Abstract
Objectives
To investigate whether amide proton transfer (APT) MR imaging can differentiate high-grade gliomas (HGGs) from low-grade gliomas (LGGs) among gliomas without intense contrast enhancement (CE).
Methods
This retrospective study evaluated 34 patients (22 males, 12 females; age 36.0 ± 11.3 years) including 20 with LGGs and 14 with HGGs, all scanned on a 3T MR scanner. Only tumours without intense CE were included. Two neuroradiologists independently performed histogram analyses to measure the 90th-percentile (APT90) and mean (APTmean) of the tumours’ APT signals. The apparent diffusion coefficient (ADC) and relative cerebral blood volume (rCBV) were also measured. The parameters were compared between the groups with Student’s t-test. Diagnostic performance was evaluated with receiver operating characteristic (ROC) analysis.
Results
The APT90 (2.80 ± 0.59 % in LGGs, 3.72 ± 0.89 in HGGs, P = 0.001) and APTmean (1.87 ± 0.49 % in LGGs, 2.70 ± 0.58 in HGGs, P = 0.0001) were significantly larger in the HGGs compared to the LGGs. The ADC and rCBV values were not significantly different between the groups. Both the APT90 and APTmean showed medium diagnostic performance in this discrimination.
Conclusions
APT imaging is useful in discriminating HGGs from LGGs among diffuse gliomas without intense CE.
Key Points
• Amide proton transfer (APT) imaging helps in grading non-enhancing gliomas
• High-grade gliomas showed higher APT signal than low-grade gliomas
• APT imaging showed better diagnostic performance than diffusion- and perfusion-weighted imaging
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gliomas are the most frequent primary brain neoplasms, varying histopathologically from low to high grades [1]. The differentiation between low-grade glioma (LGG, grade II) and high-grade glioma (HGG, grades III, IV) is clinically important, since the prognosis and the therapeutic strategy could substantially differ depending on the grade. Because of their dismal prognosis, HGGs are usually treated with surgical resection followed by adjuvant radiation therapy and chemotherapy [2]. HGGs misdiagnosed as LGGs will be treated less aggressively than necessary, and vice versa.
The preoperative imaging of diffuse gliomas with estimation of the grade is also important since there can be inherent sampling errors associated with intratumoral histological heterogeneity in gliomas. Inappropriate sampling from sites with a lower histological grade in a tumour may lead to the underestimation of the true grades. Such sampling errors could occur especially in patients who undergo biopsy. Moreover, a 2014 study revealed that a MR imaging biomarker such as the relative cerebral blood volume (rCBV) measured by dynamic susceptibility contrast (DSC) perfusion-weighted (PW) imaging better predicted the prognosis of patients with diffuse gliomas than histology [3], indicating the possibility of superior performance of imaging over histopathology in predicting glioma outcomes.
Traditionally, the presence and degree of contrast enhancement (CE) after the administration of a gadolinium-based contrast agent on MR imaging have been used as characteristics of the malignancy of diffuse gliomas [4, 5]. Typically, HGGs show moderate-to-strong CE, whereas LGGs exhibit no or mild CE. However, the degree of CE of gliomas is not always reliable for the differentiation between LGGs and HGGs, presumably because the CE of gliomas may result from a disruption of the blood-brain barrier rather than neovascularization.
The grading of nonenhancing gliomas by conventional MR imaging methods has been challenging. It was reported that approximately 20 % of LGGs enhanced after the administration of a gadolinium-based contrast agent, whereas 14 %–45 % of nonenhancing gliomas were histologically proved to be HGGs [6, 7]. It was reported that diffusion-weighted (DW) or PW imaging could differentiate HGGs from LGGs in nonenhancing gliomas [8, 9], but no consensus has been reached in light of the limited number of studies.
