Abstract
Objectives
The increasing detection of small testicular lesions by ultrasound (US) in adults can lead to unnecessary orchiectomies. This article describes their nature, reviews the available literature on this subject and illustrates some classical lesions. We also suggest recommendations to help characterization and management.
Methods
The ESUR scrotal imaging subcommittee searched for original and review articles published before May 2015 using the Pubmed and Medline databases. Key words used were ‘testicular ultrasound’, ‘contrast-enhanced sonography’, ‘sonoelastography’, ‘magnetic resonance imaging’, ‘testis-sparing surgery’, ‘testis imaging’, ‘Leydig cell tumour’, ‘testicular cyst’. Consensus was obtained amongst the members of the subcommittee, urologist and medical oncologist.
Results
Simple cysts are frequent and benign, and do not require follow up or surgery. Incidentally discovered small solid testicular lesions detected are benign in up to 80 %, with Leydig cell tumours being the most frequent. However, the presence of microliths, macrocalcifications and hypoechoic areas surrounding the nodule are findings suggestive of malignant disease.
Conclusion
Asymptomatic small testicular lesions found on ultrasound are mainly benign, but findings such as microliths or hypoechoic regions surrounding the nodules may indicate malignancy. Colour Doppler US remains the basic examination for characterization. The role of newer imaging modalities in characterization is evolving.
Key points
• Characterization of testicular lesions is primarily based on US examination.
• The role of MRI, sonoelastography, contrast-enhanced ultrasound is evolving.
• Most small non-palpable testicular lesions seen on ultrasound are benign simple cysts.
• Leydig cell tumours are the most frequent benign lesions.
• Associated findings like microliths or hypoechoic regions may indicate malignancy.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The most common palpable intra-testicular lesion in boys and young men between 15and 34 years of age is a malignant testicular carcinoma [1]. However, non-palpable cystic or solid testicular nodules may be incidentally discovered in adult men who present with scrotal pain or subfertility and undergo a scrotal ultrasound (US). Many such lesions are simple cysts, which are benign lesions that require neither surgery nor follow-up. When the incidental nodule is solid in nature, it raises the concern for a malignant tumour. Orchiectomy is often the treatment for these non-palpable intratesticular solid abnormalities but incidental solid lesions may be benign in up to 80 % of cases [2, 3], rendering orchiectomy an inappropriately aggressive treatment. The European Society of Urogenital Radiology (ESUR) scrotal imaging subcommittee addressed the challenges posed by the detection of such lesions in adult men and produced this consensus document in which the existing literature on the subject is reviewed.
Materials and methods
For this review, the ESUR scrotal imaging subcommittee searched for original and review articles published before May 2015 using Pubmed and Medline databases. Key words used were ‘testicular ultrasound’, ‘contrast-enhanced sonography’, ‘sonoelastography’, ‘magnetic resonance imaging’, ‘testis-sparing surgery’, ‘testis imaging’, ‘Leydig cell tumour‘, and ‘testis cyst’. Consensus was obtained amongst the members of the subcommittee, and guest panelists composed of urologists and medical oncologists. In this paper, we will not discuss ‘pseudo-tumorous lesions‘ such as intratesticular varicocele, adrenal rests, hematoma, splenogonadal fusion, sarcoidosis, or abscess, all entities which may occasionally be difficult to distinguish from testicular neoplasms on imaging. They have already been well described in the existing literature [4, 5].
Results
What is the reported size of an ‘incidental’ non-palpable intratesticular lesion?
The ability to palpate an intratesticular lesion depends on the volume of the testis and the volume and location of the mass. There is no published literature regarding the size at which a mass is impalpable. The reported incidence of non-palpable intratesticular lesions is based on retrospective studies by surgeons, which may cause a recruitment bias whereby ‘very small’ nodules are not included. Intratesticular lesions < 5 mm are frequently detected on scrotal US in contemporary practice due to improved resolution of US transducers. Large series reporting on scrotal US indicates that the incidence of non-palpable lesions may range between 0.8 % to 7.4 %, with the non-palpable lesions detected on US ranging between 10 to 15 mm in size [6–9]. The size of a testicular cystic lesion has not been evaluated with regards to the diagnosis.
