Abstract
Objective
To investigate the incidence rate, time-to-onset and recovery, MRI morphology and occurrence of insufficiency fractures in radiation-induced changes in the sacrum following pelvic radiotherapy.
Material and methods
410 patients with pelvic malignancies treated with radiotherapy were reviewed. Follow-up was 1–124 months (mean 22 months). Serial MRI (average four studies/patient) were analysed using a new semi-quantitative score (Radiation-Induced Sacral Changes=RISC). A size category (I/II/III), a type category for MR signal morphologies (a/b/c) and sacral insufficiency fractures (+/-) were applied.
Results
Seventy-two patients (17.6 %) were found to have new pathological signal changes. Radiation osteitis was documented in 83.3 % (60/72, RISC stage a + b), and definite osteonecrosis (stage c) in 12 patients (16.7 %, 12/72). Thirty-one patients (43.1 %) had sacral insufficiency fractures. Initial bone marrow signal changes were found 1–35 months (median 4 months) after radiotherapy. The maximum manifestation of radiation-induced signal changes occurred after 1–35 months (mean 11 months). Fifty-six cases (77.8 %) showed a significant signal recovery within 16.5 months.
Conclusion
Radiation-induced bone marrow changes appear with a high incidence at the sacrum with an early onset and frequent recovery. The majority presented a pattern of radiation osteitis, whereas osteoradionecrosis was proportionately rare.
Key Points
• Radiation-induced sacral bone marrow changes appear frequently (17.6 %) following pelvic radiotherapy.
• Insufficiency fractures are common late effects (43 %).
• Radiation osteitis develops early (4 mo), with recovery between 16.5 and 39.5 months.
• Definite radiological osteoradionecrosis is proportionately rare (3 %).
• A 3-stage classification system simplifies and standardizes the morphological disease staging.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In 2012 the incidence of cervical and corpus carcinoma in Europe was 13.4 and 19.3/100.000 women, respectively [1]. Radiotherapy (RT) is essential for the treatment of pelvic malignancies [2,3,4]. However, adverse radiation reactions in healthy, irradiated tissues are common [5, 6]. Frequent osseous complications such as radiation osteitis (RO) and osteoradionecrosis (ORN) are summarized as radiation-induced reactions (RIR) [5,6,7,8,9]. A common misinterpretation of RIR is bone metastasis [10, 11]. The discrimination of different stages of RIR has not been investigated closely with MRI, and a classification system has not been established. The sacral bone is the most common one affected by RIR due to its amount of red bone marrow within the central field of pelvic radiation [12]. The reported incidence of pelvic ORN varies widely, with a range of 2.1–34 % [7, 13, 14], depending on technique and criteria applied.
The pathophysiology of RIR in the bone is partly understood and believed to result from a toxic response leading to increased permeability of endothelial bone marrow sinus, cytoplasmatic swelling and rapid decline in bone marrow cells [15, 16], as well as impairment of bone remodelling cells [17,18,19], resulting in bone degradation [17, 18, 20, 21]. The extent of cellular and structural damage depends heavily on treatment-related factors such as the delineation of the clinical target volume, the type (external radiation and/or intracavital radiation), total dose, duration and fractionation of RT and additional concomitant and/or adjuvant therapies [18, 22, 23]. Furthermore, the extent of RIR on bone in particular depends on patient-related factors such as age, body weight, sex, skeletal co-morbidities (e.g. osteoporosis) and co-medications, primarily corticosteroids [18, 22, 24].
One important sequela of RIR in the pelvic skeleton is progressive biomechanical bone instability that may eventually lead to insufficiency fractures, with or without clinical symptoms [7, 22]. While computed tomography (CT) plays an important role in fracture detection, magnetic resonance imaging (MRI) is more sensitive to bone marrow abnormalities but has also been proven to be equally or more sensitive in detection of fractures in the pelvis [25, 26].
While the literature describes healing without therapy [7, 20] and various imaging patterns, we assumed that radiation-induced changes in pelvic bones follow a dynamic pattern with variable morphology and potential remission.
