Abstract
Objectives
To investigate the progression of coronary atherosclerosis burden by coronary CT angiography (CCTA) and to demonstrate its association with the incidence of major adverse cardiac events (MACE).
Methods
We retrospectively studied patients with stable angina who had undergone repeat CCTA due to recurrent or worsening symptoms. Lipid-rich, fibrous, calcified and total plaque burden as well as coronary diameter stenosis were quantitatively analysed. The incidence of MACE during follow-up was determined.
Results
The final cohort consisted of 268 patients (mean age 52.9 ± 9.8 years, 71 % male) with a mean follow-up period of 4.6 ± 0.9 years. Patients with lipid-rich, fibrous, calcified and total plaque burden (%) progression, as well as coronary diameter stenosis (%) progression had a significantly higher incidence of MACE than those without (all p < 0.05). The progression of lipid-rich plaque (HR = 1.601, p = 0.021), total plaque burden (HR = 2.979, p = 0.043) and coronary diameter stenosis (HR = 4.327, p <0.001) were independent predictors of MACE (all p < 0.05).
Conclusions
Patients presenting with recurrent or worsening symptoms associated with coronary artery disease who have coronary atherosclerosis progression on CCTA are at an increased risk of future MACE.
Key Points
• Repeat CCTA can provide information regarding the progression of coronary atherosclerosis.
• Coronary atherosclerosis progression at CCTA is independently associated with MACE.
• CCTA findings could serve as incremental predictors of MACE.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The insidious process of atherosclerosis can result in stenosis formation or plaque rupture within the coronary arteries, causing myocardial damage and major adverse cardiac events (MACE) [1]. Serial assessment of coronary plaque burden has contributed to our understanding of the natural history and pathophysiology of coronary artery disease (CAD). Previous studies using repeat coronary artery calcium (CAC) scans found CAC and its progression to be predictive of MACE [2]; however, calcified plaque is only one of many components involved in coronary atherosclerosis.
Non-invasive contrast-enhanced coronary computed tomography angiography (CCTA) is capable of visualising plaque composition, morphology and distribution, as well as assessing lumen stenosis severity. Its ability to quantify coronary plaque burden has rendered it a valuable modality to evaluate overall coronary atherosclerosis and its progression in low to intermediate risk patients [3, 4]. Schuhbaeck et al. [5] report that there is high reproducibility of coronary atherosclerotic plaque volume measurements using this technology. Therefore, they concluded that serial studies to determine progression of coronary atherosclerotic plaque were feasible. Recently, the SCOT-HEART trial demonstrated that, in patients with suspected CAD, CCTA could clarify the diagnosis, enable targeting of intervention and reduce the future risk of myocardial infarction [6].
With the growing application of CCTA it is not uncommon for patients to undergo more than one CCTA study, especially with recurrent or worsening symptoms. Interestingly, few studies have focused on the quantitative evaluation of coronary atherosclerosis progression, which is the evidence required to evaluate the potential role of this imaging biomarker and its effect on cardiovascular outcomes. One previous study demonstrated that plaque progression detected by serial CCTA is an independent predictor of acute coronary syndrome [7]. However, coronary atherosclerosis progression in patients with stable angina and its association with MACE is insufficiently studied. We hypothesised that coronary atherosclerosis progression at repeat CCTA is associated with a higher incidence of MACE. Therefore, we quantitatively analysed patients with stable angina who underwent repeat CCTA with recurrent or worsening symptoms to investigate coronary atherosclerosis progression and its association with MACE.
