Abstract
Purpose
To investigate staging accuracy of multidetector CT (MDCT) for pancreatic neuroendocrine tumour (PNET) and diagnostic performance for differentiation of PNET from pancreatic adenocarcinoma.
Material and methods
We included 109 patients with surgically proven PNET (NETG1 = 66, NETG2 = 31, NEC = 12) who underwent MDCT. Two reviewers assessed stage and presence of predefined CT findings. We analysed the relationship between CT findings and tumour grade. Using PNETs with uncommon findings, we also estimated the possibility of PNET or adenocarcinoma.
Results
Accuracy for T stage was 85–88 % and N-metastasis was 83–89 %. Common findings included well circumscribed, homogeneously enhanced, hypervascular mass, common in lower grade tumours (p < 0.05). Uncommon findings included ill-defined, heterogeneously enhanced, hypovascular mass and duct dilation, common in higher grade tumours (p < 0.05). Using 31 PNETs with uncommon findings, diagnostic performance for differentiation from adenocarcinoma was 0.760–0.806. Duct dilatation was an independent predictor for adenocarcinoma (Exp(B) = 4.569). PNETs with uncommon findings were associated with significantly worse survival versus PNET with common findings (62.7 vs. 95.7 months, p < 0.001).
Conclusion
MDCT is useful for preoperative evaluation of PNET; it not only accurately depicts the tumour stage but also prediction of tumour grade, because uncommon findings were more common in higher grade tumours.
Key Points
• CT accurately depicts the T stage and node metastasis of PNET.
• Uncommon findings were more common in higher grade tumours.
• CT information may be beneficial for optimal therapeutic planning.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pancreatic neuroendocrine tumours (PNETs) constitute a heterogeneous group of tumours that originate from neuroendocrine cells. PNETs are classically characterized as slow-growing, indolent tumours. However, aggressive tumours with early metastases have also been reported [1]. The incidence of PNET is as high as 10 % in pancreatic tumours. In addition, the current detection rate of incidental PNET during imaging procedures is increasing [2].
According to the recent WHO classification, PNET has a spectrum of tumour grades with respect to the mitotic count and the Ki-67 index, i.e. NET grade 1, NET grade 2 and neuroendocrine carcinoma (NEC). NET grade 1 is mostly benign and has an excellent prognosis, whereas NEC is mostly malignant with a poor prognosis [3]. Although the WHO classification is an important step toward defining the diverse tumour biology of PNET, it does not represent the tumour extent. For this reason, many medical institutions use not only the classical grading system, but also TNM staging [4–6]. The classical grading system is based on histological differentiation, whereas the TNM staging system is based on the tumour extent. Therefore, accurate assessment of the tumour stage and grade is essential when planning PNET treatment.
Contrast-enhanced CT is the primary imaging modality for evaluating the pancreas. According to previously published reports, common CT findings of PNET include a well circumscribed, hypervascular mass, lack of duct dilatation and the presence of calcification or cystic degeneration [1, 2, 7–10]. However, we occasionally find PNETs with uncommon CT findings including a hypovascular enhancement pattern or duct dilatation and image findings that closely resemble those of pancreatic ductal adenocarcinoma (PDAC) [11–14].
Although there are previous reports regarding the CT findings for PNET, to our knowledge, the accuracy of preoperative CT for determining the staging and the assessment of common and uncommon CT findings has not yet been established. The purpose of our study was to investigate the staging accuracy of MDCT for PNET as well as the common and uncommon findings. We also assessed diagnostic performance in order to differentiate PNET with uncommon CT findings from PDAC.
Materials and methods
Patients
Our institutional review board approved this retrospective study, and the requirement for informed consent was waived. From a computerized search of our hospital’s pathology files and medical records from January 2006 to January 2013, we identified 136 consecutive patients with surgically proven PNET who underwent CT before surgery. In this study, we excluded patients who had undergone a single-phase CT scan (n = 12) or non-contrast-enhanced CT (n = 5) and who had insufficient data in order to determine the pathological grade (n = 10). Finally, 109 patients with pathologically proven PNET were enrolled in this study (Fig. 1). All patients had undergone surgery, i.e. pylorus-preserving pancreaticoduodenectomy (n = 47), distal pancreatectomy (n = 44), tumour enucleation (n = 9), Whipple surgery (n = 8) and median pancreatectomy (n = 1).
