Abstract
Objective
To explore the use of two consecutive axial scans in triple-rule-out (TRO) examination on a 16 cm wide-detector CT for radiation dose reduction.
Materials and methods
Sixty TRO patients were assigned to either study group (Group A, n = 30) or control group (Group B, n = 30). Group A used a two-phasic contrast injection: 25mgI/kg/s for 12 s in 1st and at 3.0 ml/s injection rate for 7 s in 2nd phase. The pulmonary artery, coronary artery and aorta were scanned in succession with two axial scans using smart-coverage technique. Group B used the conventional protocol of scanning pulmonary arteries first in helical, followed by coronary arteries in axial and aorta in helical mode with contrast injection of 25mgI/kg/s for 14 s. All images were reconstructed with 80% ASIR-V. The qualitative and quantitative image assessment and effective dose of the two groups were statistically compared.
Results
The demographic data and quantitative measurements and qualitative image scores between the two groups were statistically the same (p > 0.05). However, Group A reduced radiation dose by 52% (2.67 ± 0.98 mSv vs. 5.65 ± 1.37 mSv) (p < 0.001).
Conclusion
Using two consecutive axial scans in triple-rule-out on a 16 cm wide-detector CT reduces radiation dose while maintaining image quality compared with the conventional TRO protocol.
Key Points
• Triple-rule-out can be performed with two-axial scans on a wide-detector CT system.
• TRO with two-axial scans maintain image quality compared with conventional protocol.
• TRO with two-axial scans reduces 52% radiation dose over conventional protocol.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chest pain is one of the most common symptoms in emergency departments. The acute coronary syndrome (ACS), pulmonary thromboembolism (PTE), and aortic dissection (AD) are the three major conditions associated with substantial morbidity and mortality for these patients, and should first be excluded [1]. The “triple-rule-out”(TRO) computed tomography angiography (CTA) for chest pain patients has recently emerged as a technique that noninvasively evaluates the coronary arteries and simultaneously visualizes the pulmonary arteries, thoracic aorta and other intrathoracic structures and its value is generally recognized [2]. CT systems with 64-slice multidetector, 128-slice multidetector and dual-source have all been used in TRO CTA examination with retrospectively ECG-gated helical scan mode for the coronary arteries. On one hand, the use of small-pitch, retrospective ECG-gated scanning for coronary arteries and with overlapped coverage for same anatomy (for ruling out different causes) in TRO examination results in a higher radiation dose, the mean radiation dose is reported to be 4.8-9.6mSv, [3,4,5] and increases cancer risks [6]. On the other hand, acute coronary syndrome, pulmonary thromboembolism or aortic dissection has a very high mortality and rapid onset, and TRO examination has demonstrated its clinical value. So how to ensure the quality of TRO images and reduce radiation dose at the same time has become the focus of many radiologists. Compared with the narrow-detector CT system, the introduction of 16 cm wide-detector CT system greatly improves the single rotation coverage to not only improve the image quality for more demanding cardiac patients [7], but also make the task of covering the entire chest with high temporal resolution at low radiation dose in two gated axial scans possible. The purpose of this study was to explore the feasibility of using two consecutive axial scans in TRO examination for chest pain patients on a 16 cm wide-detector CT system to reduce overall radiation dose and maintain image quality compared with the conventional three-scan protocol that optimized the scan timing for pulmonary arteries, coronary arteries and aorta individually for good image quality.