Histopathology of non-palpable intratesticular lesions
Determining whether a testicular lesion is cystic or solid is of prime importance. Simple cysts in the testes are quite frequent [10, 11], easy to identify and may be solitary, multiple, unilateral or bilateral. They have no malignant potential and do not require monitoring or follow-up. They may be of mesothelial origin, or arise from ectopic rete testis epithelium [12]. Complex cysts may be of several different forms such as complex mesothelial cysts arising from the tunica albuginea, lymphangiomas, epidermoid cysts, but also teratomas; the latter are considered to be malignant germ cell tumours in adults, though this has been challenged recently [13]. Published studies regarding incidental small solid or solid-like testicular nodules report that as many as 80 % of such lesions are benign [3, 14] . A review of 6 series of testis-sparing surgeries in 105 patients with nodules ranging from 0.5 to 31 mm (1 cm mean size) revealed Leydig cell tumours in 47 (45 %), germ cell tumours in 10 (10.5 %), and an assortment of lesions in 48, including epidermoid cysts, Sertoli cell tumours and lymphoma [2]. Incidental lesions in the testes are most often detected in men undergoing evaluation for subfertility. Toren reported on 49 cases (mean age 35 years) of incidentally detected hypoechoic testicular lesions in a cohort of 4418 patients of whom 39/49 (85 %) were referred for infertility. Malignancy was confirmed in only one case. Five focal lesions were Leydig cell tumours/hyperplasia. Most of the remaining lesions were followed [8]. When a testis nodule coexists with infertility, every effort should be made to avoid orchiectomy in order to preserve the potential pool of spermatozoa. In Eifler’s series comprising 145 patients referred for azoospermia (mean age 34 +/- 0.6 years), a focal US abnormality was detected in 49 (33 %) cases [15]; a karyotype abnormality was found in 8.3 % of patients, mainly represented by Klinefelter’s syndrome, which is known to be associated with benign Leydig cell tumours and Leydig cell hyperplasia, which are often seen as sub-centimetre nodules [16–19]. When azoospermic men with small-volume testes have testicular nodules on US, evaluation should be complemented by karyotyping.
The proportion of sporadic Leydig cell tumours is quite substantial in incidentally discovered testicular nodules. Although only 10 % of Leydig tumours are generally known to be malignant, differentiating benign and malignant forms even at pathological examination is challenging. The risk of malignancy is higher in larger lesions (>5 cm), and if there are associated pathological features such as necrosis, moderate or severe cellular atypia, angiolymphatic invasion, an infiltrating margin, and > 5 mitotic features per 10 high-powered fields [20]. Of these six high-risk pathological features, tumour size is the only feature that can be assessed preoperatively by imaging. In a series reporting on 48 patients with testicular sex cord stromal tumours [21], 65 % were palpable, only 5 had retroperitoneal disease, and the 3 patients who died from the disease had tumour sizes of 12 cm, 9 cm and 5.4 cm. Small incidental Leydig cell tumours are more likely to have a benign course [22]. Germ cell tumours may be discovered incidentally on scrotal US, the majority are pure seminoma and most are clinically stage I (no detectable metastases), while most non-seminomatous germ cell tumours present as palpable tumours, often with metastases [23].
Imaging in testicular lesions
Ultrasound
US is established as the appropriate first step in imaging the testes. Lesions can be differentiated based on US feature criteria as cystic (obvious if anechoic) or solid, vascularized or avascular, hypoechoic or hyperechoic, and by the presence of microliths or macrocalcifications. Some cystic lesions may show an echoic pattern. The literature correlating US characteristics of scrotal tumours to pathology is sparse but reviews do describe US findings for several testicular tumour types, as well as US–MR correlation [4, 24–26].