For comparison, we enrolled patients with PDAC. From a computerized search of our hospital’s pathology files from January 2010 to June 2010, we identified 29 consecutive patients with PDAC who underwent surgery and preoperative dynamic CT.
Multidector CT (MDCT) imaging
Seventy-nine patients underwent quadruple-phase CT and 30 patients underwent triple-phase CT. CTs were obtained using one of the following commercially available MDCT systems: four-channel CT system (Mx8000, Marconi Medical Systems, Cleveland, OH, USA; n = 12); eight-channel CT scanner (Lightspeed, GE Healthcare, Milwaukee, WI, USA; n = 17); 16-channel CT scanner (Sensation 16, Siemens Medical Solutions, Erlangen, Germany; n = 20); 64-channel CT scanner (Brilliance 64, Philips Healthcare, Cleveland, OH, USA; n = 35) and dual-source CT scanner (Somatom definition, Siemens Medical Solutions; n = 25). The imaging parameters for the four-, eight-, 16- and 64-detector CT examinations, detector collimations of 2.5 mm, 1.25 mm, 0.75 mm and 0.625 mm, and a pitch of 1.25, 1.35, 1.25 and 0.891 were used. Images with 2.5- to 3.2-mm thick sections were acquired. A section thickness of 3 mm with a 3-mm reconstruction interval, a field of view of 300–370 mm, a gantry rotation time of 0.5 s, a tube current–time product of 150–200 mAs, and a peak voltage of 120 kVp were used for the MDCT scanners. The scanning parameters for dual-source CT scanners were a detector collimation of 0.6 mm, rotation time of 0.5 s and pitch of 0.85. The reference tube current time product was set at 80 mAs for a 140-kVp tube and at 340 mAs for an 80-kVp tube. After 120 mL of nonionic contrast material (Iopromide, Ultravist 370, Schering, Berlin, Germany) was administered using a power injector (Multilevel CT, Medrad, Pittsburgh, PA, USA) at a rate of 3 mL/s through an 18-gauge, plastic, intravenous catheter placed in an antecubital vein, a 20-mL flush of sterile saline followed. Contrast enhancement was automatically calculated by placing the region-of-interest cursor over the vessel of interest, i.e. the abdominal aorta, and the level of the trigger threshold was set at an increase of 100 HU. Early arterial-phase imaging was automatically obtained 6–9 s after the trigger threshold was reached. The early and late arterial phases were acquired separately during each breath-hold using a minimum inter-scan delay of 5–9 s. The mean imaging time delay was 23 s for the early arterial phase and 37–45 s for the late arterial phase. Venous-phase imaging was obtained 70 s after triggering.
Imaging interpretation
CT scans were retrospectively analysed by two radiologists (J.H.K. and H.W.E.), each with 14 years of clinical experience in abdominal CT. The two radiologists retrospectively and independently reviewed each patient’s CT image set. Both reviewers knew that the patients had proven PNET, although they were blinded to all of the detailed pathology findings.
All phases of the CT scans were evaluated as a whole. The two radiologists assessed the T stage and lymph-node metastasis. The largest diameter of each tumour was measured using the T-staging of PNET according to the AJCC (American Joint Committee on Cancer) seventh edition [15]. Lymph nodes were considered positive when there was a short-axis diameter greater than 5 mm. The radiologists also assessed the image findings, including the tumour margin (well circumscribed vs. ill-defined), tumour enhancement (homogeneous vs. heterogeneous) during the venous phase, the presence of cystic change or calcification, contrast-enhancement patterns on the early arterial, late arterial and venous phases, and the presence of duct dilatation. When there was a discrepancy between the two radiologists’ diagnoses, a third reviewer (J.M.L.) with 19 years of clinical experience performing abdominal CT made the final decision.