Materials and methods
Patient characteristics
Our study was approved by the institutional review board and all participants gave written informed consent. One author (J.L.) is an employee of GE Healthcare, the manufacturer of the CT system used in this study. The other authors, who are not GE Healthcare employees, had control of the data and information that might have represented a conflict of interest for (J.L.). This was a prospective cohort study of consecutive patients who underwent TRO scans in the period from November 2016 to November 2017. Patients aged 18 years and older and a body-mass index (BMI) ≤30.0 kg/m2 who with clinical histories including a chief complaint of chest pain; shortness of breath; syncope or near syncope; neck, shoulder, back, or arm pain not appearing to be musculoskeletal in nature; and patients for suspicion of heart disease, pulmonary embolism, or aortic dissection were enrolled in our study. Exclusion criteria included pregnancy, traumatic injury, elevated cardiac biomarkers (troponine-I or creatine kinase-MB) in the initial blood sample, ECG changes indicating an ACS, hypotension (systolic blood pressure 90 mmHg), elevated initial cardiac enzymes, known allergy to intravenous contrast material, renal insufficiency (creatinine≤120 μmol/l), and inability to cooperate with TRO scans. According to the inclusion and exclusion criteria, a total of 60 patients (24 women, 26 men; mean age 54.5 ± 1.6 years; range 33–80 years) were enrolled and assigned randomly to either the study group (Group A, n = 30) or control group (Group B, n = 30), of whom 20 with a clinical history of hypertension and coronary heart disease at the same time; 14 patients with hyperlipidemia; 7 patients with a history of type II diabetes; 3 patients with a history of pulmonary hypertension, and 3 patients with severe Pneumonia, and the rest of the patients included had an unclear clinical history of the past.
Scan protocols
All scans were performed on a 16 cm wide-detector Revolution CT scanner (GE Healthcare, Waukesha, WI USA). Both groups used 100 kV tube voltage, 0.28 s rotation speed and automatic tube current modulation for obtaining a preset noise index of 35HU at 0.625 mm slice thickness, and contrast agent iopamidol (370 mgI/ml). The acquisition windows for cardiac scans were dependent on the patient heart rates (HR): 70-80% of the R-R interval for HR < 65 bpm; 30-50% for HR > 80 bpm and both 30-50% and 70-80% for HR between 66 bpm and 80 bpm. Scan modes and contrast injection protocols were different for the two groups.
Group A used the all-axial scan mode with the pulmonary artery, coronary artery and aorta covered with two axial scans to provide a total axial coverage of about 204 mm from the thoracic entrance to the top of the diaphragm. The Smart-coverage technique on the scanner was used to automatically determine the collimations: after the user prescribes a region to be scanned, the scanner will step through the combination of available collimations (4, 8, 10, 12, 14 and 16 cm) to find the smallest that will cover the required range. For cardiac scan mode, when the scan region requires two slabs, the scanner will use a large slab on the inferior end, to cover as much of the heart as possible, and place a smaller slab on the superior side (Fig. 1). A small scan overlap between the two axial scans was required to reduce the wide cone angle effect, and this small overlap was determined automatically by the system based on the image slice thickness and display field-of-view. A patient weight-dependent, two-phasic contrast injection protocol was used for Group A: 25mgI/kg/s for 12 s in the 1st phase and at 3.0 ml/s injection rate for 7 s in the 2nd phase. The injection rate in the 1st phase was determined based on the total contrast volume divided by the preset injection time (12 s) The pulmonary artery (in the superior portion) scan was triggered at a threshold of 220HU for descending aorta with a short delay time of 1.6-2.2 s, and the scan for coronary artery, aorta and the inferior portion of the pulmonary arteries was carried out immediately after the first scan in prospective ECG-triggering cardiac mode. The injection, triggering and scanning protocols were determined based on our preliminary studies to ensure adequate contrast enhancement in vessels.
Group B used a more conventional three-scan protocol that optimized the scan timing for pulmonary arteries, coronary arteries and aorta individually. A patient weight-dependent contrast agent injection protocol of 25mgI/kg/s for 14 s was used followed by 30 ml of saline at the same injection rate. The injection rate was determined based on the total contrast volume divided by the preset injection time (14 s). Pulmonary arteries were scanned first in a helical mode with 8 cm detector collimation, pitch 0.992:1 and 0.28 s rotation speed to provide an axial coverage from the thoracic entrance to the top of the diaphragm; scan was triggered with a threshold of 100HU for pulmonary arteries. The coronary arteries were scanned next after 8–9 s delay in a prospective ECG-triggered cardiac axial mode with 0.28 s rotation speed and up to 16 cm detector collimation covering areas from the 1 cm below tracheal carina to the bottom of the heart; followed immediately by aorta using a helical scan mode, from the supra-aortic arch to the top of the diaphragm. (Fig. 1).