Amide proton transfer (APT) imaging was developed as one of the endogenous chemical exchange saturation transfer (CEST) imaging techniques [10, 11]. Since grades of diffuse gliomas are associated with the presence of mitosis and degree of cell density, the unique amide proton-based contrast mechanism may be able to detect the increase in concentrations of proteins and peptides in higher grades of diffuse gliomas. This method may be useful in grading gliomas in a different way from previous MR methods. The APT method allows noninvasive imaging of the proton exchange between bulk-water and the amide protons (-NH) of endogenous mobile proteins and peptides [10, 11]. It was demonstrated that APT imaging is useful for grading gliomas [12–14], but no detailed analysis including other imaging findings has been performed, to our knowledge. The purpose of the present study was to determine whether APT imaging could differentiate HGGs from LGGs among gliomas without intense CE. We compared the diagnostic performance of APT imaging in this discrimination with that of DW imaging and DSC PW imaging.
Materials and methods
Patients
This retrospective study was approved by the institutional review board of Kyushu University Hospital, and the requirement for informed consent was waived. APT imaging has been a part of our routine preoperative MR imaging protocol for brain tumours since August 2011. The preoperative MR imaging data of the 91 consecutive patients with diffuse gliomas who were identified between September 2011 and July 2015 were analyzed. The histopathologic diagnosis and grades were determined with resected or biopsy specimens according to the WHO criteria by two established neuropathologists (S.O.S. and T.I. with 22 and 31 years of experience, respectively), who were blinded to the imaging findings. A total of 34 patients including 20 with LGGs (World Health Organization [WHO] grade II, 13 males and seven females; mean age 36.0 ± 11.5 years) and 14 with HGGs (WHO grade III or IV, nine males and five females; mean age 39.0 ± 13.3 years) were identified. The characteristics of patients are described in Table 1.
The patients’ histological types of gliomas were as follows: 12 diffuse astrocytomas, seven oligodendrogliomas, one oligoastrocytoma, four anaplastic astrocytomas, three anaplastic oligodendrogliomas, two anaplastic oligoastrocytomas, one gliomatosis cerebri, and four glioblastoma multiforme (GBMs). All patients underwent contrast-enhanced T1-weighted imaging, APT, DW, and DSC PW imaging in their preoperative examinations. The interval between the MR imaging and the surgery was <2 weeks in all patients.
MR imaging
MR imaging was performed on a 3T clinical scanner (Achieva TX, Philips Healthcare, Best, The Netherlands) equipped with a pencil-beam, second-order shim, using an eight-channel head coil for signal reception and two-channel parallel transmission via the body coil for radiofrequency (RF) transmission. For the APT imaging, the acquisition software was modified to alternate the operation of the two transmission channels during the RF saturation pulse, which enables long quasi-continuous RF saturation at a 100 % RF duty-cycle and a special RF shimming for the saturation homogeneity of the alternated pulse [15, 16].
On a single axial slice corresponding to a maximum cross-section area of a tumour, two- dimensional (2-D) APT imaging was performed using a saturation pulse with a duration of 2 s (40 × 50 msec, sinc-gauss-shaped elements) and a saturation power level of B1,rms = 2.0 μT [15, 16]. For acquiring a Z-spectrum, the imaging was repeated at 25 saturation frequency offsets from ω = −6 to +6 ppm with a step of 0.5 ppm as well as one far-off-resonant frequency (ω = −1560 ppm) for signal normalization.
The other imaging parameters were as follows: Fast spin-echo (FSE) readout with driven equilibrium refocusing; echo train length (ETL) = 128; sensitivity encoding factor = 2; repetition time (TR) = 5,000 msec; echo time (TE) = 6 msec; matrix = 128 × 128 (reconstructed to 256 × 256); slice thickness = 5 mm, field of view (FOV) = 230 × 230 mm; scan time = 2 min 20 s for one Z-spectrum. A ΔB0 map for off-resonance correction was acquired separately using a 2D gradient-echo with identical spatial resolution for a point-by-point ΔB0 correction [15, 16].
The DW imaging was performed in the axial plane with a single-shot spin-echo echo planar imaging sequence with the following parameters: TR = 4,136 msec; TE = 70 msec; matrix = 160 × 126 (reconstructed to 256 × 256); slice thickness = 5 mm, FOV = 230 × 230 mm; scan time = 50 s, b-values; 0 and 1000 (sec/mm2). The apparent diffusion coefficient (ADC) was calculated by mono-exponential fitting with the pair of b-values.