Methods
Study population
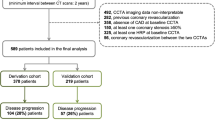
Institutional Review Board approval was obtained and a waiver of informed consent was granted. We retrospectively analysed data of 371 patients with stable angina who had undergone repeat CCTA from 2009 to 2015. Exclusion criteria included the following: (1) patients with previous coronary revascularisation (percutaneous coronary intervention [PCI] and coronary artery bypass grafting [CABG]) between serial CCTA examinations (n = 51); (2) patients with incomplete serum biochemical tests at baseline (n = 17); (3) patients with unevaluable CT images in either scan (n = 9); (4) patients with a time interval < 30 days between baseline and follow-up CCTA (n = 0) [7]. A total of 294 patients were enrolled and 26 (8.8 %) were lost to follow-up. Consequently, the final study consisted of 268 patients (see study flow chart in Fig. 1). Cardiovascular risk factors, symptoms, baseline serum biochemical results and medications were collected using electronic medical records. Incomplete serum biochemical tests were defined as a lack of any of the following markers: triglycerides, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, high-sensitivity C-reactive protein and blood glucose. To improve the validity of the study, two experienced radiologists independently evaluated the image quality of each coronary segment according to Zhang et al. [8]. All patients with an image quality rating of 1 in either of the two scans were excluded. In cases of disagreement, a joint reading session was performed to reach a consensus decision.
Coronary CT angiography protocol
All examinations were performed with a dual-source CT scanner (SOMATOM Definition or SOMATOM Definition Flash, Siemens Healthcare, Forchheim, Germany). An oral beta-blocker (metoprolol; Betaloc, AstraZeneca, Cambridge, England) was administered to all patients with a resting heart rate > 90 beats/min. After an initial non-contrast-enhanced calcium scoring CT scan, patients underwent contrast-enhanced CCTA using prospective ECG-triggering. The following parameters were used: collimation 2 × 64 × 0.6 mm by using z-flying focal spot; gantry rotation 0.28 s; tube voltage 120 kV or 100 kV; tube current 400–500 mAs; field of view (FOV) 200–250 mm. Contrast medium (iohexol (Omnipaque 350 mgI/ml, GE Healthcare, Little Chalfont, UK) or iopromide (Ultravist 370 mgI/ml, Bayer, Berlin, Germany)) was injected at a rate of 4–5 ml/s. A triple-phase contrast material injection protocol was used. CCTA images were reconstructed with a section thickness of 0.625 mm.
Quantitative analysis by coronary CT angiography
Plaque analysis was performed using an off-line three-dimensional semi-automated image analysis workstation (Vitrea Version 6.6, Vital images, Minnetonka, MN, USA) by two observers who were blinded to clinical parameters. The coronary tree was automatically extracted. Each of the major epicardial vessels (the left anterior descending, the left circumflex and the right coronary artery) were individually analysed from the ostium to the point at which the internal vessel calibre decreased to less than 2 mm. Automated longitudinal contouring of the inner lumen and outer wall was performed, and results were manually adjusted when deviations were noted [9]. We measured the same vessel with the same origins and lengths for each of the patient’s two scans. Previous studies have demonstrated the accuracy of CCTA to characterise and quantify coronary plaque and support the feasibility of using CCTA to assess atherosclerotic plaque burden [10,11,12]. As reported in Inoue’s previous study [13], the software colour codes coronary plaques and lumen based on varying HU profiles as follows: lipid-rich plaque (-100–30 HU), fibrous plaque (30–150 HU), calcified plaque (350–1,000 HU) and lumen (150–350 HU). A previous study using intravascular ultrasound (IVUS) demonstrated that an attenuation threshold of 30 HU provides a sensitivity of 91 % and specificity of 100 % for the detection of lipid-rich plaque [14]. In addition, other authors [15, 16] have established the intensity-threshold of calcified plaque to be 130 HU on non-contrast-enhanced CT examination and of 350 HU in CCTA studies. Total plaque volume included lipid-rich, fibrous and calcified plaque components of the aforementioned three major vessels. The vessel volume of each patient was defined as the combined lumen volume of the three main vessels plus the total measured plaque volume. Coronary diameter stenosis (%) was an adjunct calculation provided by the plaque burden analysis.
