Abstract
Objectives
To investigate the diagnostic performance of gadoxetic acid-enhanced MRI including diffusion-weighted imaging (DWI) for the detection of colorectal liver metastases (CRLMs) after neoadjuvant chemotherapy (NAC).
Methods
Our study population comprised 77 patients with 140 CRLMs who underwent gadoxetic acid-enhanced MRI within 1 month prior to surgery: group A (without NAC, n = 38) and group B (with NAC, n = 39). Two radiologists independently assessed all MR images and graded their diagnostic confidence for CRLM on a 5-point scale. Diagnostic accuracy, sensitivity and positive predictive values (PPV) were calculated and compared between the two groups.
Results
Diagnostic accuracy of gadoxetic acid-enhanced MRI in group B was slightly lower than in group A, but a statistically significant difference was not observed (observer 1: A z, 0.926 in group A, 0.905 in group B; observer 2: A z, 0.944 in group A, 0.885 in group B; p > 0.05). Sensitivity and PPV of group B were comparable to those of group A (observer 1: sensitivity = 93.5 % vs. 93.6 %, PPV = 95.1 % vs. 86.9 %; observer 2: sensitivity = 96.8 % vs. 91.0 %; PPV = 90.0 % vs. 89.7 %; all p > 0.05).
Conclusions
Gadoxetic acid-enhanced MRI including DWI provided good diagnostic performance with high sensitivity (>90 %) for the detection of CRLMs, regardless of the influence of NAC.
Key Points
• Gadoxetic acid-enhanced MRI including DWI shows high sensitivity for CRLMs.
• Chemotherapy does not influence the diagnostic performance of liver MRI for CRLMs.
• Gadoxetic acid-enhanced MRI can be used for evaluation of CRLMs after NAC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The liver is the most common organ of distant metastases in patients with colorectal cancer, with liver metastasis occurring at some point during the disease course in over half of all colorectal cancer patients [1, 2]. Today, with advances in chemotherapy, previously unresectable colorectal liver metastases (CRLMs) can now be considered resectable with complete resection achieved in 15–30 % of initially unresectable patients, providing a clear survival benefit [3]. Furthermore, with developments in surgical techniques, the indications of resectability for CRLMs have been expanded compared to historically considered indications [4, 5]. However, as incomplete resection of CRLMs has not been shown to increase patient survival, it is of critical importance to better select patients who may be able to show sufficient tumour response and obtain benefit from secondary surgery and to determine the exact number and segmental location of metastatic tumours [6, 7]. According to a meta-analysis by Niekel et al. [8] who investigated the diagnostic performance of computed tomography (CT), magnetic resonance imaging (MRI), fluorine-18 fluorodeoxyglucose (FDG) positron emission tomography (PET) and FDG PET/CT for the detection of CRLMs in patients who have not previously undergone therapy, MRI was shown to be the preferred first-line modality.
After neoadjuvant chemotherapy (NAC), however, the determination of the precise number and location of metastases on preoperative imaging studies becomes even more challenging as a result of changes in the treated tumours in terms of size or internal texture as well as hepatic parenchymal changes such as sinusoidal injuries or steatohepatitis [9, 10]. This has also been shown to have a clear influence on the staging accuracy of preoperative imaging modalities for CRLMs [11–14]. In a recent meta-analysis study by van Kessel et al. [15], CT, FDG-PET and FDG-PET/CT showed lower diagnostic performances for the evaluation of CRLMs in patients treated with chemotherapy compared to chemonaive patients, whereas MRI showed a high pooled sensitivity of 85.7 % in patients who underwent chemotherapy. Recently, gadoxetic acid (Gd-EOB-DTPA, Primovist®; Bayer Healthcare, Berlin, Germany), which is a hepatocyte-specific MR contrast agent, has received much attention as a promising contrast medium for the evaluation of liver metastases [16–18]. In addition, several studies have demonstrated that the combination of DWI and gadoxetic acid-enhanced MRI can improve the accuracy and sensitivity for the detection of liver metastasis, especially for small liver metastasis, as well as after preoperative chemotherapy [19–23]. However, to the best of our knowledge, there have been no large series studies regarding this issue or data on the diagnostic performance of MRI with gadoxetic acid for the detection of CRLM in patients with NAC.
Therefore, we conducted this retrospective study to investigate the diagnostic performance of gadoxetic acid-enhanced MRI including DWI for the detection of CRLM in patients who underwent NAC.