The DSC PW imaging was conducted after the administration of 0.05 mmol/kg gadolinium (Gd) contrast agent (gadopentetate dimeglumine; Bayer Health Care, Osaka, Japan or gadodiamide; Daiichi-Sankyo Pharmaceutical, Tokyo, Japan) as a preload dose to minimize T1 leakage effects [17]. During the acquisition, an additional 0.05 mmol/kg bolus was administered at 5 mL/s. The DSC PW imaging was obtained with a gradient-echo echo-planar imaging sequence in the axial plane using the following parameters: TR = 1,428 msec; TE = 35 msec; matrix = 288 × 288; slice thickness = 5 mm, FOV = 230 × 230 mm; number of slices = 22, sensitivity encoding factor 2; scan time = 1 min 26 s.
Relative cerebral blood volume (rCBV) maps were generated using an Osirix workstation (v. 5.0.2, 64 bit; http://www.osirix-viewer.com/) with IB Neuro software (ver.1; Imaging Biometrics, Elm Grove, WI) to calculate the whole-brain CBV from the DSC PW imaging data [18]. Briefly, after excluding the first four time points of each DSC PW imaging data set due to saturation effects, signal intensities were normalized to the baseline and then converted to the change in relaxivity over time [ΔR2*(t)] for the entire brain. The CBV was calculated voxel-by-voxel by integrating the area under the ΔR2*(t) curve, ending at the time point 40 s after the nadir signal intensity of the first pass-bolus. All CBV values were corrected for T1-weighted leakage with preload dosing, and a modeling algorithm was used to correct T2/T2*-weighted residual effects [19]. The CBV maps were normalized to contralateral normal-appearing white matter (NAWM) to create rCBV maps.
For reference, standard MR images, including T1-weighted (TR = 500 msec, TE = 10 msec, matrix = 256 × 271, 22 slices), T2-weighted (TR = 3,000 msec, TE = 80 msec, ETL = 8, matrix = 512 × 416, 22 slices), fluid attenuation inversion recovery (FLAIR, TR = 10,000 msec, TE = 120 msec, Inversion time = 2,700 msec, ETL = 27, matrix = 320 × 211, 22 slices), and contrast enhanced T1-weighted images (TR = 500 msec, TE = 20 msec, matrix = 256 × 271, 22 slices) were acquired in the axial plane. The FOV size (230 × 230 mm) and the slice thickness (5 mm) are identical in these images. The APT images were acquired before the administration of the gadolinium contrast agent.
Two experienced neuroradiologists (O.T. and K.Y., 15 and 13 years of experience in neuroradiology, respectively) independently reviewed and evaluated the degree of CE after the administration of a gadolinium-based contrast agent to each tumour. The degree of CE was visually graded on a six-point scale: 0, none; 1, slight; 2, mild, 3, moderate; 4, distinct; and 5; strong. Only patients with a glioma that showed no (grade 0) or slight (grade 1) CE judged by both observers were included in this study. The inter-rater agreement statistic (Kappa) for the evaluation of CE was 0.82.
APT imaging processing
The APT signal is defined as asymmetry of the magnetization transfer ratio at 3.5 ppm: MTRasym (3.5 ppm) [11].
where Ssat (−3.5 ppm), Ssat (+3.5 ppm), and S0 are the signal intensities obtained at −3.5, +3.5, and −1560 ppm, respectively [10]. All image data were analyzed with the software program ImageJ (version 1.43u; National Institutes of Health, Bethesda, MD). A plug-in was created to assess the Z-spectra and MTRasym equipped with a correction function for B0 inhomogeneity, using interpolation among the Z-spectral image data.
First, rigid body motion correction was performed using the Turboreg algorithm [20]. The local B0 field shift in Hz was obtained from the B0 map, which was created from dual-echo gradient echo images (ΔTE = 1 msec), and each voxel was corrected in image intensity for the nominal saturation frequency offset by Lagrange interpolation among the neighboring Z-spectral images. This procedure corresponds to a frequency shift along the saturation frequency offset axis according to the measured B0 shift. Finally, the APT-weighted image was calculated by Eq. (1) described above.