The objective of our study was to evaluate the incidence of alterations to bone marrow and mineralized bone in the sacrum following RT of pelvic malignancies, and to develop criteria and a classification system to discriminate between RO and ORN, by observing MRI signal changes over time, assessing the time-to-onset and bone marrow recovery, as well as conditions under which insufficiency fractures occur.
Material and methods
Data collection and cohort
The local ethics committee approved this study. Patient consent was waived due to the retrospective design of the study. To identify patients with pelvic malignancies with available imaging, we matched a list of 717 cancer patients from the local cancer registry who had undergone RT at our institution with a retrospective search in our clinical RIS/PACS for patients treated between January 2004 and July 2015 at our cancer treatment facility with curative radiotherapy. For a total of 410 patients, imaging studies were available. Their median age was 58 years (range 22–90 years). Case results from PACS were matched with a RIS database query for the same time interval using the following keywords: ‘osteoradionecrosis’, ‘ORN’, ‘osteonecrosis’, ‘marrow edema’, ‘bone edema’, ‘radiation osteitis’, ‘sacral necrosis’ and ‘insufficiency fracture’. Irrespective of whether any of these keywords were found or not, all pre- and post-radiation studies of the 410 target patients were searched for abnormalities in the pelvic skeleton.
Potential risk factors such as age, chemotherapy and osteopenia were assessed. Patients under 18 years of age and patients undergoing palliative radiotherapy were excluded, as well as patients with pre-existing bone metastases in any location (Table 1).
Data collection included age, location and tumour type, concomitant or sequential treatment (e.g. chemotherapy), acute reactions to RT such as skin rash, nausea or diarrhoea, initial MR imaging findings (t0, baseline), follow-up imaging results and time until initial bone marrow signal changes (t1), time until maximum bone marrow alterations (t2) and time until changes had resolved completely (t3).
Imaging and follow-up
For a case to be included in the cohort, a minimum of one pre-therapeutic baseline (before RT) MRI and additional CT study and a minimum of two studies following RT (average four pre- and post-therapeutic imaging studies) covering a minimum follow-up time of 6 weeks were required. A minimum of four different MRI sequences (T1wi/ T2wi/ TIRM/ T1wi with contrast (C+)) in at least two planes were required. All evaluable CT studies were reviewed for IF and structural changes. All imaging examinations included in this study were performed as standard of care. The median follow-up was 16 months (range 1–20 months). MRI and CT scans of the pelvis were performed in a routine follow-up (3 months average). 79.8 % had both CT and MRI scans (n = 327), CT scans alone were performed in 19.8 % (n = 81) of patients, MRI scans alone in 0.5 % (n = 2).
Radiation therapy
Two frequently used types of RT, 3DCRT (3D-conformation-radiotherapy, 61.7 %) and IMRT (intensity-modulated radiotherapy, 34.9%), had been used, frequently combined with brachytherapy.
Median prescribed dose of pelvic RT was 45 Gray (Gy) (range 12–65 Gy). 133/410 (32.4 %) patients received additional intra-cavity brachytherapy (ICBT). End-organ doses in the sacrum were not available and will be part of future investigations.