Plaque burden (X) was defined as plaque volume divided by the vessel volume on a per-patient level, where X stands for lipid-rich, fibrous, calcified or total plaque burden. Change in plaque burden (X) was defined as plaque burden at the second CCTA study (X) minus initial plaque burden (X).
The rate of plaque burden (X) change was defined as plaque burden at the second CCTA study minus the initial plaque burden divided by the plaque burden at the initial scan.
Significant progression was defined as a rate of change > 0.05 %. The plaque burden and the change in plaque burden were both expressed as percentages. Change of coronary diameter stenosis was defined as diameter stenosis at the second CCTA study minus diameter stenosis at the initial study and its change was also expressed by percentage.
Follow-up
Follow-up information was collected through clinical visits or telephone contact. We verified all reported events by reviewing hospital records or initiating direct contact with the respective attending physicians. Study endpoints were defined as any occurrence of cardiac events defined as cardiac death (including any death without definitive non-cardiac cause), non-fatal myocardial infarction, unstable angina pectoris requiring hospitalisation or coronary revascularisation. The definition of non-fatal myocardial infarction was based on the criteria of typical acute chest pain and persistent ST-segment elevation or positive cardiac enzymes. Unstable angina pectoris was defined as typical acute chest pain with negative cardiac enzymes if CAD could not be excluded as the cause of symptoms according to guidelines [17].
Statistical analysis
All statistical analyses were performed using SPSS software package (version 17.0, SPSS, Chicago, IL, USA). The Kolmogorov Smirnov test was used for assessing normality of data distribution. For descriptive statistics continuous variables were expressed as mean ± standard deviation or median (interquartile range (IQR)), and categorical variables were expressed as frequency (percentages). The differences between the groups were tested by the χ2 statistic and the unpaired Student’s t-test or Mann-Whitney U test. Intra-class correlation coefficients (ICCs) were used to calculate the reliability of intra-observer and inter-observer analysis. For cumulative event rates of MACE Kaplan-Meier plots were generated and log-rank test was performed. The association between clinical characteristics, CT measures and MACE were estimated by using Cox proportional hazard model. Multivariate logistic regression analysis was used to identify predictors for the clinical characteristics and CT findings. A p value <0.05 was considered statistically significant.
Results
Population characteristics
Of the 294 patients who had repeat CCTA, 268 could be contacted for follow-up, resulting in a follow-up rate of 91.2 %. There were no significant differences found in the baseline characteristics between the follow-up group and the group lost to follow-up. 193 patients of the follow-up group (71 %) were male with a mean age of 52.9 ± 9.8 years. Repeat CCTAs were performed after a mean interval time of 668 ± 323 days after the initial CCTA examination. The median effective radiation dose of each CCTA was 3.9 mSv. Baseline clinical characteristics of patients with and without MACE are presented in Table 1. In patients with MACE, diabetes mellitus, dyslipidemia, history of smoking and family history of CAD were significantly more prevalent than in those without MACE. Patients with MACE also had a significantly higher level of low-density lipoprotein cholesterol (LDL-C) at baseline (3.3 ± 1.0 vs. 3.0 ± 1.1, p = 0.046) and a lower proportion of statin therapy (27.5 % vs. 47.4 %, p = 0.047).
Cardiac events
During a mean follow-up time of 4.6 ± 0.9 years, 40 patients (14.9 %) experienced a MACE. Of the 40 patients with MACE, 32 underwent coronary revascularisation (PCI n = 27; CABG n = 5) and eight required hospitalisation due to unstable angina; however, none of the patients suffered cardiac death or non-fatal myocardial infarction. The remaining 228 (85.1 %) patients did not experience MACE during a mean follow-up of 4.7± 1.3 years.