Image analysis
The APT-weighted image, ADC map, rCBV map, and FLAIR image of each patient were co-registered to the raw image of the APT image with the rigid body registration using the Turboreg algorithm [20]. This registration procedure performed fine adjustments for locations and angles on two types of single-slice image. In this process, the distance between any pair of points images was conserved, and the voxel value did not change. On the FLAIR image, areas showing high signal intensity were manually segmented independently by the two neuroradiologists (O.T. and K.Y.) with the software program Medical Image Processing, Analysis and Visualization (MIPAV, version 7.0.1, Centre for Information Technology, U.S. National Institutes of Health, Bethesda, MD). The segmented regions were copied from the FLAIR mage and pasted to the APT-weighted image, the ADC map, and the rCBV map (Fig. 1).
Segmentation of the tumours. After the co-registration of all images, areas showing a hyperintense signal on the FLAIR image (a) were manually segmented independently by the two observers. The segmented regions were copied from the FLAIR mage and pasted to the ADC map (b), and the rCBV map (c), and the APT-weighted image (d)
The areas with obvious artifacts or signals from a blood vessel were excluded from the segmented area. The APT signal, ADC, and rCBV were measured in all pixels included in the segmented region. The 90th percentile for APT (APT90) and the 90th percentile for rCBV (rCBV90), and the 10th percentile for ADC (ADC10) were derived by the histogram approach in the segmented region. The rCBV90 and ADC10 correspond to the maximum rCBV and minimum ADC in previous region-of-interest (ROI)-based studies, respectively. The mean values for the parameters (APTmean, ADCmean, rCBVmean) were also calculated. No necrosis, hemorrhage, or cysts were identified on the MR images in any patients.
Statistical analysis
All values are expressed as mean ± standard deviation (SD). The APT signals, ADC values, and rCBV values were compared between the LGG and HGG groups using Student’s t-test. The size of segmented region was compared between the two observers using the paired t-test. The inter-observer agreement regarding the measurements by the two observers was analyzed by calculating the intra-class correlation coefficient (ICC). ICCs are considered to be excellent if >0.74 [21]. The measurements by the two observers for each patient were averaged for further analysis. The comparisons of measurements between the LGGs and HGGs were performed with Student’s t-test. Correlations among the APT signals, ADC values, and rCBV values were evaluated with Pearson’s correlation coefficient.
Receiver operating characteristic (ROC) curve analyses were conducted to evaluate the diagnostic performance of the parameters in differentiating LGGs from HGGs. We considered area under the curve (AUC) values <0.7, 0.7–0.9, and >0.9 to indicate low, medium, and high diagnostic performance, respectively. Statistical analyses were performed with a commercially available software package (SPSS, IBM 19, Armonk, NY; Prism 5.0, GraphPad Software, San Diego, CA; and MedCalc v. 13.1.2.0, Broekstraat, Mariakerke, Belgium). P-values < 0.05 were considered significant.
Results
Histogram analysis
Figure 2 demonstrates the histogram profiles over all pixels in the patients as obtained by one of the two observers. Our normalized histogram analysis of the APT signal over all pixels in tumour ROIs (LGG: 23,494 pixels, HGG: 26,884 pixels) revealed that the overall histogram profile shifted towards the higher APT signal in the HGG group in comparison with the LGG group, and a heavy-tailed distribution was observed in the HGG group. The normalized histogram analysis of the ADCs and rCBV values over all pixels in the tumour ROIs resulted in similar profiles for both groups, but there were pixels with a higher ADC or a lower rCBV in the LGG group.
