Abstract
Objective
To evaluate the accuracy, reliability and time saving potential of a novel cardiac CT (CCT)-based, automated software for the assessment of segmental left ventricular function compared to visual and manual quantitative assessment of CCT and cardiac magnetic resonance (CMR).
Methods
Forty-seven patients with suspected or known coronary artery disease (CAD) were enrolled in the study. Wall thickening was calculated. Segmental LV wall motion was automatically calculated and shown as a colour-coded polar map. Processing time for each method was recorded.
Results
Mean wall thickness in both systolic and diastolic phases on polar map, CCT, and CMR was 9.2 ± 0.1 mm and 14.9 ± 0.2 mm, 8.9 ± 0.1 mm and 14.5 ± 0.1 mm, 8.3 ± 0.1 mm and 13.6 ± 0.1 mm, respectively. Mean wall thickening was 68.4 ± 1.5 %, 64.8 ± 1.4 % and 67.1 ± 1.4 %, respectively. Agreement for the assessment of LV wall motion between CCT, CMR and polar maps was good. Bland–Altman plots and ICC indicated good agreement between CCT, CMR and automated polar maps of the diastolic and systolic segmental wall thickness and thickening. The processing time using polar map was significantly decreased compared with CCT and CMR.
Conclusion
Automated evaluation of segmental LV function with polar maps provides similar measurements to manual CCT and CMR evaluation, albeit with substantially reduced analysis time.
Key Points
• Cardiac computed tomography (CCT) can accurately assess segmental left ventricular wall function.
• A novel automated software permits accurate and fast evaluation of wall function.
• The software may improve the clinical implementation of segmental functional analysis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Measurement of left ventricular (LV) function is a well-established clinical parameter that has fundamental diagnostic, therapeutic, and prognostic implications for coronary artery disease (CAD) [1]. Echocardiography is the most utilized technique in clinical practice for the assessment of LV function despite the fact that it remains an operator-dependent modality, which is usually limited by poor reproducibility and its potential impairment due to an inadequate acoustic window [2]. Although cardiac magnetic resonance (CMR) is considered to be the gold standard for assessment of LV function [3], it is important to note its clinical limitations. CMR requires a long imaging time and can be problematic in patients with implanted devices such as pacemakers or defibrillators, or in cases of severe claustrophobia [4]. To date, several studies have reported that retrospective electrocardiography (ECG)-gated cardiac computed tomography (CCT) has potential in evaluating LV volumes, ejection fraction, and mass in a fast and reliable way [5–9], reducing the inter-observer and intra-observer variability [10] while showing a strong correlation with the gold standard represented by CMR [8]. Thus, CCT is increasingly being considered as an alternative tool for the combined assessment of the coronary anatomy and global cardiac function [11, 12], especially with the introduction of reliable, automated software to rapidly analyze the ventricular volumes and function in an easy and reproducible way [13]. Currently, CCT is able to accurately assess the segmental ventricular wall thickness and function, showing high concordance with CMR and echocardiography [8, 14]. However, this analysis is time-consuming and requires extensive training with a relatively long learning curve.
An automated software system able to perform an accurate and reliable segmental ventricular function analysis would benefit clinical practice by adding important functional information without increasing the analysis time.
Thus, the purpose of this study was to evaluate the accuracy, reliability, and time saving potential of a novel CCT-based, automated software for the assessment of segmental LV function compared to visual and manual quantitative assessment with CCT and CMR.
Materials and methods
Patient population
Forty-seven patients who underwent a retrospective ECG-gated contrast-enhanced CT were retrospectively enrolled in this study. All subjects also had a CMR examination within 1 month before or after the CCT examination. Standard exclusion criteria for CCT and CMR were applied. All patients were in sinus rhythm and had normal renal function (glomerular filtration rate (GFR) >30 mL/min). Exclusion criteria were known iodinated contrast media allergy, arrhythmia, renal insufficiency (GFR ≤30 mL/min), and any contraindications to MR scan (pacemaker or defibrillator implantation, severe claustrophobia). This study was approved by the ethics committee and all patients gave written informed consent.
