Abstract
Objectives
Our goal is to determine the ability of multi-parametric magnetic resonance imaging (mpMRI) to differentiate muscle invasive bladder cancer (MIBC) from non-muscle invasive bladder cancer (NMIBC).
Methods
Patients underwent mpMRI before tumour resection. Four MRI sets, i.e. T2-weighted (T2W) + perfusion-weighted imaging (PWI), T2W plus diffusion-weighted imaging (DWI), T2W + DWI + PWI, and T2W + DWI + PWI + dif-fusion tensor imaging (DTI) were interpreted qualitatively by two radiologists, blinded to histology results. PWI, DWI and DTI were also analysed quantitatively. Accuracy was determined using histopathology as the reference standard.
Results
A total of 82 tumours were analysed. Ninety-six percent of T1-labeled tumours by the T2W + DWI + PWI image set were confirmed to be NMIBC at histopathology. Overall accuracy of the complete mpMRI protocol was 94% in differentiating NMIBC from MIBC. PWI, DWI and DTI quantitative parameters were shown to be significantly different in cancerous versus non-cancerous areas within the bladder wall in T2-labelled lesions.
Conclusions
MpMRI with DWI and DTI appears a reliable staging tool for bladder cancer. If our data are validated, then mpMRI could precede cystoscopic resection to allow a faster recognition of MIBC and accelerated treatment pathways.
Key Points
• A critical step in BCa staging is to differentiate NMIBC from MIBC.
• Morphological and functional sequences are reliable techniques in differentiating NMIBC from MIBC.
• Diffusion tensor imaging could be an additional tool in BCa staging.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bladder cancer (BCa) is a common cancer worldwide and one of the most expensive to manage [1, 2]. This disease accounts for 6–8% of all male malignancies and 2–3% in women, reflecting aetiological factors [3, 4]. The tumour is best classified as either non-muscle invasive (NMIBC) or muscle-invasive (MIBC) as this reflects the biology and alters the treatment intent [5]. Most deaths occur from MIBC, which must be treated radically if cure is to be obtained. Whilst many NMIBC are indolent tumours, around one- third are high grade and can rapidly progress to muscle invasion and metastases [6, 7]. As such, the prognosis and treatment of patients with BCa requires accurate staging at the time of diagnosis.
Staging of BCa depends upon clinical, radiological and pathological features. The former are obtained from endoscopic appearances and patient features, whilst the latter reflect the material obtained by transurethral resection (TUR). Conflicting results between these staging tools are common. Whilst TUR is the cornerstone of BCa staging, it is recognized to both under- and overstage cancers. Up to one- third of invasive BCa are initially staged as NMIBC at TUR [8], and residual tumour rates following TUR vary considerably, reflecting user dependency of this procedure [9].
Given the limitations of current clinical staging approaches, improved radiological tools are needed. Magnetic resonance imaging (MRI) is an accurate technique that provides a high tissue contrast resolution, being able to differentiate the layers of the bladder wall without administration of radiation.
Although a number of studies have shown little difference in the accuracy of multiple detector computed tomography (MDCT) and MRI for BCa staging [10], recent improvements in MRI technology and new functional MR sequences such as diffusion- weighted imaging (DWI) and perfusion-weighted imaging (PWI) suggest it is time to review the accuracy of contemporary MRI. The aim of this current study is to examine the accuracy of morphological and functional mpMRI sequences in differentiating NMIBC from MIBC. Tested sequences include PWI, DWI and diffusion tensor imaging (DTI), with the fractional anisotropy (FA) methodology and fibre tractography (FT).
Materials and methods
Patient population
Between January and November 2016, 76 consecutive patients with suspected or confirmed bladder lesions were enrolled in the study, following institutional review board approval. All patients underwent MRI and then bladder resection within the following 2–8 weeks. We selected patients with normal renal function whose cytology, ultrasound (US) and/or cystoscopy suggested bladder cancer, and obtained written informed consent. We excluded patients with renal impairment, those who did not provide consent and those who could not attain adequate bladder distension. Prior to MRI evaluation by the readers, MRI examinations of patients with locally advanced BCa (pT3-4) and whose bladder resection did not reveal BCa were excluded.