Image reconstruction and analysis
Images were reconstructed with the new Adaptive Statistical Iterative Reconstruction (ASIR-V, GE Healthcare, Waukesha, WI USA) algorithm at 80% strength (80%ASIR-V) in both groups, and for coronary CT angiography, the best cardiac phase for generating images was automatically selected by using the Smartphase technique provided by the CT system and the Snapshot freeze (SSF) reconstruction technique was used for cardiac motion correction. All images were transferred to an Advanced Workstation (ADW4.6, GE Healthcare, Waukesha WI USA) for measurement and analysis. The volume rendering (VR) and maximum intensity projection (MIP) images were generated for image comparison. CT attenuation value and standard deviation (SD) of the thoracic aorta, pulmonary artery, coronary artery, fat and erector spinae muscle were measured on the axial images using region-of-interest (ROI) of 20-400 mm2, and contrast-to-noise ratio (CNR) for vessels were calculated. For pulmonary arteries and aorta, CNR was calculated using muscle as the background with SD of muscle as the background noise, and for coronary arteries, fat was used as the background with SD value of fat as the background noise. Two imaging radiologists with cardiovascular diagnosis experience over 8 years assessed the cardiac image quality blindly and independently for subjective image quality score based on the American Heart Association modified 15-segment classification [8] and a 5-point scoring system [9]: 5, Excellent image quality, clear vessel boundary and display, no motion artefact; 4, Good image quality, mostly clear boundary, only a small amount of motion artefacts, not affecting the diagnosis; 3, Suboptimal image quality but interpretable, can be used for diagnosis, may affecting the diagnostic accuracy; 2, Poor image quality and difficult to perform vessel analysis; 1, Non-diagnostic, poor image quality with severe motion artefacts. The lowest score of the segment in each vessel was used as the final score for the vessel. If there was disagreement for scoring between reviewers, consensus was reached after negotiation. The original scores from the two reviewers were analyzed for inter-observer consistency.
Radiation dose calculation
The volumetric CT dose index (CTDIvol) in mGy, dose-length-product (DLP) in mGy-cm of CT scan were recorded by the system after each scan and the effective dose in millisieverts (mSv) was estimated as the product of the dose-length product (DLP) in mGy-cm and a conversion coefficient of 0.014 mSv-mGy−1-cm−1) [10].
Statistical analysis
Continuous variables were expressed as means ± standard deviations (SD) and statistical analyses were performed using SPSS software (SPSS v. 21.0, IBM Corp., New York, NY). The Mann-Whitney U-test was used to compare the per-vessel image quality scores and the unpaired t-test was used to compare the continuous variables including CT value, CNR and radiation dose and contrast dose. Kappa test was used to evaluate the inter-observer consistency of subjective scores by the two reviewers, and the Kappa values were defined as follows: for κ values of 0–0.20, slight agreement; for κ values of 0.21–0.40, fair agreement; for κ values of 0.41–0.60, moderate agreement; for κ values of 0.61–0.80, substantial agreement; and for κ values of 0.81–1.00, almost perfect agreement. A p value of less than 0.05 was considered statistically significant.
Results
There was no difference in age (years) (55.7 ± 12.7 vs. 53.8 ± 10.0), heart rate (bpm) (70.2 ± 9.9 vs. 69.6 ± 10.0), weight (kg) (66.6 ± 9.2 vs. 68.6 ± 10.3), body mass index (kg/m2) (24.0 ± 2.3 vs. 24.7 ± 2.9) and the main clinical history of the patients between the two groups (p > 0.05) (Table 1). The distribution of main diagnosis was similar in both groups. The main diagnoses of the initial reports were confirmed in the consensus reading in all cases. Pulmonary embolism was found in three patients in Group A and in four patients in Group B. Coronary artery stenosis was found in four patients in both groups. Aortic dissection was diagnosed in five patients in Group A and in four patients in Group B. One case of penetrating atherosclerotic ulcer of the descending aorta was diagnosed in Group B, while there was no penetrating atherosclerotic ulcer in Group A (Table 1).