Cystic lesions
Simple cysts in the testes are anechoic, with an imperceptible wall and through transmission, without soft tissue nodularity or a thickened wall. Malignant histopathology has never been reported in simple testicular cysts. “Complex” cysts may have septae, and a solid component. The risk of a teratoma accounts for the usual surgical removal of such lesions. An onion-skin pattern with concentric layers or an echogenic homogenous well-defined avascular lesion is suggestive of an epidermoid cyst.
Mixed cystic and solid components (consisting of keratinous debris) may raise the suspicion of malignancy and lead to orchiectomy, but management should be directed towards avoidance of total orchiectomy in these benign lesions. Epidermoid cysts are avascular lesions on imaging studies. Tubular ectasia usually appears as a complex cystic lesion and may be mistaken as a tumour, leading to unnecessary orchiectomies. It must be recognized by radiologists by its typical appearance, the lack of solid areas in the lesion, and its typical location in the testicular hilum; these features can lead to a definitive diagnose and avoid the referral of the patient for an orchiectomy [27]. Examples of simple and complex cystic lesions with their histological correlation are illustrated in Fig. 1.
A–D. Cystic lesions. A: Simple cyst. B: Complex mesothelial cyst. The lesion’s growth over 10 months led to partial orchiectomy. C: Avascular heterogenous lesion in a small testis proved to be a dermoid cyst. D: Complex multilocular avascular cystic lesion with echoic component proved to be a mature teratoma
Solid lesions
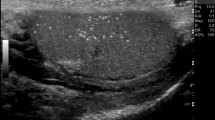
We first must define what we mean by nodules and hypoechoic areas: a testicular nodule has a spheric or ovoid shape, well-defined borders, while hypoechoic areas do not have this geometric configuration, and have poorly defined borders. Hypoechoic nodules associated with microlithiasis are highly suggestive of seminomatous tumours (Figs. 2 and 3), while heterogeneous, mixed cystic and solid hyperechoic masses are more suggestive of non-seminomatous tumours (Fig. 4) [28]. Although the significance of testicular microlithiasis is debated [29], a recent study indicated that the presence of microlithiasis with a testicular lesion was helpful to differentiate malignant from benign and non-neoplastic conditions. Absence of vascularity in a testicular solid lesion at colour Doppler may occasionally suggest the rare diagnosis of a burned-out tumour (Fig. 5) [30], which refers to a testis tumour that has shrunk [31]. In most reported cases, the testis lesion was discovered during the evaluation of a metastatic patient presenting with large retroperitoneal nodes sometimes associated with back flank pain [32], but burned-out tumours may also appear as an incidental finding [30]. If metastatic dissemination is established based on tumour marker increase or pathology, treatment and follow-up should be the same as for metastatic germ cell tumours. In case of an apparently localized burned-out cancer (no detectable metastases and normal serum tumour markers), a post-orchiectomy follow-up similar to that used in stage I germ cell tumours seems recommended.
When a solid testicular lesion is found, Leydig and Sertoli cell tumours are the main lesions to be differentiated from germ cell tumours [33]. Sporadic and incidentally found Leydig cell tumours are usually small, and less hypoechoic than seminomas; larger lesions may demonstrate well-defined, lobulated borders with peripheral vessels (Fig. 6) [9, 34]. A ‘small’ size is the best prognostic factor for benignity. Normal surrounding parenchyma, the absence of microliths, macrocalcification and the absence of hypoechoic areas are also criteria for this diagnosis. There is insufficient published data to guide management, but short-term follow-up or enucleation are generally recommended by urologists and oncologists for lesions < 5 mm while larger lesions are best treated by partial orchiectomy [22].