After completing the first interpretation session, we defined atypical PNET using uncommon CT findings. Atypical PNET showed more than three uncommon CT findings including an ill-defined border margin, heterogeneous enhancement, hypovascular on the early arterial phase, hypovascular on the late arterial phase, hypovascular on the venous phase and duct dilatation. CT images of PNET with uncommon CT findings and of PDAC were retrospectively analysed by two board-certified radiologists (J.H.K. and H.W.E.), who had attended the first interpretation session. In order to reduce the recall bias, we scheduled the second session 8 weeks after the first session. On the basis of the previously reported imaging features, the radiologists independently graded the possibility of PNET using a five-point scale: 1 – definitely PNET; 2 – probably PNET; 3 – Indeterminate; 4 – probably PDAC; and 5 – definitely PDAC. Ratings 1 and 2 assumed the presence of a PNET. When there was a discrepancy between the two radiologists’ diagnoses, the third reviewer (J.M.L.) made the final decision. We also analysed survival data of 107 patients with PNET as two patients had been lost during follow-up.
Statistical analysis
The relationship of the tumour grade and the T-staging, node metastasis, tumour size and CT findings was assessed using the Chi-square test and one-way analysis of variance (ANOVA). Statistical significance was assumed at a confidence level of 0.05. The accuracy of the T-staging and the presence of node metastasis were assessed using the Chi-square test. To assess interobserver agreement, we performed a simple κ analysis. The degree of interobserver agreement was interpreted as follows: 0–0.20 slight agreement; 0.21–0.40 fair agreement; 0.41–0.60 moderate agreement; 0.61–0.80 substantial agreement; and 0.81–1 almost perfect agreement. The diagnostic performance of CT to differentiate PNET with uncommon CT findings from PDAC was evaluated using the area under the receiver-operating-characteristic (ROC) curve (Az). Statistically significant CT findings associated with PDAC (p < 0.05) were further analysed using multivariate logistic regression analysis. Patient survival probabilities were calculated using the Kaplan-Meier method and were compared using the log-rank test. ROC curve analysis was performed using MedCalc statistical software (version 6.15, MedCalc) for Windows (Microsoft), and other statistical analyses were performed using an SPSS software package (version 14.0, SPSS).
Results
The tumour grade consisted of NET grade 1 in 66 patients, NET grade 2 in 31 patients and NEC in 12 patients. The T stage of the PNET consisted of T1 in 33 patients, T2 in 37 patients, and T3 in 39 patients. Node metastasis was confirmed in 20 patients. Table 1 summarizes the pathology results of the PNET. The T stage was correlated to the tumour grade (p < 0.001). While ten patients with NEC (83 %) and 17 patients with NET grade 2 (55 %) were of the T-3 stage, only 12 patients with NET grade 1 (18 %) were of the T-3 stage. Node metastasis was also correlated to the tumour grade (p < 0.001). The tumour size was larger according to the tumour grade, although without statistical significance except for NET grades 1 and 2. NET grade 2 (37.2 ± 23.4 mm) was significantly larger than NET grade 1 (24.9 ± 13.7 mm, p = 0.03).
Table 2 summarizes the diagnostic accuracy of CT for assessing the T stage and node metastasis. The accuracy for determining the T stage was 85 % (n = 93) for reader 1 and 88 % (n = 96) for reader 2, and with almost perfect interobserver agreement (κ = 0.847). The accuracy for determining each T stage was 92 % for T1, 91 % for T2 and 88 % for T3 for reader 1 and 93 % for T1, 92 % for T2 and 92 % for T3 for reader 2. The accuracy for determining lymph-node metastasis was 83 % for reader 1 and 89 % for reader 2 with substantial interobserver agreement (κ = 0.614).