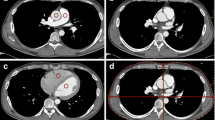
All vessels had similar CT enhancement between Group A and Group B (Table 2). The CT attenuation values (in HU) in the ascending aorta and pulmonary artery trunk of Group A were 456.8 ± 38.4 and 429.4 ± 43.2 respectively. These values were statistically the same as the respective values of 485.8 ± 73.4 and 457.5 ± 70.4 in group B (p > 0.05). The CNR values for all major vessels between the two groups were also statistically the same (p > 0.05) (Table 2). All major vessels had similar image quality scores between Group A and Group B, and there were at least substantial agreements between the two reviewers for evaluating these vessels (all κ > 0.61) (Table 3). For the right coronary artery, the image quality scores of Group A and Group B were (4.20 ± 0.60) and (4.20 ± 0.70), respectively (Z = −0.314, p = 0.753); For the left anterior descending, the scores of group A and group B were (4.10 ± 0.64) and (4.20 ± 0.61), respectively (Z = −1.215, p = 0.224); and for the left circumflex artery, the scores of group A and group B were (3.72 ± 0.41) and (4.06 ± 0.61), respectively (Z = −1.014, p = 0.397) (Figs. 2a-f)).
a–c: 63-year-old male, images were acquired with a helical scan for pulmonary arteries followed by axial scan for CCTA and helical scan for aorta. d–f: 59-year-old male, images were acquired with 2 consecutive axial scans with 16 cm wide-detector collimation. a and d Volume Rendering (VR) image, b and e axial maximum intensity projection (MIP) image and c and f sagittal MIP image
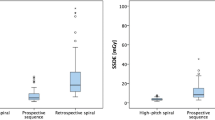
However, there was a significant difference in the total effective radiation dose between Group A (2.67 ± 0.98 mSv) and group B (5.65 ± 1.37 mSv) (p < 0.001) (Table 4), resulting in 52% effective dose reduction using the 2-consecutive axial scan mode, Moreover, the total exposure time for Group A was significantly shorter than for group B (1.35 ± 0.78 s vs. 2.58 ± 0.90s) (p < 0.001). However, to ensure adequate enhancement in the pulmonary artery imaged with the two axial scans in Group A, a second phase contrast injection of about 20 ml contrast medium was used instead of the normal saline flush, resulted in a 12% higher contrast dose than in Group B (73.9 ± 7.4 ml vs. 65.2 ± 9.7 ml).
Discussion
In our study, we evaluated the use of an all-axial scan mode for performing triple-rule-out (TRO) CT angiography for patients with acute chest pain. Our results demonstrated the feasibility of using two consecutive axial scans in TRO CT angiography examination on a 16 cm wide-detector scanner to achieve significant radiation dose reduction while maintaining good image quality compared with a three-scan TRO protocol that optimized the scan timing for pulmonary arteries, coronary arteries and aorta individually for good opacity in vessels. No significant differences were found in image quality between the two groups. Patients in the study group showed an effective dose reduction of up to 52%.
