DKI and conventional DWI for the assessment of tumour characteristics. The AUC was 0.809 (95% CI: 0.693–0.895, p < 0.001) and 0.871 (95% CI: 0.766–0.941, p < 0.001) for the assessment of well differentiated tumours using the ADC and K values, respectively, and there was no significant difference between them (p = 0.276) (Fig. 4). The optimal cut-off value was 0.959 for K (100% sensitivity and 66.67% specificity) and 0.697 × 10-3mm2/s for ADC (77.78% sensitivity and 77.19% specificity). The AUC was 0.768 (95% CI: 0.592–0.914, p = 0.04) for distinguishing T2 from T1 by K values (Fig. 5). The optimal cut-off value was 0.908, which showed relatively high sensitivity (81.1%) and specificity (80%).
The ROC was performed to assess the well differentiated tumours using the ADC (AUC = 0.809, 95% CI: 0.693–0.895) and K values 0.871 (95% CI: 0.766–0.941). The optimal cut-off value was 0.959 for K (100% sensitivity and 66.67% specificity) and 0.697×10-3mm2/s for ADC (77.78% sensitivity and 77.19% specificity)
Discussion
In our study, different slice protocols do not significantly influence ADC, K and D measurements on the 3T system. Lambregts’s [7] study also demonstrated that there are no significant differences in tumour ADC values between whole-volume and single-slice methods, which is in agreement with our results. In cases where tumours are small, the number and selection of slices may not vary for different slice protocols. Furthermore, it is fairly simple to correctly define the largest tumour axial section on the ADC map to reduce selection bias, which also helps to explain our results.
However, different ROI methods have considerable influence on diffusion parameters. Tumour ADC and D values from outline ROIs are significantly higher than those from round ROIs. In addition, K values derived from the round ROI method are significantly higher than those from the outline ROI method. Chie et al. [15] also confirmed that the ADCs from outline ROIs are relatively higher than those from round ROIs. The outline ROI covers the whole tumour signal and therefore may contain relatively more micro-liquefactive necrosis, which cannot be distinguished by the naked eye, but can increase water diffusivity, contributing to a higher ADC and D value than round ROIs. For round ROIs, which were placed within the solid tumour, there may exist a higher cell density and more complicated cell activity than for the outline ROI, which may in turn lead to higher K values.
Our study showed that the WTS-outline ROI measurement generated the highest intra- and inter-observer agreement of ADC, K and D on the 3T system. This result may be for the following reasons. First, rectal cancers normally have irregular shapes and the positioning of the round ROI within the solid tumour might vary among readers, while this variation can be minimised using an outline ROI method. Thus, there may be a difference in the absolute values among readers measured by round ROI, but not by outline ROI. Second, selection bias is further minimised using the whole tumour slice protocol. Third, analysing a larger number of pixels may result in more reproducible results for the measured parameters. We would therefore conclude that the most repeatable ROI strategy is not dependent upon diffusion-fitting model or magnetic field strength. Thus, we recommend the WTS-outline ROI strategy to measure the parameters of DKI, and we used this method for further analysis in our study.
By using WTS-outline ROI, K values in well-differentiated tumours were significantly lower than those in poorly to moderately differentiated tumours, which was consistent with the conclusion of Zhu’s study performed on a 1.5T system. While ADC values in poorly to moderately differentiated tumour were significantly lower than those in well-differentiated tumours, Akashi’s [16], Curvo-Semedo’s [17] and our previous study [18] also confirmed this finding. Compared to well differentiated tumours, poorly to moderately differentiated tumours are characterised by increased mitotic activity and varied nuclear pleomorphisms, resulting in few diffusion barriers and increased structural complexity. Thus, employing the differences in K and ADC observed in our study to reflect the differences in microstructural complexity and water diffusion between poorly to moderately and well differentiated tumours is possible. Of particular note, K values in T1 were significantly lower than in T2 and T3 tumours in our study. The AUC can distinguish T2 from T1 by K values, and K values also show relatively high sensitivity and specificity. How to identify T1 and T2 on MR before treatment remains a challenge. Our results show such K values will be useful in distinguishing T2 from T1 tumours when considering treatment strategies. The following reasons may explain the better performance of the DKI model compared with that of the DWI model. First, the conventional DWI model is based on the assumption that water diffusion within a voxel follows Gaussian behaviour, whereas the DKI model is based on another assumption that water molecules within biological tissues exhibit a non-Gaussian phenomenon, which provides a more accurate model of diffusion and captures the non-Gaussian diffusion behaviour as a reflective marker for tissue heterogeneity [19]. The vigorous cell mitosis and strong proliferative ability in more aggressive cancers can result in more a complex microstructure and increased cell density in each voxel, which in turn affects water diffusion (vs. less aggressive tumours).