CCT protocol
CCT exams were performed on a dual-source CT (Somatom Definition Flash, Siemens Medical Solutions, Forchheim, Germany). Imaging direction was cranio-caudal. Tube voltage of 120 kV and a current of 600 – 900 mAs for both tubes were employed. Full tube current was given during 30 % – 80 % of the R-R window. Detector collimation was 2 × 64 × 0.6 mm and acquisition collimation was 2x64x0.6 mm by means of z-flying focal spot. Gantry rotation time was 330 ms. Bolus tracking was performed placing a region of interest in the root of ascending aorta, and image acquisition was automatically started 6 seconds after the signal attenuation reached the predefined threshold of 100 Hounsfield units. The imaging range was from the tracheal bifurcation to just below the diaphragm. The contrast agent was injected by, a dual-head power injector (Stellant D, Medrad, Indianola, PA) using an 18 – 20 gauge intravenous needle placed on the right antecubital vein. A biphasic protocol was used. In total, 70 – 80 mL of contrast agent (Ultravist, 370 mgI/mL iopromide, Bayer, Wayne, NJ) was injected, followed by 30 mL saline (0.9 % sodium chloride) as bolus chaser. The injection rate for all phases was 5.5 – 6 mL/s.
CMR protocol
All CMR scans were performed on a 1.5 T MR scanner (Magnetom Avanto, Siemens Medical Solutions, Erlangen, Germany) with a 6-element phased array coil. After localization of the heart using three-plane and oblique survey images, a series of short-axis cine images were acquired to cover the entire left ventricle from base to apex. Images were acquired using an electrocardiography (ECG)-gated steady-state free precession (SSFP) sequence during serial breath holds. Twenty cine phases were acquired using retrospective gating with a temporal resolution varying between 25 – 50 ms. Section thickness was 8 mm with no interval increment. Imaging parameters were repetition time 3.1 ms, echo time 1.3 ms, flip angle 80°, field of view 280x375 mm, and image matrix of 156x192 pixels.
Image post-processing
For CCT left ventricular function analysis, all data were reconstructed using 30 – 80 % R-R with 10 % interval, section thickness 1.5 cm with 1 cm interval, and kernel B26f. All CCT and CMR imaging data were loaded on a separate workstation (syngo.via, version VA20, Siemens Healthcare), equipped with a dedicated software application for cardiac function (CCT and CMR cardiac function). Segmental LV function including wall thickening and wall motion of the myocardium were assessed based on the American Heart Association/American College of Cardiology (AHA/ACC) segment model [15]. We used the 16-segment model, because it is more appropriate for evaluation of wall motion abnormalities, as the tip of the normal apex (segment 17) does not move [16].
A cardiac polar map is a 2-D polar display of the LV surface in which the apex is projected on the midpoint and the base of the heart is on the edge of the polar map. The software uses the 17 segment model, which was recommended in 2002 by the American Heart Association (AHA) for all imaging modalities [15]. In this model, area 17 relates to the apex, the rings with the areas 1-6, 7-12 and 13-16 relate to the basal, mid-ventricular, and apical regions, respectively. Based on automatically segmented multiphase CT data, the borders of the endocardium and the epicardium are segmented and correspondences between the endocardial and epicardial surfaces are established. The software calculates local values for myocardial wall thickening and wall thickness within each of the 17 regions using point-correspondences without the need of any manual anatomical landmarks placement. A manual correction of endocardial and epicardial borders can be performed if needed. The quantitative results for all three parameters (wall thickness during end-diastole, wall thickness during end-systole, and wall thickening) are visualized in the 17 segment image as a color-coded polar map (syngo.via VA20 product SW) and the average value for each of the segments can be exported (Cardiac function protoype SW).
CCT, CMR and automated polar map analysis were performed by two readers (R.W. and C.N.D.C.) blinded to the clinical indications and the CCT and CMR findings. CCT, CMR, and polar map data sets were analyzed with a minimum of a 1-week interval to avoid recall bias. Disagreement was resolved by a consensus. The processing time from the axial image reconstruction to the display of the different LV parameters was recorded for each analysis tool.