Atherosclerosis progression and major adverse cardiac events (MACE)
Atherosclerotic plaque characteristics and progression are given in Table 2. At baseline, the burden (%) of lipid-rich, fibrous, calcified and total plaque were 17.4 ± 2.6, 27.8 ± 5.2, 4.6 (2.4–7.4) and 50.9 ± 8.2, respectively. Coronary diameter stenosis (%) was 60.5 (53.0–83.0). There were no significant differences in these quantitative parameters between patients with and without MACE (p > 0.05 for all). At follow-up, quantitative parameters of total plaque burden and coronary diameter stenosis were all significantly higher in patients with MACE than those without MACE.
After combining serial CCTA data, the plaque progression (%) of lipid-rich, fibrous, calcified and total plaque were 0.3 (-9.4–9.1), -1.8 (-9.5–7.1), 7.7 (-30.7–75.3) and 0.24 (-7.9–8.01), respectively. Coronary diameter stenosis progression was 10.7 (0–28.5) %. There was significant progression of lipid-rich plaque burden (%), fibrous plaque burden (%), calcified plaque burden (%), and total plaque burden (%) in patients with MACE compared with those without MACE (6.5 (-3.9–11.6) vs. -0.9 (-10.1–8.3), p = 0.023;3.9 (-4.8–13.0) vs. -2.6 (-10.0–6.9), p =0.009; 34.4 (-4.8–97.7) vs. 3.3 (-33.6–66.0), p = 0.005; and 6.6 (2.5–12.6) vs. -0.8 (-8.8–6.9), p < 0.001; respectively). Coronary diameter stenosis (%) progression was 17.5 (1.8–29.8) in patients with MACE and 7.1 (0–15.7) in patients without MACE (p = 0.021).
Cox proportional hazard models
Table 3 gives risk-adjusted predictors of clinical factors and CCTA findings for predicting MACE. The prevalence of diabetes mellitus (hazard ratio [HR] = 1.698, p = 0.043), history of smoking (HR = 2.033, p = 0.002), family history of CAD (HR = 1.206, p = 0.026), progression of lipid-rich plaque burden (HR = 1.601, p = 0.021), progression of total plaque burden (HR = 2.979, p = 0.043), and progression of coronary diameter stenosis (HR = 4.327, p < 0.001) were associated with MACE. Figure 2 depicts the Kaplan-Meier curves of MACE according to change of total plaque burden (A), lipid-rich plaque burden (B) and coronary diameter stenosis (C). Patients with a progression in their lipid-rich plaque burden, total plaque burden and coronary diameter stenosis all exhibited a higher event rate (all p < 0.05, log-rank test) than patients with non-progression of atherosclerotic plaque.
Kaplan-Meier survival curves of patients without plaque progression (green line) and patients with plaque progression (red line) of (a) lipid-rich plaque burden, (b) total plaque burden and (c) coronary diameter stenosis; log rank p < 0.05. Patient follow-up was performed after a mean period of 4.6 ± 0.9 years. MACE included coronary revascularisation (PCI and CABG), cardiac death and hospitalisation due to unstable angina. We observed significantly more MACE in the cohort of patients with progression of lipid plaque burden (HR = 1.601, p = 0.021), total plaque burden (HR = 2.929, p = 0.043) and coronary diameter stenosis (HR = 4.327, p <0.001) when compared to patients who did not have any plaque progression. MACE major adverse cardiac events, PCI percutaneous coronary intervention, CABG coronary artery bypass grafting, HR hazard ratio
Multivariate regression of plaque progression
Progression of lipid-rich plaque burden, total plaque burden, coronary diameter stenosis and their association with baseline clinical and laboratory characteristics are shown in Table 4. The progression of lipid-rich plaque burden was associated with baseline dyslipidemia (odds ratio [OR] = 3.131, p < 0.001), statin therapy (OR = 0.348, p = 0.001) and baseline LDL-C (OR = 2.522, p = 0.015). Progression of total plaque burden was associated with dyslipidemia (OR = 1.847, p = 0.028) and statin therapy (OR = 0.596, p = 0.022). Progression of coronary diameter stenosis was associated with the occurrence of diabetes mellitus (OR = 1.489, p = 0.043), dyslipidemia (OR = 1.277, p = 0.026), history of smoking (OR = 1.437, p = 0.044) and statin therapy (OR = 0.544, p < 0.001). Figures 3 and 4 are examples of plaque burden regression and progression during repeat CCTA, respectively.