Histogram profiles over all pixels in the patients. The normalized histogram analysis of the APT signal (a) over all pixels in tumour ROIs (LGG: 23,494 pixels, HGG: 26,884 pixels) revealed that the overall histogram profile shifted towards the higher APT signal in the HGG group in comparison with the LGG group, and a heavy-tailed distribution was observed in the HGG group. The normalized histogram analysis of the ADCs (b) and rCBV (c) values over all pixels in the tumour ROIs resulted in similar profiles for both groups, but there were pixels with a higher ADC or a lower rCBV in the LGG group
Inter-observer agreement
The mean size of segmented regions by observer 1 (1212 ± 779 mm2) was significantly smaller than that by observer 2 (1456 ± 886 mm2). Table 2 shows the ICCs of the measurements by the two observers. Excellent agreement was observed for APT90, rCBV90, APTmean, ADCmean, and rCBVmean, but not for ADC10.
Comparisons of the parameters between LGGs and HGGs
Table 3 shows the parameter measurements in the LGG and HGG groups. The APT90 (P = 0.001) and APTmean (P = 0.0001) were significantly larger in the HGG group compared to the LGG group. The ADC10, ADCmean, rCBV90, and rCBVmean values were not significantly different between the two groups. The diameter of HGG (56.4 ± 20.1 mm) was significantly larger than that of LGG (43.8 ± 14.1 mm).
Correlation among the parameters
The APT90 significantly correlated with the rCBV90 (r = 0.42, P = 0.01). The other comparisons among the APT signal, ADC and rCBV did not show significant correlations. The diameter of the tumour did not correlate with the APT signal, ADC, and rCBV.
Diagnostic performance in differentiating HGGs from LGGs
Table 4 and Fig. 3 summarize the diagnostic performance of the parameters as determined by the ROC analyses. Medium diagnostic performance was achieved by the APT90, with the AUC of 0.811, and by the APTmean with the AUC of 0.886. APT90 showed 85.7 % sensitivity and 70.0 % specificity with the cutoff value of 2.92 %. APTmean showed 71.4 % sensitivity and 95.0 % specificity with the cutoff value of 2.56 %. The ADC10, ADCmean, rCBV90, and rCBVmean resulted in low diagnostic performance. The comparisons of ROC curves revealed that the AUC of APT90 was significantly higher than those of ADC10 and rCBV90 (P < 0.05), and the AUC of APTmean was significantly higher than those of ADCmean and rCBVmean (P < 0.05)
Receiver operating characteristic curve analyses in differentiating LGGs from HGGs. (a) APT90 showed medium diagnostic performance, whereas ADC10 and rCBV90 showed low diagnostic performance. (b) APTmean showed medium diagnostic performance, whereas ADCmean and rCBVmean showed low diagnostic performance
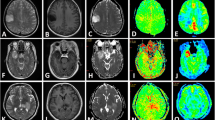
Figures 4 and 5 show representative cases of grade II (diffuse astrocytoma) and grade III (anaplastic oligodendroglioma), respectively. Although little CE was seen in both cases, the grade III glioma had a higher APT signal than the grade II glioma. Note that a high APT signal was extensively observed within the tumour, which resulted in a higher APTmean value.
A 38-year-old male with a diffuse astrocytoma. (a) The post-contrast T1-weighted image showed no contrast enhancement (CE) in the tumour in the right frontal lobe. (b) The ADC map revealed a high ADC value (ADC10 = 0.99 × 10−3 mm2/s) of the tumour compared to the normal white matter. (c) The rCBV map showed low rCBV (rCBV90 = 2.78) in the tumour. (d) The APT-weighted image showed only a mild increase in APT signal (APT90 = 2.15 %) in the tumour
A 47-year-old male with an anaplastic oligodendroglioma. (a) A post-contrast T1-weighted image showed only slight CE in the tumour in the left insula. (b) The ADC map revealed a high ADC value (ADC10 = 1.48 × 10−3 mm2/s) of the tumour compared to the normal white matter. (c) The rCBV map showed reduced rCBV (rCBV90 = 2.34) in the tumour. (d) The APT-weighted image revealed an increase in the APT signal (APT90 = 4.19 %) in the tumour
Discussion
The results of the present study demonstrated the diagnostic performance of APT imaging for grading diffuse gliomas with no or little CE. We found a significant increase in the APT90 and APTmean values in the HGGs compared to the LGGs.
