Abstract
Molecular imaging aims to improve the identification and characterization of pathological processes in vivo by visualizing the underlying biological mechanisms. Molecular imaging techniques are increasingly used to assess vascular inflammation, remodeling, cell migration, angioneogenesis and apoptosis. In cardiovascular diseases, molecular magnetic resonance imaging (MRI) offers new insights into the in vivo biology of pathological vessel wall processes of the coronary and carotid arteries and the aorta. This includes detection of early vascular changes preceding plaque development, visualization of unstable plaques and assessment of response to therapy. The current review focuses on recent developments in the field of molecular MRI to characterise different stages of atherosclerotic vessel wall disease. A variety of molecular MR-probes have been developed to improve the non-invasive detection and characterization of atherosclerotic plaques. Specifically targeted molecular probes allow for the visualization of key biological steps in the cascade leading to the development of arterial vessel wall lesions. Early detection of processes which lead to the development of atherosclerosis and the identification of vulnerable atherosclerotic plaques may enable the early assessment of response to therapy, improve therapy planning, foster the prevention of cardiovascular events and may open the door for the development of patient-specific treatment strategies.
Key Points
• Targeted MR-probes allow the characterization of atherosclerosis on a molecular level.
• Molecular MRI can identify in vivo markers for the differentiation of stable and unstable plaques.
• Visualization of early molecular changes has the potential to improve patient-individualized risk-assessment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Molecular imaging represents a technique for the visualisation of molecular and cellular processes in vivo. Molecular imaging techniques such as magnetic resonance imaging (MRI), computed tomography (CT), ultrasound (US), positron emission tomography (PET), single photon emission computed tomography (SPECT) and optical imaging are increasingly used to characterise vascular inflammation, remodeling, cell migration, angioneogenesis and apoptosis. Ongoing advances have contributed to the development and use of multimodality imaging tools, both in the preclinical and the clinical settings. Hybrid PET/CT systems have emerged as the preferred system for clinical nuclear medicine, and PET/MRI systems are subject to modern molecular imaging studies.
Cardiovascular disease, including atherosclerosis of the aorta, carotid and coronary arteries, is still the leading cause of morbidity and mortality worldwide [1]. In the arterial vessel wall, smoldering inflammatory processes result in either a slow narrowing of the arterial lumen or, in the case of unstable plaque, in a sudden narrowing of the lumen [2]. X-ray angiography currently represents the clinical gold standard for the evaluation of atherosclerotic stenosis. Traditional invasive and non-invasive techniques (e.g. CT-angiography) are focused on the grading of luminal narrowing. The degree of luminal narrowing is, however, not a parameter that allows for the differentiation between stable or vulnerable plaques [3–5]. This represents one of the main limitations of traditional imaging approaches. As plaque rupture occurs, in most cases, without preceding warning signs, this type of plaque causes the most immediate life-threatening cardiovascular events [6]. Therefore, there is a clinical need to introduce novel techniques and imaging approaches for the early detection of vulnerable plaques and for the characterization of plaque composition into the clinical setting. In the past, considerable achievements have been made using 18 F-fluorodeoxyglucose (18 F-FDG) and sodium-18 F-fluoride (18 F-NaF) PET/CT for characterizing plaque biology, inflammation and vulnerability [7–9]. Other more widely available techniques such as contrast-enhanced US successfully contributed to the visualisation of vulnerable carotid plaque by imaging intraplaque neovascularization [10]. Molecular MRI has shown potential for the characterization of plaque composition, progression and mechanisms associated with plaque destabilization in human studies [11, 12]. Techniques for the visualisation and quantification of plaque components of the carotid, aortic and coronary arteries with traditional non-specific extracellular gadolinium-based agents have already been introduced into the clinical setting [13–15]. In terms of molecular MR-probes, a wide range of specific and non-specific agents have been validated in animal models and initial human trials. The most common molecular MR-probes can be broadly divided into two categories: (1) T1-shortening paramagnetic gadolinium-(Gd)-based-probes and (2) T2-shortening superparamagnetic-(iron-oxide-based)-nanoparticles. Previous studies in small/large animal models and initial human studies demonstrated the potential of these molecular probes to assess plaque biology (e.g. the expression of endothelial adhesion molecules) [16], plaque lipid and macrophage content [17–20], neovascularisation [17, 21] and plaque thrombosis [22–26]. In summary, specific molecular MR-probes allow the visualisation and characterization of pathological processes at a molecular and cellular level, which is the key for early disease detection and the characterization of vulnerable atherosclerotic plaques.
The aim of this review is to outline the most promising molecular MR imaging strategies and targeted MR-probes for the characterization of atherosclerotic vessel wall disease.
Pathophysiology of atherosclerosis
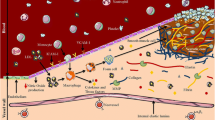
As atherosclerotic plaques develop and progress slowly in most cases, it can take decades until initial clinical symptoms become apparent [27]. Key steps in the inflammatory cascade of early plaque development include endothelial dysfunction, activation of cell adhesion molecules, docking of monocytes to activated endothelium, influx of low density lipoprotein (LDL) into the subendothelial tissue and differentiation of monocytes into macrophages (Fig. 1) [27, 28]. Subsequent steps include activation of platelets and smooth muscle cell proliferation followed by expression of extracellular matrix (ECM) components, e.g. elastin and collagen, which results in positive and negative vascular remodelling [5]. Positive vascular remodeling is a process that is defined by an outward increase of the ECM. An increase in positive vascular remodeling was shown to be associated with vulnerable plaque [29]. This type of remodeling cannot be detected using traditional imaging techniques relying on the angiographic visualisation of the arterial lumen. Negative vascular remodeling, on the other hand, refers to a process that is associated with a decrease of the luminal area [5]. This type of vascular remodeling can be detected and graded by traditional luminographic imaging techniques. Continous negative remodeling of the arterial vessel wall progressively narrows the arterial lumen and can cause a flow-relevant constriction in advanced stages of plaque development. Further crucial steps during the development of stable and unstable atherosclerotic plaques include extracellular matrix degradation, apoptosis and angiogenesis [27]. In the past, landmark intravascular ultrasound (IVUS) studies of the coronary arteries have identified distinct characteristics of vulnerable plaques such as large plaque volume, large necrotic core, thin fibrous cap, positive vascular remodeling and high density of neovascularization in patients [29].