MR image acquisition
Prior to the MRI, patients received bowel preparation for 24 h and intramuscular administration of Buscopan® 1 ml (20 mg/ml) just before imaging. Patients voided urine 1 h before the MRI and then drank water until the scan. Studies were performed at a 3 T MRI scanner (Discovery MR750, GE Healthcare, Milwaukee, WI, USA) equipped with a 32-channel pelvic phased-array coil (HD Body, GE Healthcare). Patients were positioned with the bladder at the coil isocentre. The entire pelvis was imaged from the aortic bifurcation to the inferior margin of the pubic symphysis.
MR sequences
Turbo spin-echo T2-weighted (T2W) images (TR/TE, 8,000/120 ms; matrix, 320x320; section thickness, 3 mm; field of view, 23 cm;) were obtained in axial and sagittal planes. DW images were obtained during free-breathing in the axial plane by using a single-shot spin-echo echoplanar sequence with chemical shift-selective fat-suppression techniques (b, 0-500-800-1,000 s/mm2; TR/TE, 5,300/60 ms; matrix, 128x96; section thickness, 3 mm; field of view, 24 cm). PWI images were acquired with a temporal resolution of 5 s (TR/TE, 2.9/1.3 ms; flip angle, 15°; matrix, 192x138; section thickness, 3 mm), 20–180 s after intravenous administration of 0.1 mmol/kg of gadobutrol (Gadovist, Bayer Schering Pharma, Berlin, Germany) at a rate of 3 ml/s. T1-weighted images were obtained post contrast medium injection with a fat-suppressed 3D volumetric spoiled gradient-echo sequence (TR/TE, 16.4/4.7 ms; flip angle, 15°; section thickness, 3 mm; matrix, 352x352) aimed at complete pelvic examination. DTI data were acquired using a sequence with the following parameters: b-value of 0-1,000 s/mm2; 16 different gradient directions, TR/TE, 2,000/80 ms; slice thickness, 3 mm; matrix size, 96x96; field of view, 24 cm.
MRI data analysis
Images were independently analysed in two reading sessions (2 weeks apart) by two radiologists with 10 and 3 years of urogenital experience, respectively. Disagreements were resolved by consensus. Four image sets (T2W plus PWI, T2W plus DWI, T2W plus DWI plus PWI and T2W plus DWI plus PWI plus DTI) for each patient were interpreted qualitatively without knowledge of the surgical or histological findings.
During the first session, each radiologist interpreted T2W plus PWI images according to criteria previously described in the literature [11, 12] without formulating a definitive diagnosis of muscular invasion. On T2W images the bladder wall was considered to be intact (stage T1 or lower) when the low SI line which represents the normal bladder wall was present. On contrast-enhanced images, an intact submucosal linear enhancement adjacent to a tumour and no enhancement of the muscle layer, which is indicative of a normal wall, was regarded as indicative of stage T1 or lower. In the second session, the remaining images were analysed, including DWI sequences and DTI imaging results, which were assessed both qualitatively and quantitatively. DWI and the corresponding apparent diffusion coefficient (ADC) maps were interpreted using staging criteria described in the literature. At DWI, a focal high signal intensity (SI) area corresponds to the tumour. A high SI tumour with a low SI submucosa [13] was considered as stage T1 or lower; a high SI tumour without a low SI submucosal lining was classified as T2 or higher. When a stalk is present, in a T1 lesion it is shown as a low SI structure. DTI data were assessed qualitatively using FT images, where non-invasive cancer shows even colours and regular arrangement of muscle fibres; invasive cancer shows irregular arrangement and directions of muscle fibres. DTI was used where there was inconsistency between PWI and DWI: if detrusor muscle invasion was suspected on FT images, a lesion was categorized as T2, otherwise it was labelled as T1. Detrusor muscle invasion was scored according to criteria described in the literature as follows: 1, probably absent; 2, possibly absent; 3, slightly suspicious; 4, possibly present; or 5, probably present.
Quantitative analysis was based on the results of the third image set (T2W + PWI + DWI), which was used to formulate the definitive report. All cases considered at least as “slightly suspicious” (i.e. all cases with a score ≥ 3) after reading the aforementioned image set (34 lesions) were analysed separately from the others (48 lesions). Three regions of interest (ROIs) were considered, placed respectively: on the intravesical tumour (ROI 1), at the bladder wall immediately below the lesion (ROI 2: detrusor/tumour interface) between the bladder mucosa and detrusor muscle, and on a normal detrusor muscle remote from the lesion (ROI 3).
