Abstract
Objectives
To investigate the impact of background parenchymal enhancement (BPE), amount of fibroglandular tissue (FGT) and menopausal status on apparent diffusion coefficient (ADC) values in differentiation between malignant and benign lesions.
Methods
In this HIPAA-compliant study, mean ADC values of 218 malignant and 130 benign lesions from 288 patients were retrospectively evaluated. The differences in mean ADC values between benign and malignant lesions were calculated within groups stratified by BPE level (high/low), amount of FGT (dense/non-dense) and menopausal status (premenopausal/postmenopausal). Sensitivities and specificities for distinguishing malignant from benign lesions within different groups were compared for statistical significance.
Results
The mean ADC value for malignant lesions was significantly lower compared to that for benign lesions (1.07±0.21 x 10−3 mm2/s vs. 1.53±0.26 x 10−3 mm2/s) (p<0.0001). Using the optimal cut-off point of 1.30 x 10−3 mm2/s, an area under the curve of 0.918 was obtained, with sensitivity and specificity both of 87 %. There was no statistically significant difference in sensitivities and specificities of ADC values between different groups stratified by BPE level, amount of FGT or menopausal status.
Conclusions
Differentiation between benign and malignant lesions on ADC values is not significantly affected by BPE level, amount of FGT or menopausal status.
Key Points
• ADC allows differentiation between benign and malignant lesions.
• ADC is useful for breast cancer diagnosis despite different patient characteristics.
• BPE, FGT or menopause do not significantly affect sensitivity and specificity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Magnetic resonance imaging (MRI) is a quintessential tool for breast cancer diagnosis. Due to its high sensitivity, it has several established clinical indications including pre-operative staging, evaluation of treatment response in patients receiving neoadjuvant therapy, differentiation between scar tissue and tumour recurrence, examination of patients with breast implants, and screening of high-risk patients [1, 2]. Currently, dynamic contrast-enhanced MRI (DCE-MRI) is the most sensitive test for the diagnosis of breast cancer with good specificity [3,4,5]. Moreover, in the past few decades, efforts have been made to further improve the diagnostic accuracy of DCE-MRI [6], with several functional MRI parameters having been investigated for their utility in improving the diagnostic accuracy of DCE-MRI [7, 8]. Diffusion-weighted imaging (DWI) has emerged as the most reliable MRI parameter for an improved diagnosis and characterization of breast cancer in a multiparametric setting [9, 10].
DWI is a non-contrast MRI technique, sensitive to the random motion of water molecules in tissues [11]. Water diffusivity can be quantified using the apparent diffusion coefficient (ADC), which varies according to the amount of cellularity, intracellular and extracellular oedema, viscosity and fibrosis [12,13,14]. Numerous studies have evaluated DWI with ADC mapping for the diagnosis of breast tumours. It has been shown that ADC as a quantitative imaging biomarker can be used to differentiate malignant from benign lesions with high sensitivity and specificity of up to 98 % and 93 %, respectively [9, 10, 15,16,17,18,19].
Although DWI with ADC mapping is being implemented in clinical routine care, it is still unclear which patient characteristics may affect ADC values and what their impact is on the diagnostic accuracy of DWI. A few preliminary reports have addressed the impact of different patient characteristics, including background parenchymal enhancement (BPE), amount of fibroglandular tissue (FGT) and menopausal status on ADC values but with conflicting results [20,21,22,23,24,25,26,27,28]. In addition, it has also been reported that breast tissue composition can influence intravoxel signals of breast tumours and alter their ADC values [29]. To date, however, there are no data on whether BPE, FGT and menopausal status can affect the diagnostic accuracy of ADC for breast cancer detection.
Therefore, the goal of the study was to investigate the impact of BPE, FGT and menopausal status on ADC measurements and cancer diagnosis in patients with breast tumours.
Materials and methods
This single-institution retrospective study was compliant with Health Insurance Portability and Accountability Act guidelines and approved by the Institutional Review Board with a waiver of informed consent.