Pathogenesis of plaque development. Endothelial dysfunction initiates inflammatory processes and leads to the migration of immune cells and low density lipoprotein (LDL) into the subendothelial tissue leading to the differentiation of monocytes into macrophages which transform, with increasing intake of lipids and cholesterol, into foam cells. Subsequently atherosclerotic plaques develop, which are characterised by activation of platelets and smooth muscle cells, followed by deposition of extracellular connective matrix (ECM) components and endothelial proliferation and the formation of lipid-rich necrotic cores. Vulnerable plaques are characterised by a large plaque volume, a large necrotic core, a thin fibrous cap, positive vascular remodeling and a high density of neovascularization and are responsible for the majority of acute cardiovascular events, e.g. myocardial infarction due to intravascular thrombus formation. EEM external elastic membrane, ICAM1 intercellular adhesion molecule-1 IEM internal elastic membrane. Adapted from [84]
In the following sections we will outline which probes can be used to target and visualise the different steps during the development of atherosclerotic vessel wall disease.
Probes for molecular magnetic resonance imaging (MRI)
T1-shortening MR probes
To increase signal intensity on T1-weighted imaging sequences, MR probes can be applied to shorten local T1 relaxation times. Gadolinium-(Gd)-based MR agents represent the most commonly administered agents in the clinical setting [30]. The detection limit of MRI for Gd-chelates is significantly higher compared to nuclear probes such as 18 F-FDG. Greater knowledge of the structurally related relaxation mechanisms constantly fosters the development of new chelate designs to improve signal enhancement and therefore the sensitivity for the detection of probes [31]. To target key molecules or proteins involved in plaque development, T1-shortening Gd-based probes are conjugated with a specific vector, e.g. antibodies, peptides or more Gd-chelates. Such an approach was used with targeted probes loaded with high amounts of amphipathic Gd-chelates embedded in their outer membrane: liposomes [32], perfluorocarbon lipid emulsions [33] and micelles [34] (see below).
T2-shortening MR probes
Recently, much research has been conducted regarding the synthesis and application of iron oxide particles, which shorten the local T2/T2* relaxation time and thus result in a focal hypointensity on T2/T2*-weighted images [35]. This effect is especially pronounced in the liver, spleen and bone marrow as a result of uptake of iron oxide particles by cells of the reticuloendothelial system (RES), usually 24–48 hours after administration of the probe [36]. In the past years, superparamagnetic iron oxide MR probes have been optimized for a variety of applications [36]. The size of iron oxide particles also has an effect on the extravasation and biodistribution of probes. Their size ranges from monocrystalline iron oxide nanoparticles (MIONs) with 3 nm, ultrasmall superparamagnetic iron oxide particles (USPIO) with 15–30 nm, superparamagnetic iron oxide particles (SPIO) with 60–180 nm to micro-sized iron oxide particles (MPIO) up to 10 μm. In vivo, SPIOs aggregate in solution and are rapidly cleared by cells of the reticuloendothelial system (RES), which can limit their clinical application. In order to realize a longer blood-circulation half-life, superparamagnetic iron oxide nanoparticles have been stabilized with either hydrophilic (e.g. dextran or derivates of glucose such as the P904 ultrasmall superparamagnetic iron oxide nanoparticles (USPIO) or electrostatic coating (e.g. citrate) [18]. USPIOs are among the more predominantly used particles for molecular imaging in atherosclerosis [36]. Various studies in hyperlipidaemic rabbits and humans have measured USPIO accumulation in macrophages of vulnerable atherosclerotic plaques [34, 37–39]. In contrast to directly targeted molecular MR probes with a specific targeting ligand, T2-shortening iron oxide nanoparticles target phagocytic cells passively by unselective phagocytic uptake. Both the uptake of iron oxide particles by macrophages as well as increased influx of nanoparticles in inflamed tissues due to increased permeability and neovascularization are the basis for the most commonly used strategy to image macrophages in plaque [36]. This molecular imaging strategy has been successfully translated into the clinic setting [40].
Molecular vessel wall imaging in atherosclerosis and clinical applications
Endothelial dysfunction and imaging of surface molecules
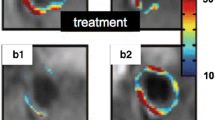
Initial inflammatory vessel wall processes lead to an impaired vasodilatation by a decreased bioavailability of nitric oxide, which is thought to increase VEGF-induced endothelial permeability [41]. Endothelial dysfunction induces the expression of specific adhesion-molecules on the endothelial surface, e.g. E-selectin and vascular adhesion molecule (VCAM)-I, and correlates significantly with local plaque burden, thus representing an interesting target in early stages of atherosclerotic disease [42]. Gadofosveset trisodium, an MRI probe binding to albumin, has successfully been used to visualise endothelial dysfunction in a mouse model of atherosclerosis (Fig. 2) [43, 44]. It is assumed that gadofosveset bound to albumin enters plaques through leaky endothelium and subsequently accumulates in the plaque matrix [43]. Furthermore, several promising animal studies demonstrated a considerable uptake of VCAM-1 specific nanoparticles in the aortic root of mice in early stages of atherosclerosis (Fig. 3) [16, 45]. These VCAM-1-binding nanoparticles consist of linear peptides conjugated in multivalent form to VINP-28 (VCAM-1 internalizing peptide-28 nanoparticles), which is responsible for target specificity [16].