As regards quantitative evaluation of PWI, we considered the k-trans value as a robust parameter since it allows evaluation of capillary permeability, which is indicative of the underlying tissue physiology, particularly the rate of tumour neoangiogenesis [14, 15]. It is also relatively independent from the method of measurement. On PWI images the software calculated the k-trans for each ROI automatically.
On ADC maps, each ROI was measured three times, and an average ADC was used for statistical analysis.
DTI images data were obtained as follows: T2-DWI fusion images were used in order to put the ROIs on the same level as the ADC maps. For each ROI, ADC and FA were calculated and expressed as medians with interquartile ranges (IQRs).
Statistical analysis
Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy in detecting muscle invasion were calculated for all image sets, and receiver operating characteristic (ROC) curves were generated. Muscular invasion was defined using histological material resected from the bladder. Comparisons between PWI, ADC and DTI quantitative parameters (k-trans, ADC value and FA) for the three different ROIs were performed by the Friedman test. Post-hoc tests were performed by the Wilcoxon signed-rank test with Bonferroni correction for the following comparisons: intra-cavity tumour versus detrusor muscle invasion (ROI1 vs. ROI2) and detrusor muscle invasion versus normal remote detrusor muscle (ROI2 vs. ROI3). ROC curves were used to examine the diagnostic performance of DTI parameters versus ADC values to distinguish cancerous areas versus non-cancerous areas within the detrusor muscle (ROI2 vs. ROI3). All statistical analyses were two-sided and performed within MedCalc software for Windows v.14.8.1 (MedCalc Software, Ostend, Belgium). Statistical significance was defined as p < 0.05.
Results
Patients and tumours
In total, 76 patients entered the study, including 46 undergoing TUR, four undergoing partial cystectomy and 26 undergoing radical cystectomy. Two patients were excluded for lack of BCa on histology and four because tumour stage could not be accurately defined histologically. The remaining patients included 56 men and 14 women, with an age range of 45–80 years (mean ± SD: 66 ± 1). A total of 92 lesions were resected, 54 from solitary tumours and 38 from multifocal BCa. Solitary tumours were staged as pTa-1 (n = 23), pT2 (n = 25) pT3 (n = 5) and pT4 (n = 1). Sixteen patients had multifocal BCa, staged as follows: pTa-1 (n = 30; six patients had two foci and six patients had three foci); pT2 (n = 4, two patients with two lesions each); pT4 (n = 4, two patients had two tumours). We excluded ten pT3-4 tumours, leaving a final total of 82 lesions for analysis.
MRI findings: overview
MR images were scored from 1 to 5 according to radiological likelihood of muscle invasion and compared to histological findings (Fig. 1a, b and Table 1). Agreement between the two radiologists for muscle invasion was excellent (kappa values of 0.904 for T2W plus PWI, 0.924 for T2W plus DWI, 0.908 for T2W plus PWI plus DWI and 0.953 for T2W plus PWI plus DWI plus DTI).
Multiparametric magnetic resonance imaging (mpMRI) of the bladder. (a) Invasive bladder cancer depicted respectively on A morphological T2W image as a loss of wall continuity and on diffusion-weighted image as a focal interruption of the low SI of the muscular wall. A related apparent diffusion coefficient (ADC) map for quantitative evaluation is shown. Diffusion tensor imaging (DTI) reconstruction demonstrates uneven fibre arrangement in the bladder wall; note in the same zone a strong and focal enhancement involving detrusor muscle layer on perfusion imaging. (b) Non-invasive bladder cancer depicted respectively on a morphological T2W image as a conserved wall continuity and on diffusion-weighted imaging (DWI) as homogeneous cell density of muscle layer, consistent with integrity of detrusor muscle. A related ADC map for quantitative evaluation is shown. DTI reconstruction shows even fibres in the wall. Focal and strong enhancement of the lesion with homogeneous and diffuse wall enhancement is shown on perfusion imaging. In both Figs. 1a and 1b, quantitative data concerning DWI (ADC), PWI (KTrans) and DTI (FA) are reported, calculated from three regions of interest (ROIs) placed respectively on: the intravesical tumour (ROI 1), at the tumour/bladder wall interface (ROI 2) and on normal detrusor muscle remote from the lesion (ROI 3)
For the third image set (T2W + PWI + DWI), there were only five discrepancies between the two readers: to formulate the definitive report, disagreements were resolved assuming the most experienced radiologist’s interpretation as the correct one, except for obvious oversights.