A, B: Pathologically proven examples of Leydig cell tumours. A: Benign Leydig cell tumour (5 mm) found incidentally. The nodule is slightly hypoechoic, with peripheral vessels with normal surrounding testicular parenchyma. This lesion was removed by partial orchiectomy. B: A 9-mm Leydig cell tumour treated by partial orchiectomy
Calcifications and microlithiasis in the testes have been extensively studied. Clustered macrocalcifications associated with hypoechoic areas or hypoechoic nodules suggest germ cell tumours, especially seminomas. A diffuse echo texture abnormality may also represent malignancy, and here too, the presence of microlithiasis is a “marker” of malignant disease.
Klinefelter’s syndrome is often undiagnosed when infertile men first present for evaluation. The presence of nodules associated with microlithiasis in the testes on US may lead to an erroneous diagnosis of germ cell tumours, but the very small testes volume and the symmetric appearance should raise the possibility of this diagnosis and help prevent inappropriate orchiectomies. A karyotyping is then required, as mentioned above; the testicular nodules represent Leydig cell hyperplasia or Leydig cell tumours in such men in most cases (Fig. 7).
Contrast-enhanced sonography (CEUS)
CEUS optimizes enhancement in lesions which are apparently avascular at colour Doppler (Fig. 8). The rate of the wash-in and the wash-out of contrast may help to differentiate malignant from benign tumours. Leydig cell tumours have been reported to demonstrate a prolonged wash-out in one study [9], and a shorter filling time than germ cell tumours in another [35]. Unfortunately, there are only a few published papers which describe scrotal CEUS in patients with testicular lesions [9, 35–38] and, therefore, the routine use of CEUS to determine management of incidentally discovered testicular masses cannot currently be recommended for clinical management. We illustrate CEUS of a histologically proven case of Leydig tumour in Fig. 9.
Sonoelastography
The role of elastography in differentiating between malignant and benign nodules in the testes is currently still unclear, with only a few reports in the literature [35, 39–43]. Increased tissue stiffness has been reported in testicular tumours. Real-time elastography, semi-quantitative elastography, and shearwave elastography have all been studied but their role in the evaluation of testicular tumours remains in evolution. Examples of malignant and benign tumours examined with shear wave elastography are illustrated (Fig. 10).
MRI
Scrotal MRI is mainly a problem-solving tool in the assessment of testicular pathology [24]. Most testicular tumours are of low signal intensity on T2-WIs, iso-intense to testicular parenchyma on T1-WIs, and demonstrate enhancement with contrast (Figs. 11 and 12). Non-seminomatous germ cell tumours are typically heterogeneous, while teratomas may show a cystic or a fatty component. Leydig cell hyperplasia has been described to be of low signal intensity on T2-WIs with mild enhancement after contrast administration. Scrotal MRI has also been used to evaluate lesions that are hypoechoic on US and may potentially represent a burned-out tumour (Fig. 13) [44].
A–C: MRI of testicular seminomatous germ cell tumour (same patient as in Fig. 3). A: T2-WI: the tumour had low signal intensity. B: T1-WI: very slightly high signal. C: T1-WI post contrast: persistent enhancement with a rim sign on delayed phase imaging
In an effort to distinguish between different lesions, different types of time-intensity curves have been defined on dynamic contrast-enhanced MRI. Fernandez reported the MRI findings of three Leydig cell tumours with an early enhancement [45]. Tsili also studied the enhancement of several tumours, and most of them were germ cell tumours with an early enhancement. No Leydig cell tumours were included in this study [46]. Manganaro reported recently a prospective study of 44 testicular lesions, including 21 sex cord stromal tumours. He concluded that well-defined margins, rapid and marked wash-in followed by slow and late wash-out should orient to the diagnostics of Leydig cell tumours [47]. It remains to be seen whether the type of enhancement displayed by different tumours will allow differentiation between different lesions.
Diffusion-weighted MRI has been used to evaluate testicular lesions and non-tumoral parenchyma [48, 49]. Benign and malignant lesions in the testes cannot accurately be distinguished by the ADC values as significant variations and overlap exist between different tumour subtypes; tumour size and degree of necrosis can also influence the ADC values.