Plaque burden, endothelial dysfunction and uptake of Gd-based gadofosveset in plaque in an ApoE−/− mouse model. Mice on antiatherosclerotic therapy show a significantly lower delayed vessel wall enhancement in contrast-enhanced T1-weighted magnetic resonance (MR) sequences (A3, A4), and thus reduced plaque burden, in comparison to untreated mice (A2) following gadofosveset administration. Corresponding R1 maps demonstrate lower gadofosveset concentrations after treatment with minocycline and ebselen (B3, B4) compared to untreated mice (B2); yellow signal intensity indicates high gadofosveset concentrations. DE-MRI delayed-enhancement magnetic resonance imaging, R1 maps relaxation rate maps, HFD high-fat diet. Adapted from Phinikaridou et al. [44]
In vivo imaging of angiogenesis in the aortic wall of cholesterol-fed rabbits after administration of Gd-based avß3-targeted nanoparticles combined with drug delivery of fumagilin (A1, B1) and without fumagillin (A2, B2). The magnetic resonance (MR) signal intensity is depicted as a coloured overlay of percent signal enhancement on T1-weighted MR images at the time of treatment (A1, A2) and 1 week after treatment (B1, B2). Drug delivery of fumagilin to the target results in significantly reduced signal enhancement after 1 week of treatment (B1), indicating a decrease of the inflammatory plaque burden. Adapted from Winter et al. [75]
Targeting extracellular plaque components
Vascular remodeling involves the synthesis, degradation and reorganization of ECM proteins, which are one of the major components of atherosclerotic lesions [46]. Smooth muscle cells and macrophages induce ECM formation, e.g. collagen and elastin [27]. ECM protein expression is associated with neoangiogenesis and unstable plaques [47]. Positive vascular remodeling of coronary atherosclerotic plaques has been found postmortem in the majority of patients with a myocardial infarction [48]. To detect and characterise these mechanisms, an elastin-specific Gd-labeled low-molecular-weight probe (855.95 Da) has been successfully used to target elastin expression in initial stages of coronary artery disease in a mouse and swine model of atherosclerosis (Fig. 4) [49–51]. Using electron microscopic mapping of gadolinium, it was shown that the probe co-localizes with elastic fibres. The exact binding mechanism of the probe is, however, not fully elucidated yet. ESMA additionally allows for the quantification of elastin content in the matrix of atherosclerotic plaques by measuring the signal intensity derived from this molecular probe. This enables the quantification of the overall plaque burden with a high signal-to-noise ratio. In clinical studies the overall plaque burden was shown to be one of the most important markers for plaque instability [50]. Gadofluorine M, a Gd-based macrocyclic probe, was shown to co-localize with the ECM of lipid-rich plaques in the aortic wall of hyperlipidemic rabbits [52, 53]. It was observed that gadofluorine-M accumulates in lipid-rich regions of atherosclerotic plaques due to its inherit lipophilic properties [53]. The exact mechanism responsible for gadofluorine-M accumulation into plaque is not fully elucidated yet.
Plaque imaging in a mouse model of atherosclerosis using a small molecular weight gadolinium-based elastin-binding contrast agent (ESMA). (a) Chemical structure of the elastin-binding contrast agent. (b) Transmission electronmicroscopy (left) and mapping of gadolinium distribution in the vessel wall sample (right) successfully targeting elastin expression. (c) High-resolution T1-weighted delayed-enhancement cross-sectional views (upper row) and time-of-flight images (lower row) of the brachiocephalic artery in a mouse model of apolipoprotein E–knockout mice demonstrate a significant increase in plaque burden after 12 weeks of HFD in comparison to the control group. Treatment with statins resulted in a significant decrease in plaque burden. Ph phenylalanine. Adapted from Makowski et al. [50]
Targeting fibrin in atherosclerotic plaques
Fibrin plays a central role in thrombus formation and is mainly found in the matrix of the thrombus and within fissures on the surface of vulnerable atherosclerotic plaques [54]. Different fibrin-specific MR probes have been developed to assess intravascular thrombosis and early plaque detection. Gd-labeled fibrin avid nanoparticles [55] and other small peptides (e.g. EP-2104R) have been successfully used for imaging of thrombus formation in the jugular vein, aorta, pulmonary and coronary arteries in animal studies [22, 24, 26, 56] (Fig. 5). EP-2104R was among the first molecular MR probes to be translated into and evaluated in clinical trials where it was demonstrated to directly image thrombi in the cardiovascular system [56].
In vivo imaging of subacute thrombosis after plaque rupture in hyperlipidaemic New Zealand White rabbits by administration of fibrin-targeting Gd-based EP-1873 (a) In-plane view of the baseline T1-weighted image does not show an increase in signal intensity which would indicate thrombus formation. T1-weighted images after administration of EP-1873 (b) at 30 min, 60 min and 20 h (c-e) show a well-delineated intramural thrombus subsequently increasing in size and signal intensity. Matched histological section confirms the results (f). Adapted from Botnar et al. [26]
Imaging macrophages
The presence of macrophages within atherosclerotic lesions can be imaged using different molecular imaging approaches. Iron oxide nanoparticles target phagocytic cells passively by unselective phagocytic uptake, which has been demonstrated predominantly using SPIO and USPIO in hyperlipidaemic rabbits [18, 38, 57–61], mice (Fig. 6) [62] and in human studies [39, 63--65]. Macrophages have a considerable impact on plaque vulnerability and represent an attractive target in both early as well as advanced stages of arterial vessel wall disease. Thus, it is clinically relevant to monitor macrophage infiltration in the fibrous cap, allowing for example the assessment of plaque inflammation in response to therapy. To reach a higher specificity in detection of SPIO- and USPIO-labelled cells, significant progress in the development of advanced positive-contrast imaging techniques has been made. Among the specific imaging sequences are: spectrally selective inversion RF pulses to pre-saturate on resonant water (IRON) [66, 67], golden angle radial sparse parallel (GRASP) [68] and susceptibility gradient mapping (SGM) techniques [69]. Other molecular imaging techniques for the detection of macrophages are based on gadolinium-loaded micelles targeting scavenger receptors [34], gadolinium-loaded LDL-based nanoparticles [70] and modified HDL nanoparticles [19]. These MR probes have, however, not been tested in a clinical setting.