Materials and methods
Study population
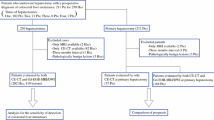
This study was approved by our institutional review board, and written informed consent was waived as the MR image data was retrospectively obtained from routine liver MRI examinations. From July 2008 to June 2013, 144 consecutive patients underwent hepatic resection and were pathologically confirmed to have CRLMs at our institution. Of these 144 patients, 97 underwent gadoxetic acid-enhanced MRI within 1 month prior to surgery. Among them, four patients were excluded as they had multiple liver metastases (at least ten in one patient and at least five in one segment in three patients) or had poor image quality due to susceptibility artefacts owing to portal vein coil embolization (n = 1). We also excluded 16 patients whose time interval between the last date of chemotherapy and the MR examination was more than 3 months and less than 1 year, as the remnant effect of chemotherapy was uncertain in these patients. The remaining 77 patients (51 men, 26 women; mean age, 61.43 years; range, 29–84 years) and 40 randomly selected patients without liver metastasis for the purposes of control were included in this study.
A flow chart showing the enrolment of the study population is presented in Fig. 1. The 77 patients with CRLM were divided into two groups according to the presence of NAC: group A (patients without NAC) and group B (patients with NAC). Group A consisted of 38 patients (23 men, 15 women; mean age, 63.23 years; range, 41–84 years), 30 patients who did not undergo NAC and eight patients who underwent chemotherapy more than 1 year ago (mean interval between chemotherapy and MRI, 652.88 days; range, 399–1,121 days). Group B consisted of 39 patients (28 men, 11 women; mean age, 59.82 years; range, 29–84 years) who underwent NAC within 3 months prior to the MR examination (mean interval between chemotherapy and MRI, 27.10 days; range, 0–82 days). In group B, the chemotherapeutic regimens used were FOLFOX (n = 19), FOLFIRI (n = 11), XELOX (n = 4), 5-FU (n = 1) and others (S-1, TSOX, XELIRI, XELODA, each n = 1). Mean time interval between chemotherapy and surgery was 39.28 ± 13.81 days (range, 18–85 days). Detailed demographic and clinical features of the study patients are shown in Table 1.
To serve as the control group, through a radiological and medical database search of 2011, we randomly selected 40 patients without liver metastasis (19 men, 21 women; mean age, 63.48 years; range 36–79 years) and they were randomly designated into groups A and B (20 patients each). These patients had undergone gadoxetic acid-enhanced liver MRI for the preoperative evaluation of colorectal cancer, stomach cancer and breast cancer. However, no liver metastasis was found on follow-up liver CT and MRI for at least 2 years after the initial MR examination.
Standard of reference
The 77 patients with liver metastasis underwent hepatic resection and a total of 137 CRLMs were identified pathologically. Thereafter, post-operative contrast-enhanced CT and MRI were added as the standard of reference to review non-resected lesions and to evaluate the presence of any missed lesions. Through this imaging review which was performed by the study coordinator (J.M.L. with 18 years of experience in interpreting abdominal CT and MRI), additional three lesions (0.5 cm, 0.6 cm and 1.6 cm in diameter) in three patients were also observed to be liver metastases. These lesions showed typical imaging findings of metastasis on MRI [14, 24], and interval growth in the longest axial diameter of at least 20 % was seen on post-operative follow-up contrast-enhanced CT and MR examinations (mean follow-up interval, 6.7 months; range, 1–11 months). Two lesions were considered as false-negative lesions on initial preoperative image interpretation as a result of their small size. The last one was not resected as it was confused with a nearby small hepatic cyst during the operation. In the remaining patients, there was no evidence of recurring metastasis on post-operative follow-up contrast-enhanced CT and MR examinations for a minimum of 6 months. Therefore, a total of 140 CRLMs (mean diameter, 2.44 ± 2.06 cm; range, 0.4–14.3 cm; ≤1 cm, n = 38; >1 cm, n = 102) were included in this study. The mean number of liver metastasis per patient was 1.82 ± 1.2 (range, 1–8). There were 62 liver metastases (mean diameter, 2.79 ± 2.32 cm; range, 0.5–14.3; ≤1 cm, n = 11; >1 cm, n = 51) in group A. In group B, there were 78 liver metastases (mean diameter, 2.16 ± 1.80 cm; range, 0.4–7.5; ≤1 cm, n = 27; >1 cm, n = 51) (Table 1). In 77 patients with liver metastasis and 40 patients without liver metastasis, 93 benign hepatic lesions were identified including cysts (n = 59), haemangiomas (n = 13), pseudo-lesions due to partial volume artefact or parenchymal change (n = 11), parenchymal defects previously treated with radiofrequency ablation (n = 8), focal nodular hyperplasia (n = 1) and embolization coil (n = 1). Benign liver lesions were diagnosed on the basis of their characteristic MRI findings with stable features on follow-up imaging studies as well as the clinical lack of evidence of the disease.