Image analysis, findings and classification
Following a 2-week image training period for detecting sacral pathologies (degenerative, inflammatory, necrosis) a radiology resident reviewed all 410 cases considering the above-mentioned pathologies. All cases with suspicious findings in the sacrum or pelvic bone were marked and reviewed independently by a musculoskeletal radiologist with 10 years of experience, who was blinded to the original study interpretation and report, but in knowledge of the clinical tumour history, age and gender. All MR sequences were scrutinized for subtle signal changes, using CT alongside, whenever available, to depict structural bone alterations and early IF. Osteopenia was defined semi-quantitatively as a significantly rarefied osseous trabecular structure of the sacral massa lateralis in baseline CT-studies. Degenerative changes (DJD) of the sacroiliac joint (SIJ) and related focal bone marrow signal changes were documented. Follow-up findings were reviewed in a side-by-side fashion. After reviewing 20 patients in follow-up with initial signs of RIR in the sacrum, a definitive dynamic MRI pattern was recognized, starting with localized punctuated contrast enhancement that eventually becomes more diffuse over time, followed by recurrent enhancement. Since a uniform classification for sacral bone/marrow changes following radiotherapy does not exist in the literature, we decided to introduce a semi-quantitative three-stage scoring system for sacral pathologies, based on MRI studies with and without contrast (T1wi TSE, T2wi TSE, TIRM, T1wi+contrast), and termed it ‘RISC’-classification (Radiation-Induced Sacral Changes). The RISC-classification consists of three different categories accounting for the extent of alterations, the type of signal change, and the presence or absence of fractures (Tables 2 and 3, Figs. 1 and 2). The rationale for the classification was to prove or disprove correlations between (1) signal size, (2) signal morphology and (3) fractures of the sacrum, which required a system that combines this information. In general, RO is defined as an inflammatory reaction with a bone marrow oedema pattern, without morphological evidence of necrosis, indicated by punctuated or confluent hyperintensities on T1wi +contrast and TIRM sequences. ORN is defined as a longer-lasting detrimental damage to bone marrow cells and marrow reticulum with one or more non-enhancing, necrotic parts on MRI.
Schematic representation of pathological MR signal changes in the sacrum with respect to size and expansion, type, morphology and fracture documentation according to the ‘RISC-Score’. (a) Size category (RISC I-III) dividing the sacral ala in three thirds. (b) Type category (RISC a) representing initial bone marrow signal changes with punctuated or curvy-linear morphology. (c) Type category (RISC b) representing generally larger, more intense sacral signal change without definite signs of osteonecrosis. (d) Type category (RISC c) representing full osteonecrosis with a central region of non-enhancing tissue. (e) Fracture category (RISC +/-) documenting insufficiency fractures on one or both side of the sacrum (yellow lines)
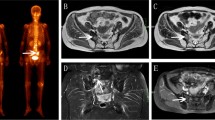
MR case examples according to the ‘RISC-Score’ in Fig. 1. (a) Coronal T1wi +C representing initial signal abnormalities according to RISC Ia-, often very subtle, generally arising laterally (arrow), adjacent to the SIJ. (b) MR case example (coronal T1wi +C) for RISC Ia- showing a more areal distribution with high signal intensity. (c) MR case example (coronal T1wi +C) for RISC IIb- showing a more intense and geographically outlined enhancement. (d) MR case example (coronal T1wi +C) for RISC IIc+ demonstrating a central necrosis (white arrows) and a small fracture line (black arrow). (e) MR case example for RISC IIb+ with a sacral fracture line parallel to the SIJ on the right seen on axial T1wi (VIBE)
Statistical analysis
Log-rank-test, cox-regression and Fisher’s exact test were used for comparison of the data between two groups. Significant statistical difference was defined by p<0.05.
Results
Incidence of RO and ORN
The overall incidence of all radiation-induced reactions (RIRs) combined in pelvic bones following RT of pelvic malignancies was 17.6 % (72/410 cases); 83.3 % (60/72) were classified as radiation osteitis (RO, type-categories >a< and >b< combined), while 16.7 % (12/72) were classified as osteoradionecrosis (ORN, category >c<), thus radiological ORN was 4–5 times less frequent than RO. Median age of patients with RO and ORN was 55 years (range 28–84 years) and 60 years (range 43–84 years), respectively.
Median latency time between termination of RT and first onset of RIR on MRI was 4 months (range 1.5–35 months) and median latency time between termination of RT and the maximum of RIR was 8.5 months (range 1.5–35 months). The predominant site of involvement was the sacrum (43.1 %, 31/72 patients), with extra-sacral bone involvement in 54.2 % (39/72), most frequently the acetabulum and adjacent to the symphysis (Electronic Supplemental Material, Fig. 1). Fatty conversion of the bone marrow within the irradiated field was seen in all patients.