One major concern for the triple-rule-out protocols is radiation dose, especially when retrospectively ECG-gated helical scan of the entire thorax is required. There has been a lot of effort to reduce radiation dose in the TRO scans. This includes the use of ECG-gated tube current modulation, [11] adaptive detector collimation for prospectively ECG-triggered coronary CT angiography with third-generation dual-source CT to minimize unnecessary exposure beyond heart [12]. Others used either non-gated, ultra-high pitch CT scan for the evaluation of pulmonary embolism and visualization of cardiac structures [13] or ECG-gated, high-pitch dual spiral technique with dual-source CT to cover the chest in one short scan to reduce overall radiation dose in TRO examinations [14]. However, the high pitch dual spiral technique often requires patients with low and stable heart rates. In our study we proposed an all-axial scan mode that used two consecutive axial scans to cover the pulmonary arteries, coronary arteries and aorta, fully took advantage of the 16 cm wide-detector coverage of the Revolution CT scanner. The image quality and radiation dose of this protocol was compared with the conventional protocol which requires the use of helical scan mode for pulmonary arteries and aorta and axial scan for the heart which results in overlapped scans for the same anatomy. Our results indicated that the proposed all-axial scan protocol provided similar qualitative image quality scores and the quantitative enhancement and contrast-noise-ratio in the pulmonary arteries, coronary arteries and aorta as the conventional TRO-CTA protocol. On the other hand, total radiation dose was reduced by 52% from 5.65 mSv to 2.67 mSv. This was due to the following two reasons. First, it was due to the difference of the total exposure ranges between the two groups. The scans in Group A with the two-axial scans were sequential in nature, there was no repeated exposure for the same anatomy (except for the small scan overlap required for the two consecutive axial scans), and the images for the pulmonary arteries of the entire chest came from the sum of the two consecutive scans: one for the upper chest and one for the lower chest that included the heart. The small scan overlap for the two consecutive axial scans with the wide-detector CT system in Group A depends on the image slice thickness and display field of view (DFOV) for the scan to compensate for the cone angles and was about 1.5 cm in our study; The scans in the conventional Group B required repeated overlapped exposures to the same organs: the helical scan for pulmonary arteries also covered the heart and aorta. Second, in Group B covering pulmonary arteries and aorta with helical scanning mode, there was over-scan associated with helical scan mode, resulting in effective dose addition compared with the use of axial scan mode [15].
It needs to be noted that in this study, even though the radiation dose for the TRO CTA in Group B with the more conventional protocol was higher than the proposed axial scan protocol, the total radiation dose of only 5.65 ± 1.37 mSv in Group B itself was greatly reduced compared with the most recent published TRO CTA results with the median dose of 9.1 mSv, [16] taking advantage of the prospectively ECG triggered one-beat coronary CT angiography with the 16 cm detector collimation and advanced iterative reconstruction algorithm. The radiation dose was further reduced in Group A to 2.67 ± 0.98 mSv with the use of two-axial scans. Finally, in Group A with the proposed CT protocol, the pulmonary arteries were scanned at a delayed phase than in the conventional three-scan TRO protocol, an additional contrast agent injection was needed based on our preliminary experience to ensure adequate contrast enhancement. We therefore modified the contrast agent injection protocol to ensure adequate enhancement for the pulmonary arteries during the second axial scan for CCTA, and We replaced the traditional saline flush with a second phase contrast injection of about 20 ml at an injection rate of 3.0 ml/s. This resulted in a slightly higher contrast dose than Group B. However, the total contrast volume of about 74 ml in Group A was still substantially less than the reported 120 ml by Takx et al. [17].
The study still has some limitations: This study reflects our preliminary experience with a small number of patients, and only patients with BMI ≤ 30.0 kg/m2 were included in the study, further studies with large patient cohort is required. Also, this study was focused on evaluating the technical feasibility and image quality of the proposed approach for TRO, the clinical significance and applications of TRO itself with the proposed approach needs to be fully studied in the future. Finally, the contrast injection protocol and triggering scheme in the study group were based on our preliminary experience, further optimization is needed to further reduce contrast dose.
In conclusion, the use of two consecutive axial scans in triple-rule-out (TRO) CTA on a 16 cm wide-detector CT substantially reduces radiation dose while maintaining image quality compared with the conventional three-scan TRO scanning protocol.