MR examination
MRI was performed on either a 1.5-T or 3-T system using an 8-channel, a 16-channel or a 32-channel phased-array coil. Our routine liver MRI protocol consisted of a breath-hold fat-saturated T2-weighted fast spin echo or turbo spin echo sequence, a breath-hold T1-weighted dual-echo (in-phase and opposed-phase) sequence, dynamic 3D fat-saturated T1-weighted sequences, and respiratory triggered DWI using a single-shot echo planar imaging sequence. Dynamic 3D fat-saturated T1-weighted sequences were performed both before and after intravenous administration of the standard dose of contrast agent (Primovist® 0.025 mmol/kg) at a rate of 1 ml/s, immediately followed by a 20-ml saline flush using a power injector (Spectris Solaris® EP, MEDRAD Inc., Warrendale, PA, USA). Following contrast administration, real-time MR fluoroscopic monitoring technique was used to determine the scanning delay times for arterial phase imaging. The arterial phase was scanned 7 s after the contrast media had arrived at the thoracic aorta, and the portal-venous phase, delayed phase and hepatobiliary phases were subsequently scanned 50 s, 3 min and 20 min, respectively, after starting contrast medium injection. Among the study patients (n = 117), DWI was available in 110 patients with 132 CRLMs and was not available in the remaining seven patients (group A, n = 5; group B, n = 2). DWI was acquired using the conventional method or intravoxel incoherent motion (IVIM) diffusion method. Conventional DWI was obtained in 38 patients and for each patient the repetition time was matched to the length of the respiratory cycle; every patient had b values of 0 s/mm2 and 500 s/mm2 (n = 33) or 0 s/mm2 and 800 s/mm2 (n = 5). All sequences were obtained in the axial plane. In the remaining 72 patients, IVIM-DWI was obtained using 10 b values (from 0 to 1,000 s/mm2). Detailed scanning parameters of the MR equipment used are summarized in Table 2.
Image analysis
Image interpretation was performed to evaluate the diagnostic performance of gadoxetic acid-enhanced MRI for the detection of CRLMs. Two clinically experienced abdominal radiologists (M.H.Y. and B.Y.H. with 8 and 5 years of clinical experience in abdominal imaging, respectively) independently interpreted each patient’s liver MR images which were randomly allocated. They were aware that the patients had or were suspected of having liver metastases, but were blinded to the number and location of the lesions as well as the patient group.
For each hepatic lesion identified, each observer recorded the size and location of the hepatic lesion and graded the possibility of liver metastasis using a 5-point confidence scale as follows: 1, definitely benign lesion; 2, probably benign lesion; 3, indeterminate lesion; 4, probably metastasis; 5, definitely metastasis. The observers were informed that lesions assigned to grades 4 or 5 were regarded as positive for liver metastasis. When a patient had multiple lesions, they recorded the lesion number and any remarkable comments regarding the lesion location using Couinaud segments and major vascular landmarks, such as the segmental branches of the portal vein or hepatic veins, to the review sheet so as to avoid confusion in data analysis. All image analyses were performed using picture archiving and communication system (PACS) software (Maroview 5.4, Infinitt, Seoul, Korea) running on a workstation with monitors having a spatial resolution of 1,600 × 1,200. If any false-positive or false-negative results were observed in a reader’s interpretation, the study coordinator also assessed the reason for the misinterpretation by reviewing the MR images.