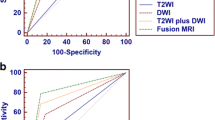
The greatest accuracy for invasion was seen with all MR sequences (T2W, PWI, DWI and DTI: AUROC 0.99), compared to only T2W with PWI (AUROC 0.72) (Fig. 2a).
Both readers overstaged 25/53 NMIBC on the T2W with PWI image set. DTI helped to correctly categorize four suspicious pT2 lesions as NMIBC and gave more confidence in categorizing four pT2 tumours in which muscle invasion was regarded as ‘slightly suspicious’ (Table 1).
MRI findings: vascular permeability in the tumour and detrusor muscle
Quantitative analysis in three different ROIs for vascular permeability (k-trans) in the PWI images (after reading T2W + PWI + DWI images) are shown in Fig. 3. The greatest permeability was present within the intra-luminal tumour (ROI 1) and in the detrusor wall (ROI 2) for muscle invasive cancers (Friedman test p < 0.001). No significant difference in k-trans values was seen for the detrusor/tumour interface or distant detrusor for pTa-1 tumours. ADC values across three different ROIs are shown in Fig. 4. Statistical analysis revealed that the distribution of ADCs differed significantly among the three ROIs (P < 0.001) for pT2 tumours. Post-hoc corrected testing showed no statistically significant differences across ROIs placed in detrusor muscle for pTa-1 tumours. FA values across three different ROIs in two groups of lesions are shown in Fig. 5. Similar to ADC results, the overall tests indicated that the distributions of FA differed among the three ROIs in the two groups. The post-hoc analysis showed that FA was significantly higher at the interface between bladder mucosa and detrusor muscle than in normal muscle in T2 lesions (median ROI 2 = 0.36; IQR [0.30-0.41] vs. median ROI 3 = 0.13; IQR [0.11-0.16]), while in T1 tumours there was no such difference.
Both ADC and FA values were significantly different in NMI and MIBC for ROI 2 (tumour/detrusor interface). ROC analysis suggested (Fig. 2b) FA was significantly superior to ADC (AUC for FA = 0.985 [95% CI 0.914–1.000] vs. ADC = 0.889 [95% CI 0.782–0.955]).
Discussion
BCa diagnosis and staging currently relies on a combination of non-invasive and invasive procedures. Whilst for many patients this approach works well, others are left with undue delays in obtaining TUR samples [16, 17], risk for dissemination of tumour cells at the time of TUR [18] and there can be confusion about the need for radical treatment due to conflicts between staging tools. To improve patient care, we evaluated contemporary functional and anatomical mpMRI sequences. Even though there is no accepted algorithm for radiological imaging of bladder tumour, in recent years it has become evident that MRI can match or be better than MDCT in diagnosing and staging bladder tumours by using standard MRI protocols that rely on single sequences [10, 19]. Further studies have shown that DWI is a reliable technique in bladder imaging [20, 21] and several authors demonstrated that adding functional sequences to traditional protocols improves MRI performance, reaching an accuracy of 92% [13] to 94% [22] for protocols that included T2W, PWI and DWI. Overall, we found that combined protocols offered greatest accuracy and that these outperformed T2W plus PWI sequences. Our results are equal to those in previous reports as far as T2W, DWI and PWI are concerned [23], since the best accuracy is obtained by interpreting the three sequences together.
We found only five cases that were misclassified by the MRI examination. Of these, two T2 lesions had early microscopic invasion at histopathology (T2a) and were classified as NMIBC, whilst three pT1 lesions showing a very similar signal intensity of tumour and sub-mucosa at PWI were labelled as MIBC.
The innovation of the multiparametric evaluation in this study is the introduction of the DTI imaging. In cases where DWI and ADC sequences were inconclusive and an interpretative doubt about bladder invasion existed, we resorted to DTI as decisive tool, because it is also a water diffusion elaboration. This study demonstrates the need to integrate all the sequences information for an optimal bladder evaluation. The inclusion of DTI into an mp-MRI protocol allowed us to reach a diagnostic accuracy of 94% in differentiating MIBC from NMIBC. In this study we also demonstrate that the diffusion tensor parameters of invasive and non-invasive bladder cancer differ significantly. The FA values were not significantly different in normal muscle tissue and interface between tumour and bladder wall in NMIBC. The FA value was significantly higher at the interface between tumour and bladder wall in MIBC. A higher FA value is probably due to disruption of muscle fibres organization where they are intersected by neoplastic tissue; therefore it appears to be indicative of muscular invasion. If these assumptions are confirmed, the calculation of anisotropy would become another non-invasive instrument for discriminating between invasive and non-invasive bladder cancer forms, making it possible to plan the most suitable therapeutic option.