Discussion
Imaging may be useful in different settings in patients with small, non-palpable testicular nodules
Despite the absence of approved recommendations, the practice of urologists has evolved to using serial US monitoring to follow small, incidental testicular lesions. Eifler proposed an algorithm based on tumour markers, size and vascularity of the lesions, wherein a lesion < 5 mm and characterized by absence of vascularity and negative tumour markers could be followed by serial US monitoring [15]. Therefore, monitoring patients every three months for 12 months and then annually may be useful in clinical practice. Surgery is indicated for a lesion that shows increasing volume at follow up. Imaging also plays an important role in patients undergoing partial orchiectomy. Intraoperative US can help to choose the most accurate route for the removal of a nodule after surgical exposure of the testis, and is also useful to confirm the absence of residual nodules after surgical resection. Some surgeons may use US to guide pre-operative needle placement into a nodule to facilitate enucleationy [3].
Macroscopic findings during surgery may help the surgeon determine the possible nature of a testicular lesion. For example, a golden brown appearance and very well-defined margins are suggestive of a Leydig cell tumour [50] while whitish lesions are more suggestive of seminoma. The availability of frozen section analysis during surgery to remove testicular nodules is key for the management of small tumours and emphasizes the need for specialized centres where such services can be offered by trained pathologists. Percutaneous biopsies are routinely used in the management of lesions of an uncertain nature in parenchymal organs, but percutaneous biopsy of testicular tumours is considered to be an inappropriate practice, due to fear of tumour seeding of the scrotal tissues, with the possibility of involvement of inguinal lymph nodes. However, percutaneous biopsy has been suggested by some authors as a useful procedure in patients with testicular lesions of an indeterminate nature [51]. No biopsy-related complications are reported. The use of this technique in selected cases may be of value but there is no consensus yet amongst oncologists and urologists about the role of this management option in guiding management of equivocal testicular lesions.
Recommendations of the ESUR scrotal imaging subcommittee are as follows for non-palpable testicular nodules
-
1.
Testis tumour characterization is still primarily based on US features.
-
2.
Simple intra-testicular cysts are benign and require neither follow-up nor surgery.
-
3.
The association of hypoechoic spherical or ovoid nodules, hypoechoic areas, and grouped microliths are strongly suggestive of seminomatous germ cell tumours in non-Klinefelter men and require specialist input. It is important to recognize that tumour markers can be negative in many seminomatous germ cell tumours.
-
4.
Non-palpable, testicular, solid, single, sporadic nodules without any microliths are often benign. In such cases, the report should avoid advice leading to the removal of the entire testis. US follow-up can be an alternative to orchiectomy in young and/or infertile men if the lesion is < 5 mm and tumour markers are negative. The 5-mm size threshold is not an absolute and applies if no sinister findings are evident Table 1.
Table 1 Indicative reassuring and sinister patterns based on ultrasound (US) findings
Conclusion
Many non-palpable, small, solid, testicular nodules are benign, making orchiectomy an inappropriately aggressive therapy for management of all such lesions. Leydig cell tumours are the most common benign solid tumour when small non-palpable testicular nodules are discovered in infertile men, particularly those with Klinefelter’s syndrome. US monitoring is increasingly the preferred option for following small homogenous lesions with normal surrounding parenchyma and normal tumour markers. In selected cases, partial removal with intraoperative US guidance is indicated but this less invasive approach requires the availability of expertise in both imaging and in histological analysis; the availability of frozen section analysis is critical to guide the surgeons in organ-sparing management. There is also a necessity for dialogue and consensus regarding the role of percutaneous biopsies amongst urological and oncological societies. Further studies are needed to determine if advanced MRI and US techniques will contribute to accurate preoperative lesion characterization of small testicular nodules.