Patient selection
We queried our institutional database for all consecutive patients who underwent multiparametric 3.0T MRI of the breast combining T2-weighted, DCE-MRI and DWI imaging between January 2011 and January 2013. The search yielded 854 results. We then applied the following exclusion criteria: (1) Breast Imaging Reporting and Data System (BI-RADS®) 0–3 classification on the MRI report; (2) treatment received prior to MRI examination; (3) histopathological verification of suspicious imaging findings by either image-guided or surgical biopsy unavailable; (4) lesions measuring less than 0.8 cm on contrast-enhanced T1-weighted sequence; and (5) DWI images hampered by artifacts or suboptimal fat suppression. Thus, a total of 288 patients were included in our study.
MRI acquisition
MR imaging was performed on a 3.0T system (Discovery MR750; GE Healthcare, WI, USA) using the body coil as a transmitter and a dedicated 16-channel phased-array receiver coil (Sentinelle Vanguard, Toronto, Canada). Conventional T1-weighted (3D T1-weighted gradient echo VIBRANT sequence; TR/TE 4.3/2.1 ms; flip angle 10o; matrix size 320x192; field of view (FOV) 30 cm; slice thickness 1 mm; number of excitations (NEX) 1; acquisition time ~1 min) as well as T2-weighted (fast spin-echo sequence with TR/TE 4,384/102 ms; auto flip angle 111o; matrix size 288x224; FOV 30 cm; slice thickness 3 mm; NEX 2; acquisition time ~3.5 min) images were acquired with and without fat suppression.
Axial DWI MR imaging was performed using 2D, DW single-shot, dual spin echo-planar imaging (EPI) sequences (TR 6,000 ms; minimum TE; flip angle 90°; acquisition matrix: 98x98 or 128x128; reconstructed matrix: 256x256; FOV 28–38 cm; slice thickness: 4 or 5 mm; NEX 3; slice gap: 0–1 mm; fat suppression: enhanced; parallel imaging: ASSET; acquisition time ~2 min for 2 b-values). In all the patients, DWI was acquired at b-values of 0 and 600 s/mm2.
DCE-MRI images were acquired using a sagittal 3D T1-weighted sequence before and at three points at 60-s intervals after an injection of 0.1 mmol/kg of gadopentetate dimeglumine (Magnevist; Bayer HealthCare, Hanover, NJ, USA). Subsequently, axial 3D T1-weighted gradient echo VIBRANT delayed contrast-enhanced imaging was performed.
Data and image evaluation
All MRI studies and patient medical records were reviewed by a single dedicated breast radiologist (M.D.) who had 7 years of experience in breast MRI. The radiologist had access to patient data, including age, menopausal status and imaging study reports, but was blinded to pathological data. Menopausal status was classified into premenopausal and postmenopausal, defined as at least 1 year of amenorrhoea. The information acquired was annotated and compiled using a standardized form.
MRI
Images were analysed on a GE Advantage Workstation (GE Healthcare). Lesions were identified on MRI using DCE-MRI images and the longest transverse axis was measured as the lesion size. BPE was assessed on the first post-contrast sequence and the amount of FGT was assessed on T1 images without fat saturation. Both BPE and FGT classifications were performed according to the 5th edition BI-RADS [30]. BPE was classified as minimal, mild, moderate or marked. The amount of FGT was classified as almost entirely fat, scattered, heterogeneous or extreme FGT. When there was asymmetry of BPE or amount of FGT, the breast with the largest lesion was considered the reference standard.
DWI
The slice location with the largest transverse axis on T1-weighted DCE-MRI was used to identify the closest image location of the lesion on DWI. High b-value DWI images were visually assessed for hyperintensities matched to enhancing lesions on DCE-MRI. Subsequently, parametric quantitative ADC maps were generated using READY View software (GE Healthcare). Mean ADC values were calculated by manually selecting a 2D region of interest covering most parts of the lesion, but avoiding cystic or necrotic portions, surrounding normal tissue and susceptibility artifacts due to the presence of post-biopsy clips or changes.
Histopathological analysis
In all patients, histopathology was used as the standard of reference. Histopathological reports of the breast lesions were reviewed and breast lesions were classified as either malignant or benign, which included high-risk lesions. Histopathology from surgical specimens was considered the reference standard, and when it was not available, histopathology from breast biopsy samples were used.
Statistical analysis
For statistical analysis, BPE and FGT classifications were dichotomized. Breasts with minimal or mild BPE were considered as ‘low’ BPE and breasts with moderate or marked BPE were considered as ‘high’ BPE. Breasts with almost entirely fat or scattered FGT composition were considered as ‘non-dense’ and breasts with heterogeneous or extreme FGT composition were considered as ‘dense’.