In vivo imaging of intraplaque macrophages using susceptibility gradient mapping in an ApoE−/− mouse model of atherosclerosis after administration of iron oxide particles (USPIO). Transverse T2*-weighted images show considerable USPIO uptake (signal void) in the plaque in mice on HFD which can be observed as a positive signal on susceptibility gradient images (lower line; arrow heads). In contrast, the control group does not show any signal alteration after iron oxide injection. A matched histological section confirms the results. HFD high fat diet. Adapted from Cormode et al. [62]
Imaging neovascularisation
It has been shown that neovascularization promotes plaque instability and can result in intraplaque haemorrhage (IPH) [71, 72]. More recently, neovessel-rich regions have been successfully visualised with gadofosveset trisodium, an albumin-binding MR probe, in rabbit aortic plaques [21, 43, 73]. In contrast to healthy tissues, activated endothelial cells in areas of neovascularity characteristically express unique surface marker proteins such as αvβ3-integrin, a heterodimeric protein that is expressed in human atherosclerotic plaques. To visualise this process, an MR probe based on gadolinium-loaded liposomes targeting αvβ3-integrin has been successfully used to detect angioneogenesis in a rabbit model of atherosclerosis [74]. Αvβ3 expression can be imaged in early atherosclerotic lesions by lipid-encapsulated gadolinium-coated perfluorocarbon nanoparticles conjugated with an arginine lycine aspartic acid (RGD) peptidometic molecule, which specifically binds to αvβ3-integrin expressed on activated endothelial cells [33, 74]. The αvβ3-targeted probe showed persistent plaque signal enhancement on in vivo MR images in cholesterol-fed rabbits with intimal hyperplasia [33, 74]. A different study using Gd-based αvß3-targeted nanoparticles demonstrated the potential of combining molecular imaging with drug delivery to the target side and thereby monitoring the local response-to-treatment [75]. In detail, αvß3-targeted nanoparticles have been used to assess the inflammatory plaque burden, specifically neovascularization, in an animal model of cholesterol-fed rabbits, in which fumagilin could be successfully delivered with αvb3-targeted nanoparticles to the target, resulting in a decrease of neovascularization after treatment (Fig. 3). Avß3-targeted MR nanoparticles have so far not been tested in humans.
Targeting proteolytic enzymes and imaging of apoptosis
Another approach to image inflammatory mechanisms in early plaque progression is targeting proteolytic enzymes such as matrix metalloproteinases (MMPs), which are known to be a major contributor to plaque destabilization [46, 76]. In vivo MR imaging of MMP-rich plaques was successfully tested in animal models of atherosclerosis with an MMP-inhibitor conjugated to a Gd-chelate (P947) [77, 78]. Further evidence has emerged that other members of the heme peroxidase superfamily, such as myeloperoxidase (MPO), represent a major factor of tissue damage during initiation and acute complication phases of atherosclerosis [79]. It has been shown that significant MPO-activity can be detected in early atherosclerotic plaques of hypercholesterolaemic rabbits by non-invasive MR imaging using bis-5-hydroxytryptamide-diethylenetriamin-pentaacetate-gadolinium (MPO-Gd) [80]. MPO-Gd co-localized with regions of MPO-expressing macrophages and thus showed high focal signal intensities in the aortic wall of hypercholesterolemic rabbits [52]. Furthermore, apoptosis is known to correlate with plaque vulnerability and instability [81]. In this case especially annexin-A5 can be used as a molecular target to detect cells that express phosphatidylserine and phosphatidylethanolamine on their cell surface. Therefore, MR imaging of apoptosis in atherosclerotic lesions has been successfully validated in a mouse model of atherosclerosis with small micellar fluorescent nanoparticles, which are composed of Gd-labeled and PEGylated lipids conjugated with annexin-A5 for target specificity [82]. The small size of these annexin-A5-functionalized nanoparticles allows fast extravasation in areas with enhanced endothelial permeability such as atherosclerotic lesions [82]. This molecular probe has so far not been tested in humans.
Targeting lipid content in atherosclerotic plaques
One of the initiating events in the formation of atherosclerotic lesions is the accumulation of cholesterol, lipoproteins and macrophages. Since lipoproteins represent major cardiovascular risk factors, the high lipid content of atherosclerotic plaques represents an attractive target for molecular imaging. As described earlier gadofluorine-M showed a high accumulation in atherosclerotic plaques in hyperlipidaemic rabbits [53]. Moreover, recombinant HDL-like nanoparticles (rHDL), conjugated with a carboxyfluoresceine-labelled apolipoprotein E-derived lipopeptide P2fA2, were used for non-invasive molecular MR imaging of atheroslerotic plaques in a mouse model of atherosclerosis [20]. These modified HDL-particles can cross the endothelial border and were shown to co-localize with macrophage-rich regions in atherosclerotic plaques in mice [19, 20, 83].
An overview about the most promising molecular MR-probes is given in supplementary Table 1.