RIR-related factors
Patients with N0 commonly underwent 3DCRT, while patients with N1-3 generally received IMRT, with an extra irradiation boost to pathologic lymphatic nodes (p = .036, hazard ratio (HR) 5.5). Patients who received irradiation with IMRT had a significantly higher risk for pathological sacral signal changes compared to 3DCRT technique (p = .000). Patients with lymph node metastases (N1-3, 52.4 %) showed an increased risk with a HR of 2.4 to develop RIR compared to patients without lymph node metastases (N0, 47.6 %).
RIR frequently occurred in patients with RT receiving a threshold dose >40 Gy (177/374, p=0.034, HR 4.0) (Fig. 6).
Patients with acute (clinically apparent) RT reactions (45.6 %) showed an increased risk for RIR (p=0.014, HR 1.8).
Patients with osteopenia combined with SIJ degeneration had a statistically significant risk for RIR compared to patients without bone loss or degenerative disease (p=0.002, HR 4.9).
Patients with additional chemotherapy demonstrated a statistically non-significant higher risk to develop RIR (p=.069).
The statistically non-significant characteristics of RO and ORN are listed in Table 4.
Classification system
The MR signal changes would frequently start laterally, close to the sacroiliac joint line (Fig. 2a) and then extend medially with increasing size. If bilateral RIR were present, the highest category was used for the classification (Fig. 2d). In our study the RIR in the sacrum were classified into three different categories and its stages using MRI studies (I / II / III, a / b / c, - / +). Additional CT-studies were used to diagnose IF in a majority of patients (Electronic Supplemental Material, Fig. 2). In the size category, a relatively equal distribution for stages I, II and III, with 23/72 (31.9 %), 26/72 36.1 %, 21/72 (29.2 %) and 2/72 (2.8 %) with extra sacral changes were observed, respectively.
The early stage Ia- (Fig. 2a), representative of minor RO without fractures, occurred with 25.7 % (18/70), while full expression of ORN with involvement of the entire sacrum and insufficiency fractures – stage IIIc+ – occurred in 11.4 % (8/70). Of the patients, 15.7 % (11/70) were classified as IIa- (Fig. 3a) and 14.3 % (10/70) as IIIb+ (Fig. 3c). Stage IIb+ was diagnosed in 7.1 % (5/70) and stage Ib- in 5.7 % (4/70) of cases. Stages IIa+, IIb- (Fig. 3b) and IIc+ developed in 4.3 % (3/70) of patients. Stages Ia+, IIc-, IIIa-, IIIa+ and IIIb- each occurred in one patient only (1.4 %, 1/70). Stages Ib+, Ic-, Ic+ and IIIc- were not seen. Two patients with exclusively extra-sacral involvement (Electronic Supplemental Material, Fig. 1) were not categorized.
MR follow-up (including CT and scintigraphy in Electronic Supplemental Material, Figs. 2–4) in a 66-year-old patient with a history of corpus carcinoma (T1aN0M0) following radiation therapy (RT) of the small pelvis with a total of 45 Gy. (a) Nine months after RT, axial T1wi +C shows initial punctuated and linear signal abnormalities (RISC IIa-) in the sacrum (arrows). (b) Five months after RT, there are increasing areas with bilateral contrast enhancement on coronal T1wi +C (RISC IIb-) medially to the SIJ (arrows). (c) Eight months after RT, the sacral alterations typical of radiation osteitis (RO) have reached their maximum (RISC IIIb+). (d) In the same study as image (c) a fracture line on the left side parallel to the SIJ is seen uniquely on axial T1wi (arrows) (Electronic Supplemental Material, Fig. 2)
Insufficiency fractures
Of patients with pathological sacral RIR, 43.1 % (31/72) were diagnosed with one or more IF, 25 % (18/72) with a single IF and 18.1 % (13/72) with multiple, bilateral IF (Fig. 3d) (Electronic Supplemental Material, Fig. 2). Of the cases, 23 % had additional bone scintigraphies during follow-up (Electronic Supplemental Material, Fig. 3). As a side finding, only cases with fracture-adjacent sclerosis in CT showed a positive uptake in scintigraphy, indicating increased bone remodelling during fracture healing, which has an important implication in the interpretation of historic data from studies using scintigraphy only for facture detection. Forty-one patients (56.9 %) had no evidence of IF (Fig. 5). The median time period from the end of radiotherapy to first appearance of fractures was 10 months.
