Abstract
Objective
To explore the feasibility of sentinel lymph node (SLN) identification by contrast-enhanced ultrasound (CEUS) in pre-operative breast cancer patients and the value of enhancement patterns for diagnosing lymph node metastases and characterising axillary nodal burden.
Methods
110 consecutive breast cancer patients were enrolled. Before the surgery, microbubbles were injected intradermally. The lymphatic drainage pathway was detected to identify the SLNs. Blue dye and indocyanine green (ICG) fluorescence were used to trace SLNs during the operation. The enhancement patterns of SLNs were recorded and compared with the final pathological diagnosis.
Results
SLN detection rate was 96.4 % of 110 patients, 134 SLNs were detected in total. The sensitivity, specificity, positive-predictive value, negative-predictive value and accuracy of predicting SLNs metastases by CEUS enhancement patterns were 100 %, 52.0 %, 43.4 %, 100 % and 64.9 %, respectively. No metastatic SLNs were presented as homogeneous enhancement. Low nodal burden with 0–2 SLN metastases in 92.5 % nodes presented as heterogeneous enhancement. No enhancement pattern was proved to be SLN metastases in 100 % patients.
Conclusions
CEUS is a feasible approach for SLN identification. CEUS enhancement patterns can be helpful in recognising metastatic SLNs and nodal burden.
Key Points
• CEUS is a feasible approach for SLN identification and characterisation.
• The enhancement patterns on CEUS can be helpful in recognising metastasised SLNs.
• Homogeneous enhancement pattern has the highest negative-predictive value.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Axillary lymph node (ALN) status is an important prognostic factor in patients with breast cancer [1]. Sentinel lymph node (SLN) biopsy has replaced ALN dissection as the standard procedure for nodal staging in breast cancer with clinical unapparent nodes, while ALN dissection is associated with significant morbidity [2]. The definition of an SLN is an initial lymph node that receives lymphatic drainage from the primary tumour [3]; thus, its histological status can represent the entire lymph node basin.
American Society of Clinical Oncology Guidelines from 2014 [4] recommend clinicians should not perform ALND for women with early-stage breast cancer and one or two SLN metastases who will undergo breast-conserving therapy (BCS) with conventionally fractionated whole-breast radiotherapy. This recommendation is based on the ACOSOG Z2011 [5, 6] randomized trial, which suggests that ALND does not improve local recurrence rates or overall survival for patients with T1/T2 tumours with limited SLN metastases treated with BCS. Thus, differentiation of axillary nodal burden in the diagnostic period remains clinically important, which would aid surgical decision making [7].
The conventional methods for SLN biopsy include radioactive colloids and the blue dye technique [8]. The identification rate of the combination is up to 96 %, and the false-negative rate is between 5 % and 10 % [9, 10]. Nevertheless, radioisotopes (such as 99mTc) are expensive and difficult to manage; dyes are cheap and easy to use but constitute a more invasive method, the need for great care in finding lymphatic channels may raise problems [11, 12].
Contrast-enhanced ultrasound (CEUS) is a new feasible technique for SLN biopsy that was originally used in a swine melanoma model [13, 14]. Ultrasound contrast agents consist of sulphur hexafluoride microbubbles with a mean diameter of 2.5 mm; therefore, they can cross the blood capillaries and lymphatic microvessels easily [15]. Many studies [9, 16,17,18,19] have confirmed CEUS as a safe and reliable method for localisation of SLNs. CEUS can identify 89 % of the SLNs in breast cancer patients [20]. However, there are few data [12, 17] from prospective studies assessing intradermal CEUS-enhancement patterns for the diagnosis of SLNs.
The aim of our study was to investigate the effectiveness of CEUS in the identification of SLNs and explore the value of enhancement patterns in diagnosing SLN metastases and characterising nodal burden in early breast cancer.
Materials and methods
Patients
Between April 2015 and December 2016, 110 consecutive patients with newly pathologically diagnosed breast cancer were recruited for the study. The exclusion criteria were as follows: patients who had proven ALN involvement, contraindications for the use of the ultrasound contrast agent, a history of previous ipsilateral breast cancer with axillary surgery or radiotherapy, and no axillary surgery planned. The ethics committee of Peking Union Medical College Hospital approved the study, and all participants provided signed informed consent.