Semi-quantitative analysis
Segmental LV function analysis was performed on four-chamber views, vertical long-axis views, and short-axis cine loops. In order to make sure that CCT and CMR assessments were based on the same level, the distance for each basal, mid-ventricular, and apical section from the most basal slice was recorded and the anatomic position was checked against the papillary muscle attachment. Each LV segment was assigned a score on a 3-point scale: score 1 = normal contraction, score 2 = hypokinetic, and score 3 = akinetic [9]. On the polar map each segment was assigned a different colour based on the score, red colour for normal (score 1), yellow for hypokinetic (score 2), and blue for akinetic (score 3).
Quantitative analysis
For quantitative analysis of cardiac function, the CCT cardiac function analysis software automatically detected the end-diastolic (ED) and end-systolic (ES) images at 30 – 40 % and 70 – 80 % of cardiac cycle, respectively. In the CMR analysis, the image with the smallest LV cavity was considered to be ES, and then the contrary was ED. The wall thickness of each segment was manually measured on three short-axis views (the basal, midventricular, and apical positions along the long axis) of both ED and ES images on CCT and CMR modalities. Each segment wall thickness at both ED and ES was measured twice, and the mean value was recorded. The wall-thickening rate was calculated as (wall thickness ES - wall thickness ED) / wall thickness ED. For the automatically generated polar maps, the value of LV wall thickness on both ED and ES, the wall-thickening rate, and the regional LV wall motion of each segment was automatically provided and exported as a mean value by the software (syngo.via Cardiac Function prototype).
Statistical analysis
Continuous variables were described as mean ± standard deviation (SD). Segmental LV functional parameters from the automated polar map were compared with the results calculated from the CCT and CMR. Cohen kappa (κ) statistics were used to evaluate the agreement of semi-quantitative analysis for the segmental wall motion assessment between pairs of the three methods and interpreted as follows: ≤ 0.20 slight or poor agreement, 0.21 – 0.40 fair agreement, 0.41 – 0.60 moderate agreement, 0.61 – 0.80 good agreement, and > 0.80 excellent agreement.
For linear correlation analysis the Spearman rank correlation coefficient (ρ) was determined. Correlation was defined as poor (ρ = 0), minimal (ρ = 0.1 – 0.40), moderate (ρ = 0.41 – 0.60), good (ρ = 0.61 – 0.80), and excellent (ρ = 0.81 – 1.0). To assess the degree of agreement between the results of CCT and MRI for each pair of left ventricular values, Bland and Altman plots including mean differences and limits of agreement were generated.
The interclass-correlation coefficient (ICC) was also calculated for each quantitatively measured variable. The ICC can range from 0 to 1, with an ICC of zero representing no agreement and an ICC of 1 meaning perfect reliability. Multivariate calculations with repeated measures of analysis of variance (ANOVA) were employed to calculate differences in left ventricular parameters for respective CCT, CMR, and polar map values.
A p-value of 0.05 or less was considered statistically significant. Data analysis was performed using commercially available statistical software (MedCalc, Version 9.3.0.0. MedCalc Software; Mariakerke, Belgium).
Results
Patient characteristics
Patient characteristics are reported in Table 1. The mean age was 58 ± 11 years (range 28 – 89 years), and 16 of the patients were female. Fourteen patients had a previous myocardial infarction and 32 had a prior revascularization procedure (stent or CABG). All studies were of adequate quality for functional assessment allowing optimal manual segmentation of the left ventricle.
Semi-quantitative assessment of LV segmental wall movement
A total of 752 segments were subjectively analyzed in this study. CMR demonstrated 673 out of 752 LV segments as normal, 34 as hypokinetic, and 45 as akinetic. CCT demonstrated 669 out of 752 LV segments as normal, 45 as hypokinetic, and 38 as akinetic. Six hundred and forty-five out of 752 LV segments were regarded as normal on the polar map, 41 as hypokinetic, and 66 as akinetic. Kappa test showed that the agreement between pairs of the three methods was good (Table 2, Fig. 1).