Bone marrow recovery
Fifty-six cases (56/72, 77.8 %) showed a significant recovery towards a normal bone marrow signal, six (6/72, 8.3 %) even returned to normal (Electronic Supplemental Material, Fig. 4). Ten patients (10/72, 13.9 %) showed no recovery on MRI.
The median time from occurrence of RIR until first signs of recovery from RIR in MRI was 16.5 months (range 1–74 months), the time to complete return to normal was 39.5 months (range 14–113 months) (Fig. 4). The occurrence of IF did not influence the time to bone marrow recovery, and healing of fractures without therapeutic measures was observed.
The median age was 63.5 years (range 43–84 years) for patients without any recovery to physiological bone marrow signal, 56 years (range 28–84 years) for those with a partial recovery, and 48 years (range 35–59 years) for those whose bone marrow signal returned to normal as imaged by MRI (Figs. 5 and 6).
Interestingly, acute clinical RT-related side effects had not only a statistically significant positive correlation with time to first occurrence of RIR but also with regard to recovery to physiological bone marrow signal (p=.011; p=0.036, respectively).
Discussion
In our study on radiation-induced reactions (RIRs) of the pelvic skeleton we observed a repetitive sequence of pathological changes in the sacrum on MRI, from the frequently observed radiation-osteitis (~83 %) leading to the occasional end-stage of osteoradionecrosis (~16 %) with frequently associated insufficiency fractures (43 %). All radiation-induced reactions were temporary and resolved over time (~21 months average). A new classification system (RISC) helped to structure disease stages.
A number of publications exist investigating the incidence of pelvic insufficiency fractures related to irradiation [12, 24, 27,28,29], but to date, there is no study that evaluates the differences in occurrence and MR morphology of potential precursor stages: radiation osteitis (RO) and osteoradionecrosis (ORN). Also, morphological criteria and imaging modalities to describe RO, ORN and IF vary widely [7, 24, 30]. As a result of these inconsistencies, the reported incidence of pelvic RIR in the literature ranges from 0.44 % to 34 % [7, 12,13,14, 24, 30, 31]. We therefore suggest a new classification (abbreviated RISC), to improve standardization and comparison of post-irradiation MR signal changes in the sacrum.
In two CT studies, the time-to-onset of symptomatic ORN or IF was between 11 and 44 months [7, 24]. Median time following RT to MR diagnosis of RIR was 25 months average in the Ugurluer et al. study [12]. In our study, initial and maximal RIR presented earlier, after 5.9 and 10.7 months, respectively, after RT, which could be explained by different follow-up regimens and evaluation of asymptomatic patients in our cohort in contrast to Ugurluer et al. [12] .
IF of pelvic bones are common complications of ORN and occur frequently in the sacrum [25, 32, 33]. High loading forces through the sacrum in combination with osteopenia increases the risk for biomechanical failure and IF [30, 34]. Patients in our cohort with osteopenia had a higher risk (p = .008, hazard ratio 3.6) for developing IF than patients without.
According to literature, the incidence of radiation-induced IF in the sacrum ranges from 1.7 % to 89 % [12, 14, 29, 32, 35,36,37]. Tai et al. demonstrated a 5-year cumulative incidence rate of 2.1 % for symptomatic patients [14]. Ugurluer et al. reported that 41.2 % of patients with IF had clinical symptoms [12]. In our study, IF was diagnosed in 31 of 410 patients, resulting in a cumulative incidence of 7.6 %. The difference is probably due to the fact that the combined imaging modalities in our study were more sensitive for IF, and that our cohort included asymptomatic patients. IF did not significantly prolong time to recovery from RO or ORN, and all IF showed signs of healing without treatment during follow-up, which is important for clinical management. This wide range may reflect varying protocols, definitions and methods applied, but may also be influenced by imaging methods used in follow-up as well as the radiologist’s attention to such findings.