Contrast-enhanced ultrasound (CEUS) examination and image analysis
All sonographic examinations were performed with an Acuson S2000 (Siemens Medical Systems, Erlangen, Germany) equipped with high-frequency linear array probes (18L6HD, 9L4) and contrast pulse sequences (CPS). Low mechanical index (MI) values were applied (MI: 0.06) to reduce microbubble destruction. Microbubbles (Sonovue, Bracco Imaging, Milan, Italy) were used as the ultrasound contrast agent. Sonovue dry powder was mixed with 2 ml sterile saline.
The CEUS procedure was performed according to Sever et al. [15, 20, 21]. In the early stage of research, Sever had ensured that microbubbles could successfully enter breast lymphatics and accumulate in architecturally defined axillary nodes by intradermal injection. The CEUS-identified SLNs were localised with guidewires or clips to confirm SLNs identified with blue dye, and when excised were also evaluated with CEUS [21, 22]. Briefly, approximately 0.4 ml of ultrasound contrast agent was injected intra-dermally into the periareolar area, and the injection area was massaged for 10–30 s. Subcutaneous lymphatic channels could be visualised immediately on CPS after injection. Enhanced lymph nodes could be detected by moving the probe along the channels. Grey scale or live dual images were used to confirm the presence of an architecturally defined SLN. If the lymphatic channel or lymph node was not detected successfully, one or two additional injections could be performed. Once identified, lymphatic duct and SLNs were marked on the skin surface. This serves as a road map so that the SLNs can be identified easily by surgeons. Then the size and number of SLNs were recorded.
All ultrasound imaging was evaluated by two experienced ultrasound physicians (ZJ and ZQL), who were blinded to the patients’ clinical data, mammographic findings and patient history. Initially, the examiner who performed CEUS gave the assessment on-site. Then the reader gave an independent assessment by reviewing image loops. If there was disagreement about the enhancement pattern, consensus was reached by discussion.
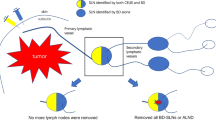
The pattern of enhancement of SLNs can be classed into three types: type I, homogeneous enhancement; type II, inhomogeneous enhancement; and type III, no enhancement [12] (Fig. 1). In this study, type I SLNs were considered negative nodes, and type II or III SLNs were considered positive nodes. The results were compared with the final pathological diagnosis.
Surgical management of the axillary lymph nodes
Immediately after anaesthesia induction, 0.1 ml of blue dye and 0.1 ml of indocyanine green (ICG) were intradermally injected into the periareolar upper outer quadrant region. The draining fluorescent lymphatic duct was visualised using the Photodynamic Eye, and the incision line was determined 1–2 cm away from the end of the lymphatic duct localisation of SLNs. The surgeon also found the draining blue-stained lymphatic ducts, then all blue-stained lymph nodes were excised. The remaining nodes were re-evaluated by Photodynamic Eye and harvested if ICG fluorescence was detected [23,24,25]. All excised SLNs were submitted to standard histological analysis. The number of SLN metastases at the end of surgical treatment was also determined for each patient.
Statistical analysis
SPSS software version 15.0 (SPSS Inc. Chicago, IL, USA) was used for the data analysis. Univariate analysis was done using Fisher’s exact test for categorical variables, and the independent samples t-test was used to compare means of the continuous normal data. The diagnostic indices including sensitivity, specificity, positive-predictive value, negative-predictive value and accuracy. The k coefficient was calculated to determine the inter-reader agreement. A p < 0.05 was considered to indicate a statistically significant difference.
Results
Baseline characteristics of the patients
There were 109 females and one male with a median age of 49 years (range, 28–76 years). The stages of these patients were 0–IIB. The clinical features of these patients are summarised in Table 1.
The median number of injections in 110 cases was one (range 1–3). In 81 of 106 patients (81/106, 71.4 %), SLN could be successfully localised by the first injection.
The identification rate of SLNs by CEUS was 96.4 %. In four of 110 patients, CEUS failed to observe SLN preoperatively, while the median number of SLNs detected by blue dye/ICG was 3.5 nodes. In the remaining 106 patients, the median number of SLNs detected by CEUS and blue dye/ICG was 1.2 and 3.6, respectively.
Enhancement pattern of sentinel lymph nodes (SLNs)
By comparing the enhancement pattern with the pathological diagnosis of SLNs, we found that type I nodes were all non-involved SLNs (51/51); 62 % of type II nodes were non-involved SLNs (47/76) and 38 % of type II nodes were involved SLNs (29/76) (Fig. 2); all type III nodes were involved SLNs (7/7) (Fig. 3).
