Abstract
Objectives
We aimed to examine the impact of corticospinal tract (CST) involvement in acute ischaemic stroke (AIS) patients on functional outcome and the interaction with reperfusion.
Methods
We retrospectively examined data in consecutive anterior circulation AIS patients undergoing thrombolysis. MR perfusion (time to maximum of tissue residue function, Tmax) and apparent diffusion coefficient (ADC) images were transformed into standard space and the volumes of CST involvement by Tmax > 6 s (CST-Tmax) and ADC < 620 × 10−6 mm2/s (CST-ADC) lesions were calculated. Good outcome was defined as modified Rankin scale ≤ 2 at 3 months. Reperfusion was defined as a reduction in Tmax > 6 s lesion volume of ≥70% between baseline and 24 h.
Results
82 patients were included. Binary logistic regression revealed that both CST-Tmax and CST-ADC volume at baseline were significantly associated with poor outcome (p < 0.05). The 24-h CST-ADC volume was correlated with baseline CST-ADC volume in patients with reperfusion (r = 0.79, p < 0.001) and baseline CST-Tmax volume in patients without reperfusion (r = 0.67, p < 0.001). In patients with CST-Tmax volume > 0 mL and CST-ADC volume < 3 mL, the rate of good outcome was higher in patients with reperfusion than those without (70.4% vs 38.1%, p = 0.04).
Conclusions
The use of CST-Tmax in combination with CST-ADC provides prognostic information in patients considered for reperfusion therapies.
Key Points
• Examine the impact of corticospinal tract involvement in acute ischaemic stroke patients.
• Spatially registered Tmax images can identify corticospinal tract hypoperfusion injury.
• Corticospinal tract salvage through reperfusion is associated with improved outcome.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In acute ischaemic stroke (AIS), penumbra is defined as hypoperfused tissue with loss of electric activity, which is potentially salvageable with timely reperfusion [1, 2]. Successful reperfusion of penumbra is of great significance to reversal of neurological deficits, leading to good outcome [3]. The DEFUSE (Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution) [4] and EPITHET (Echoplanar Imaging Thrombolysis Evaluation Trial) [5] studies used the perfusion parameter time to maximum of tissue residue function (Tmax) on MRI to identify hypoperfused tissue, and documented that a Tmax > 6 s identified ischaemic tissue that was likely to progress to irreversible injury in the absence of reperfusion.
The majority of current imaging analyses in AIS for reperfusion therapy focus on the overall extent of ischaemic injury. However, infarct localization and fibre tract integrity play critical roles in functional impairment [6, 7]. Studies have demonstrated that the severity of functional motor deficit after AIS is highly dependent on the extent of lesion involved in corticospinal tract (CST), but not the lesion size [7]. In 2010, diffusion tensor imaging (DTI)-derived probabilistic maps of CST were developed, and the extent of lesion along the CST was shown to strongly correlate with motor impairment in chronic stroke patients [8, 9]. Most recently, the volume of diffusion lesion within the CST (defined using a probabilistic atlas derived from healthy elderly control subjects) was shown to strongly correlate with post-stroke motor outcomes at 3 months [10].
We hypothesized that timely reperfusion of hypoperfused but diffusion-negative CST would be associated with improved functional outcome and applied the atlas-based assessment of CST involvement to Tmax maps, in conjunction with assessment of reperfusion.
Materials and methods
Patient selection
We retrospectively reviewed our prospectively collected database for AIS patients who received thrombolytic therapy between March 2009 and December 2014. We included patients who (1) had a diagnosis of anterior circulation AIS confirmed by diffusion-weighted imaging (DWI); (2) received intravenous recombinant tissue-type plasminogen activator (rt-PA) within 6 h of symptom onset; (3) underwent T1-weighted imaging (T1WI), DWI and magnetic resonance perfusion (MRP) before and 24 h after rt-PA infusion; (4) had complete follow-up at 3 months. We excluded patients with poor image quality due to severe head motion, and movement artefacts on MRI were assessed by an experienced neuroradiologist (Z.W.).
Ethics statement
The study was approved by our local human ethics committee. All clinical investigations were conducted according to the principles expressed in the Declaration of Helsinki. Informed consent was obtained from all patients.