Statistical analysis
Demographic and clinical findings of the two groups were compared using the Mann–Whitney U test, independent t test and Fisher’s exact test. Based on the two observers’ reviews, an alternative free receiver operating characteristic (AFROC) curve was performed on a lesion-by-lesion basis [25]. The AFROC curve was fitted to each observer’s confidence rating using a maximum likelihood estimation program (ROCKIT 1.1B, CE Metz and University of Chicago, Chicago, IL) [26]. Diagnostic accuracy of MRI for each observer was assessed by calculating the area under the receiver operating characteristic curve (A index, A z). Differences between the two groups for each observer were compared using the two-tailed Student’s t test for unpaired data. Thereafter, the sensitivities and positive predictive values (PPVs) on a per-lesion basis were calculated. Sensitivities and PPVs of the two groups were compared using the generalized estimating equation and Fisher’s exact test, respectively. On a per-patient basis, sensitivities, PPVs and specificities of the two groups were also calculated. Weighted kappa statistics was used to evaluate interobserver agreement on a per-lesion basis. A p value less than 0.05 was considered to indicate statistical significance. Statistical analysis was performed using SPSS version 19.0 software (SPSS Inc., Chicago, IL, USA) and MedCalc version 10.4.0.0 software for Windows (MedCalc Software, Mariakerte, Belgium).
Results
Clinical findings of study patients
Detailed demographic and clinical data of the study patients are shown in Table 1. There were no significant differences in this data between the two groups (p > 0.05, respectively). The presence of chemotherapy-induced hepatic injuries was observed in group B. Sinusoidal injury was diagnosed in 25.6 % (10/39) of patients on the basis of the intraoperative finding (the so-called blue liver) and the pathology report (sinusoidal dilatation and vascular congestion). All patients with sinusoidal injury showed characteristic findings on gadoxetic acid-enhanced MRI, i.e. diffuse reticular hypointensity on HBP imaging [27] (Figs. 2 and 3). Hepatic steatosis was also found in 20.5 % (8/39) on the basis of the pathology report. However, the degree of steatosis was mild, and sinusoidal injury was accompanied in three of the eight patients.
A 29-year-old man with colorectal liver metastasis and history of neoadjuvant chemotherapy (capecitabine and oxaliplatin) in group B. Gadoxetic acid-enhanced MR images demonstrate small liver metastasis (arrows) in segment 8 of the liver. This metastasis shows high signal intensity on the T2-weighted image (a) and peripheral enhancement on the arterial phase image (b). Despite the diffuse reticular hypointensity (arrowhead) in the liver parenchyma suggesting findings of chemotherapy-induced sinusoidal injury, this lesion is shown as a well-defined clear hypointense defect on the hepatobiliary phase image (c). This diffuse reticular hypointensity is well correlated with the hepatic congestive area (arrowhead) in the peripheral portion of the surgical specimen (d)
A 70-year-old man with colorectal liver metastasis and history of neoadjuvant chemotherapy (folinic acid, fluorouracil and oxaliplatin) in group B. There is a small nodule (arrows) in segment 4 of the liver. It shows low signal intensity with subtle peripheral enhancement on both the arterial phase image (a) and portal phase image (b) of gadoxetic acid-enhanced MRI. Despite the small size of this lesion and diffuse reticular hypointensity suggesting findings of chemotherapy-induced sinusoidal injury, the hepatobiliary phase image (c) reveals this lesion as a discrete well-defined hypointense nodule. Diffusion-weighted images (d) also demonstrate this lesion with strong diffusion restriction. The surgical specimen (e) revealed a 0.8-cm-sized, metastatic adenocarcinoma in the congestive change of the background liver. The dilatation of sinusoids and vascular congestion is observed in the background liver on pathology slide (f) (×12.5)
Diagnostic performance for the detection of CRLMs
Diagnostic accuracy of gadoxetic acid-enhanced MRI for the detection of CRLMs is presented in Table 3. The diagnostic accuracy in group B was slightly lower than that in group A; however, a statistically significant difference was not observed (observer 1: A z, 0.926 in group A, 0.905 in group B; observer 2: A z, 0.944 in group A, 0.885 in group B; p > 0.05). Interobserver agreement between the two observers for the possibility of CRLM was substantial (κ = 0.721).
Table 4 shows the sensitivity and PPV of MRI for the detection of CRLM in the two groups on per-lesion analysis. In total, the sensitivity of MRI for the detection of CRLM was 93.6 % (131/140) for the two observers. The PPV was 90.3 % (131/145 positive interpretations) for observer 1 and 89.7 % (131/146 positive interpretations) for observer 2. With regard to the comparison between the two groups, the sensitivity and PPV in group B were slightly lower than or similar to those in group A, and no statistically significant difference was observed (sensitivity = 93.5 % [58/62] vs. 93.6 % [73/78], PPV = 95.1 % [58/61] vs. 86.9 % [73/84] for observer 1; sensitivity = 96.8 % [60/62] vs. 91.0 % [71/78]; PPV = 90.0 % [60/67] vs. 89.7 % [71/79] for observer 2; all p > 0.05). On per-patient analysis, the sensitivities, specificities and PPVs also showed comparable results between groups A and B (Table 5).