As suggested by EAU guidelines on bladder cancer, if a suspicious lesion is identified at imaging or endoscopy, it should be followed by cystoscopy and/or resection. According to the same guidelines, selected patients with MIBC can be considered for multimodality bladder sparing therapy and MRI can be used for local staging. Since mpMRI, including DTI, has high accuracy in determining detrusor muscle invasion, we suggest it could be included in the diagnostic evaluation of patients suspected of having BCa. If mpMRI findings suggest muscle invasion, one wonders whether in the future TURB could be excluded, as is being tested by a new trial in the UK (HTA – 14/08/60). This could theoretically expedite radical treatment and allow the urologist to avoid unnecessary invasive procedures.
Our study had several limitations. The sample population was relatively small and the distribution of T stages was uneven, with a higher number of Ta-1 lesions compared to T2. This reflects the BCa disease spectrum, but limits the power in our report. DTI has some inherent limitations, such as the difficulty in acquiring images with high spatial resolution. This leads to possible partial volume effects so that ‘fibres’ with a different spatial orientation can be found inside a single voxel, reducing the information that tractographic processing is able to provide on finer structural correlations. This may affect the ability to examine fine structures, such as the detrusor/mucosa interface. Studies investigating normal bladder architecture in vivo using DTI are lacking; nevertheless promising results have been obtained in other body areas [24, 25].
In conclusion our approach suggests promising results for the local staging of BCa using mpMRI. If our findings are confirmed by others, we propose that mpMRI could become the key diagnostic and staging tool of choice for BCa and could allow urologist to alternate treatment pathways for patients with the highest need.
References
Chavan S, Bray F, Lortet-Tieulent J, Goodman M, Jemal A (2014) International variations in bladder cancer incidence and mortality. Eur Urol 66:59–73
Svatek RS, Hollenbeck BK, Holmang S, Lee R, Kim SP, Stenzl A et al (2014) The economics of bladder cancer: costs and considerations of caring for this disease. Eur Urol 66:253–62
Cumberbatch MGK, Cox A, Teare D, Catto JWF (2015) Contemporary occupational carcinogen exposure and bladder cancer: a systematic review and meta-analysis. JAMA Oncol 1:1282–90
Cumberbatch MG, Rota M, Catto JWF, La Vecchia C (2016) The role of tobacco smoke in bladder and kidney carcinogenesis: a comparison of exposures and meta-analysis of incidence and mortality risks. Eur Urol 70:458–66
Babjuk M, Bohle A, Burger M, Capoun O, Cohen D, Comperat EM, et al. EAU Guidelines on Non-Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. Eur Urol. 2016.