MR acquisition
All subjects underwent MRI on a 3.0-T system (Signa Excite HD, General Electric Medical System, WI, USA) equipped with an 8-channel phased array head coil. The MRI protocol included an axial isotropic diffusion-weighted echo-planar spin-echo sequence and bolus-tracking MRP. DWI was performed with a spin echo-planar sequence (field of view = 240 mm, slice thickness = 5 mm, number of slices = 18, slice gap = 1 mm, acquisition matrix = 160 × 160). MRP was performed with gradient echo-planar imaging (field of view = 240 mm, repetition time = 1500 ms, echo time = 30 ms, acquisition matrix = 128 × 128, total repetitions = 50, gadolinium dose = 15 mL, injection speed = 4–5 mL/s, scan duration = 1 min 15 s). Conventional T1WI parameters were repetition time = 1900 ms, echo time = 25 ms and slice thickness = 5.0 mm.
Image analysis
The CST-Tmax and CST-ADC were assessed using similar approaches to previous publications [8, 10, 11]. The specific steps were as follows: (1) MIStar (Apollo Medical Imaging Technology, Melbourne, Vic., Australia) was used to generate Tmax from MRP and apparent diffusion coefficient (ADC) images from DWI. The Tmax map was produced using standard singular value deconvolution (SVD) without delay correction (no arterial input function (AIF) delay). The AIF was automatically selected, which should be a global AIF from a normal artery. (2) Both Tmax and ADC images were spatially coregistered with the concurrently acquired high-resolution T1WI for each subject using SPM12 (Wellcome Department of Neurology, University College of London, UK). T1WI was spatially registered into the Montreal Neurology Institute (MNI) standard brain space using the segmentation-based spatial normalization algorithm implemented in SPM12. ADC and Tmax images were subsequently warped into the MNI space using the T1WI-derived spatial normalization transformation. We applied cost function masking (CFM) [12] first and used the state-of-art new segmentation-based brain normalization method, a new upgrade to the unified normalization [13], to register an individual brain into the MNI space. (3) Infarct lesion was defined on the normalized ADC images in MRIcron software (http://www.nitrc.org/projects/mricron) with a threshold of ADC < 620 × 10−6 mm2/s [14]. Hypoperfusion was identified by MRIcron with a threshold of Tmax > 6 s on the normalized Tmax images. Then the automatically segmented infarct and hypoperfusion lesions were manually checked and corrected [including two steps: (1) correction of non-cerebral parenchyma areas including ventricles and leptomeninges being labelled as hypoperfusion or infarct lesion; (2) correction of cerebral parenchymal areas in the unaffected hemisphere being labelled as hypoperfusion or infarct lesion] by one experienced neurologist (S.Z.) and another radiologist (Z.W.) who were blinded to all other imaging and clinical data. Both lesions were overlaid with the canonical CST [8, 10] to determine CST-ADC and CST-Tmax. The volumes of CST-ADC or CST-Tmax were calculated by adding up the volumes of ADC or Tmax lesions overlaid with the posterior limb of internal capsule and the upper extent of corona radiata.
Outcome measures
The National Institute of Health Stroke Scale (NIHSS) and the motor subscore of the NIHSS (mNIHSS, the limb-related elements of the NIHSS) were collected at baseline, 24 h and 7 days after stroke onset. Overall reperfusion rate (RR) was defined as (baseline hypoperfusion volume − 24-h hypoperfusion volume)/baseline hypoperfusion volume, and overall reperfusion was defined as RR ≥ 70% with baseline hypoperfusion volume ≥ 10 mL [15]. CST reperfusion rate (CRR) was defined as (baseline CST-Tmax volume − 24-h CST-Tmax volume)/baseline CST-Tmax volume, and CST reperfusion was defined as CRR ≥ 70% with baseline CST-Tmax volume ≥ 0.2 mL. The modified Rankin Scale (mRS) was collected at 3 months after stroke onset, which was used to define good outcome (mRS ≤ 2) or poor outcome (mRS > 2). In the study, we defined good motor outcome as mNIHSS ≤ 4 and poor motor outcome as mNIHSS > 4. Haemorrhage transformation (HT) and symptomatic HT (SHT) were defined according to the European Cooperative Acute Stroke Study (ECASS) II trial [16].