Type II identification by contrast-enhanced ultrasound (CEUS) image with corresponding pathology. a CEUS image of a sentinel lymph node (SLN) that presented with inhomogeneous enhancement; b corresponding histological image of the SLN in (a) (haematoxylin-eosin stain; original magnification × 4). The pathology results show metastasis (marked with ‘M’) in the SLN; (c) CEUS image of another type II SLN; (d) corresponding histological image of the SLN in (c) (haematoxylin-eosin stain; original magnification × 4). The pathology results showed no metastasis in the SLN, but expansion of the lymphatic sinus was observed
Type III enhancement with corresponding pathology. a The sentinel lymph node (SLN) shows no enhancement on contrast-enhanced ultrasound (CEUS); b corresponding histological image of the SLN (haematoxylin-eosin stain; original magnification × 4). The pathology results show metastasis (marked with ‘M’) in almost the entire SLN, and only a small amount of normal lymphoid tissue is distributed to the periphery of the SLN (marked with ‘ ✩ ‘)
In 126 out of 134 lesions (94.0 %), the two readers agreed with each other on the classification of enhancement patterns. In the remaining eight lesions with disagreement, the variation in differences included homogeneous, heterogeneous and no enhancement patterns. The k coefficient was 0.886, indicating good inter-reader agreement (Table 2).
When we considered the type I SLNs as negative nodes and type II or III SLNs as positive nodes, the sensitivity of the diagnosis of SLN metastasis was 100 %, the specificity was 52.0 %, and the accuracy rate was 64.9 %. The positive predictive value was 43.4 %, and the negative predictive value was 100 % (Table 3).
Nodal burden of T1-T2 breast cancer
A total of 90 patients had T1-T2 breast cancer with normal grey-scale axillary ultrasound. Of them, 62 patients had no metastatic SLN. In the remaining 28 patients with SLN metastases, eight patients had a high axillary nodal burden (≥3 SLN metastases) while 20 patients had a low axillary nodal burden (1–2 SLN metastases) according to surgical histopathology (Table 4).
When we considered ≥3 SLN metastases as positive, 0–2 SLN metastases as negative, the sensitivity of CEUS enhancement pattern diagnosis SLN status was 100 %, the specificity was 34.2 %, and the accuracy rate was 37.9 %. The positive predictive value was 13.3 %, and the negative predictive value was 100 % (Table 5).
The mean tumour size in the SLN negative group, SLN low nodal burden group and SLN high nodal burden group was 1.66 cm, 1.70 cm and 1.85 cm, respectively. There was a tendency toward size increment among the three groups, but the difference did not reach statistical significance (p=0.576). No statistically significant differences were found in either tumour histology (p=0.907) or molecular subtype (p=0.157).
Discussion
SLN biopsy has become the standard procedure for axillary staging of early breast cancer. Clinicians should not recommend ALND for women with early-stage breast cancer and one or two SLN metastases, but should receive BCS with conventionally fractionated whole-breast radiotherapy [4, 26]. Thus, the accurate evaluation of the SLN status and axillary nodal burden is essential for prognosis. Apart from the conventional methods (e.g. blue dye and radioisotopes), CEUS using different sonographic agents and techniques has gained increasing interest in recent years [13,14,15,16, 20, 27].
The mean number of SLNs identified by CEUS was 1.2, less than the 3.6 retrieved by blue dye/ICG. The blue dye/ICG method could detect more SLNs than CEUS as the blue dye regimen can pass through the SLN to adjacent lymph nodes [11, 20].
In this study, all type I SLNs were non-involved lymph nodes. The sensitivity was 100 %, and the negative predictive value was 100 %. This result is consistent with previous research [28], which confirmed that homogeneous enhancement has a high negative predictive value in diagnosing SLN metastasis, with a high Kappa value, resulting in a greater potential for clinical application.
Of the type II nodes, 29 SLNs were involved lymph nodes, including those with regional and diffused inhomogeneous enhancement (Fig. 2a and b). Many studies have reported that filling defects in enhanced lymph nodes are significantly associated with tumour infiltration [13, 14], and tumour cells are invaded by afferent lymphatic vessels and first implanted in the local cortex lymphoid sinus. With continuous proliferation, tumour cells accumulate in the lymph nodes and obstruct or destroy the small lymphatic vessels, forming areas that do not enhance on CEUS [29]; thus, the SLNs presented with inhomogeneous enhancement.