Current limitations and challenges of molecular MRI
The main challenge of molecular MRI and the translation of molecular MR probes into clinical practice compared to nuclear techniques, such as PET, is the lower sensitivity of MRI for the detection of molecular probes (in the range of 10−3–10−5). Most of the molecular probes described, which bear the potential to be translated into a clinical setting, have not gained approval from the respective American or European agencies. One of the reasons is that molecular MR-probes have to be administered in higher doses, compared to PET probes, which is associated with potential side effects. Therefore, molecular MR probes have to be evaluated in extensive and expensive toxicology trials. Regarding translation, this is a major drawback, as a substantial financial investment is required before these probes can be tested in human trials. Such high investments are usually beyond the financial capabilities of academic institutions; in most cases support from industrial partners is required. One suggested path for the development of targeted MR probes is the evaluation of these probes linked to a nuclear PET carrier for a first in vivo evaluation. If these results are promising such a tracer can be developed into an MR probe with the advantages of for example the high spatial resolution and the lack of ionizing radiation. A further potential challenge for MRI, compared to PET is that MRI is a more focused imaging technique, which is used to target to a specific vascular bed with high temporal and spatial resolution. PET on the other hand is more suitable for whole body imaging, enabling the visualisation of almost all vascular beds in a single scan. However, PET only achieves a relatively low spatial resolution, which can be especially problematic for the visualisation of the coronary arteries and the detection of initial atherosclerotic vessel wall changes and small atherosclerotic plaques.
Conclusion and future directions
The field of molecular MR imaging is constantly expanding due to the ongoing development of novel molecular MR probes and demonstrated great potential for the non-invasive characterization of arterial vessel wall disease without the need for ionizing radiation. Molecular MRI using targeted molecular probes specifically enables the visualisation and quantification of proteins and cells in the atherosclerotic vessel wall with high spatial resolution. This spatially localized molecular information may represent the key for the noninvasive differentiation of stable and unstable plaques. Several recent studies investigated the feasibility of molecular MRI techniques for the characterization of early atherosclerotic changes, positive vascular remodelling and plaque burden of the aorta, carotid and coronary arteries in patients with subclinical and advanced arterial vessel wall disease. The direct visualisation of early molecular changes may foster patient-individualized risk assessment and open the door for a more sensitive, specific and cost-effective diagnostic assessment and therapy of atherosclerosis in all vascular beds. Regarding the translation of novel molecular probes into clinical practice, probes with comparable molecular size and biodistribution to already clinically approved contrast agents (small molecular weight gadolinium-based agents, iron oxide agents), have the highest probability to be translated into clinical practice in the short term. Regarding early disease detection, follow-up and the monitoring of response to treatment it will be important to define a suitable target to visualise these processes in vivo. In conclusion, molecular MRI of the arterial vessel wall has unique potential for the non-invasive characterization of atherosclerosis on a morphological, functional and biological level. The urgent need for early detection of atherosclerotic plaques susceptible to rupture drives molecular MRI toward a further clinical translation.
References
Roger VL, Go AS, Lloyd-Jones DM et al (2011) Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation 123:e18–e209
Singh RB, Mengi SA, Xu YJ, Arneja AS, Dhalla NS (2002) Pathogenesis of atherosclerosis: A multifactorial process. Exp Clin Cardiol 7:40–53
Ambrose JA, Tannenbaum MA, Alexopoulos D et al (1988) Angiographic progression of coronary artery disease and the development of myocardial infarction. J Am Coll Cardiol 12:56–62
Varnava AM, Mills PG, Davies MJ (2002) Relationship between coronary artery remodeling and plaque vulnerability. Circulation 105:939–943
Glagov S, Weisenberg E, Zarins C, Stankunavicius R, Kolettis G (1987) Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med 316:1371–1375
Burke AP, Farb A, Malcom GT, Liang Y, Smialek J, Virmani R (1998) Effect of risk factors on the mechanism of acute thrombosis and sudden coronary death in women. Circulation 97:2110–2116
Cocker MS, Mc Ardle B, Spence JD et al (2012) Imaging atherosclerosis with hybrid [18F]fluorodeoxyglucose positron emission tomography/computed tomography imaging: what Leonardo da Vinci could not see. J Nucl Cardiol 19:1211–1225
Dweck MR, Chow MW, Joshi NV et al (2012) Coronary arterial 18F-sodium fluoride uptake: a novel marker of plaque biology. J Am Coll Cardiol 59:1539–1548
Rosenbaum D, Millon A, Fayad ZA (2012) Molecular imaging in atherosclerosis: FDG PET. Curr Atheroscler Rep 14:429–437
Coli S, Magnoni M, Sangiorgi G et al (2008) Contrast-enhanced ultrasound imaging of intraplaque neovascularization in carotid arteries: correlation with histology and plaque echogenicity. J Am Coll Cardiol 52:223–230