In our study ORN (category c) with an extended sacral involvement (category III) was always associated with IF and vice versa (p = 0.000, HR 6.8), as opposed to early stages (I and II). The mechanism of action is likely related to a decreased elasticity of necrotic bone prone to failure. Also, ORN was significantly correlated with higher radiation doses as compared to radiation osteitis, although doses of 50 Gy or higher, as stated by Ramalho et al., had not been reached in all cases [38, 39].
Few studies describe the evolution of radiological features of RIR in bones. Daldrup-Link et al. describe subtle MR signal changes related to RO, hyperintense on T2w, which we observed in 50 % (RISC type >a<) of all affected cases [15; 16]. Our findings demonstrated that initial findings most frequently grow in size and intensity suggesting a variable course of pathobiological evolution from RO to ORN to IF that may halt at any stage during the development. Confounding factors (e.g. chemotherapy) have yet to be studied. Previously published studies indicated that most pathological osseous signal changes after pelvic RT would eventually demonstrate partial bone marrow recovery [7, 30, 33]. Time to remission was in a range of 3 and 30 months, depending on the study and presence or absence of IF [13, 30, 37, 39]. Holler et al. demonstrated a partial or complete remission of IF in 71.4 %, which is comparable to our study with a partial or complete remission of RIR in 77.8 %, independent of the presence of IF [7].
Limitations
Due to the retrospective character of this study, protocols for follow-up examinations were variable. We believe that our exclusion criteria for insufficient imaging were adequate for a thorough image analysis before and after RT and that the cohort size created valuable follow-up data. Another limiting factor may be a gender bias due to a predominance of gynaecological tumours. Due to the retrospective character of this study, no pathological confirmation could be obtained. Furthermore, the diagnosis of DJD and osteopenia was based on CT findings. ORN may have been underestimated in RISC stage >b<, but we believe that the strict application of objective MR parameters increases overall standardization and reliability for MR follow-up comparisons. Lastly, the relevance of the received organ dose to bone and influence of radiation technique is yet unknown and the subject of further investigation.
Conclusion
Radiation-induced sacral bone marrow changes following pelvic radiotherapy appear with a high incidence in about one out of six patients. Insufficiency fractures are common, self-limiting late effects with no delayed healing. The majority of patients presented a pattern of limited radiation osteitis with an early onset and bone marrow recovery within 1–2 years, whereas persisting osteoradionecrosis is proportionately rare.