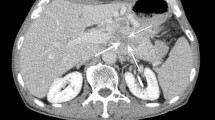
Common CT findings of PNET included its being well circumscribed, homogeneously enhanced and hypervascular as seen on the arterial and venous phases, which were more common in lower grade tumours (p < 0.05) (Fig. 2). Alternatively, uncommon CT findings included the PNET being ill-defined, heterogeneously enhanced and hypovascular on the arterial and venous phases as well as there being duct dilation, all of which were more common in higher grade tumours (p < 0.05) (Fig. 3). Sixty-three patients with NET grade 1 (95 %) and 27 patients with NET grade 2 (87 %) had tumours with well circumscribed borders, whereas seven patients with NEC (58 %) had tumours with ill-defined borders. Fifty-three and 43 patients with NET grade 1 (53/66, 80 %; 43/66, 65 %) had tumours with hypervascularity seen on the late arterial and venous phases, whereas eight and six patients with NEC (8/12, 66 %; 6/12, 50 %) had tumours with hypovascularity seen on the late-arterial and venous phases. Bile duct or pancreatic duct dilatations were common in higher grade tumours (p = 0.008). Although CT is useful for predicting the tumour grade, NET grade 2 showed mixed findings. Sixteen patients with NET grade 2 (16/31, 52 %) showed tumours with hypervascularity on the late arterial phase, whereas 14 patients (14/31, 45 %) showed tumours with hypovascularity on the late arterial phase. Thirteen patients with NET grade 2 (13/31, 42 %) showed bile duct or pancreatic duct dilatation (Fig. 4). In addition, there were no independent CT findings to predict higher grade PNET on multivariate logistic regression analysis (p < 0.05). Table 3 summarizes the PNET CT findings according to the tumour grade.
A 69-year-old woman with pancreatic neuroendocrine tumour grade 1. (a, b) On pancreatic (a) and venous (b) phases of dynamic contrast-enhanced CT images, a well circumscribed mass located in the tail of the pancreas shows hypervascular enhancement on pancreatic (arrows) phase and homogeneous enhancement on venous phase. There is no definite duct dilatation in the upstream pancreatic parenchyma (arrowheads). (c) Cross section of specimen shows well defined white to yellow mass in the tail of the pancreas. (d) Ki-67 immunostaining shows positive in 0.4 % of tumour cells (original magnification × 100)
A 40-year-old man with pancreatic neuroendocrine carcinoma. (a-d) On pancreatic (a) and venous (b, c) phases of dynamic contrast-enhanced CT images, a well circumscribed mass located in the head of the pancreas shows hypovascular enhancement on pancreatic (arrow) and homogeneous iso attenuation on venous (arrow) phase. The dilated bile duct and pancreatic duct are noted (arrowheads). (d) Ki-67 immunostaining shows positive in 40 % of tumour cells (original magnification × 100)
A 53-year-old woman with pancreatic neuroendocrine tumour grade 2. (a-c) On pancreatic (a) and venous (b) phases of dynamic contrast-enhanced CT images, an ill-defined mass located in the head of the pancreas shows homogeneous hypovascular enhancement on pancreatic (arrow) and venous (arrow) phase. Biliary stent is noted (arrowhead). On venous phases (c), the dilated pancreatic duct and air-biliary gram in the dilated bile duct are noted (arrowheads). There is an enlarged lymph node in the portocaval space (arrow). (d) Ki-67 immunostaining shows positive in 13.6 % of tumour cells (original magnification × 100)
Thirty-one patients (31/109, 28 %) with PNET showed more than three uncommon CT findings. PNET with uncommon CT findings indicated nine patients with NET grade 1 (9/66, 14 %), 14 patients with NET grade 2 (14/31, 45 %) and eight patients with NEC (8/12, 67 %). Az for the differentiation of atypical PNET from PDAC were 0.806 and 0.760 with excellent agreement (κ = 0.831) (Fig. 5). Pancreatic duct dilatation (10/31 in PNET vs. 19/29 in PDAC, P = 0.019) and homogeneous enhancement (12/31 PNET vs. 20/29 in PDAC, P = 0.01) were more common in PDAC than PNET. Table 4 summarizes the CT findings of atypical PNET and PDAC. Pancreatic duct dilatation was an independent CT predictor for PDAC (Exp(B) = 4.569, 95.0 % CI = 1.445–14.453, p = 0.01). PNET with uncommon CT findings were associated with a significantly worse overall survival rate compared to PNET with common CT findings (mean survival; 62.7 ± 5.8 (51.229–74.308) months vs. 95.7 ± 1.8 (92.197–99.349) months, p < 0.001) (Fig. 6).