ADC values were presented as mean ± standard deviation (SD) measured in units of 10−3 mm2/s. ADC values were measured on a ‘lesion-by-lesion’ basis and bilateral lesions in the same patient were assumed to be non-correlated. All ADC values were examined for normality and comparisons were made using two-sample t-tests or non-parametric tests as necessary. The overall sensitivity and specificity of ADC for identifying malignant lesions was plotted using a receiver operating characteristic (ROC) curve and the optimal cut-off point for ADC was identified using Youden’s index [31]. To determine the impact of BPE, FGT and menopausal status on ADC sensitivity and specificity, we used this cut-off point in a chi-square test to compare groups of patients stratified according to the respective dichotomized categories.
All statistical tests were two-tailed and the significance was established at p < 0.05. The analysis for this study was generated using SAS v9.4 software (SAS Institute Inc., Cary, NC, USA).
Results
A total of 348 lesions in 288 women were identified. The mean patient age was 49 years (range, 23–81 years). There were 218 malignant and 130 benign lesions, including 23 high-risk and 107 other benign lesions, with a mean size of 2.4 cm (range 0.8–12 cm). The patient characteristics are demonstrated in Table 1 and the characteristics of the lesions are summarized in Table 2.
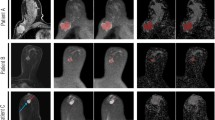
The mean ADC (± SD) value for malignant lesions was lower compared to that for benign lesions (1.07 ± 0.21 x 10−3 mm2/s vs. 1.53 ± 0.26 x 10−3 mm2/s), with a statistically significant difference (p < 0.0001). Representative images of DCE-MRI, DWI and ADC mapping images for malignant and benign lesions are demonstrated by Figs. 1 and 2. ROC curve analysis yielded an optimal ADC cut-off value of 1.30 x 10−3 mm2/s with an area under the curve (AUC) of 0.918 (Fig. 3).
A 49-year-old postmenopausal woman with dense breasts and low background parenchymal enhancement. In the left breast, an irregular mass with rim enhancement is noted on T1-weighted contrast enhanced (a), diffusion-weighted imaging (DWI) (b) and ADC map (c) images. The mean ADC value obtained was 0.949 x 10-3 mm2/s. Biopsy yielded invasive ductal carcinoma
A 39-year-old premenopausal woman with dense breasts and low background parenchymal enhancement. In the left breast, a 1.7 cm heterogeneously enhancing mass
with circumscribed margins is noted on T1-weighted contrast enhanced (a), diffusion-weighted imaging (DWI) (b) and ADC map (c) images. The mean ADC value obtained was 1.670 x 10-3 mm2/s. Biopsy yielded fibroadenoma
The overall and stratified ADC sensitivities and specificities are summarized in Table 3. There were no statistically significant differences in sensitivity and specificity for ADC in differentiating malignant and benign lesions in groups of patients separated by BPE level, amount of FGT or menopausal status.
Table 4 summarizes the mean ADC values for benign and malignant breast lesions stratified by BPE, FGT and menopausal status. When patients were stratified as low and high BPE, there was a significant difference in the ADC values of benign and malignant lesions (p < 0.0001 for both) with an AUC of 0.903 and 0.937, respectively (Fig. 4). Similarly, when patients were stratified based on amount of FGT, the difference between benign and malignant lesion ADC values was statistically significant among non-dense and dense breasts (p = 0.0003 and p < 0.0001, respectively) with an AUC of 0.887 and 0.924, respectively (Fig. 5). When stratified by menopausal status, differentiation of benign and malignant lesions was equally possible (p < 0.0001 for both) with an AUC of 0.938 in premenopausal and 0.892 in postmenopausal women (Fig. 6).
Discussion
Our findings show that DWI with ADC mapping distinguishes benign and malignant lesions with good sensitivity and specificity (87 % for both), and was not significantly influenced by the patient characteristics such as FGT, BPE and menopausal status. When patients were stratified according to BPE, FGT and menopausal status, there remained a significant difference in ADC values between benign and malignant lesions: malignant lesions consistently demonstrated significantly lower ADC values compared with benign lesions. Thus, our findings indicate that DWI with ADC mapping is equally accurate in breast cancer diagnosis regardless of different patient characteristics, and support its use as part of a standard imaging protocol for improved cancer diagnosis.