Abbreviations
- ACS:
-
Acute coronary syndrome
- AD:
-
Aortic dissection
- ASIR:
-
Adaptive statistical iterative reconstruction
- CNR:
-
Contrast-to-noise ratio
- CTA:
-
Computed tomography angiography
- CTDI:
-
CT dose index
- DFOV:
-
Display field of view
- DLP:
-
Dose-length product
- MIP:
-
Maximum intensity projection
- mSv:
-
Millisievert
- PTE:
-
Pulmonary thromboembolism
- ROI:
-
Region-of-interest
- SD:
-
Standard deviation
- SSF:
-
Snapshot freeze
- TRO:
-
Triple-rule-out
- VR:
-
Volume rendering
References
Wnorowski AM, Halpern EJ (2016) Diagnostic yield of triple-rule-out CT in an emergency setting. AJR Am J Roentgenol 207(2):295–301
Kim HS, Kim SM, Cha MJ et al (2017) Triple rule-out CT angiography protocol with restricting field of view for detection of pulmonary thromboembolism and aortic dissection in emergency department patients: simulation of modified CT protocol for reducing radiation dose. Acta Radiol 58(5):521–527
Manheimer ED, Peters MR, Wolff SD et al (2011) Comparison of radiation dose and image quality of triple-rule-out computed tomography angiography between conventional helical scanning and a strategy incorporating sequential scanning. Am J Cardiol 107(7):1093–1098
Ayaram D, Bellolio MF, Murad MH et al (2013) Triple rule-out computed tomographic angiography for chest pain: a diagnostic systematic review and meta-analysis. Acad Emerg Med 20(9):861–871
Krissak R, Henzler T, Prechel A et al (2012) Triple-rule-out dual-source CT angiography of patients with acute chest pain: dose reduction potential of 100 kV scanning. Eur J Radiol 81(12):3691–3696
Mayo JR, Aldrich J, Muller NL et al (2003) Radiation exposure at chest CT: a statement of the Fleischner society. Radiology 228(1):15–21
Andreini D, Pontone G, Mushtaq S et al (2017) Image quality and radiation dose of coronary CT angiography performed with whole-heart coverage CT scanner with intra-cycle motion correction algorithm in patients with atrial fibrillation. Eur Radiol. https://doi.org/10.1007/s00330-017-5131-2
Wong DTL, Soh SY, Ko BS et al (2014) Superior CT coronary angiography image quality at lower radiation exposure with second generation 320-detector row CT in patients with elevated heart rate: a comparison with first generation 320-detector row CT. Cardiovasc Diagn Ther 4(4):299–306
Hong C, Becker CR, Huber A et al (2001) ECG-gated reconstructed multi-detector row CT coronary angiography: effect of varying trigger delay on image quality. Radiology 220(3):712–717
European guideline on quality criteria for computed tomography (1999) Brussels, Belgium Report EUR 16262 EN
Frauenfelder T, Appenzeller P, Karlo C et al (2009) Triple rule-out CT in the emergency department: protocols and spectrum of imaging findings. Eur Radiol 19:789–799
Messerli M, Dewes P, Scholtz J et al (2017) Evaluation of an adaptive detector collimation for prospectively ECG-triggered coronary CT angiography with third-generation dual-source CT. Eur Radiol. https://doi.org/10.1007/s00330-017-5177-1
Hou DJ, Tso DK, Davison C et al (2013) Clinical utility of ultra high pitch dual source thoracic CT imaging of acute pulmonary embolism in the emergency department: are we one step closer towards a non-gated triple rule out? Eur J Radiol 82(10):1793–1798
Sommer WH, Schenzle JC, Becker CR et al (2009) Saving dose in triple-rule-out computed tomography examination using a high-pitch dual spiral technique. Invest Radiol 45:64–71
Halpern EJ, Levin DC, Zhang S et al (2009) Comparison of image quality and arterial enhancement with a dedicated coronary CTA protocol versus a triple rule-out coronary CTA protocol. Acad Radiol 16(9):1039–1048
Burris AC, Boura JA, Raff GL (2015) Triple rule out versus coronary CT angiography in patients with acute chest pain: results from the ACIC consortium. J Am Coll Cardiol Img 8(7):817–825
Takx RAP, Krissak R, Fink C et al (2017) Low-tube-voltage selection for triple-rule-out CTA: relation to patient size. Eur Radiol 27(6):1–6
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Taiping He.
Conflict of interest
The authors of this manuscript declare no conflict of interest exits in the submission of this manuscript.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• Prospective
• randomized controlled trial
• performed at one institution
Rights and permissions
About this article
Cite this article
Chen, Y., Wang, Q., Li, J. et al. Triple-rule-out CT angiography using two axial scans with 16 cm wide-detector for radiation dose reduction. Eur Radiol 28, 4654–4661 (2018). https://doi.org/10.1007/s00330-018-5426-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5426-y