In addition, 47 type II SLNs were non-involved lymph nodes (Fig. 2c and d), and this mechanism needs to be further researched. We hypothesise that this result may be associated with lymphatic reflux or lymph node structure. Wang et al. [30] reported that a histopathology examination of SLNs showed partial enhancement. They showed the proliferation of lymphatic follicles or the lymphatic sinus, while normal lymphatic tissue was observed in completely enhanced SLNs. Lymph nodes consist of multiple lymphoid lobules surrounded by lymph-filled sinuses and are separated by trabeculae (larger lymph nodes that contain more lobules) [28]. The microbubbles pass from afferent lymphatic channels to the cortical sinuses, then to the medullary sinuses, and finally to the efferent channels. Our study showed that non-involved SLNs that presented as type II exhibited expansion of the lymphatic sinus. This region may show slower enhancement; thus, SLNs may manifest as heterogeneous enhancement.
Macdonald et al. [31] showed that lymphatic flow was positively correlated with regional tissue pressure and negatively correlated with lymphatic reflux pressure. The wall of collective lymphatic channels consisted of smooth muscle with spontaneous contraction frequency. If the massage frequency was greater than the spontaneous contraction frequency of the initial lymphatic vessels, the lymphatic flow could increase. Therefore, when inhomogeneous enhanced SLNs include involved and non-involved lymph nodes, the overlap between benign and malignant nodes is large. Solely relying on the enhancement patterns cannot accurately predict the SLN status.
Type III SLNs were all positive lymph nodes, which may have occurred due to the following reasons: (1) tumour cells proliferated in the SLNs and infiltrated almost the entire lymph node (Fig. 3); (2) tumour cells infiltrated part of the lymph node but obstructed the main lymphatic channels, causing the SLNs to present with no enhancement. In this study, the sample size of type III SLNs was low, and greater numbers are needed to evaluate their predictive value.
Enhancement pattern could be helpful for characterisation of nodal burden. All patients (27/27, 100 %) with a homogeneous enhancement pattern had no SLN metastases, and may be ideally suited for axillary conservation. Although no statistically difference was reached, heterogeneous enhancement pattern in SLN seems to be a low nodal burden entity. Most patients (49/53, 92.5 %) with a heterogeneous enhancement pattern in this study had 0–2 SLN metastases, while only four patients (4/53, 8.5 %) had ≥3 SLN metastases. No enhancement pattern implied a high nodal burden. In this study, 100 % of them had involved SLNs, four patients (4/7, 57.1 %) had more than two SLN metastases, while three patients (3/7, 42.9 %) had 1–2 SLN metastases. In this scenario, the detection of the precise number of metastatic axillary nodes should be evaluated histopathologically. No diagnostic method (ultrasound, ultrasound plus biopsy, MR) is suitable for the entity of node involvement at present [32, 33]. Thus, a SLN biopsy is warranted when preoperative CEUS non-enhancement is suspected.
Theoretically, there was a positive correlation between tumour size and nodal burden, with a larger tumour there was a higher nodal burden. Unfortunately, there was a tendency toward size increment from low groups to high groups, but the difference did not reach statistical significance (p=0.576). Furthermore, in the current study the most common type of tumour histology and molecular subtype was invasive ductal carcinoma and luminal B subtype, respectively. No statistically significant differences were found either in tumour histology (p=0.907) or in molecular subtype (p=0.157). We consider that more multicentre data are needed to validate the true correlations between CEUS and tumour size, histology and molecular subtype.
There was good inter-reader agreement on the classification of enhancement patterns (k coefficient: 0.886). The two readers agreed with each other in 94.0 % of SLNs (126/134). In the remaining eight SLNs with disagreement, the variation in differences included homogeneous (5/8), heterogeneous (2/8) and no enhancement (1/8) patterns. The inter-reader agreement data have not been described earlier reports [12, 17]. The reproducibility of the CEUS in this study is high. This implies that the classification pattern in the study is simple and appropriate training could ensure that this technique could be readily applied to the real clinical setting.
Technical limitations remained in CEUS method. In this study, CEUS failed to identify SLN in four patients and three SLNs could be identified after three consecutive injections in two patients. However, the median number of injections used in the population was 1 (range 1–3). In 81 of 106 patients (81/106, 71.4 %), SLN could be successfully localised by the first injection. Combined with a high identification rate, CEUS could be a valuable technique for clinical application.