Automated plaque detection in CCTA study of a 50-year-old man without MACE reveals stability or slight regression of total plaque burden. Red represents lipid-rich plaque, blue represents fibrous plaque, yellow represents calcified plaque, and green represents vessel lumen. Initial CCTA (a) was performed in 2012 with a total plaque burden within the LAD of 44.2 %. The same patient underwent a second CCTA study (b) in 2014 where the total plaque burden within the LAD had decreased to 42.5 %. PCI percutaneous coronary intervention, CCTA coronary computed tomography angiography, LAD left-anterior descending artery
Automated plaque detection in CCTA study of a 64-year-old man who eventually underwent PCI demonstrates progression of total plaque burden. Red represents lipid plaque, blue represents fibrous plaque, yellow represents calcified plaque, and green represents vessel lumen. Initial CCTA (a) was performed in 2010 with a total plaque burden within the LAD of 55.4 %. The same patient underwent a second CCTA study (b) in 2013 where the total plaque burden within the LAD had increased to 64.2 %. PCI percutaneous coronary intervention, CCTA coronary computed tomography angiography, LAD left-anterior descending artery
Reproducibility
A subsequent re-analysis of 30 randomly selected patients demonstrated validation of this software in analysing atherosclerosis plaque on a patient level. The R-R interval range was 71–75 % and the mean difference of the rate of plaque burden change was < 0.05 % (total plaque burden: 0.01 ± 0.02 %; lipid-rich plaque burden: 0.01 ± 0.01 %; fibrous plaque burden: 0.01 ± 0.02 %; calcified plaque burden: 0.01 ± 0.03 %). Intra-observer and interobserver reliability for vessel volume, any plaque volume detection and coronary diameter stenosis were excellent for the first CCTA (ICC = 0.92, 95 % CI: 0.90–0.95) and the second CCTA (ICC = 0.93, 95 % CI: 0.91–0.96).
Discussion
This study demonstrated that repeat CCTA examinations are an effective tool to evaluate coronary atherosclerosis progression and monitor changes in coronary stenoses, both of which were also shown to be associated with MACE. The ability to assess the progression of luminal stenosis and atherosclerotic plaque burden is a major benefit of repeat CCTA analysis in patients presenting with recurrent symptoms related to CAD. CCTA’s reproducibility as a reliable method to assess coronary atherosclerosis progression has already been established [10,11,12]. Previous studies have highlighted the feasibility of repeat CCTA examinations to monitor the progression of CAD, including the association of plaque progression with future episodes of accurate coronary syndrome [7, 13]. In addition, plaque characteristics derived from CCTA datasets have been shown to be in good accordance with IVUS [18].
One meta-analysis demonstrated that the sensitivity and specificity of CCTA to detect coronary plaque compared with IVUS were 93 % and 92 %, respectively, with an area under the receiver-operating curve of 0.97 [19]. Our study evaluated plaque burden progression using serial CCTA datasets that were acquired in the same cardiac phase. Plaque parameters were analysed on a per-patient level (total plaque burden) rather than a per-plaque level because it would have been unreasonable to evaluate changes in plaque burden of individual plaques. Furthermore, considering that total plaque burden is a composite of lipid-rich, fibrous and calcified plaque, total plaque burden provides a more robust analysis to demonstrate its predictive value of MACE. The PROSPECT study found that IVUS interrogated lesions with a plaque burden > 70 % at baseline had a strong association with future clinical events [20]. However, the association between the change of atherosclerosis burden and clinical events is currently unknown. Our study included patients with stable angina who underwent repeat CCTA examinations due to recurrent or worsening symptoms, and demonstrated that patients with plaque and stenosis progression had a higher incidence of MACE than those without plaque or stenosis progression. The interval time between CCTA scans ranged between 256 and 1,551 days. Our study excluded patients with < 30 days between CCTA examinations in accordance with Motoyama et al. [7]. Although 80 % of patients had approximately 2–3 years between CCTA examinations, a prospective cohort study with a greater patient population may be needed to identify the most appropriate interval time for symptomatic patients to undergo a repeat CCTA.