Statistical analysis
Statistical analysis was performed using SPSS 17.0 (SPSS Inc., Chicago, USA). All metric and normally distributed variables were reported as mean ± standard deviation; non-normally distributed variables were reported as median (25th–75th percentile). Categorical variables were presented as frequency (percentage). Comparisons between groups were assessed by using Student’s t test for data that followed a normal distribution, Mann–Whitney U test for data that did not follow a normal distribution and Fisher’s exact test for categorical data. Binary logistic regression analysis was used in analysis of clinical outcome. Patients were divided into reperfusion group and non-reperfusion group for correlation analyses, which also were derived from overall data. Pearson correlation analysis was used to analyse the relationship of volumes between CST-ADC and CST-Tmax. The optimal volume thresholds for CST-ADC and CST-Tmax to define good outcome were calculated using receiver operating characteristic (ROC) analysis, and the threshold was derived from analysis from overall data of the included patients. A p value of less than 0.05 was considered to be statistically significant.
Results
Subject characteristics
A total of 82 patients were included after exclusion of six who had poor image quality. The mean age was 67.5 ± 12.3 years and 55 (67.1%) were male. Baseline NIHSS score was 7.0 (4.0–14.0) and the median time from onset to treatment was 219.5 (174.3–261.5) min. Good motor outcome occurred in 61 (74.4%) patients at 7 days. Good outcome occurred in 55 (67.1%) patients at 3 months. Overall reperfusion was analysed in 58 (70.7%) patients, of whom 33 (56.9%) patients achieved reperfusion at 24 h, 40 (69.0%) patients had good motor outcome and 34 (58.6%) patients had good outcome. CST reperfusion was analysed in 54 (65.9%) patients, of whom 28 (51.9%) patients achieved CST reperfusion at 24 h, 35 (64.8%) patients had good motor outcome and 30 (55.6%) patients had good outcome.
Relationship between CST-Tmax volume and neurological outcome
In univariate analysis, patients with poor outcome were older and had higher baseline NIHSS score, longer time from onset to treatment, larger baseline CST-ADC volume and CST-Tmax volume than the patients with good outcome (Table 1). Binary logistic regression showed that baseline CST-Tmax volume was significantly associated with poor outcome (odds ratio (OR) 1.4, 95% confidence interval (CI) 1.1–1.8, p = 0.003) and poor motor outcome (OR 1.5, 95% CI 1.2–1.9, p < 0.001) after adjusting for age and onset to treatment time. The areas under the curves in ROC analysis for poor outcome and poor motor outcome were 0.76 (95% CI 0.66–0.87, p < 0.001) and 0.79 (95% CI 0.67–0.90, p < 0.001), respectively, larger than those of overall hypoperfusion volume which were 0.73 (95% CI 0.60–0.85, p = 0.001) and 0.73 (95% CI 0.60–0.87, p = 0.002), respectively.
As shown in Table 2, all 16 patients with CST-Tmax volume = 0 mL had good outcome, while only 59.1% (39/66) patients with CST-Tmax volume > 0 mL had good outcome (p = 0.001). In contrast, patients with overall hypoperfusion volume < 10 mL on MRP had a lower rate of good outcome (21/24, 87.5%).
We analysed reperfusion status in the patients (n = 51) who had baseline CST-Tmax volume > 0 mL and found that the rate of good outcome was significantly higher in patients with overall reperfusion than those without reperfusion (67.9% vs 34.8%, p = 0.026).
Relationship between reperfusion and CST-ADC volume and CST-Tmax volume
The baseline CST-ADC volume was correlated with baseline CST-Tmax volume in patients with overall reperfusion (r = 0.50, p = 0.003) (Fig. 1b) and not correlated with baseline CST-Tmax volume in those without overall reperfusion (r = 0.31, p = 0.134) (Fig. 1a), as well as in patients without (r = 0.24, p = 0.230) and with CST reperfusion (r = 0.33, p = 0.088) (Fig. 1c, d). The 24-h CST-ADC volume was larger than baseline CST-ADC volume (0.334 (0.051–1.430) vs 0.113 (0–0.546) mL, p = 0.001; 1.317 (0.334–7.656) vs 0.413 (0.013–1.561) mL, p = 0.001) both in patients with and without overall reperfusion. This difference was also found in patients with and without CST reperfusion (p = 0.027, p = 0.001).