The sources of the APT signal and contrast are a matter of controversy. The high APT signal in tumours can be attributed to abundant concentration of mobile proteins and peptides in cellular cytoplasm. Higher cell density reported in HGGs in a human study [22] and animal study [23] would be a cause of higher concentration of mobile proteins and peptides. The present results revealed that the ADC did not differ between the high and low grades, indicating that the cell density was not very different between the grades whereas the APT signals were clearly different between the grades. This is consistent with the previous study which demonstrated that APT signal shows a weak correlation with cell density in diffuse gliomas [13]. These results suggested that the concentrations of mobile proteins and peptides included in the intracellular space are increased in HGGs compared to LGGs. The proteins and peptides in the extracellular space (such as interstitial fluid, mucin, or microcysts) could contribute to the increase in the APT signal in gliomas, if they are different between the grades. The APT signal could be also affected by changes in the nuclear Overhauser effect (NOE) in tissue [24, 25]. Thus, overall, further investigations are needed to determine the mechanisms of the APT contrast in tumours.
Regarding the differentiation of low and high grades in nonenhancing gliomas, Lee et al. reported that the minimum ADC was useful although substantial overlaps were observed between LGGs and HGGs [8]. In our present study, however, ADC10 failed in this discrimination. The inter-observer agreement for ADC10 was not high, despite the excellent agreement for ADCmean. This result indicated that ADC10 originated from the tumour boundary area, rather than the tumour core, in which the location of the regions-of-interest could differ between the two observers. The boundary area consists of the mixture of infiltrating glioma cells and normal brain tissue. Since normal white matter has lower ADCs than most types of tumorous tissue, ADC10 might reflect the content of normal brain tissue rather than the cell density of gliomas. This could be a limitation of the histogram analysis of the ADC in evaluating infiltrating gliomas.
Falk et al. reported using DSC PW imaging; they observed that rCBV90 was significantly higher in grade III compared to grade II nonenhancing gliomas (P = 0.04) [9]. Although two of our present HGG cases had rCBV values >10.00 and although the rCBV tended to be higher in our HGG group, no significant difference was found between the two groups. These parameters could still be valuable to investigate in a larger cohort.
We found that the APT90 significantly correlated with the rCBV90. This correlation might indicate that the increase in cytoplasmic proteins and peptides in glioma cells is associated with neoangiogenesis.
In the CEST imaging, long saturation pulses are necessary to accumulate saturated protons in bulk water; however, the length of the saturation pulse was limited to <1 s in previous studies, due to typical limitations of the RF amplifiers in clinical MRI systems. This problem was solved in the present study by using the two amplifiers of a parallel transmission system in alternation [15, 16], enabling 2 s of saturation at 100 % duty-cycle. A 2012 study showed that the APT contrast obtained with the 2-s saturation was higher than the corresponding values obtained with 0.5-s or 1-s saturation in various types of human brain tumours including gliomas [26]. Since CEST imaging is highly sensitive to B0 inhomogeneity, shimming and correction for B0 inhomogeneity in the post-processing are crucial. The accuracy and reproducibility of the APT imaging scheme used in the present study was confirmed in patients with various types of brain tumour [16].
The present study has several limitations. First, the cohort is relatively small, especially that of the HGGs (n = 14) which usually show CE. The sample size was not large enough to perform separate analyses of astrocytomas and oligodendrogliomas. Second, single slice acquisition was employed in the APT imaging sequence due to the limitation of the total acquisition time in the clinical patient scans. Thus, it was not possible to cover the entire tumour. However, a recent study showed that diagnostic performance of a single slice APT imaging was equivalent to that of multi-slice imaging in grading gliomas [14]. Protocols with fast three-dimensional (3-D) coverage are now under development to better characterize the typical tumour tissue heterogeneity in all dimensions. Finally, the histopathological diagnoses were based on a biopsy in three patients with HGG, and all of the other patients’ diagnoses were based on resected specimens. The amount or fraction of high grade component within a tumour might have influenced the results in the biopsy cases. However, we expect that the histogram approach should have been still effective in those cases.