Several studies investigating the effect of statin treatment on the plaque progression using repeat CCTA examinations have been published [13, 21]. These studies demonstrated that statin therapy prevented plaque progression by reducing the volume of low-density plaque. Likewise, others have focused on risk factors related to the progression of coronary atherosclerosis and showed that a history of diabetes mellitus and dyslipidemia are correlated with the plaque progression [22]. However, these studies did not include details of medical treatment and follow-up after repeat CCTA. Becker et al. [23] and the MESA study [24] showed that MACE were associated with the presence of CAC, but these studies did not relate these findings to lipid-rich plaque, fibrous plaque or total plaque burden. Our study used calcified plaque burden instead of CAC score. CAC is not included in our study because the calcified plaque burden parameter provides similar information to that of CAC. Additionally, the specificity of CAC to diagnose obstructive CAD is low due to a high false-positive rate [25].
We found that the progression of plaque burden and the progression of coronary stenosis on repeat CCTA are associated with MACE. It is worth mentioning that there were no cardiac-related deaths in either of the study cohorts. This could be attributed to the fact that our patient population had CCTA twice. Repeated visits with their treating physician could indicate that this population has an active interest in their health. A larger population with more follow-up should be considered for future investigations. Baseline plaque burden was not associated with MACE, which is most likely due to the lack of statistical significance between the number of patients with MACE and without MACE at baseline.
The guidelines of the American College of Cardiology (ACC)/American Heart Association (AHA) state that the treatment of high cholesterol can reduce the risk of atherosclerosis in patients with cardiovascular diseases [26]. The 2014 recommendations of the National Lipid Association (NLA) for the patient-centred management of dyslipidemia also concluded that the LDL-C is the root cause of atherosclerosis [27]. Statin therapy is recommended for patients experiencing dyslipidemia in order to prevent the progression of coronary atherosclerosis [28]. In our investigation, statin therapy was associated with reduced lipid-rich plaque progression and that elevated LDL-C and dyslipidemia were associated with lipid-rich plaque progression. Diabetes mellitus has previously been associated with higher coronary plaque burden [29]. Bamberg et al. reported that cardiovascular risk factors have been linked to the presence of coronary atherosclerotic plaque in a cross-sectional study [30]. Other studies have reinforced that changes in cardiac risk factors, such as diabetes mellitus or tobacco use, affects the outcome of patients with CAD [31, 32]. Our study was in agreement with these conclusions, demonstrating that diabetes mellitus and smoking were associated with MACE and correlated with coronary plaque burden. However, almost all pharmacological treatments including statins, aspirin, beta-blockers and antidiabetic drugs were prescribed less in the population that experienced MACE. We should emphasise that medications were recorded as what was prescribed to patients at the time of the initial CCTA examination.
Limitations
This study included a relatively small cohort and was not free from selection bias due to the retrospective design. Our results should be confirmed in larger, prospective, multicentre investigations. We only considered baseline cardiovascular risk factors and medical therapies – changes in cardiac risk and in medical therapies were not taken into consideration.
In conclusion, repeat CCTA examinations are an effective way to monitor the progression of coronary atherosclerotic plaque burden. In light of the fact that patients with progressive atherosclerosis plaque have a greater risk of experiencing future MACE, repeat CCTA examinations could be indicated in patients presenting with recurrent or worsening symptoms related to CAD.