False-negative and false-positive lesions
There were nine false-negative lesions for both observers (observer 1, 4 in group A and 5 in group B; observer 2, 2 in group A and 7 in group B) (Table 6). For observer 1, six lesions were not detected because of either their small tumour size (≤1 cm, n = 4) or subcapsular or dome location (n = 2). The remaining three lesions were identified but were considered to be haemangiomas (grade 2: probably benign lesions) owing to their T2 bright high signal intensity. Those three lesions were metastases from mucinous adenocarcinoma of the rectum. For observer 2, seven lesions were not detected because of either small tumour size (≤1 cm, n = 6) or dome location (n = 1). The other two lesions were misinterpreted as a probable benign or indeterminate lesion (grades 2 and 3) because of poor enhancement (n = 1) and T2 bright high signal intensity (n = 1).
As for false-positive lesions, a total of 22 false-positive lesions were detected (8 in group A and 14 in group B) (Table 6). Observer 1 detected 14 false-positive lesions (3 in group A and 11 in group B) and observer 2 detected 15 false positive lesions (7 in group A and 8 in group B). Small (≤1 cm) haemangiomas (n = 5) and cysts (n = 3) were misinterpreted as true metastasis (grade 4 or 5) by the two observers. Other false-positive lesions were parenchymal defects (n = 3) previously treated with radiofrequency ablation and pseudo-lesions (n = 11) owing to partial volume artefacts or parenchymal change.
Discussion
Our study results demonstrate that gadoxetic acid-enhanced MRI including DWI shows good diagnostic performance for the detection of CRLMs with a high sensitivity of 93.6 %, regardless of the presence of NAC. Furthermore, despite the presence of chemotherapy-induced hepatic injuries in patients with NAC, the diagnostic performance of gadoxetic acid-enhanced MRI was observed to be comparable to that in patients without NAC. According a recent meta-analysis study [15], a pooled sensitivity of 85.7 % for MR was reported for the detection of CRLM in the neoadjuvant setting. In a more recent study, Macera et al. [23] reported the diagnostic accuracy of 89.2 % and sensitivity of 91 % for the combination of DWI with gadoxetic acid-enhanced MRI in patients with CRLM treated with preoperative chemotherapy. Our study results agree well with those of the above studies. However, considering that there were a relatively small number of lesions smaller than 1 cm in diameter in our study, the high overall sensitivity of gadoxetic acid-enhanced MRI compared with previous studies could be partly due to the lesion conspicuity of liver metastases larger than 1 cm in diameter.
Interestingly, there were no significant differences in the diagnostic performance of gadoxetic acid-enhanced MRI for the detection of CRLMs between patients who received NAC and those who did not undergo NAC. It is well known that chemotherapy-related changes result in a significant decline in the diagnostic performance of post-chemotherapy imaging, especially for CT and PET [12, 13, 28]. Similarly, we originally expected that the diagnostic performance of gadoxetic acid-enhanced MRI would decline somewhat following chemotherapy, since sinusoidal injury may impair the detection of small focal liver lesions on MRI by hiding the lesion or leading to a false-positive lesion as a result of the typical diffuse reticular hypointensity seen on the HBP image [27]. However, contrary to our expectation, even though diffuse reticular hypointensity on the HBP image was observed in all 25.6 % (10/39) of the study patients with sinusoidal injury, no lesions were missed by the two observers, including even small (≤1 cm) CRLMs, but rather these were depicted quite well on the HBP image despite the diffuse reticular hypointensity. Our study results were in good agreement with a previous study [29] which reported that although the chemotherapy-induced changes slightly decreased lesion conspicuity on HBP imaging, there was no significant reduction of lesion detection owing to the excellent relative contrast between focal liver lesions and the background liver parenchyma. Furthermore, because DWI does not seem to be seriously affected by chemotherapy-induced sinusoidal injury and has strengths in the detection of small metastases [21, 30], the use of DWI may contribute to the high diagnostic performance of MRI in the neoadjuvant chemotherapy group. On the basis of our study results, we believe that chemotherapy-induced sinusoidal injury may not significantly influence the diagnostic performance of gadoxetic acid-enhanced MRI with DWI for the detection of CRLMs.