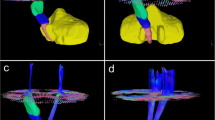
Relationship between reperfusion and CST-ADC volume and CST-Tmax volume. Scatter plot comparing baseline CST-ADC volume and baseline CST-Tmax volume in patients a without and b with overall reperfusion; and c without and d with CST reperfusion. Scatter plot comparing 24-h CST-ADC volume and baseline CST-Tmax volume in patients e without and f with overall reperfusion; and g without and h with CST reperfusion. Scatter plot comparing 24-h CST-ADC volume and baseline CST-ADC volume in patients i without and j with overall reperfusion; and k without and l with CST reperfusion. CST corticospinal tract, CST-ADC volumes of CST involvement by apparent diffusion coefficient < 620 × 10−6 mm2/s, CST-Tmax volumes of CST involvement by time to maximum of tissue residue function > 6 s
The 24-h CST-ADC volume was correlated with baseline CST-Tmax volume in patients without overall reperfusion (r = 0.67, p < 0.001) and baseline CST-ADC volume in those with overall reperfusion (r = 0.79, p < 0.001) (Fig. 1e, j). The 24-h CST-ADC volume was correlated with baseline CST-Tmax volume in patients without CST reperfusion (r = 0.67, p < 0.001) and baseline CST-ADC volume in those with CST reperfusion (r = 0.70, p < 0.001) (Fig. 1g, l).
Relationship between CST-ADC volume and neurological outcome
Baseline CST-ADC volume was significantly associated with poor outcome (OR 5.1, 95% CI 2.1–12.8, p < 0.001) and poor motor outcome (OR 2.9, 95% CI 1.5–5.7, p = 0.003) after adjusting for age and onset to treatment time in binary logistic regression. In ROC analysis the area under curve was 0.79 (95% CI 0.68–0.90, p < 0.001) for poor outcome and 0.81 (95% CI 0.70–0.92, p < 0.001) for poor motor outcome, larger than that of overall infarct volume of 0.66 (95% CI 0.53–0.79, p = 0.020) and 0.61 (95% CI 0.45–0.76, p = 0.147), respectively. Additionally, the threshold of baseline CST-ADC volume to predict poor outcome was 3 mL, with a specificity of 100% in ROC analysis.
Relationship between the combined CST-Tmax and CST-ADC imaging profiles and good outcome
We analysed the relationship between reperfusion and good outcome (Table 3) and good motor outcome (Table 4). Patients were divided into three groups using CST-Tmax volume and CST-ADC volume at baseline: (1) no hypoperfusion lesion (CST-Tmax volume = 0 mL, n = 16), (2) large infarct lesion (CST-ADC volume ≥ 3 mL, n = 3) and (3) target lesion profile (CST-Tmax volume > 0 mL combined with CST-ADC volume < 3 mL, n = 63). We found that overall reperfusion (70.4% vs 38.1%, p = 0.04) and CST reperfusion (74.1% vs 41.7%, p = 0.025) led to higher rates of good outcome than those without CST reperfusion in patients with target lesion profile. Overall reperfusion (85.2% vs 42.9%, p = 0.005) and CST reperfusion (85.2% vs 45.8%, p = 0.006) also led to higher rates of good motor outcome than those without overall reperfusion or CST reperfusion in patients with target lesion profile. Representative examples are shown in Figs. 2 and 3. Moreover, there was no association between reperfusion and HT or SHT in three groups as shown in Tables 1 and 2 in the Supplemental Material.
Representative examples of two patients. Infarct lesion (violet) and hypoperfusion lesion (green) were overlaid onto the probabilistic fibre map (red). The images in the left column were obtained at baseline, images in the right column were obtained at 24 h after thrombolysis. For each patient, images before and after normalization were presented separately. Patient A had relatively large CST-ADC volume and did poorly despite reperfusion. Patient B had a relatively small CST-Tmax volume and therefore reperfusion status was unlikely to influence motor outcome. CST corticospinal tract, CST-ADC volumes of CST involvement by apparent diffusion coefficient < 620 × 10−6 mm2/s, CST-Tmax volumes of CST involvement by time to maximum of tissue residue function > 6 s, mNIHSS limb-related elements of the National Institute of Health Stroke Scale, mRS modified Rankin Scale
See caption of Fig. 2 for full explanation and abbreviations. Representative examples of two patients. In patients with a target profile (CST-Tmax volume > 0 mL and CST-ADC volume < 3 mL), the outcome at 3 months was significantly better in patients with reperfusion (patient C) than those without reperfusion (patient D)
Additionally, the areas under the curve in ROC analysis of CST reperfusion rate for good outcome and good motor outcome were 0.71 (95% CI 0.55–0.86, p = 0.018) and 0.76 (95% CI 0.60–0.92, p = 0.005), respectively, larger than those of overall reperfusion rate which were 0.68 (95% CI 0.51–0.85, p = 0.042) and 0.72 (95% CI 0.56–0.87, p = 0.008), respectively.