In conclusion, the APT90 and APTmean were significantly higher in the HGGs than in the LGGs, whereas no significant differences were found in ADCs and rCBV values in these gliomas without intense CE. The APT90 and APTmean showed medium diagnostic performance in the discrimination of the HGG and LGG groups. APT imaging, which produces an endogenous contrast based on saturation transfer, may be useful in the prediction of the histopathological malignancy of diffuse gliomas without CE.
Abbreviations
- APT:
-
Amide proton transfer
- LGG:
-
Low-grade gliomas
- HGG:
-
High-grade glioma
- CE:
-
Contrast enhancement
- ADC:
-
Apparent diffusion coefficient
- rCBV:
-
relative cerebral blood volume
- ROC:
-
Receiver operating characteristic
- DSC:
-
Dynamic susceptibility contrast
- PW:
-
Perfusion-weighted
- DW:
-
Diffusion-weighted
- CEST:
-
Chemical exchange saturation transfer
- WHO:
-
World Health Organization
- GBM:
-
Glioblastoma multiforme
- RF:
-
Radiofrequency
- TR:
-
Repetition time
- TE:
-
Echo time
- FOV:
-
Field of view
- 2D:
-
Two dimensional
- NAWM:
-
Normal-appearing white matter
- FLAIR:
-
Fluid attenuation inversion recovery
- MTRasym :
-
Asymmetry of the magnetization transfer ratio
- ROI:
-
Region-of-interest
- ICC:
-
Intra-class correlation coefficient
- AUC:
-
Area under the curve
- 3D:
-
Three-dimensional
References
Daumas-Duport C, Scheithauer B, O'Fallon J, Kelly P (1988) Grading of astrocytomas. A simple and reproducible method. Cancer 62:2152–2165
Law M, Yang S, Babb JS et al (2004) Comparison of cerebral blood volume and vascular permeability from dynamic susceptibility contrast-enhanced perfusion MR imaging with glioma grade. AJNR Am J Neuroradiol 25:746–755
Hilario A, Sepulveda JM, Perez-Nunez A et al (2014) A prognostic model based on preoperative MRI predicts overall survival in patients with diffuse gliomas. AJNR Am J Neuroradiol 35:1096–1102
Pierallini A, Bonamini M, Bozzao A et al (1997) Supratentorial diffuse astrocytic tumours: proposal of an MRI classification. Eur Radiol 7:395–399
Mihara F, Numaguchi Y, Rothman M, Sato S, Fiandaca MS (1995) MR imaging of adult supratentorial astrocytomas: an attempt of semi-automatic grading. Radiat Med 13:5–9
Fan GG, Deng QL, Wu ZH, Guo QY (2006) Usefulness of diffusion/perfusion-weighted MRI in patients with non-enhancing supratentorial brain gliomas: a valuable tool to predict tumour grading? Br J Radiol 79:652–658
McKnight TR, Lamborn KR, Love TD et al (2007) Correlation of magnetic resonance spectroscopic and growth characteristics within Grades II and III gliomas. J Neurosurg 106:660–666
Lee EJ, Lee SK, Agid R, Bae JM, Keller A, Terbrugge K (2008) Preoperative grading of presumptive low-grade astrocytomas on MR imaging: diagnostic value of minimum apparent diffusion coefficient. AJNR Am J Neuroradiol 29:1872–1877
Falk A, Fahlstrom M, Rostrup E et al (2014) Discrimination between glioma grades II and III in suspected low-grade gliomas using dynamic contrast-enhanced and dynamic susceptibility contrast perfusion MR imaging: a histogram analysis approach. Neuroradiology 56:1031–1038
Zhou J, Payen JF, Wilson DA, Traystman RJ, van Zijl PC (2003) Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat Med 9:1085–1090
Zhou J, Lal B, Wilson DA, Laterra J, van Zijl PC (2003) Amide proton transfer (APT) contrast for imaging of brain tumors. Magn Reson Med 50:1120–1126
Zhou J, Blakeley JO, Hua J et al (2008) Practical data acquisition method for human brain tumor amide proton transfer (APT) imaging. Magn Reson Med 60:842–849
Togao O, Yoshiura T, Keupp J et al (2014) Amide proton transfer imaging of adult diffuse gliomas: correlation with histopathological grades. Neuro Oncol 16:441–448