Receiver operating characteristic curve for diagnostic performance of CT regarding the differentiation of PNET with uncommon CT findings from pancreatic adenocarcinoma in reader 1 and reader 2. The area under the curve is 0.806 for reader 1 and 0.760 for reader 2 with excellent agreement (κ = 0.831)
Overall survival after diagnosis of PNET, according to the common and uncommon CT findings. Pancreatic neuroendocrine tumours (PNETs) with uncommon CT findings were associated with a significantly worse overall survival rate compared to PNETs with common CT findings (mean survival: 62.7 ± 5.8 months vs. 95.7 ± 1.8 months, p < 0.001)
Discussion
Our study results show that the accuracy of CT for determining the T stage was 85 % and 88 % and node metastasis was 83 % and 89 %. The common findings of PNET included a well circumscribed, homogeneously enhanced and hypervascular appearance, which were more common in lower grade tumours (p < 0.05). On the other hand, uncommon findings included an ill-defined, heterogeneously enhanced and hypovascular appearance and duct dilation, which were more common in higher grade tumours (p < 0.05). Using 31 PNET with uncommon findings, the diagnostic performance of CT for differentiating from PDAC was 0.806 and 0.760. Pancreatic duct dilatation was an independent predictor for PDAC (Exp(B) = 4.569). In addition, PNET with uncommon CT findings was associated with a significantly worse survival rate.
PNETs are heterogeneous in their morphological and biological features. Due to their rarity and heterogeneity, accurate diagnosis before surgery has been challenging. According to our results, CT accurately depicts the T stage as well as node metastasis. CT findings are also helpful for predicting the tumour grade. According to previously published reports, common CT findings of PNET include a well circumscribed mass with early enhancement, no ductal dilatation and lack of vascular encasement [1, 2, 7–10]. These results are similar to those of our study. In previous reports, common findings, especially the early enhancement, have been described in approximately 61–80 % of PNETs. On the other hand, uncommon imaging findings, including hypo-enhancement, were only seen in a small portion of PNETs [11–14, 16]. In our study, 31 patients (31/109, 28 %) with PNET showed more than three uncommon CT findings including them being ill-defined, heterogeneously enhanced and hypovascular, as seen on arterial- and venous-phase images, as well as duct dilation, and which were usually seen in higher grade tumours (p < 0.05). Although CT is useful for predicting the tumour grade, NET grade 2 showed mixed findings. In our study, 12 (39 %) patients with NET grade 2 showed tumours with homogeneous enhancement, whereas 19 (61 %) patients showed tumours with heterogeneous enhancement. Sixteen (52 %) patients with NET grade 2 showed tumours with hypervascularity, whereas 14 (45 %) patients showed tumours with hypovascularity. Thirteen (42 %) patients with NET grade 2 showed duct dilatation. In NET grade 2 tumours, CT only has a limited role in predicting the tumour grade.
As PNET with uncommon CT findings closely resembles PDAC, PNETs are often misdiagnosed as PDAC. In our study, the diagnostic performance for differentiating PNET with uncommon findings from PDAC was 0.760 to 0.806. Tummala at al. [17] investigated the incidence of pancreatic duct dilatation in malignant tumours. They confirmed that 152 of 187 (81.2 %) patients with focal, pancreatic, solid lesions with duct dilatation had malignant tumour. Among these 152 patients, 134 (88 %) were confirmed with PDAC and 14 (9 %) were confirmed to have PNET. Therefore, pancreatic duct dilatation is considered to be one of the most important CT findings indicating PDAC. Our results are analogous with their results in that in our study pancreatic duct dilatation was an independent predictor for PDAC (Exp(B) = 4.569).