Abbreviations
- CABG:
-
Coronary artery bypass grafting
- CAC:
-
Coronary artery calcium
- CAD:
-
Coronary artery disease
- CCTA:
-
Coronary CT angiography
- CI:
-
Confidence interval
- HR:
-
Hazard ratio
- IVUS:
-
Intravascular ultrasound
- ICC:
-
Intra-class correlation coefficients
- MACE:
-
Major adverse cardiac events
- OR:
-
Odds ratio
- PCI:
-
Percutaneous coronary intervention
References
Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM (2000) Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol 20:1262–1275
Budoff MJ, Hokanson JE, Nasir K et al (2010) Progression of coronary artery calcium predicts all-cause mortality. JACC Cardiovasc Imaging 3:1229–1236
Rodriguez K, Kwan AC, Lai S et al (2015) Coronary plaque burden at coronary CT angiography in asymptomatic men and women. Radiology 277:73–80
Lehman SJ, Schlett CL, Bamberg F et al (2009) Assessment of coronary plaque progression in coronary computed tomography angiography using a semiquantitative score. JACC Cardiovasc Imaging 2:1262–1270
Schuhbaeck A, Dey D, Otaki Y et al (2014) Interscan reproducibility of quantitative coronary plaque volume and composition from CT coronary angiography using an automated method. Eur Radiol 24:2300–2308
The SCOT-HEART investigators (2015) CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet 385:2383–2391
Motoyama S, Ito H, Sarai M et al (2015) Plaque characterization by coronary computed tomography angiography and the likelihood of acute coronary events in mid-term follow-up. J Am Coll Cardiol 66:337–346
Zhang LJ, Qi L, Wang J et al (2014) Feasibility of prospectively ECG-triggered high-pitch coronary CT angiography with 30 mL iodinated contrast agent at 70 kVp: initial experience. Eur Radiol 24:1537–1546
Kwan AC, May HT, Cater G et al (2014) Coronary artery plaque volume and obesity in patients with diabetes: the factor-64 study. Radiology 272:690–699
Voros S, Rinehart S, Qian Z et al (2011) Prospective validation of standardized, 3-dimensional, quantitative coronary computed tomographic plaque measurements using radiofrequency backscatter intravascular ultrasound as reference standard in intermediate coronary arterial lesions: results from the ATLANTA (Assessment of Tissue Characteristics, Lesion Morphology, and Hemodynamics by Angiography With Fractional Flow Reserve, Intravascular Ultrasound and Virtual Histology, and Noninvasive Computed Tomography in Atherosclerotic Plaques) I study. J Am Coll Cardiol Intv 4:198–208
Papadopoulou SL, Neefjes LA, Schaap M et al (2011) Detection and quantification of coronary atherosclerotic plaque by 64-slice multidetector CT: a systematic head-to-head comparison with intravascular ultrasound. Atherosclerosis 219:163–170
Hoffmann U, Moselewski F, Nieman K et al (2006) Noninvasive assessment of plaque morphology and composition in culprit and stable lesions in acute coronary syndrome and stable lesions in stable angina by multidetector computed tomography. J Am Coll Cardiol 47:1655–1662
Inoue K, Motoyama S, Sarai M et al (2010) Serial coronary CT angiography-verified changes in plaque characteristics as an end point: evaluation of effect of statin intervention. JACC Cardiovasc Imaging 3:691–698
Motoyama S, Kondo T, Anno H et al (2007) Atherosclerotic plaque characterization by 0.5-mm-slice multislice computed tomographic imaging. Circ J 71:363–366
Hong C, Becker CR, Schoepf UJ, Ohnesorge B, Bruening R, Reiser MF (2002) Coronary artery calcium: absolute quantification in nonenhanced and contrastenhanced multi-detector row CT studies. Radiology 223:474–480
Kruk M, Noll D, Achenbach S et al (2014) Impact of coronary artery calcium characteristics on accuracy of CT angiography. JACC: Cardiovascular Imaging 7:49–58
Anderson JL, Adams CD, Antman EM et al (2011) 2011 ACCF/AHA focused update incorporated into the ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 123:e426–e579
Papadopoulou SL, Neefjes LA, Garcia-Garcia HM et al (2012) Natural history of coronary atherosclerosis by multislice computed tomography. JACC Cardiovasc Imaging 5:S28–S37