The role of DWI in breast imaging has been previously investigated, with several studies demonstrating that ADC is a useful tool for characterizing breast lesions, even though optimal thresholds varied among these studies [9, 10, 15,16,17,18,19]. In a meta-analysis by Tsushima et al. [18], an ADC threshold value of 1.23 x 10−3 mm2/s for the differentiation of benign and malignant lesions was obtained with a pooled sensitivity and specificity of 89 % and 77 %, respectively. In our study, using a derived optimal threshold of 1.30 x 10−3 mm2/s, we achieved a sensitivity and specificity of 87 %, which is in good agreement with previously published reports [9, 10, 16, 17, 19].
To the best of our knowledge, this is the first study to investigate the impact of FGT, BPE as well as menopausal status on the diagnostic accuracy of DWI with ADC mapping for breast cancer diagnosis in one patient collective.
Several studies have had mixed findings for the effect of BPE on DWI ADC values, but none have evaluated the subsequent impact on diagnostic accuracy. When assessing the relationship of BPE and ADC of normal fibroglandular tissue, studies have shown conflicting results [22, 25, 27]. For example, in patients with normal breast DWI MRIs, McDonald et al. [27] found that whereas mammographic breast density and ADC of FGT are positively correlated, BPE and ADC of FGT are not. Similar correlations were observed by Iacconi et al. [25] in a population composed mainly of patients with breast cancer. Cho et al. [22] showed that patients with breast cancer exhibited significantly higher BPE values, but like the other studies BPE values were not correlated with ADC of FGT. On the other hand, different results were obtained by Kawamura et al. [26], who found that BPE was inversely correlated with ADC of FGT. In our study, the sensitivity and specificity of DWI with ADC mapping in differentiating benign from malignant lesions was not statistically significantly affected by BPE.
In the current study, we employed a fat-suppressed sequence and did not observe a significant impact of amount of FGT on ADC values of both benign and malignant breast lesions. Previously, Partridge et al. assessed the influence of intravoxel fat signal on ADC values obtained using DWI with and without fat suppression for both tumour and normal FGT in a group of 21 women with diagnosed breast cancer [29]. In this study, there were systematic underestimations of ADCs for both tumour and normal breast tissue due to intravoxel contribution from fat signal on non-fat-suppressed DWI but not when adequate fat suppression was used. This was more pronounced for normal tissue than for tumours and was worse in women with mammographic low density than those with extremely dense breasts. These findings confirm the importance of a robust fat suppression for optimal diagnostic accuracy.
Studies that have investigated the impact of FGT or mammographic breast density on ADC values of healthy breast tissue in breast cancer patients or healthy volunteers have not yet evaluated its diagnostic accuracy for breast cancer diagnosis [22, 23, 25,26,27]. O’Flynn et al. [28], who investigated BRCA 1/2 mutation carriers and patients who had undergone supradiaphragmatic radiotherapy, showed that patients who had received radiation therapy to the breast had higher ADC values of FGT, which could improve the detection of malignant tumours. Fat necrosis induced by radiation therapy could be attributed to the higher ADC values found in that population. The FGT ADC also correlated with mammographic breast density in their study, being higher in dense breasts and lower in fatty ones. Even though the amount of fat in the breast may influence DWI, in our study the sensitivity and specificity of ADC in differentiating benign from malignant lesions were not statistically significantly different between dense and non-dense breasts.
Some studies have also evaluated the relationship between menstrual cycle and menopausal status with DWI [20, 21, 24]. A previous study by Clendenen et al. [24] demonstrated that the FGT ADC could be affected by the menstrual cycle. On the other hand, studies by El Khouli et al. [20] and Kim et al. [21], which evaluated the ADC values of benign and malignant lesions, did not find significant differences in the ADCs of premenopausal and postmenopausal women, similar to what was observed in our study. In our study, the accuracy for breast cancer diagnosis was not significantly different in pre- and postmenopausal patients.
Limitations
There were several limitations to our retrospective study. Due to the low spatial resolution of DWI, lesions smaller than 0.8 cm were not included. Therefore, insights into this specific subgroup are limited. Since the study was conducted in a comprehensive cancer centre, our population presented with a relatively high number of malignant compared to benign lesions. Additionally, a greater number of dense than non-dense breasts were found in our study population, which was composed mostly of premenopausal women under the age of 50 years.