In conclusion, CEUS is easy to operate and has high reproducibility. CEUS can accurately identify the SLNs in preoperative breast cancer patients. The enhancement patterns on CEUS may be helpful for further recognising metastatic SLNs and axillary nodal burden. CEUS could be a promising technique for localisation and characterisation of SLNs in pre-operative breast cancer.
Abbreviations
- CEUS:
-
Contrast-enhanced ultrasound
- ICG:
-
Indocyanine green
- SLN:
-
Sentinel lymph node
References
Fisher B, Bauer M, Wickerham DL, Redmond CK, Fisher ER, Cruz AB et al (1983) Relation of number of positive axillary nodes to the prognosis of patients with primary breast cancer. An NSABP update. Cancer 52:1551–1557
Veronesi U, Paganelli G, Viale G, Luini A, Zurrida S, Galimberti V et al (2003) A Randomized Comparison of Sentinel-Node Biopsy with Routine Axillary Dissection in Breast Cancer. New England Journal of Medicine 349:546–553
Morton DL, Wen DR, Wong JH, Economou JS, Cagle LA, Storm FK et al (1992) Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg 127:392–399
Lyman GH, Temin S, Edge SB, Newman LA, Turner RR, Weaver DL et al (2014) Sentinel lymph node biopsy for patients with early-stage breast cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 32:1365–1383
Giuliano AE, Hunt KK, Ballman KV, Beitsch PD, Whitworth PW, Blumencranz PW et al (2011) Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. Jama 305:569–575
Giuliano AE, McCall L, Beitsch P, Whitworth PW, Blumencranz P, Leitch AM et al (2010) Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg 252:426–432 discussion 432-423
Cox K, Weeks J, Mills P, Chalmers R, Devalia H, Fish D et al (2016) Contrast-Enhanced Ultrasound Biopsy of Sentinel Lymph Nodes in Patients with Breast Cancer: Implications for Axillary Metastases and Conservation. Ann Surg Oncol 23:58–64
Albertini JJ, Lyman GH, Cox C, Yeatman T, Balducci L, Ku N et al (1996) Lymphatic mapping and sentinel node biopsy in the patient with breast cancer. Jama 276:1818–1822
Kim T, Giuliano AE, Lyman GH (2006) Lymphatic mapping and sentinel lymph node biopsy in early-stage breast carcinoma: a metaanalysis. Cancer 106:4–16
Barone JE, Tucker JB, Perez JM, Odom SR, Ghevariya V (2005) Evidence-based medicine applied to sentinel lymph node biopsy in patients with breast cancer. Am Surg 71:66–70
Gkegkes ID, Iavazzo C (2016) Contrast Enhanced Ultrasound (CEU) Using Microbubbles for Sentinel Lymph Node Biopsy in Breast Cancer: a Systematic Review. Acta Chirurgica Belgica 115:212–218
Xie F, Zhang D, Cheng L, Yu L, Yang L, Tong F et al (2015) Intradermal microbubbles and contrast-enhanced ultrasound (CEUS) is a feasible approach for sentinel lymph node identification in early-stage breast cancer. World J Surg Oncol 13:319–326
Mattrey RF, Kono Y, Baker K, Peterson T (2002) Sentinel lymph node imaging with microbubble ultrasound contrast material. Acad Radiol 9:S231–S235
Goldberg BB, Merton DA, Liu JB, Thakur M, Murphy GF, Needleman L et al (2004) Sentinel lymph nodes in a swine model with melanoma: contrast-enhanced lymphatic US. Radiology 230:727–734
Sever A, Broillet A, Schneider M, Cox K, Jones S, Weeks J et al (2010) Dynamic visualization of lymphatic channels and sentinel lymph nodes using intradermal microbubbles and contrast-enhanced ultrasound in a swine model and patients with breast cancer. J Ultrasound Med 29:1699–1704
Omoto K, Matsunaga H, Take N, Hozumi Y, Takehara M, Omoto Y et al (2009) Sentinel node detection method using contrast-enhanced ultrasonography with sonazoid in breast cancer: preliminary clinical study. Ultrasound Med Biol 35:1249–1256
Dellaportas D, Koureas A, Contis J, Lykoudis PM, Vraka I, Psychogios D et al (2015) Contrast-Enhanced Color Doppler Ultrasonography for Preoperative Evaluation of Sentinel Lymph Node in Breast Cancer Patients. Breast Care (Basel) 10:331–335