Esposito L, Saam T, Heider P et al (2010) MRI plaque imaging reveals high-risk carotid plaques especially in diabetic patients irrespective of the degree of stenosis. BMC Med Imaging 10:27
Saam T, Underhill HR, Chu B et al (2008) Prevalence of American Heart Association type VI carotid atherosclerotic lesions identified by magnetic resonance imaging for different levels of stenosis as measured by duplex ultrasound. J Am Coll Cardiol 51:1014–1021
Botnar RM, Stuber M, Kissinger KV, Kim WY, Spuentrup E, Manning WJ (2000) Noninvasive coronary vessel wall and plaque imaging with magnetic resonance imaging. Circulation 102:2582–2587
Fayad ZA, Fuster V, Fallon JT et al (2000) Noninvasive in vivo human coronary artery lumen and wall imaging using black-blood magnetic resonance imaging. Circulation 102:506–510
Corti R, Fuster V, Fayad ZA et al (2005) Effects of aggressive versus conventional lipid-lowering therapy by simvastatin on human atherosclerotic lesions: a prospective, randomized, double-blind trial with highresolution magnetic resonance imaging. J Am Coll Cardiol 46:106–112
Nahrendorf M, Jaffer FA, Kelly KA et al (2006) Noninvasive vascular cell adhesion molecule-1 imaging identifies inflammatory activation of cells in atherosclerosis. Circulation 114:1504–1511
Sirol M, Moreno P, Purushothaman K et al (2009) Increased Neovascularization in Advanced Lipid-Rich Atherosclerotic Lesions Detected by Gadofluorine-M-Enhanced MRI: Implications for Plaque Vulnerability. Circ Cardiovasc Imaging 2:391–396
Wagner S, Schnorr J, Ludwig A et al (2013) Contrast-enhanced MR imaging of atherosclerosis using citrate-coated superparamagnetic iron oxide nanoparticles: calcifying microvesicles as imaging target for plaque characterization. Int J Nanomedicine 8:767–779
Cormode DP, Frias JC, Ma Y et al (2009) HDL as a contrast agent for medical imaging. Clin Lipidol 4:493–500
Chen W, Vucic E, Leupold E et al (2008) Incorporation of an apoE-derived lipopeptide in high-density lipoprotein MRI contrast agents for enhanced imaging of macrophages in atherosclerosis. Contrast Media Mol Imaging 3:233–242
Pedersen SF, Thrysoe SA, Paaske WP et al (2011) CMR assessment of endothelial damage and angiogenesis in porcine coronary arteries using gadofosveset. J Cardiovasc Magn Reson 13:10
Andia ME, Saha P, Jenkins J et al (2014) Fibrin-Targeted Magnetic Resonance Imaging Allows In Vivo Quantification of Thrombus Fibrin Content and Identifies Thrombi Amenable for Thrombolysis. Arterioscler Thromb Vasc Biol. doi:10.1161/ATVBAHA.113.302931
Wu X, Balu N, Li W et al (2013) Molecular MRI of atherosclerotic plaque progression in an ApoE(−/−) mouse model with a CLT1 peptide targeted macrocyclic Gd(III) chelate. Am J Nucl Med Mol Imaging 3:446–455
Vymazal J, Spuentrup E, Cardenas-Molina G et al (2009) Thrombus imaging with fibrin-specific gadolinium-based MR contrast agent EP-2104R: results of a phase II clinical study of feasibility. Investig Radiol 44:697–704
Botnar RM, Buecker A, Wiethoff AJ et al (2004) In vivo magnetic resonance imaging of coronary thrombosis using a fibrin-binding molecular magnetic resonance contrast agent. Circulation 110:1463–1466
Botnar RM, Perez AS, Witte S et al (2004) In vivo molecular imaging of acute and subacute thrombosis using a fibrin-binding magnetic resonance imaging contrast agent. Circulation 109:2023–2029
Libby P, Ridker PM, Hansson GK (2011) Progress and challenges in translating the biology of atherosclerosis. Nature 473:317–325
Choudhury RP, Fuster V, Fayad ZA (2004) Molecular, cellular and functional imaging of atherothrombosis. Nat Rev Drug Discov 3:913–925
Stone GW, Maehara A, Lansky AJ et al (2011) A prospective natural-history study of coronary atherosclerosis. N Engl J Med 364:226–235
Caravan P, Ellison JJ, McMurry TJ, Lauffer RB (1999) Gadolinium(III) Chelates as MRI Contrast Agents: Structure, Dynamics, and Applications. Chem Rev 99:2293–2352
Caravan P (2006) Strategies for increasing the sensitivity of gadolinium based MRI contrast agents. Chem Soc Rev 35:512
Maiseyeu A, Mihai G, Kampfrath T et al (2009) Gadolinium-containing phosphatidylserine liposomes for molecular imaging of atherosclerosis. J Lipid Res 50:2157–2163
Caruthers SD, Cyrus T, Winter PM, Wickline SA, Lanza GM (2009) Anti-angiogenic perfluorocarbon nanoparticles for diagnosis and treatment of atherosclerosis. Wiley Interdiscip Rev Nanomed Nanobiotechnol 1:311–323
Amirbekian V, Lipinski MJ, Briley-Saebo KC et al (2007) Detecting and assessing macrophages in vivo to evaluate atherosclerosis noninvasively using molecular MRI. Proc Natl Acad Sci U S A 104:961–966
Weissleder R, Elizondo G, Wittenberg J, Rabito CA, Bengele HH, Josephson L (1990) Ultrasmall superparamagnetic iron oxide: characterization of a new class of contrast agents for MR imaging. Radiology 175:489–493
Zhao X, Zhao H, Chen Z, Lan M (2014) Ultrasmall superparamagnetic iron oxide nanoparticles for magnetic resonance imaging contrast agent. J Nanosci Nanotechnol 14:210–220
Briley-Saebo KC, Mani V, Hyafil F, Cornily JC, Fayad ZA (2008) Fractionated Feridex and positive contrast: in vivo MR imaging of atherosclerosis. Magn Reson Med 59:721–730
Ruehm SG, Corot C, Vogt P, Kolb S, Debatin JF (2001) Magnetic resonance imaging of atherosclerotic plaque with ultrasmall superparamagnetic particles of iron oxide in hyperlipidemic rabbits. Circulation 103:415–422
Kooi ME, Cappendijk VC, Cleutjens KB et al (2003) Accumulation of ultrasmall superparamagnetic particles of iron oxide in human atherosclerotic plaques can be detected by in vivo magnetic resonance imaging. Circulation 107:2453–2458