Many pathological parameters have been identified which predict the prognosis following PNET resection, including tumour grade, Ki-67 index and mitotic count [18, 19]. However, accurate prediction of the prognosis before surgery has been challenging [11–14]. Worhunsky et al. [14] investigated the correlation between the degree of tumour enhancement on CT and the overall patient survival. In their study, they analysed the CT images of 118 patients with PNET. The majority had hyperenhancing tumours (68 %) or isoenhancing tumours (10 %). Hypoenhancement was noted in 22 % of the PNETs. In their study, hypoenhancing PNETs were associated with a significantly worse overall patient survival rate than hyperenhancing or isoenhancing tumours (5-year, 54 % vs. 93 % vs. 89 %). On multivariate analysis, only hypoenhancement (HR = 2.32, p = 0.02) was independently associated with patient survival. Rodallec et al. [12] also confirmed the correlation between hypoenhancing tumours and poor differentiation as well as the decreased survival rate. D’Assignies et al. [11] found tumour blood flow, as measured with perfusion CT, to be associated with microvessel density and tumour differentiation. It was proposed that enhancement during the arterial phase is a surrogate parameter for vascularization and perfusion and may indicate tumour differentiation in PNET. Our findings are analogous with the findings of previous such studies. PNETs with uncommon CT findings, including hypo-enhancement, were associated with a significantly worse overall survival rate compared to PNETs with typical CT findings (62.7 vs. 95.7 months). PNETs with uncommon CT findings would, therefore, be one of the predictors of a poor outcome.
Our study has several possible limitations. First, it had a retrospective design and there was a possibility of selection bias. As we could only include patients with surgically resected PNETs, our study population did not reflect the entire spectrum of PNET. Second, contrast-enhanced CT was performed with different CT scanners and 30 patients did not undergo early arterial-phase scanning. However, despite this heterogeneity, all patients underwent contrast-enhanced, multiphasic CT including late arterial phase, the so-called pancreatic parenchymal phase, which is the most important phase for evaluation of the enhancement patterns of focal pancreatic lesions [20, 21] . In addition to this, in our study, early arterial enhancement showed similar enhancement patterns to those of the late arterial phase in each patient. We also used the accepted protocol as well as acceptable and adequate CT image quality.
In conclusion, our results show that preoperative CT accurately depicts the T stage and node metastasis of PNETs. The common findings were more common in lower grade tumours, whereas uncommon findings were more common in higher grade tumours. PNETs with uncommon findings were also associated with a significantly worse overall survival. Although CT is useful for predicting the tumour grade, NET grade 2 showed mixed findings. As it is sometimes difficult to differentiate PNET with uncommon CT findings from PDAC, clinicians should be particularly aware. CT information is beneficial for optimal therapeutic planning using a multidisciplinary team approach.
References
Ehehalt F, Saeger HD, Schmidt CM, Grützmann R (2009) Neuroendocrine tumors of the pancreas. Oncologist 14:456–467
Owen N, Sohaib S, Peppercorn P et al (2001) MRI of pancreatic neuroendocrine tumours. Br J Radiol 74:968–973
Bosman FT, Carneiro F, Hruban RH, Theise ND (2010) WHO classification of tumours of the digestive system. World Health Organization
Ekeblad S, Skogseid B, Dunder K, Öberg K, Eriksson B (2008) Prognostic factors and survival in 324 patients with pancreatic endocrine tumor treated at a single institution. Clin Cancer Res 14:7798–7803
Rindi G, Klöppel G, Alhman H et al (2006) TNM staging of foregut (neuro) endocrine tumors: a consensus proposal including a grading system. Virchows Arch 449:395–401