Sakata A, Okada T, Yamamoto A et al (2015) Grading glial tumors with amide proton transfer MR imaging: different analytical approaches. J Neurooncol 122:339–348
Keupp J, Baltes C, Harvey P, Van den Brink J (2011) Parallel RF transmission based MRI technique for highly sensitive detection of amide proton transfer in the human brain at 3T. Proc Int Soc Magn Reson Med 19:710
Togao O, Hiwatashi A, Keupp J et al (2015) Scan-rescan reproducibility of parallel transmission based amide proton transfer imaging of brain tumors. J Magn Reson Imaging 42:1346–1353
Boxerman JL, Schmainda KM, Weisskoff RM (2006) Relative cerebral blood volume maps corrected for contrast agent extravasation significantly correlate with glioma tumor grade, whereas uncorrected maps do not. AJNR Am J Neuroradiol 27:859–867
Hu LS, Eschbacher JM, Heiserman JE et al (2012) Reevaluating the imaging definition of tumor progression: perfusion MRI quantifies recurrent glioblastoma tumor fraction, pseudoprogression, and radiation necrosis to predict survival. Neuro Oncol 14:919–930
Paulson ES, Schmainda KM (2008) Comparison of dynamic susceptibility-weighted contrast-enhanced MR methods: recommendations for measuring relative cerebral blood volume in brain tumors. Radiology 249:601–613
Thevenaz P, Ruttimann UE, Unser M (1998) A pyramid approach to subpixel registration based on intensity. IEEE Trans Image Process 7:27–41
Shrout PE, Fleiss JL (1979) Intraclass correlations: uses in assessing rater reliability. Psychol Bull 86:420–428
Wen Z, Hu S, Huang F et al (2010) MR imaging of high-grade brain tumors using endogenous protein and peptide-based contrast. Neuroimage 51:616–622
Salhotra A, Lal B, Laterra J, Sun PZ, van Zijl PC, Zhou J (2008) Amide proton transfer imaging of 9L gliosarcoma and human glioblastoma xenografts. NMR Biomed 21:489–497
Xu J, Zaiss M, Zu Z et al (2014) On the origins of chemical exchange saturation transfer (CEST) contrast in tumors at 9.4 T. NMR Biomed 27:406–416
Heo HY, Zhang Y, Lee DH, Hong X, Zhou J (2015) Quantitative assessment of amide proton transfer (APT) and nuclear overhauser enhancement (NOE) imaging with extrapolated semi-solid magnetization transfer reference (EMR) signals: application to a rat glioma model at 4.7 tesla. Magn Reson Med. doi:10.1002/mrm.25581
Togao O, Yoshiura T, Keupp J et al (2012) Effect of saturation pulse length on parallel transmission based Amide Proton Transfer (APT) imaging of different brain tumor types. Proc Int Soc Magn Reson Med 21:744
Acknowledgments
The scientific guarantor of this publication is Hiroshi Honda.
The authors of this manuscript declare relationships with the following companies: Jochen Keupp is an employee of Philips Reseach Europe, and Masami Yoneyama is an employees of Philips Electronics Japan. This study has received funding by the Japanese Society of Neuroradiology, Japanese Radiological Society, the Fukuoka Foundation for Sound Health Cancer Research Fund, and JSPS KAKENHI Grants-in-Aid for Scientific Research nos. 26461827, 26293278, 26670564 and 22591340. No complex statistical methods were necessary for this paper. Institutional Review Board approval was obtained. Written informed consent was waived by the Institutional Review Board.
Methodology: retrospective, diagnostic or prognostic study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Togao, O., Hiwatashi, A., Yamashita, K. et al. Grading diffuse gliomas without intense contrast enhancement by amide proton transfer MR imaging: comparisons with diffusion- and perfusion-weighted imaging. Eur Radiol 27, 578–588 (2017). https://doi.org/10.1007/s00330-016-4328-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-016-4328-0