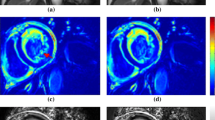
A 49-year-old male patient with chest pain underwent contrast enhanced cardiac CT (CCT) and cardiac MR (CMR). Polar map showed abnormalities of regional LV with akinesia of segments 10 – 11 (a – d, yellow arrows) (a: wall thickness ES [mm]; b: wall thickness ED [mm]; c: wall motion [mm]; d: wall thickening [%]). The short-axis view of left ventricle in both systolic (e, g) and diastolic (f, h) phases of CCT and CMR demonstrated akinetic regional left ventricular function in segments 10 – 11 (red arrows). ES: end-systolic, ED: end-diastolic
Quantitative assessment of LV segmental wall thickness and thickening
The mean diastolic and systolic wall thickness, and wall thickening are summarized in Table 3. The mean diastolic thickness assessed on CCT and CMR was 8.9 ± 0.1 and 8.3 ± 0.1, whereas the mean value extrapolated from the automated polar map was 9.2 ± 0.1. The mean systolic thickness measured from CCT and CMR was 14.5 ± 0.1 and 13.6 ± 0.1 respectively, whereas the mean value derived from the polar map was 14.9 ± 0.2. Finally, the percentage of wall thickening calculated from CCT and CMR was 64.8 ± 1.4 % and 67.1 ± 1.4 %, whereas the percentage calculated from the automated polar map was 68.5 ± 1.5 %.
There was a good correlation between CMR and automated polar map measurements of the diastolic thickness (ρ = 0.642), and between CCT and CMR both for the diastolic and systolic thickness (ρ = 0.698 and ρ = 0.651, respectively). In all other cases a moderate correlation was observed. A systematic slight overestimation of the diastolic and systolic wall thickness as well as the wall thickening by automated software when compared with CCT and CMR was observed (Table 3).
Bland-Altman plots and ICC indicated good agreement between CCT, CMR, and automated polar maps of the diastolic and systolic segmental wall thickness and thickening (Fig. 2). Results are shown in Table 4.
A significant reduction in mean processing time (p < 0.001) was observed between the automated software (230 ± 27 sec) in comparison with CCT (509 ± 39 sec) and CMR (464 ± 40 sec), representing a 55 % and 50 % time reduction, respectively (Table 3). No manual correction of endocardial and epicardial borders was needed for polar maps generation with the automated software.
Discussion
In this study, we assessed the performance of an automated software tool for the analysis of segmental LV function and wall thickness on CCT data-sets, in comparison with manual assessment using CCT and CMR. Our results demonstrated that the automated software could provide a reliable and accurate assessment of segmental LV function with a significant reduction in time.
Automated measurements of the diastolic and systolic thickness show a good correlation with the visual assessment of both CCT and CMR for the detection of segmental contraction abnormalities. A significant difference between diastolic and systolic thickness was observed between automated software values and manual CCT/CMR measures, with a slight overestimation of the thickness and thickening. However, as reported in Table 3, this difference was smaller than 1 mm for the diastolic phase, and approximately 1.3 mm for the systolic thickness, meaning a maximum of 3 % difference in the overall wall thickening. These subtle differences seem to be of little if any clinical significance, considering similar physiological variation in thickness can be observed in manual intra-reader analysis.
Moreover, considering the good correlation observed between manual CCT and CMR segmental function analysis, CCT can be confirmed as a robust technique for the assessment of wall motion. These findings seem to be directly correlated to the improved temporal resolution (83 ms) of the dual-source technique, which allows imaging without beta-blocking and provides more accurate LVEF measurement [17]. In previous studies using scanners with a temporal resolution higher than 83 ms, it has been reported that the main discrepancy occurred in the interventricular septum distinguishing normal from hypokinetic segments and in the lateral LV wall differentiating hypokinetic from akinetic segments [16]. In our study, these discrepancies between CCT and CMR were not observed; this result is probably due to the improved temporal resolution, which approaches the temporal resolution of the CMR method. In addition, the application of a 10-phase image reconstruction in CCT is sufficient to provide a reliable evaluation of the segmental LV function, as previously reported by Ko et al. [9] for LV volumes and EF.