Discussion
This study has demonstrated that CST salvage through reperfusion is associated with improved outcome, independent of overall salvage. We normalized Tmax and ADC images to canonical brain and confirmed that patients with target lesion (CST-Tmax volume > 0 mL and CST-ADC volume < 3 mL) benefit from reperfusion, indicating that an early estimate of potentially salvageable tissue and ischaemic core specifically related to the motor pathway would more accurately predict imaging and clinical outcomes and potentially guide therapeutic interventions.
Minor stroke poses a dilemma for thrombolysis decision-making with many patients deemed “too good to treat” [17]. However, without reperfusion therapy, 25% minor stroke patients deteriorate and are left disabled or even dead [18]. It is difficult to predict which patients will deteriorate [19,20,21]. Hypoperfusion volume < 10 mL has been proposed as an imaging marker for minor stroke [22, 23]. However, the location of the perfusion lesion is also an important consideration. In our study, the absence of CST involvement in the perfusion lesion (CST-Tmax volume = 0 mL) was associated with good outcome in all patients, compared to only 87.5% good outcome in patients with hypoperfusion volume < 10 mL. Therefore, the use of CST-Tmax volume may provide an important clue for guiding reperfusion therapy in minor stroke patients.
The “mismatch” principle is that baseline ischaemic core volume will approximate the final infarct volume in patients who achieve reperfusion, whilst the baseline hypoperfusion volume will predict the final infarct volume in “non-reperfusers” [24]. We observed that this principle held true when applied specifically to the corticospinal tract. The 24-h CST-ADC volume was well correlated with baseline CST-ADC volume in patients who achieved reperfusion and baseline CST-Tmax volume in patients without reperfusion, indicating that hypoperfused CST tissue may be salvaged by timely reperfusion, whereas CST tissue exhibiting diffusion restriction at baseline was not salvageable. However, without reperfusion, the hypoperfusion CST tissue converted to irreversible injury.
Previous studies have demonstrated that the severity of motor function damage after AIS is highly dependent on the volume of CST-ADC rather than the overall lesion size [10], which was also found in our study. Besides, all patients with baseline CST-ADC volume ≥ 3 mL had poor outcome even if reperfusion was achieved. Therefore, we proposed a cut-off of 3 mL for CST-ADC volume, which might be helpful to exclude patients who would not be eligible for reperfusion therapy. Since we did not obtain detailed information about the amount or intensity of rehabilitation in our patients and did not use it as a covariate in the multivariate regression analysis, future studies may be needed to determine whether patients with large CST-ADC volume would benefit more from rehabilitation therapy than reperfusion therapy.
On the basis of the above findings, we gave a definition of target lesion and found that the rate of good outcome was higher in patients with reperfusion than those without reperfusion. Patients with target lesion had relatively large CST-Tmax (large hypoperfused tissue which may die without reperfusion) and small CST-ADC lesion (already dead tissue), and might achieve functional recovery from reperfusion therapy. Therefore, the combination of CST-Tmax and CST-ADC might be useful to select eligible patients who would benefit from reperfusion therapy, and to monitor the therapeutic response to acute interventions.
Our use of CST hypoperfusion and infarct using diffusion and perfusion MR has advantages over other more specialized sequences such as functional MRI and DTI, which can be challenging to acquire in acute stroke patients because of their long acquisition time. Although a decrease in fractional anisotropy derived from DTI was associated with poor motor outcomes in stroke patients at chronic phase [25], it offered minimal predictive value in the acute phase [6]. Some studies also showed that DTI might provide inaccurate assessment of the CST lesion as a tract distal to a lesion may still appear structurally intact in the acute phase, despite already being irreversibly damaged [10]. In future, our method to evaluate CST injury in acute phase would refine selection of reperfusion therapy in AIS patients. Despite of the overall lesion size, it is rational to give reperfusion therapy in patients with target CST lesion. Recent study has showed that it was feasible and practical to achieve the benchmark of door-to-needle time ≤60 min, by using MRI as the routine screening modality before reperfusion therapy. With the post-processing of DWI/PWI, our method provides more details about motor function and could be integrated into acute workflow when waiting for the patient’s consent form. In addition, fibre tracts have been suggested to have greater ischaemic tolerance than other regions in recent studies [26]. For example, a recent study demonstrated that immediate remote ischaemic postconditioning after ischaemia could protect cerebral white matter but not grey matter in piglets [27].