Abbreviations
- US:
-
Ultrasound
- MRI:
-
Magnetic resonance imaging :
- CEUS:
-
Contrast-enhanced ultrasound
- ESUR:
-
European Society of Urogenital Radiology
- WI:
-
Weighted image
- ADC:
-
Apparent diffusion coefficient
References
Woodward PJ, Sohaey R, O’Donoghue MJ, Green DE (2002) From the archives of the AFIP: tumors and tumorlike lesions of the testis: radiologic-pathologic correlation. Radiogr Rev Publ Radiol Soc N Am Inc 22:189–216
Brunocilla E, Gentile G, Schiavina R et al (2013) Testis-sparing Surgery for the Conservative Management of Small Testicular Masses: An Update. Anticancer Res 33:5205–5210
Dieckmann K-P, Frey U, Lock G (2013) Contemporary diagnostic work-up of testicular germ cell tumours. Nat Rev Urol 10:703–712
Dogra VS, Gottlieb RH, Oka M, Rubens DJ (2003) Sonography of the scrotum. Radiology 227:18–36
Bhatt S, Jafri SZH, Wasserman N, Dogra VS (2011) Imaging of non-neoplastic intratesticular masses. Diagn Interv Radiol Ank Turk 17:52–63
Avci A, Erol B, Eken C, Ozgok Y (2008) Nine cases of nonpalpable testicular mass: an incidental finding in a large scale ultrasonography survey. Int J Urol Off J Jpn Urol Assoc 15:833–836
Connolly SS, D’Arcy FT, Gough N et al (2006) Carefully selected intratesticular lesions can be safely managed with serial ultrasonography. BJU Int 98:1005–1007, discussion 1007
Toren PJ, Roberts M, Lecker I et al (2010) Small incidentally discovered testicular masses in infertile men-is active surveillance the new standard of care? J Urol 183:1373–1377
Isidori AM, Pozza C, Gianfrilli D, et al. (2014) Differential Diagnosis of Nonpalpable Testicular Lesions: Qualitative and Quantitative Contrast-enhanced US of Benign and Malignant Testicular Tumors. Radiology 273:606–619
Martínez-Berganza MT, Sarría L, Cozcolluela R et al (1998) Cysts of the tunica albuginea: sonographic appearance. AJR Am J Roentgenol 170:183–185
Leung ML, Gooding GA, Williams RD (1984) High-resolution sonography of scrotal contents in asymptomatic subjects. AJR Am J Roentgenol 143:161–164
Nistal M, Paniagua R (2008) Non-neoplastic disease of the testis. In: Bostwick D, Liang C (eds) Urologic Surgical Pathology, 2nd edn. Edinburgh, Mosby Elsevier, p 622–623
Zhang C, Berney DM, Hirsch MS et al (2013) Evidence supporting the existence of benign teratomas of the postpubertal testis: a clinical, histopathologic, and molecular genetic analysis of 25 cases. Am J Surg Pathol 37:827–835
Giannarini G, Dieckmann K-P, Albers P et al (2010) Organ-sparing surgery for adult testicular tumours: a systematic review of the literature. Eur Urol 57:780–790
Eifler JB Jr, King P, Schlegel PN (2008) Incidental testicular lesions found during infertility evaluation are usually benign and may be managed conservatively. J Urol 180:261–264, discussion 265
Westlander G, Ekerhovd E, Granberg S et al (2001) Testicular ultrasonography and extended chromosome analysis in men with nonmosaic Klinefelter syndrome: a prospective study of possible predictive factors for successful sperm recovery. Fertil Steril 75:1102–1105
Ekerhovd E, Westlander G (2002) Testicular sonography in men with Klinefelter syndrome shows irregular echogenicity and blood flow of high resistance. J Assist Reprod Genet 19:517–522
Rock A, Marcelli F, Robin G et al (2014) [Clinical and paraclinical features of Klinefelter syndrome consulting for male infertility.]. Progres En Urol J Assoc Francaise Urol Soc Francaise Urol 24:757–763