Yilmaz A, Dengler MA, van der Kuip H et al (2013) Imaging of myocardial infarction using ultrasmall superparamagnetic iron oxide nanoparticles: a human study using a multi-parametric cardiovascular magnetic resonance imaging approach. Eur Heart J 34:462–475
Fukumura D, Gohongi T, Kadambi A et al (2001) Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc Natl Acad Sci U S A 98:2604–2609
Hays AG, Hirsch GA, Kelle S, Gerstenblith G, Weiss RG, Stuber M (2010) Noninvasive Visualization of Coronary Artery Endothelial Function in Healthy Subjects and in Patients With Coronary Artery Disease. J Am Coll Cardiol 56:1657–1665
Lobbes MB, Heeneman S, Passos VL et al (2010) Gadofosveset-enhanced magnetic resonance imaging of human carotid atherosclerotic plaques: a proof-of-concept study. Investig Radiol 45:275–281
Phinikaridou A, Andia ME, Protti A et al (2012) Noninvasive magnetic resonance imaging evaluation of endothelial permeability in murine atherosclerosis using an albumin-binding contrast agent. Circulation 126:707–719
McAteer MA, Schneider JE, Ali ZA et al (2008) Magnetic resonance imaging of endothelial adhesion molecules in mouse atherosclerosis using dual-targeted microparticles of iron oxide. Arterioscler Thromb Vasc Biol 28:77–83
Katsuda S, Kaji T (2003) Atherosclerosis and extracellular matrix. J Atheroscler Thromb 10:267–274
Korol RM, Canham PB, Liu L et al (2011) Detection of altered extracellular matrix in surface layers of unstable carotid plaque: an optical spectroscopy, birefringence and microarray genetic analysis. Photochem Photobiol 87:1164–1172
Jeremias A, Spies C, Herity NA et al (2000) Coronary artery compliance and adaptive vessel remodelling in patients with stable and unstable coronary artery disease. Heart 84:314–319
von Bary C, Makowski M, Preissel A et al (2011) MRI of Coronary Wall Remodeling in a Swine Model of Coronary Injury Using an Elastin-Binding Contrast Agent. Circ Cardiovasc Imaging 4:147–155
Makowski MR, Wiethoff AJ, Blume U et al (2011) Assessment of atherosclerotic plaque burden with an elastin-specific magnetic resonance contrast agent. Nat Med 17:383–388
Makowski MR, Preissel A, von Bary C et al (2012) Three-Dimensional Imaging of the Aortic Vessel Wall Using an Elastin-Specific Magnetic Resonance Contrast Agent. Investig Radiol 47:438–444
Ronald JA, Chen Y, Belisle AJ et al (2009) Comparison of gadofluorine-M and Gd-DTPA for noninvasive staging of atherosclerotic plaque stability using MRI. Circ Cardiovasc Imaging 2:226–234
Sirol M, Itskovich VV, Mani V et al (2004) Lipid-rich atherosclerotic plaques detected by gadofluorineenhanced in vivo magnetic resonance imaging. Circulation 109:2890–2896
Tavora F, Cresswell N, Li L, Ripple M, Burke A (2010) Immunolocalisation of fibrin in coronary atherosclerosis: implications for necrotic core development. Pathology 42:15–22
Yu X, Song SK, Chen J et al (2000) High-resolution MRI characterization of human thrombus using a novel fibrin-targeted paramagnetic nanoparticle contrast agent. Magn Reson Med 44:867–872
Spuentrup E, Botnar RM, Wiethoff A et al (1911) (2008) MR imaging of thrombi using EP-2104R, a fibrin specific contrast agent: initial results in patients. Eur Radiol 18:1995–2005
Durand E, Raynaud JS, Bruneval P et al (2007) Magnetic resonance imaging of ruptured plaques in the rabbit with ultrasmall superparamagnetic particles of iron oxide. J Vasc Res 44:119–128
Morishige K, Kacher DF, Libby P et al (2010) High-resolution magnetic resonance imaging enhanced with superparamagnetic nanoparticles measures macrophage burden in atherosclerosis. Circulation 122:1707–1715
Schmitz SA, Coupland SE, Gust R et al (2000) Superparamagnetic iron oxide-enhanced MRI of atherosclerotic plaques in Watanabe hereditable hyperlipidemic rabbits. Investig Radiol 35:460–471
Sigovan M, Boussel L, Sulaiman A et al (2009) Rapid-clearance iron nanoparticles for inflammation imaging of atherosclerotic plaque: initial experience in animal model. Radiology 252:401–409
Smith BR, Heverhagen J, Knopp M et al (2007) Localization to atherosclerotic plaque and biodistribution of biochemically derivatized superparamagnetic iron oxide nanoparticles (SPIONs) contrast particles for magnetic resonance imaging (MRI). Biomed Microdevices 9:719–727
Makowski MR, Varma G, Wiethoff A et al (2011) Non-Invasive Assessment of Atherosclerotic Plaque Progression in ApoE−/− Mice Using Susceptibility Gradient Mapping. Circ Cardiovasc Imaging. doi:10.1161/CIRCIMAGING.110.957209
Howarth SP, Tang TY, Trivedi R et al (2009) Utility of USPIO-enhanced MR imaging to identify inflammation and the fibrous cap: a comparison of symptomatic and asymptomatic individuals. Eur J Radiol 70:555–560
Tang TY, Howarth SP, Miller SR et al (2007) Comparison of the inflammatory burden of truly asymptomatic carotid atheroma with atherosclerotic plaques contralateral to symptomatic carotid stenosis: an ultra small superparamagnetic iron oxide enhanced magnetic resonance study. J Neurol Neurosurg Psychiatry 78:1337–1343
Trivedi RA, Mallawarachi C, U-King-Im JM et al (2006) Identifying inflamed carotid plaques using in vivo USPIOenhanced MR imaging to label plaque macrophages. Arterioscler Thromb Vasc Biol 26:1601–1606
Howarth SP, Li ZY, Tang TY, Graves MJ, U-King-Im JM, Gillard JH (2008) In vivo positive contrast IRON sequence and quantitative T(2)* measurement confirms inflammatory burden in a patient with asymptomatic carotid atheroma after USPIO-enhanced MR imaging. J Vasc Interv Radiol 19:446–448
Korosoglou G, Weiss RG, Kedziorek DA et al (2008) Noninvasive detection of macrophage-rich atherosclerotic plaque in hyperlipidemic rabbits using "positive contrast" magnetic resonance imaging. J Am Coll Cardiol 52:483–491