Linton KD, Rosario DJ, Thomas F, Rubin N, Goepel JR, Abbod MF et al (2013) Disease specific mortality in patients with low risk bladder cancer and the impact of cystoscopic surveillance. J Urol 189:828–33
Thomas F, Noon AP, Rubin N, Goepel JR, Catto JWF (2013) Comparative outcomes of primary, recurrent, and progressive high-risk non-muscle-invasive bladder cancer. Eur Urol 63:145–54
Kulkarni GS, Hakenberg OW, Gschwend JE, Thalmann G, Kassouf W, Kamat A et al (2010) An updated critical analysis of the treatment strategy for newly diagnosed high-grade T1 (previously T1G3) bladder cancer. Eur Urol 57:60–70
Brausi M, Collette L, Kurth K, van der Meijden AP, Oosterlinck W, Witjes JA et al (2002) Variability in the recurrence rate at first follow-up cystoscopy after TUR in stage Ta T1 transitional cell carcinoma of the bladder: a combined analysis of seven EORTC studies. Eur Urol 41:523–31
Kim B, Semelka RC, Ascher SM, Chalpin DB, Carroll PR, Hricak H (1994) Bladder tumor staging: comparison of contrast-enhanced CT, T1- and T2-weighted MR imaging, dynamic gadolinium-enhanced imaging, and late gadolinium-enhanced imaging. Radiology 193:239–45
Hayashi N, Tochigi H, Shiraishi T, Takeda K, Kawamura J (2000) A new staging criterion for bladder carcinoma using gadolinium-enhanced magnetic resonance imaging with an endorectal surface coil: a comparison with ultrasonography. BJU Int 85:32–6
Tekes A, Kamel I, Imam K, Szarf G, Schoenberg M, Nasir K et al (2005) Dynamic MRI of bladder cancer: evaluation of staging accuracy. AJR Am J Roentgenol 184:121–7
Takeuchi M, Sasaki S, Ito M, Okada S, Takahashi S, Kawai T et al (2009) Urinary bladder cancer: diffusion-weighted MR imaging--accuracy for diagnosing T stage and estimating histologic grade. Radiology 251:112–21
Naish JH, McGrath DM, Bains LJ, Passera K, Roberts C, Watson Y et al (2011) Comparison of dynamic contrast-enhanced MRI and dynamic contrast-enhanced CT biomarkers in bladder cancer. Magn Reson Med 66:219–26
Lankester KJ, Taylor JN, Stirling JJ, Boxall J, d’Arcy JA, Collins DJ et al (2007) Dynamic MRI for imaging tumor microvasculature: comparison of susceptibility and relaxivity techniques in pelvic tumors. J Magn Reson Imaging 25:796–805
Kulkarni GS, Urbach DR, Austin PC, Fleshner NE, Laupacis A (2009) Longer wait times increase overall mortality in patients with bladder cancer. J Urol 182:1318–24
Fahmy NM, Mahmud S, Aprikian AG (2006) Delay in the surgical treatment of bladder cancer and survival: systematic review of the literature. Eur Urol 50:1176–82
Blaschke S, Koenig F, Schostak M. Hematogenous Tumor Cell Spread Following Standard Transurethral Resection of Bladder Carcinoma. Vol. 70, European urology. Switzerland; 2016. p. 544–5.
de Haas RJ, Steyvers MJ, Futterer JJ (2014) Multiparametric MRI of the bladder: ready for clinical routine? AJR Am J Roentgenol 202:1187–95
Abou-El-Ghar ME, El-Assmy A, Refaie HF, El-Diasty T (2009) Bladder cancer: diagnosis with diffusion-weighted MR imaging in patients with gross hematuria. Radiology 251:415–21
Avcu S, Koseoglu MN, Ceylan K, Bulut MD, Unal O (2011) The value of diffusion-weighted MRI in the diagnosis of malignant and benign urinary bladder lesions. Br J Radiol 84:875–82
Wang H-J, Pui MH, Guan J, Li S-R, Lin J-H, Pan B, et al. (2016) Comparison of early submucosal enhancement and tumor stalk in staging bladder urothelial carcinoma. AJR Am J Roentgenol.1–7
Wang H, Pui MH, Guo Y, Li S, Guan J, Zhang X et al (2015) Multiparametric 3-T MRI for differentiating low-versus high-grade and category T1 versus T2 bladder urothelial carcinoma. AJR Am J Roentgenol 204:330–4
Zhang L, Liu A, Zhang T, Song Q, Wei Q, Wang H (2015) Use of diffusion tensor imaging in assessing superficial myometrial invasion by endometrial carcinoma: a preliminary study. Acta Radiol 56:1273–80
Panebianco V, Barchetti F, Sciarra A, Marcantonio A, Zini C, Salciccia S et al (2013) In vivo 3D neuroanatomical evaluation of periprostatic nerve plexus with 3T-MR Diffusion Tensor Imaging. Eur J Radiol 82:1677–82
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Valeria Panebianco.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Funding
The authors state that this work has not received any funding.
Statistics and biometry
Giovanni Barchetti kindly provided statistical advice for this manuscript.
Ethical approval
Institutional Review Board approval was obtained.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Methodology
• prospective
• experimental
• performed at one institution
Rights and permissions
About this article
Cite this article
Panebianco, V., De Berardinis, E., Barchetti, G. et al. An evaluation of morphological and functional multi-parametric MRI sequences in classifying non-muscle and muscle invasive bladder cancer. Eur Radiol 27, 3759–3766 (2017). https://doi.org/10.1007/s00330-017-4758-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-017-4758-3