Accardo G, Vallone G, Esposito D, et al. (2014) Testicular parenchymal abnormalities in Klinefelter syndrome: a question of cancer? Examination of 40 consecutive patients. Asian J Androl 17:154-158
Kim I, Young RH, Scully RE (1985) Leydig cell tumors of the testis. A clinicopathological analysis of 40 cases and review of the literature. Am J Surg Pathol 9:177–192
Silberstein JL, Bazzi WM, Vertosick E et al (2014) Clinical Outcomes for Local and Metastatic Testicular Sex Cord-Stromal Tumors. J Urol 192:415–419
Nicolai N, Necchi A, Raggi D et al (2015) Clinical Outcome in Testicular Sex Cord Stromal Tumors: Testis Sparing vs Radical Orchiectomy and Management of Advanced Disease. Urology 85:402–406
de Wit R, Fizazi K (2006) Controversies in the management of clinical stage I testis cancer. J Clin Oncol Off J Am Soc Clin Oncol 24:5482–5492
Kim W, Rosen MA, Langer JE et al (2007) US MR imaging correlation in pathologic conditions of the scrotum. Radiogr Rev Publ Radiol Soc N Am Inc 27:1239–1253
Coursey Moreno C, Small WC, Camacho JC et al (2015) Testicular tumors: what radiologists need to know-differential diagnosis, staging, and management. Radiogr Rev Publ Radiol Soc N Am Inc 35:400–415
Bertolotto M, Derchi LE, Secil M et al (2015) Grayscale and color Doppler features of testicular lymphoma. J Ultrasound Med Off J Am Inst Ultrasound Med 34:1139–1145
Dogra VS, Gottlieb RH, Rubens DJ, Liao L (2001) Benign intratesticular cystic lesions: US features. Radiogr Rev Publ Radiol Soc N Am Inc 21(Spec No):S273–S281
McDonald MW, Reed AB, Tran PT, Evans LA (2012) Testicular tumor ultrasound characteristics and association with histopathology. Urol Int 89:196–202
Richenberg J, Belfield J, Ramchandani P et al (2015) Testicular microlithiasis imaging and follow-up: guidelines of the ESUR scrotal imaging subcommittee. Eur Radiol 25:323–330
Miacola C, Colamonico O, Bettocchi C et al (2014) Burned-out in a mixed germ cell tumor of the testis: The problem of pT0. Case report. Arch Ital Urol Androl Organo Uff Soc Ital Ecogr Urol E Nefrol Assoc Ric Urol 86:389–390
Tasu J-P, Faye N, Eschwege P et al (2003) Imaging of burned-out testis tumor: five new cases and review of the literature. J Ultrasound Med Off J Am Inst Ultrasound Med 22:515–521
Balalaa N, Selman M, Hassen W (2011) Burned-out testicular tumor: a case report. Case Rep Oncol 4:12–15
Philips S, Nagar A, Dighe M et al (2012) Benign non-cystic scrotal tumors and pseudotumors. Acta Radiol Stockh Swed 1987 53:102–111
Maizlin ZV, Belenky A, Kunichezky M et al (2004) Leydig cell tumors of the testis: gray scale and color Doppler sonographic appearance. J Ultrasound Med Off J Am Inst Ultrasound Med 23:959–964
Lock G, Schröder C, Schmidt C, et al. (2014) Contrast-Enhanced Ultrasound and Real-Time Elastography for the Diagnosis of Benign Leydig Cell Tumors of the Testis - A Single Center Report on 13 Cases. Ultraschall Med Stuttg Ger 1980. doi: 10.1055/s-0034-1385038
Patel K, Sellars ME, Clarke JL, Sidhu PS (2012) Features of Testicular Epidermoid Cysts on Contrast-Enhanced Sonography and Real-time Tissue Elastography. J Ultrasound Med 31:115–122
Lung PFC, Jaffer OS, Sellars ME et al (2012) Contrast-enhanced ultrasound in the evaluation of focal testicular complications secondary to epididymitis. AJR Am J Roentgenol 199:W345–W354