Abnormalities of regional LV wall motion are important markers of myocardial ischemia [8] and improve diagnostic accuracy for acute coronary syndrome (ACS) in patients with acute chest pain, and, thus, may be helpful to guide further management in patients at intermediate risk for ACS [1]. Moreover, in patients undergoing CCT for detection of CAD, a measurement of LVEF can be used as an alternative method, particularly in case of equivocal or suboptimal echocardiography. Thus, CCT is increasingly being considered as an alternative tool for the combined assessment of the coronary anatomy and cardiac function parameters [11, 12]. The ability to obtain reliable information, and not only the ventricular volumes and global function, but also of segmental wall function, would further increase the accuracy of CCT for the detection of structural abnormalities.
In this study, processing time of the polar map resulted in timesavings of approximately 50 % when compared to manual measurement on CCT or CMR. This significant reduction of required time provides the potential for implementation in the clinical routine without any detrimental effect on the clinical workflow.
Our findings should be viewed in the light of their limitations. First, only a relatively small group of patients were examined, and the data need to be validated by larger patient cohorts. Secondly, the software was tested in subjects with normal function or with ischemic heart disease showing an alteration of contractility. We did not test the software in difficult cases where rhythm abnormality and inter- or intra-ventricular dyssynchrony could reduce the software accuracy. Third, the 1-month interval time between CCT and CMR studies may have influenced the cardiac function evaluation between the two imaging techniques. Likewise, the influence of CCT reconstruction parameters (slice thickness and number of phases) on automated-software performance has not been evaluated in our study. Finally, the achievement of a complete levogram with an empty right ventricle from any residual contrast medium could represent a challenge for the automatic detection of right ventricular endocardial borders in particular at the level of the interventricular septum. In all our patients, the presence of residual contrast medium in the right ventricle allowed the automatic detection of endocardial borders without the need of any manual adjustment. For the aforementioned reasons, further investigation of the software performance in these challenging populations is needed in order to confirm our findings.
In summary, the findings of this study confirm that automated evaluation of segmental LV function provides similar measurements to manual CCT and CMR evaluation, albeit with substantially reduced analysis time. The automated software may improve the implementation of segmental functional analysis in clinical routine.
Abbreviations
- CAD:
-
Coronary artery disease
- CCT:
-
Cardiac computed tomography
- CMR:
-
Cardiac magnetic resonance
- CABG:
-
Coronary artery bypasses graft
- ED:
-
End-diastolic
- ES:
-
End-systolic
- GFR:
-
Glomerular filtration rate
- ICC:
-
Interclass-correlation coefficient
- LVEF:
-
Left ventricular ejection fraction
- SSFP:
-
Steady-state free precession
References
Seneviratne SK, Truong QA, Bamberg F, Rogers IS, Shapiro MD, Schlett CL et al (2010) Incremental diagnostic value of regional left ventricular function over coronary assessment by cardiac computed tomography for the detection of acute coronary syndrome in patients with acute chest pain: from the ROMICAT trial. Circ Cardiovasc Imaging 3(4):375–383
Malm S, Frigstad S, Sagberg E, Larsson H, Skjaerpe T (2004) Accurate and reproducible measurement of left ventricular volume and ejection fraction by contrast echocardiography: a comparison with magnetic resonance imaging. J Am Coll Cardiol 44(5):1030–1035
Bellenger NG, Burgess MI, Ray SG, Lahiri A, Coats AJ, Cleland JG et al (2000) Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance; are they interchangeable? Eur Heart J 21(16):1387–1396
Petersen JW, Forder JR, Thomas JD, Moye LA, Lawson M, Loghin C et al (2011) Quantification of myocardial segmental function in acute and chronic ischemic heart disease and implications for cardiovascular cell therapy trials: a review from the NHLBI-Cardiovascular Cell Therapy Research Network. JACC Cardiovasc Imaging 4(6):671–679