Conclusion
In conclusion, our study demonstrated that DWI with ADC mapping enables the differentiation of benign from malignant lesions with high sensitivity and specificity. The diagnostic accuracy of DWI with ADC mapping is not significantly impacted by different levels of BPE, FGT and menopausal status. Therefore, data indicate that DWI with ADC can be added to a standard imaging protocol regardless of different patient characteristics for an improved breast cancer diagnosis.
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- AUC:
-
Area under the curve
- BIRADS:
-
Breast Imaging Reporting and Data System
- BPE:
-
Background parenchymal enhancement
- DWI:
-
Diffusion-weighted imaging
- DCE:
-
Dynamic contrast-enhancement
- FGT:
-
Fibroglandular tissue
- MRI:
-
Magnetic resonance imaging
- ROC:
-
Receiving operator characteristic
- SD:
-
Standard deviation
References
Mann RM, Balleyguier C, Baltzer PA et al (2015) Breast MRI: EUSOBI recommendations for women's information. Eur Radiol 25:3669–3678
Sardanelli F, Boetes C, Borisch B et al (2010) Magnetic resonance imaging of the breast: recommendations from the EUSOMA working group. Eur J Cancer 46:1296–1316
Kuhl CK, Strobel K, Bieling H, Leutner C, Schild HH, Schrading S (2017) Supplemental Breast MR Imaging Screening of Women with Average Risk of Breast Cancer. Radiology 283:361–370
Lehman CD, Smith RA (2009) The role of MRI in breast cancer screening. J Natl Compr Canc Netw 7:1109–1115
Lourenco AP, Donegan L, Khalil H, Mainiero MB (2014) Improving outcomes of screening breast MRI with practice evolution: initial clinical experience with 3T compared to 1.5T. J Magn Reson Imaging 39:535–539
Partridge SC, DeMartini WB, Kurland BF, Eby PR, White SW, Lehman CD (2009) Quantitative diffusion-weighted imaging as an adjunct to conventional breast MRI for improved positive predictive value. AJR Am J Roentgenol 193:1716–1722
Pinker K, Helbich TH, Morris EA (2017) The potential of multiparametric MRI of the breast. Br J Radiol 90:20160715
European Society of R (2010) White paper on imaging biomarkers. Insights Imaging 1:42–45
Tan SL, Rahmat K, Rozalli FI et al (2014) Differentiation between benign and malignant breast lesions using quantitative diffusion-weighted sequence on 3 T MRI. Clin Radiol 69:63–71
Guo Y, Cai YQ, Cai ZL et al (2002) Differentiation of clinically benign and malignant breast lesions using diffusion-weighted imaging. J Magn Reson Imaging 16:172–178
White NS, McDonald CR, Farid N et al (2014) Diffusion-Weighted Imaging in Cancer: Physical Foundations and Applications of Restriction Spectrum Imaging. Cancer Res 74:4638–4652
Matsubayashi RN, Fujii T, Yasumori K, Muranaka T, Momosaki S (2010) Apparent diffusion coefficient in invasive ductal breast carcinoma: correlation with detailed histologic features and the enhancement ratio on dynamic contrast-enhanced MR images. J Oncol 2010:1–6. https://www.hindawi.com/journals/jo/2010/821048/
Martincich L, Deantoni V, Bertotto I et al (2012) Correlations between diffusion-weighted imaging and breast cancer biomarkers. Eur Radiol 22:1519–1528
Arlinghaus LR, Li X, Rahman AR et al (2011) On the relationship between the apparent diffusion coefficient and extravascular extracellular volume fraction in human breast cancer. Magn Reson Imaging 29:630–638
Partridge SC, Mullins CD, Kurland BF et al (2010) Apparent diffusion coefficient values for discriminating benign and malignant breast MRI lesions: effects of lesion type and size. AJR Am J Roentgenol 194:1664–1673
Caivano R, Villonio A, D’ Antuono F et al (2015) Diffusion weighted imaging and apparent diffusion coefficient in 3 tesla magnetic resonance imaging of breast lesions. Cancer Invest 33:159–164
Suo S, Cheng F, Cao M et al (2017) Multiparametric diffusion-weighted imaging in breast lesions: Association with pathologic diagnosis and prognostic factors. J Magn Reson Imaging 46:740–750