Sellner F, Thalhammer S, Stättner S, Karner J, Klimpfinger M (2011) TNM stage and grade in predicting the prognosis of operated, non‐functioning neuroendocrine carcinoma of the pancreas—a single‐institution experience. J Surg Oncol 104:17–21
Poultsides GA, Huang LC, Chen Y et al (2012) Pancreatic neuroendocrine tumors: radiographic calcifications correlate with grade and metastasis. Ann Surg Oncol 19:2295–2303
Procacci C, Carbognin G, Accordini S et al (2001) Nonfunctioning endocrine tumors of the pancreas: possibilities of spiral CT characterization. Eur Radiol 11:1175–1183
Rha SE, Jung SE, Lee KH, Ku YM, Byun JY, Lee JM (2007) CT and MR imaging findings of endocrine tumor of the pancreas according to WHO classification. Eur J Radiol 62:371–377
Rodallec M, Vilgrain V, Zins M, Couvelard A, Ruszniewski P, Menu Y (2002) Helical CT of pancreatic endocrine tumors. J Comput Assist Tomogr 26:728–733
d'Assignies G, Couvelard A, Bahrami S et al (2009) Pancreatic endocrine tumors: tumor blood flow assessed with perfusion CT reflects angiogenesis and correlates with prognostic factors1. Radiology 250:407–416
Rodallec M, Vilgrain V, Couvelard A et al (2006) Endocrine pancreatic tumours and helical CT: contrast enhancement is correlated with microvascular density, histoprognostic factors and survival. Pancreatology 6:77–85
Tatsumoto S, Kodama Y, Sakurai Y, Shinohara T, Katanuma A, Maguchi H (2013) Pancreatic neuroendocrine neoplasm: correlation between computed tomography enhancement patterns and prognostic factors of surgical and endoscopic ultrasound-guided fine-needle aspiration biopsy specimens. Abdom Imaging 38:358–366
Worhunsky DJ, Krampitz GW, Poullos PD et al (2014) Pancreatic neuroendocrine tumours: hypoenhancement on arterial phase computed tomography predicts biological aggressiveness. HPB 16:304–311
Edge SB, Byrd D, Compton C, Fritz A, Greene FL, Trotti A (2010) AJCC cancer staging manual, 7th edn. Springer, New York, pp 285–296
Kim DW, Kim HJ, Kim KW et al (2014) Neuroendocrine neoplasms of the pancreas at dynamic enhanced CT: comparison between grade 3 neuroendocrine carcinoma and grade 1/2 neuroendocrine tumour. Eur Radiol 25(5):1375–1383
Tummala P, Rao S, Agarwal B (2013) Differential diagnosis of focal non-cystic pancreatic lesions with and without proximal dilation of pancreatic duct noted on CT scan. Clin Transl Gastroenterol 4:e42
Fischer L, Kleeff J, Esposito I et al (2008) Clinical outcome and long‐term survival in 118 consecutive patients with neuroendocrine tumours of the pancreas. Br J Surg 95:627–635
Martin RC, Kooby DA, Weber SM et al (2011) Analysis of 6,747 pancreatic neuroendocrine tumors for a proposed staging system. J Gastrointest Surg 15:175–183
Hammerstingl RM, Vogl TJ (2005) Abdominal MDCT: protocols and contrast considerations. Eur Radiol Suppl 15:e78–e90
Marin D, Nelson RC, Barnhart H et al (2010) Detection of pancreatic tumors, image quality, and radiation dose during the pancreatic parenchymal phase: Effect of a low-tube-voltage, high-tube-current CT technique—preliminary results 1. Radiology 256:450–459
Acknowledgments
We thank Bonnie Hami, South Euclid, Ohio, and Jeehyun Kim, Berkeley, California, for editorial assistance in the preparation of this manuscript.
The scientific guarantor of this publication is Joon Koo Han, M.D. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding, and have no conflicts of interest to declare. Young Jae Kim M.D. has significant statistical expertise and no complex statistical methods were necessary for this paper. Institutional Review Board approval was obtained (IRB No. 1304-052-480). Written informed consent was waived by the Institutional Review Board. No study subjects or cohorts have been previously reported. Methodology: retrospective, diagnostic study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, J.H., Eun, H.W., Kim, Y.J. et al. Pancreatic neuroendocrine tumour (PNET): Staging accuracy of MDCT and its diagnostic performance for the differentiation of PNET with uncommon CT findings from pancreatic adenocarcinoma. Eur Radiol 26, 1338–1347 (2016). https://doi.org/10.1007/s00330-015-3941-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-3941-7