Fischer C, Hulten E, Belur P et al (2013) Coronary CT angiography versus intravascular ultrasound for estimation of coronary stenosis and atherosclerotic plaque burden: a meta-analysis. J Cardiovasc Comput Tomogr 7:256–266
Stone GW, Maehara A, Lansky AJ et al (2011) A prospective natural-history study of coronary atherosclerosis. N Engl J Med 364:226–235
Hoffmann H, Frieler K, Schlattmann P et al (2010) Influence of statin treatment on coronary atherosclerosis visualised using multidetector computed tomography. Eur Radiol 20:2824–2833
Ayad SW, ElSharkawy EM, ElTahan SM, Sobhy MA, Laymouna RH (2015) The role of 64/128-slice multidetector computed tomography to assess the progression of coronary atherosclerosis. Clin Med Insights Cardiol 9:47–52
Becker CR (2005) Estimation of cardiac event risk by MDCT. Eur Radiol 15:B17–B22
Joshi PH, Patel B, Blaha MJ et al (2016) Coronary artery calcium predicts cardiovascular events in participants with a low lifetime risk of cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis 246:367–373
Greenland P, Bonow RO, Brundage BH et al (2007) ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) developed in collaboration with the Society of Atherosclerosis Imaging and Prevention and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol 49:378–402
Stone NJ, Robinson JG, Lichtenstein AH et al (2014) 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 63:2889–2934
Adhyaru BB, Jacobson TA (2016) New cholesterol guidelines for the management of atherosclerotic cardiovascular disease risk: a comparison of the 2013 American College of Cardiology/American Heart Association Cholesterol Guidelines with the 2014 National Lipid Association Recommendations for Patient-Centered Management of Dyslipidemia. Endocrinol Metab Clin North Am 45:17–37
Puri R, Nissen SE, Ballantyne CM et al (2013) Factors underlying regression of coronary atheroma with potent statin therapy. Eur Heart J 34:1818–1825
Maffei E, Seitun S, Nieman K et al (2011) Assessment of coronary artery disease and calcified coronary plaque burden by computed tomography in patients with and without diabetes mellitus. Eur Radiol 21:944–953
Bamberg F, Dannemann N, Shapiro MD et al (2008) Association between cardiovascular risk profiles and the presence and extent of different types of coronary atherosclerotic plaque as detected by multidetector computed tomography. Arterioscler Thromb Vasc Biol 28:568–574
UKPDS Group (1998) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 352:837–853
Wilson K, Gibson N, Willan A, Cook D (2000) Effect of smoking cessation on mortality after myocardial infarction: meta-analysis of cohort studies. Arch Intern Med 160:939–944
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantors of this publication are Bin Lu and Ximing Wang.
Conflict of interest
Dr. Schoepf receives institutional research support from Astellas, Bayer, GE, and Siemens and received consulting fees from Bayer, Guerbet, and Siemens.
Funding
This work had been supported by the key special Grant of Chinese Government (2016YFC1300400 and 2007BAI05B02), and National Natural Science Foundation of China (81371548, 81571672 and 81171343) and a Taishan Scholar Projection.
Statistics and biometry
Dr Richard Takx from Utrecht University was the expert in statistics or biometry in the preparation of this manuscript.
Ethical approval
Institutional Review Board approval was obtained.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Methodology
• retrospective
• cross-sectional study
• performed at one institution
Rights and permissions
About this article
Cite this article
Gu, H., Gao, Y., Hou, Z. et al. Prognostic value of coronary atherosclerosis progression evaluated by coronary CT angiography in patients with stable angina. Eur Radiol 28, 1066–1076 (2018). https://doi.org/10.1007/s00330-017-5073-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-017-5073-8