Our study had limitations related to its retrospective nature and moderate sample size. Spatially normalized brain images may contain distortions due to large lesions, oedema or cerebral ventricle. However, solutions such as masking large lesions were used to improve the accuracy of spatial normalization. Besides, we had not discussed the involvement of other specific eloquent areas other than CST in this study considering the various complex functional area. Motor function is not the sole determinant of functional outcome and other key abilities such as language could be similarly studied in future analyses. Posterior circulation ischaemic stroke was not included in our study and perfusion parameters may differ from the anterior circulation. Confirmation and extension in larger and multicentre cohorts is needed.
Conclusion
Spatially registered Tmax and ADC images could identify CST hypoperfusion and irreversible injury in the acute phase of ischaemic stroke. The combined information from CST-Tmax and CST-ADC could improve outcome prediction and potentially refine selection of reperfusion therapy.
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- AIF:
-
Arterial input function
- AIS:
-
Acute ischaemic stroke
- CI:
-
Confidence interval
- CRR:
-
Corticospinal tract reperfusion rate
- CST:
-
Corticospinal tract
- CST-ADC:
-
Volumes of corticospinal tract involvement by apparent diffusion coefficient < 620 × 10−6 mm2/s
- CST-Tmax:
-
Volumes of corticospinal tract involvement by time to maximum of tissue residue function > 6 s
- DTI:
-
Diffusion tensor imaging
- DWI:
-
Diffusion-weighted imaging
- HT:
-
Haemorrhage transformation
- MNI:
-
Montreal Neurology Institute
- mNIHSS:
-
Motor subscore of the National Institute of Health Stroke Scale
- mRS:
-
Modified Rankin scale
- MRP:
-
Magnetic resonance perfusion
- NIHSS:
-
National Institute of Health Stroke Scale
- OR:
-
Odds ratio
- ROC:
-
Receiver operating characteristic
- RR:
-
Reperfusion rate
- rt-PA:
-
Recombinant tissue-type plasminogen activator
- SVD:
-
Singular value deconvolution
- SHT:
-
Symptomatic haemorrhage transformation
- T1WI:
-
T1-weighted imaging
- Tmax:
-
Time to maximum of tissue residue function
References
Schlaug G, Benfield A, Baird AE et al (1999) The ischemic penumbra: operationally defined by diffusion and perfusion MRI. Neurology 53:1528–1537
Rohl L, Ostergaard L, Simonsen CZ et al (2001) Viability thresholds of ischemic penumbra of hyperacute stroke defined by perfusion-weighted MRI and apparent diffusion coefficient. Stroke 32:1140–1146
Inoue M, Mlynash M, Straka M et al (2013) Clinical outcomes strongly associated with the degree of reperfusion achieved in target mismatch patients: pooled data from the Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution studies. Stroke 44:1885–1890
Albers GW, Thijs VN, Wechsler L et al (2006) Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol 60:508–517
Davis SM, Donnan GA, Parsons MW et al (2008) Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurol 7:299–309
Puig J, Pedraza S, Blasco G et al (2011) Acute damage to the posterior limb of the internal capsule on diffusion tensor tractography as an early imaging predictor of motor outcome after stroke. AJNR Am J Neuroradiol 32:857–863
Lee JS, Han MK, Kim SH, Kwon OK, Kim JH (2005) Fiber tracking by diffusion tensor imaging in corticospinal tract stroke: topographical correlation with clinical symptoms. NeuroImage 26:771–776
Zhu LL, Lindenberg R, Alexander MP, Schlaug G (2010) Lesion load of the corticospinal tract predicts motor impairment in chronic stroke. Stroke 41:910–915
Lindenberg R, Zhu LL, Ruber T, Schlaug G (2012) Predicting functional motor potential in chronic stroke patients using diffusion tensor imaging. Hum Brain Mapp 33:1040–1051
Feng W, Wang J, Chhatbar PY et al (2015) Corticospinal tract lesion load: an imaging biomarker for stroke motor outcomes. Ann Neurol 78:860–870