Bertolotto M, Derchi LE, Sidhu PS et al (2011) Acute segmental testicular infarction at contrast-enhanced ultrasound: early features and changes during follow-up. AJR Am J Roentgenol 196:834–841
Huang DY, Sidhu PS (2012) Focal testicular lesions: colour Doppler ultrasound, contrast-enhanced ultrasound and tissue elastography as adjuvants to the diagnosis. Br J Radiol 85(Spec No 1):S41–S53
Aigner F, De Zordo T, Pallwein-Prettner L et al (2012) Real-time sonoelastography for the evaluation of testicular lesions. Radiology 263:584–589
Goddi A, Sacchi A, Magistretti G et al (2012) Real-time tissue elastography for testicular lesion assessment. Eur Radiol 22:721–730
Pastore AL, Palleschi G, Maceroni P et al (2014) Correlation between semiquantitative sonoelastography and immunohistochemistry in the evaluation of testicular focal lesions. Cancer Imaging Off Publ Int Cancer Imaging Soc 14:29
Correas JM, Drakonakis E, Isidori AM et al (2013) Update on ultrasound elastography: miscellanea. Prostate, testicle, musculo-skeletal. Eur J Radiol 82:1904–1912
Patel MD, Patel BM (2007) Sonographic and magnetic resonance imaging appearance of a burned-out testicular germ cell neoplasm. J Ultrasound Med Off J Am Inst Ultrasound Med 26:143–146
Fernández GC, Tardáguila F, Rivas C et al (2004) MRI in the diagnosis of testicular Leydig cell tumour. Br J Radiol 77:521–524
Tsili AC, Argyropoulou MI, Astrakas LG et al (2013) Dynamic contrast-enhanced subtraction MRI for characterizing intratesticular mass lesions. AJR Am J Roentgenol 200:578–585
Manganaro L, Vinci V, Pozza C, et al. (2015) A prospective study on contrast-enhanced magnetic resonance imaging of testicular lesions: distinctive features of Leydig cell tumours. Eur Radiol (in press)
Tsili AC, Argyropoulou MI, Giannakis D et al (2012) Diffusion-weighted MR imaging of normal and abnormal scrotum: preliminary results. Asian J Androl 14:649–654
Tsili AC, Sylakos A, Ntorkou A et al (2015) Apparent diffusion coefficient values and dynamic contrast enhancement patterns in differentiating seminomas from nonseminomatous testicular neoplasms. Eur J Radiol. Eur J Radiol 84:1219–1226
Al-Agha OM, Axiotis CA (2007) An in-depth look at Leydig cell tumor of the testis. Arch Pathol Lab Med 131:311–317
Shaida N, Berman LH (2012) Percutaneous testicular biopsy for indeterminate testicular lesions. Br J Radiol 85:S54–S58
Acknowledgments
The scientific guarantor of this publication is Jonathan Richenberg. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. No complex statistical methods were necessary for this paper. Institutional review board approval was obtained. This manuscript is a review but institutional review board approval was obtained to publish radiological data. Written informed consent was waived by the institutional review board. Methodology: review. We thank Vincent Izard, our guest specialized urologist for his contribution. We thank Mustafa Cecil, Pietro Pavlica, Michal Studniarek for their active participation in the group. We thank Valentine Sallerin for her initial English revision.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rocher, L., Ramchandani, P., Belfield, J. et al. Incidentally detected non-palpable testicular tumours in adults at scrotal ultrasound: impact of radiological findings on management Radiologic review and recommendations of the ESUR scrotal imaging subcommittee. Eur Radiol 26, 2268–2278 (2016). https://doi.org/10.1007/s00330-015-4059-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-4059-7