The results of other analyses, including ADC, K and D values between lymph node, perineural invasion and LVI-based subgroups did not show statistical significance and might be limited by sample size and imbalanced population distributions. Larger numbers of patients are needed to further explore the performance of ADC, K and D values in predicting histopathological characteristics.
Our study has some limitations. First, restaging MRI after NCRT was excluded in our study. The reason for exclusion was the number of the patients who underwent diffusion imaging after NCRT was very small and the regressive lesions were scarcely visible in diffusion images. Second, we did not use spasmolytic and endorectal gel to reduce susceptibility artifacts for diffusion imaging. The usage of spasmolytic and endorectal gel could increase the drawing accuracy of the outline ROI along the border of the tumour and increase the reliability of our conclusions. Third, the patient distribution in our study was uneven with only two patients exhibiting poor differentiation. Finally, we did not use an area-weighted averaging method to calculate the mean values of the measured parameters in TS and WTS protocols, which might influence the accuracy of our results. Thus, further study with a more accurate averaging method, a larger and more evenly distributed patient population, and the usage of spasmolytic and endorectal gel is needed to verify our results.
In conclusion, our study confirmed that using a 3T scanner, the WTS-outline ROI approach could obtain the most repeatable measurements for both non-Gaussian and Gaussian diffusion models. By using the WTS-outline method, DKI also has considerable diagnostic value for the estimation of rectal cancer characteristics, in addition to the conventional DWI model.
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- AJCC:
-
American Joint Committee on Cancer
- AUC:
-
Area under curve
- BA-LA:
-
Bland-Altman limits of agreements
- CRM:
-
Circumferential resection margin
- D:
-
Corrected diffusion coefficient
- DKI:
-
Diffusion kurtosis imaging
- DWI:
-
Diffusion weighted imaging
- EPI:
-
Single-shot echo-planar imaging
- ICC:
-
Interclass correlation coefficient
- K:
-
Diffusion kurtosis coefficient
- LVI:
-
Lymphovascular invasion
- MRI:
-
Magnetic resonance imaging
- NCRT:
-
Neoadjuvant chemoradiotherapy
- ROC:
-
Receiver operating characteristic
- ROI:
-
Region of interest
- SD:
-
Standard deviation
- SNR:
-
Signal-to-noise ratio
- SS:
-
Single slice
- T2WI:
-
T2-weighted images
- TS:
-
Three slices
- TSE:
-
Turbo spin echo
- WHO:
-
World Health Organization
- WTS:
-
Whole tumour slice
References
Van Cauter S, Veraart J, Sijbers J et al (2012) Gliomas: diffusion kurtosis MR imaging in grading. Radiology 263:492–501
Rosenkrantz AB, Sigmund EE, Johnson G et al (2012) Prostate cancer: feasibility and preliminary experience of a diffusional kurtosis model for detection and assessment of aggressiveness of peripheral zone cancer. Radiology 264:126–135
Rosenkrantz AB, Sigmund EE, Winnick A et al (2012) Assessment of hepatocellular carcinoma using apparent diffusion coefficient and diffusion kurtosis indices: preliminary experience in fresh liver explants. Magn Reson Imaging 30:1534–1540
Yu J, Xu Q, Song JC et al (2017) The value of diffusion kurtosis magnetic resonance imaging for assessing treatment response of neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Eur Radiol 27:1848–1857
Hu F, Tang W, Sun Y et al (2017) The value of diffusion kurtosis imaging in assessing pathological complete response to neoadjuvant chemoradiation therapy in rectal cancer: a comparison with conventional diffusion-weighted imaging. Oncotarget. https://doi.org/10.18632/oncotarget.17491