Revenaz A, Ruggeri M, Lagana M et al (2016) A semi-automated measuring system of brain diffusion and perfusion magnetic resonance imaging abnormalities in patients with multiple sclerosis based on the integration of coregistration and tissue segmentation procedures. BMC Med Imaging 16:4
Brett M, Leff AP, Rorden C, Ashburner J (2001) Spatial normalization of brain images with focal lesions using cost function masking. NeuroImage 14:486–500
Ashburner J, Friston KJ (2005) Unified segmentation. NeuroImage 26:839–851
Purushotham A, Campbell BC, Straka M et al (2015) Apparent diffusion coefficient threshold for delineation of ischemic core. Int J Stroke 10:348–353
Zhang S, Tang H, Yu YN, Yan SQ, Parsons MW, Lou M (2015) Optimal magnetic resonance perfusion thresholds identifying ischemic penumbra and infarct core: a Chinese population-based study. CNS Neurosci Ther 21:289–295
Larrue V, Von KRR, Müller A, Bluhmki E (2001) Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European-Australasian Acute Stroke Study (ECASS II). Stroke 32:438
Yu AY, Hill MD, Coutts SB (2015) Should minor stroke patients be thrombolyzed? A focused review and future directions. Int J Stroke 10:292–297
Smith EE, Abdullah AR, Petkovska I, Rosenthal E, Koroshetz WJ, Schwamm LH (2005) Poor outcomes in patients who do not receive intravenous tissue plasminogen activator because of mild or improving ischemic stroke. Stroke 36:2497–2499
Living AOD (1995) Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med 333:1581–1587
Shi L, Zhang M, Liu H et al (2014) Safety and outcome of thrombolysis in mild stroke: a meta-analysis. Med Sci Monit 20:2117–2124
Hacke W, Kaste M, Bluhmki E et al (2008) Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 359:1317–1329
Lansberg MG, Lee J, Christensen S et al (2011) RAPID automated patient selection for reperfusion therapy: a pooled analysis of the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET) and the Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution (DEFUSE) Study. Stroke 42:1608–1614
Campbell BC, Mitchell PJ, Yan B et al (2014) A multicenter, randomized, controlled study to investigate EXtending the time for Thrombolysis in Emergency Neurological Deficits with Intra-Arterial therapy (EXTEND-IA). Int J Stroke 9:126–132
Albers GW, Goyal M, Jahan R et al (2016) Ischemic core and hypoperfusion volumes predict infarct size in SWIFT PRIME. Ann Neurol 79:76–89
Lindenberg R, Renga V, Zhu LL, Betzler F, Alsop D, Schlaug G (2010) Structural integrity of corticospinal motor fibers predicts motor impairment in chronic stroke. Neurology 74:280–287
Tekkok SB, Ransom BR (2004) Anoxia effects on CNS function and survival: regional differences. Neurochem Res 29:2163–2169
Ezzati M, Bainbridge A, Broad KD et al (2015) Immediate remote ischemic postconditioning after hypoxia ischemia in piglets protects cerebral white matter but not grey matter. J Cereb Blood Flow Metab. doi:10.1177/0271678X15608862
Acknowledgments
We are grateful for the support from our patients.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Min Lou.
Conflict of interest
The authors of this manuscript declare relationships with the following companies: Dr. Liebeskind is among the consultant/advisory board (modest) of Stryker and Covidien. Other authors have no actual or potential conflicts of interest to disclose.
Funding
This study has received funding by the National Natural Science Foundation of China (81622017 & 81471170) and the National Key Research and Development Program of China (2016YFC1301500).
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional review board approval was obtained.
Methodology
• retrospective
• observational
• performed at one institution
Additional information
Ying Zhou and Ruiting Zhang contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 17 kb)
Rights and permissions
About this article
Cite this article
Zhou, Y., Zhang, R., Zhang, S. et al. Impact of perfusion lesion in corticospinal tract on response to reperfusion. Eur Radiol 27, 5280–5289 (2017). https://doi.org/10.1007/s00330-017-4868-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-017-4868-y