Arraiza M, Azcarate PM, De Cecco CN, Viteri G, Simon-Yarza I, Hernandez-Estefania R et al (2012) Assessment of left ventricular parameters in orthotopic heart transplant recipients using dual-source CT and contrast-enhanced echocardiography: comparison with MRI. Eur J Radiol 81(11):3282–3288
Bastarrika G, Arraiza M, De Cecco CN, Broncano J, Mastrobuoni S, Ubilla M et al (2009) Dual-source CT in heart transplant recipients: quantification of global left ventricular function and mass. J Thorac Imaging 24(2):103–109
Bastarrika G, Arraiza M, De Cecco CN, Mastrobuoni S, Ubilla M, Rabago G (2008) Quantification of left ventricular function and mass in heart transplant recipients using dual-source CT and MRI: initial clinical experience. Eur Radiol 18(9):1784–1790
Cury RC, Nieman K, Shapiro MD, Butler J, Nomura CH, Ferencik M et al (2008) Comprehensive assessment of myocardial perfusion defects, regional wall motion, and left ventricular function by using 64-section multidetector CT. Radiology 248(2):466–475
Ko SM, Kim YJ, Park JH, Choi NM (2010) Assessment of left ventricular ejection fraction and regional wall motion with 64-slice multidetector CT: a comparison with two-dimensional transthoracic echocardiography. Br J Radiol 83(985):28–34
Tsai IC, Huang YL, Liao WC, Kao KH, Chen MC (2009) Left ventricular myocardial volumes measured during arterial and delayed phases of multidetector row computed tomography: a study on intra- and interobserver variability. Int J Cardiovasc Imaging 25(Suppl 1):55–63
Asferg C, Usinger L, Kristensen TS, Abdulla J (2012) Accuracy of multi-slice computed tomography for measurement of left ventricular ejection fraction compared with cardiac magnetic resonance imaging and two-dimensional transthoracic echocardiography: a systematic review and meta-analysis. Eur J Radiol 81(5):e757–e762
Belge B, Coche E, Pasquet A, Vanoverschelde JL, Gerber BL (2006) Accurate estimation of global and regional cardiac function by retrospectively gated multidetector row computed tomography: comparison with cine magnetic resonance imaging. Eur Radiol 16(7):1424–1433
Aviram G, Sirota-Cohen C, Steinvil A, Keren G, Banai S, Sosna J et al (2012) Automated volumetric analysis of four cardiac chambers in pulmonary embolism: a novel technology for fast risk stratification. Thromb Haemost 108(2):384–393
Raman SV, Shah M, McCarthy B, Garcia A, Ferketich AK (2006) Multi-detector row cardiac computed tomography accurately quantifies right and left ventricular size and function compared with cardiac magnetic resonance. Am Heart J 151(3):736–744
Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK et al (2002) Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 105(4):539–542
Fischbach R, Juergens KU, Ozgun M, Maintz D, Grude M, Seifarth H et al (2007) Assessment of regional left ventricular function with multidetector-row computed tomography versus magnetic resonance imaging. Eur Radiol 17(4):1009–1017
Bruners P, Mahnken AH, Knackstedt C, Luhmann N, Spuntrup E, Das M et al (2007) Assessment of global left and right ventricular function using dual-source computed tomography (DSCT) in comparison to MRI: an experimental study in a porcine model. Investig Radiol 42(11):756–764
Acknowledgments
The scientific guarantor of this publication is U. Joseph Schoepf. The authors of this manuscript declare relationships with the following companies: UJS is a consultant for and receives research support from Bayer, Bracco, GE Medrad, and Siemens. The other authors declare that they have no financial disclosures. The authors state that this work has not received any funding. No complex statistical methods were necessary for this paper. Institutional Review Board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. No study subjects or cohorts have been previously reported. Methodology: retrospective, diagnostic study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, R., Meinel, F.G., Schoepf, U.J. et al. Performance of Automated Software in the Assessment of Segmental Left Ventricular Function in Cardiac CT: Comparison with Cardiac Magnetic Resonance. Eur Radiol 25, 3560–3566 (2015). https://doi.org/10.1007/s00330-015-3767-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-3767-3