Zhu L, Pan Z, Ma Q et al (2017) Diffusion Kurtosis Imaging Study of Rectal Adenocarcinoma Associated with Histopathologic Prognostic Factors: Preliminary Findings. Radiology 284:66–76
Lambregts DM, Beets GL, Maas M et al (2011) Tumour ADC measurements in rectal cancer: effect of ROI methods on ADC values and interobserver variability. Eur Radiol 21:2567–2574
Chao M, Guo X, Li L et al (2017) Effect of region of interest size on ADC measurements in pancreatic adenocarcinoma. Cancer Imaging 17:13
Colagrande S, Pasquinelli F, Mazzoni LN, Belli G, Virgili G (2010) MR-diffusion weighted imaging of healthy liver parenchyma: repeatability and reproducibility of apparent diffusion coefficient measurement. J Magn Reson Imaging 31:912–920
Bickel H, Pinker K, Polanec S et al (2017) Diffusion-weighted imaging of breast lesions: Region-of-interest placement and different ADC parameters influence apparent diffusion coefficient values. Eur Radiol 27:1883–1892
Sun K, Chen X, Chai W et al (2015) Breast cancer: diffusion kurtosis mr imaging—diagnostic accuracy and correlation with clinical-pathologic factors. Radiology 277:46–55
Jensen JH, Helpern JA (2010) MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR Biomed 23:698–710
Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K (2005) Diffusional kurtosis imaging: The quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med 53:1432–1440
Edge SB, Compton CC (2010) The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 17:1471–1474
Inoue C, Fujii S, Kaneda S et al (2014) Apparent diffusion coefficient (ADC) measurement in endometrial carcinoma: effect of region of interest methods on ADC values. J Magn Reson Imaging JMRI 40:157–161
Akashi M, Nakahusa Y, Yakabe T et al (2014) Assessment of aggressiveness of rectal cancer using 3-T MRI: correlation between the apparent diffusion coefficient as a potential imaging biomarker and histologic prognostic factors. Acta Radiol 55:524–531
Curvo-Semedo L, Lambregts DM, Maas M et al (2012) Diffusion-weighted MRI in rectal cancer: Apparent diffusion coefficient as a potential noninvasive marker of tumor aggressiveness. J Magn Reson Imaging 35:1365–1371
Sun Y, Tong T, Cai S et al (2014) Apparent Diffusion Coefficient (ADC) Value: A Potential Imaging Biomarker That Reflects the Biological Features of Rectal Cancer. PloS One 9:e109371
Wu EX, Cheung MM (2010) MR diffusion kurtosis imaging for neural tissue characterisation. NMR Biomed 23:836–848
Funding
This study has received funding by the National Natural Science Foundation of China (Grant No. 81501437).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Yajia Gu.
Conflict of interest
The authors of this manuscript declare relationships with the following companies: Siemens Shenzhen Magnetic Resonance Ltd., Shenzhen, China and MR Collaboration NE Asia, Siemens Healthineers, Shanghai, China
Statistics and biometry
Huixun Jia, MD, Department of Clinical Statistics Center, Fudan University Shanghai Cancer Center; Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China kindly provided statistical advice for this manuscript.
Informed consent
This was a retrospective study and did not require informed consent.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• diagnostic or prognostic study
• performed at one institution
Rights and permissions
About this article
Cite this article
Sun, Y., Xiao, Q., Hu, F. et al. Diffusion kurtosis imaging in the characterisation of rectal cancer: utilizing the most repeatable region-of-interest strategy for diffusion parameters on a 3T scanner. Eur Radiol 28, 5211–5220 (2018). https://doi.org/10.1007/s00330-018-5495-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5495-y