Mani V, Briley-Saebo KC, Itskovich VV, Samber DD, Fayad ZA (2006) Gradient echo acquisition for superparamagnetic particles with positive contrast (GRASP): sequence characterization in membrane and glass superparamagnetic iron oxide phantoms at 1.5T and 3T. Magn Reson Med 55:126–135
Dahnke H, Liu W, Herzka D, Frank JA, Schaeffter T (2008) Susceptibility gradient mapping (SGM): a new postprocessing method for positive contrast generation applied to superparamagnetic iron oxide particle (SPIO)-labeled cells. Magn Reson Med 60:595–603
Yamakoshi Y, Qiao H, Lowell AN et al (2011) LDL-based nanoparticles for contrast enhanced MRI of atheroplaques in mouse models. Chem Commun (Camb) 47:8835–8837
Russell DA, Abbott CR, Gough MJ (2008) Vascular endothelial growth factor is associated with histological instability of carotid plaques. Br J Surg 95:576–581
Virmani R, Kolodgie FD, Burke AP et al (2005) Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol 25:2054–2061
Lobbes MB, Miserus RJ, Heeneman S et al (2009) Atherosclerosis: contrast-enhanced MR imaging of vessel wall in rabbit model--comparison of gadofosveset and gadopentetate dimeglumine. Radiology 250:682–691
Winter PM, Morawski AM, Caruthers SD et al (2003) Molecular imaging of angiogenesis in early-stage atherosclerosis with alpha(v)beta3-integrin-targeted nanoparticles. Circulation 108:2270–2274
Winter PM, Neubauer AM, Caruthers SD et al (2006) Endothelial alpha(v)beta3 integrin-targeted fumagillin nanoparticles inhibit angiogenesis in atherosclerosis. Arterioscler Thromb Vasc Biol 26:2103–2109
Gough PJ, Gomez IG, Wille PT, Raines EW (2006) Macrophage expression of active MMP-9 induces acute plaque disruption in apoE-deficient mice. J Clin Invest 116:59–69
Lancelot E, Amirbekian V, Brigger I et al (2008) Evaluation of matrix metalloproteinases in atherosclerosis using a novel noninvasive imaging approach. Arterioscler Thromb Vasc Biol 28:425–432
Hyafil F, Vucic E, Cornily JC et al (2011) Monitoring of arterial wall remodelling in atherosclerotic rabbits with a magnetic resonance imaging contrast agent binding to matrix metalloproteinases. Eur Heart J 32:1561–1571
Nicholls SJ, Hazen SL (2005) Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vasc Biol 25:1102–1111
Ronald JA, Chen JW, Chen Y et al (2009) Enzyme-sensitive magnetic resonance imaging targeting myeloperoxidase identifies active inflammation in experimental rabbit atherosclerotic plaques. Circulation 120:592–599
Jaffer FA, Libby P, Weissleder R (2006) Molecular and cellular imaging of atherosclerosis: emerging applications. J Am Coll Cardiol 47:1328–1338
van Tilborg GA, Vucic E, Strijkers GJ et al (2010) Annexin A5-functionalized bimodal nanoparticles for MRI and fluorescence imaging of atherosclerotic plaques. Bioconjug Chem 21:1794–1803
Frias JC, Ma Y, Williams KJ, Fayad ZA, Fisher EA (2006) Properties of a versatile nanoparticle platform contrast agent to image and characterize atherosclerotic plaques by magnetic resonance imaging. Nano Lett 6:2220–2224
Makowski MR, Botnar RM (2013) MR imaging of the arterial vessel wall: molecular imaging from bench to bedside. Radiology 269:34–51
Acknowledgments
The scientific guarantor of this publication is D. Noerenberg.
The authors (B.H.) of this manuscript declare relationships with the following companies:
Abbott, Actelion Pharmaceuticals, Bayer Schering Pharma, Bayer Vital, BRACCO Group, Bristol-Myers Squibb, Chante research organisation GmbH, Deutsche Krebshilfe, Dt. Stiftung für Herzforschung, Essex Pharma, EU Programmes, Fibrex Medical Inc., Focused Ultrasound Surgery Foundation, Fraunhofer Gesellschaft,Guerbet, INC Research, lnSightec Ud., IPSEN Pharma, Kendlel MorphoSys AG, Lilly GmbH, Lundbeck GmbH, MeVis Medical Solutions AG, Nexus Oncology, Novartis, Parexel CRO Service, Perceptive, Pfizer GmbH, Philipps, sanofis-aventis S.A, Siemens, Spectranetics GmbH, Terumo Medical Corporation, TNS Healthcare GMbH, Toshiba, UCB Pharma, Wyeth Pharma, Zukunftsfond Berlin (TSB), Amgen, AO Foundation, BARD, BBraun (Sponsoring eines Workshops), Boehring Ingelheimer, Brainsgate, PPD (CRO), CELLACT Pharma, Celgene, CeloNova BioSciences, Covance, DC Devices, Inc. USA, Ganymed, Gilead Sciences, Glaxo Smith Kline, ICON (CRO), Jansen, LUX Biosciences, MedPass (CRO), Merck, Mologen, Nuvisan, Pluristem, Quintiles (CRO), Roche, Schumacher GmbH (Sponsoring eines Workshops), Seattle Genetics, Symphogen, TauRx Therapeutics Ud,, Accovion, AIO: Arbeitsgemeinschaft Internistische Onkologie, ASR Advanced sleep research, Astellas, Theradex, Galena Biopharma, Chiltern, PRAint, lnspiremd, Medronic, Respicardia, Silena Therapeutics, Spectrum Pharmaceuticals, St, Jude Medical, TEVA, Theorem, abbvie, Aesculap, biotronik, Inventivhealth, ISA Therapeutics, LYSARC, MSD, novocure, Ockham oncology, Premier-research, psi-cro, tetec-ag, winicker-norimed.
The authors state that this work has not received any funding. No complex statistical methods were necessary for this paper. Institutional Review Board approval was not required. Methodology: retrospective/review article.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
(DOCX 800 kb)
Supplementary Table 1
(DOCX 73 kb)
Rights and permissions
About this article
Cite this article
Nörenberg, D., Ebersberger, H.U., Diederichs, G. et al. Molecular magnetic resonance imaging of atherosclerotic vessel wall disease. Eur Radiol 26, 910–920 (2016). https://doi.org/10.1007/s00330-015-3881-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-3881-2