Cox K, Sever A, Jones S, Weeks J, Mills P, Devalia H et al (2013) Validation of a technique using microbubbles and contrast enhanced ultrasound (CEUS) to biopsy sentinel lymph nodes (SLN) in pre-operative breast cancer patients with a normal grey-scale axillary ultrasound. Eur J Surg Oncol 39:760–765
Sever AR, Mills P, Weeks J, Jones SE, Fish D, Jones PA et al (2012) Preoperative needle biopsy of sentinel lymph nodes using intradermal microbubbles and contrast-enhanced ultrasound in patients with breast cancer. AJR Am J Roentgenol 199:465–470
Sever AR, Mills P, Jones SE, Mali W, Jones PA (2012) Sentinel node identification using microbubbles and contrast-enhanced ultrasonography. Clin Radiol 67:687–694
Sever A, Jones S, Cox K, Weeks J, Mills P, Jones P (2009) Preoperative localization of sentinel lymph nodes using intradermal microbubbles and contrast-enhanced ultrasonography in patients with breast cancer. Br J Surg 96:1295–1299
Wang Y, Zhou W, Li C, Gong H, Li C, Yang N et al (2017) Variation of sentinel lymphatic channels (SLCs) and sentinel lymph nodes (SLNs) assessed by contrast-enhanced ultrasound (CEUS) in breast cancer patients. World J Surg Oncol 15:127–133
Wishart GC, Loh SW, Jones L, Benson JR (2012) A feasibility study (ICG-10) of indocyanine green (ICG) fluorescence mapping for sentinel lymph node detection in early breast cancer. Eur J Surg Oncol 38:651–656
Hojo T, Nagao T, Kikuyama M, Akashi S, Kinoshita T (2010) Evaluation of sentinel node biopsy by combined fluorescent and dye method and lymph flow for breast cancer. Breast 19:210–213
Ahmed M, Purushotham AD, Douek M (2014) Novel techniques for sentinel lymph node biopsy in breast cancer: a systematic review. The Lancet Oncology 15:e351–e362
Carlson RW, Allred DC, Anderson BO, Burstein HJ, Carter WB, Edge SB et al (2009) Breast cancer. Clinical practice guidelines in oncology. J Natl Compr Canc Netw 7:122–192
Goldberg BB, Merton DA, Liu JB, Murphy G, Forsberg F (2005) Contrast-enhanced sonographic imaging of lymphatic channels and sentinel lymph nodes. J Ultrasound Med 24:953–965
Sainte-Marie G, Peng FS, Belisle C (1982) Overall architecture and pattern of lymph flow in the rat lymph node. Am J Anat 164:275–309
Galie M, D'Onofrio M, Montani M, Amici A, Calderan L, Marzola P et al (2005) Tumor vessel compression hinders perfusion of ultrasonographic contrast agents. Neoplasia 7:528–536
Wang Y, Cheng Z, Li J, Tang J (2010) Gray-scale contrast-enhanced ultrasonography in detecting sentinel lymph nodes: an animal study. Eur J Radiol 74:e55–e59
Macdonald AJ, Arkill KP, Tabor GR, Mchale NG, Winlove CP (2008) Modeling flow in collecting lymphatic vessels: one-dimensional flow through a series of contractile elements. American Journal of Physiology Heart & Circulatory. Physiology 295:H305–H313
Pilewskie M, Jochelson M, Gooch JC, Patil S, Stempel M, Morrow M (2016) Is Preoperative Axillary Imaging Beneficial in Identifying Clinically Node-Negative Patients Requiring Axillary Lymph Node Dissection? J Am Coll Surg 222:138–145
van Wely BJ, de Wilt JH, Francissen C, Teerenstra S, Strobbe LJ (2015) Meta-analysis of ultrasound-guided biopsy of suspicious axillary lymph nodes in the selection of patients with extensive axillary tumour burden in breast cancer. Br J Surg 102:159–168
Funding
This study has received funding by the National Natural Science Foundation of China (81371557 and 81201112).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Yu-Xin Jiang.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• prospective
• cross-sectional study
• performed at one institution
Rights and permissions
About this article
Cite this article
Zhao, J., Zhang, J., Zhu, QL. et al. The value of contrast-enhanced ultrasound for sentinel lymph node identification and characterisation in pre-operative breast cancer patients: A prospective study. Eur Radiol 28, 1654–1661 (2018). https://doi.org/10.1007/s00330-017-5089-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-017-5089-0