Tsushima Y, Takahashi-Taketomi A, Endo K (2009) Magnetic resonance (MR) differential diagnosis of breast tumors using apparent diffusion coefficient (ADC) on 1.5-T. J Magn Reson Imaging 30:249–255
Kul S, Eyuboglu I, Cansu A, Alhan E (2014) Diagnostic efficacy of the diffusion weighted imaging in the characterization of different types of breast lesions. J Magn Reson Imaging 40:1158–1164
Ei Khouli RH, Jacobs MA, Mezban SD et al (2010) Diffusion-weighted imaging improves the diagnostic accuracy of conventional 3.0-T breast MR imaging. Radiology 256:64–73
Kim JY, Suh HB, Kang HJ et al (2016) Apparent diffusion coefficient of breast cancer and normal fibroglandular tissue in diffusion-weighted imaging: the effects of menstrual cycle and menopausal status. Breast Cancer Res Treat 157:31–40
Cho GY, Moy L, Kim SG et al (2015) Comparison of contrast enhancement and diffusion-weighted magnetic resonance imaging in healthy and cancerous breast tissue. Eur J Radiol 84:1888–1893
Choi YJ, Chen JH, Yu HJ, Li Y, Su MY (2017) Impact of Different Analytic Approaches on the Analysis of the Breast Fibroglandular Tissue Using Diffusion Weighted Imaging. Biomed Res Int 2017:1094354
Clendenen TV, Kim S, Moy L et al (2013) Magnetic resonance imaging (MRI) of hormone-induced breast changes in young premenopausal women. Magn Reson Imaging 31:1–9
Iacconi C, Thakur SB, Dershaw DD, Brooks J, Fry CW, Morris EA (2014) Impact of fibroglandular tissue and background parenchymal enhancement on diffusion weighted imaging of breast lesions. Eur J Radiol 83:2137–2143
Kawamura A, Satake H, Ishigaki S et al (2015) Prediction of background parenchymal enhancement on breast MRI using mammography, ultrasonography, and diffusion-weighted imaging. Nagoya J Med Sci 77:425–437
McDonald ES, Schopp JG, Peacock S et al (2014) Diffusion-weighted MRI: association between patient characteristics and apparent diffusion coefficients of normal breast fibroglandular tissue at 3 T. AJR Am J Roentgenol 202:W496–W502
O'Flynn EA, Wilson RM, Allen SD, Locke I, Scurr E, deSouza NM (2014) Diffusion-weighted imaging of the high-risk breast: Apparent diffusion coefficient values and their relationship to breast density. J Magn Reson Imaging 39:805–811
Partridge SC, Singer L, Sun R et al (2011) Diffusion-weighted MRI: influence of intravoxel fat signal and breast density on breast tumor conspicuity and apparent diffusion coefficient measurements. Magn Reson Imaging 29:1215–1221
Morris EA, Comstock CE, Lee CH, et al. (2013) ACR BI-RADS® magnetic resonance imaging. In: ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. American College of Radiology, Reston, VA
Youden WJ (1950) Index for rating diagnostic tests. Cancer 3:32–35
Acknowledgements
We would like to thank Joanne Chin for technical editing.
Funding
This study has received funding by Memorial Sloan Kettering Cancer Center Support Grant / NIH Core Grant (P30 CA008748), DOD BCRP W81XWH-09-1-0042 grant, and the Breast Cancer Research Foundation grant of Memorial Sloan Kettering Cancer Center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Sunitha B. Thakur.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise (Sujata Patil).
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
Some study subjects or cohorts have been previously reported in Durando M, Gennaro L, Cho GY et al (2016) Quantitative apparent diffusion coefficient measurement obtained by 3.0Tesla MRI as a potential noninvasive marker of tumor aggressiveness in breast cancer. Eur J Radiol 85:1651-1658. However, different data were used in a different context.
Methodology
• retrospective
• observational
• performed at one institution
Rights and permissions
About this article
Cite this article
Horvat, J.V., Durando, M., Milans, S. et al. Apparent diffusion coefficient mapping using diffusion-weighted MRI: impact of background parenchymal enhancement, amount of fibroglandular tissue and menopausal status on breast cancer diagnosis. Eur Radiol 28, 2516–2524 (2018). https://doi.org/10.1007/s00330-017-5202-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-017-5202-4