Abbreviations
- 3DCRT:
-
3D-conformation-radiotherapy
- CT:
-
Computer tomography
- DJD:
-
Degenerative joint disease
- ICBT:
-
Intra-cavity brachytherapy
- IF:
-
Insufficiency fracture
- IMRT:
-
Intensity-modulated radiotherapy
- MRI:
-
Magnetic resonance imaging
- ORN:
-
Osteoradionecrosis
- RIR:
-
Radiation-induced reactions
- RISC:
-
Radiation-induced sacral changes
- RO:
-
Radiation osteitis
- RT:
-
Radiotherapy
- SIJ:
-
Sacroiliac joint
- TIRM:
-
Turbo inversion recovery magnitude
- TSE:
-
Turbo spin echo
- WI:
-
Weighted images
References
Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J et al (2013) Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 49:1374–1403
Zeng SY, Li LY, Shu KY, Pan M, Li HP, Luo B (2008) Concurrent chemoradiotherapy versus radiotherapy in advanced cervical carcinoma. Ai Zheng 27:942–946
Meyer LA, Bohlke K, Wright AA (2016) Postoperative Radiation Therapy for Endometrial Cancer: American Society of Clinical Oncology Clinical Practice Guideline Endorsement of the American Society for Radiation Oncology Evidence-Based Guideline. J Oncol Pract 12:182–185
Ghosn M, Kourie HR, Abdayem P, Antoun J, Nasr D (2015) Anal cancer treatment: current status and future perspectives. World J Gastroenterol 21:2294–2302
Dalinka MK, Mazzeo VP Jr (1985) Complications of radiation therapy. Crit Rev Diagn Imaging 23:235–267
Bluemke DA, Fishman EK, Scott WW Jr (1994) Skeletal complications of radiation therapy. RadioGraphics 14:111–121
Holler U, Hoecht S, Wudel E, Hinkelbein W (2001) Osteoradionecrosis after radiotherapy for gynecologic tumors. Strahlenther Onkol 177:291–295
Ewing J (1926) Radiation Osteitis. Acta Radiol os-6:399–412
Bostel T (2015) Imaging of complications and toxicity following tumor therapy: bone marrow and chemotherapyimaging of complications and toxicity following tumor therapy: bone marrow and chemotherapy. Springer Verlag, p 17–42
Wood J, Ver Halen J, Samant S, Florendo N (2015) Radiation-induced sarcoma masquerading as osteoradionecrosis: case report and literature review. J Laryngol Otol 129:279–282
Kanberoglu K, Mihmanli I, Kurugoglu S, Ogut G, Kantarci F (2001) Bone marrow changes adjacent to the sacroiliac joints after pelvic radiotherapy mimicking metastases on MRI. Eur Radiol 11:1748–1752
Ugurluer G, Akbas T, Arpaci T, Ozcan N, Serin M (2014) Bone complications after pelvic radiation therapy: Evaluation with MRI. J Med Imaging Radiat Oncol 58:334–340
Abe H, Nakamura M, Takahashi S, Maruoka S, Ogawa Y, Sakamoto K (1992) Radiation-induced insufficiency fractures of the pelvis: evaluation with 99mTc-methylene diphosphonate scintigraphy. AJR Am J Roentgenol 158:599–602
Tai P, Hammond A, Dyk JV et al (2000) Pelvic fractures following irradiation of endometrial and vaginal cancers-a case series and review of literature. Radiother Oncol 56:23–28
Daldrup-Link HE, Henning T, Link TM (2007) MR imaging of therapy-induced changes of bone marrow. Eur Radiol 17:743–761
Daldrup HE, Link TM, Blasius S et al (1999) Monitoring radiation-induced changes in bone marrow histopathology with ultra-small superparamagnetic iron oxide (USPIO)-enhanced MRI. J Magn Reson Imaging 9:643–652
Zhuang Q, Zhang Z, Fu H, He J, He Y (2011) Does radiation-induced fibrosis have an important role in pathophysiology of the osteoradionecrosis of jaw? Med Hypotheses 77:63–65
Shah KN, Racine J, Jones LC, Aaron RK (2015) Pathophysiology and risk factors for osteonecrosis. Curr Rev Musculoskelet Med 8:201–209
Burgener FAKM (1991) Osteopenia. Differential diagnosis in Conventional Radiology, vol 21. Thieme Medical Publishers Inc, New York, pp 10–11
Fu AL, Greven KM, Maruyama Y (1994) Radiation osteitis and insufficiency fractures after pelvic irradiation for gynecologic malignancies. Am J Clin Oncol 17:248–254
Hopewell JW (2003) Radiation-therapy effects on bone density. Med Pediatr Oncol 41:208–211
Kim HJ, Boland PJ, Meredith DS et al (2012) Fractures of the sacrum after chemoradiation for rectal carcinoma: incidence, risk factors, and radiographic evaluation. Int J Radiat Oncol Biol Phys 84:694–699
Oh D, Huh SJ, Nam H et al (2008) Pelvic insufficiency fracture after pelvic radiotherapy for cervical cancer: analysis of risk factors. Int J Radiat Oncol Biol Phys 70:1183–1188
Feltl D, Vosmik M, Jirasek M, Stahalova V, Kubes J (2006) Symptomatic osteoradionecrosis of pelvic bones in patients with gynecological malignancies-result of a long-term follow-up. Int J Gynecol Cancer 16:478–483
Cabarrus MC, Ambekar A, Lu Y, Link TM (2008) MRI and CT of insufficiency fractures of the pelvis and the proximal femur. AJR Am J Roentgenol 191:995–1001
Krestan C, Hojreh A (2009) Imaging of insufficiency fractures. Eur J Radiol 71:398–405
Bluemke DA, Fishman EK, Kuhlman JE, Zinreich ES (1991) Complications of radiation therapy: CT evaluation. RadioGraphics 11:581–600
Schmeler KM, Jhingran A, Iyer RB et al (2010) Pelvic fractures after radiotherapy for cervical cancer: implications for survivors. Cancer 116:625–630
Konski A, Sowers M (1996) Pelvic fractures following irradiation for endometrial carcinoma. Int J Radiat Oncol Biol Phys 35:361–367
Kwon JW, Huh SJ, Yoon YC et al (2008) Pelvic bone complications after radiation therapy of uterine cervical cancer: evaluation with MRI. AJR Am J Roentgenol 191:987–994
Ioffe YJ, Hillen TJ, Zhou G et al (2014) Postradiation damage to the pelvic girdle in cervical cancer patients: is intensity-modulated radiation therapy safer than conventional radiation? Int J Gynecol Cancer 24:806–812
Ikushima H, Osaki K, Furutani S et al (2006) Pelvic bone complications following radiation therapy of gynecologic malignancies: clinical evaluation of radiation-induced pelvic insufficiency fractures. Gynecol Oncol 103:1100–1104
Higham CE, Faithfull S (2015) Bone Health and Pelvic Radiotherapy. Clin Oncol (R Coll Radiol) 27:668–678
Lourie H (1982) Spontaneous osteoporotic fracture of the sacrum. An unrecognized syndrome of the elderly. JAMA 248:715–717
Tokumaru S, Toita T, Oguchi M et al (2012) Insufficiency fractures after pelvic radiation therapy for uterine cervical cancer: an analysis of subjects in a prospective multi-institutional trial, and cooperative study of the Japan Radiation Oncology Group (JAROG) and Japanese Radiation Oncology Study Group (JROSG). Int J Radiat Oncol Biol Phys 84:e195–e200
Blomlie V, Rofstad EK, Talle K, Sundfor K, Winderen M, Lien HH (1996) Incidence of radiation-induced insufficiency fractures of the female pelvis: evaluation with MR imaging. AJR Am J Roentgenol 167:1205–1210
Huh SJ, Kim B, Kang MK et al (2002) Pelvic insufficiency fracture after pelvic irradiation in uterine cervix cancer. Gynecol Oncol 86:264–268
Ramalho J, Castillo M (2015) Radiotherapy induced changes in spine and spinal contents. In: Kauczor HU, Bäuerle T (eds) Imaging of complications and toxicity following tumor therapy. Springer Verlag, Berlin
Uezono H, Tsujino K, Moriki K et al (2013) Pelvic insufficiency fracture after definitive radiotherapy for uterine cervical cancer: retrospective analysis of risk factors. J Radiat Res 54:1102–1109
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Prof. Dr. Stefan Delorme.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
Thomas Hielscher, from the Department of Biostatistics at the DKFZ kindly provided statistical advice for this manuscript.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• Cross-sectional study
Electronic supplementary material
ESM 1
(DOCX 3433 kb)
Rights and permissions
About this article
Cite this article
Meixel, A.J., Hauswald, H., Delorme, S. et al. From radiation osteitis to osteoradionecrosis: incidence and MR morphology of radiation-induced sacral pathologies following pelvic radiotherapy. Eur Radiol 28, 3550–3559 (2018). https://doi.org/10.1007/s00330-018-5325-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5325-2