In our study, there were nine false-negative lesions for each of the two observers. The major cause of false-negative lesions was their small tumour size (≤1 cm, n = 4 for observer 1 and n = 6 for observer 2). Although there were no statistically significant differences in the diagnostic performance of MRI between the chemonaive group and the chemotherapy group, the slightly lower diagnostic performance of gadoxetic acid-enhanced MRI in the chemotherapy group may be mainly attributed to the larger number of smaller (≤1 cm) lesions in the chemotherapy group (n = 27 in the chemotherapy group vs. n = 11 in the chemonaive group). In a recent study by Han et al. [31], chemotherapy-associated hepatic lesions such as sinusoidal dilatation, peliosis and nodular regenerative hyperplasia were referred to as chemotherapy-induced focal hepatopathy, in which their radiologic appearance mimicked hepatic metastasis. However, sinusoidal dilatation, peliosis and nodular regenerative hyperplasia mimicking hepatic metastasis were not confirmed in this study. As for false-positive lesions, small (≤1 cm) haemangiomas (n = 5) and cysts (n = 2) were the most common cause of their misinterpretation as true metastases (grades 4 or 5). When the lesion size is less than 1 cm, the distinction between metastasis and haemangioma may be more difficult with gadoxetic acid-enhanced MRI with DWI, as all lesions may show peripheral enhancement on the arterial phase and hypointensity on the HBP image [32]. Further technological refinement of the techniques of high resolution T2-weighted imaging and DWI, which can help avoid the partial volume averaging effect for small benign or malignant liver lesions, would be necessary to avoid false-positive or false-negative interpretations on gadoxetic acid-enhanced MR imaging.
There are several limitations in this study. First, as this study was of retrospective design, there may have been unavoidable selection bias. Second, the DWI sequence was not obtained in all study patients and it was unavailable in seven patients. Last, our study patients were limited to patients eligible for hepatic resection in order to use a strict standard of reference for the presence of CRLMs in this study. Since we did not include patients who were not eligible for operation, we did not estimate the diagnostic performance of gadoxetic acid-enhanced MRI with DWI in patients who did not undergo surgery.
In conclusion, the diagnostic performance of gadoxetic acid-enhanced MRI including DWI showed a high sensitivity of over 90 % for the detection of CRLM, regardless of the influence of NAC, comparable to that in patients who did not undergo chemotherapy. Therefore, gadoxetic acid-enhanced MRI including DWI can be used as a first-line imaging modality for the preoperative evaluation of CRLM even after NAC.
Abbreviations
- CRLM:
-
Colorectal liver metastases
- DWI:
-
Diffusion-weighted imaging
- HBP:
-
Hepatobiliary phase
- NAC:
-
Neoadjuvant chemotherapy
- PPV:
-
Positive predictive value
References
Jemal A, Siegel R, Xu J, Ward E (2010) Cancer statistics, 2010. CA Cancer J Clin 60:277–300
Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM (2006) Epidemiology and management of liver metastases from colorectal cancer. Ann Surg 244:254–259
Leonard GD, Brenner B, Kemeny NE (2005) Neoadjuvant chemotherapy before liver resection for patients with unresectable liver metastases from colorectal carcinoma. J Clin Oncol 23:2038–2048
Abdalla EK, Adam R, Bilchik AJ, Jaeck D, Vauthey JN, Mahvi D (2006) Improving resectability of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol 13:1271–1280
Pawlik TM, Choti MA (2007) Surgical therapy for colorectal metastases to the liver. J Gastrointest Surg 11:1057–1077
Adam R, Delvart V, Pascal G et al (2004) Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg 240:644–657, discussion 657–648
Adam R, Pascal G, Castaing D et al (2004) Tumor progression while on chemotherapy: a contraindication to liver resection for multiple colorectal metastases? Ann Surg 240:1052–1061, discussion 1061–1054
Niekel MC, Bipat S, Stoker J (2010) Diagnostic imaging of colorectal liver metastases with CT, MR imaging, FDG PET, and/or FDG PET/CT: a meta-analysis of prospective studies including patients who have not previously undergone treatment. Radiology 257:674–684
Robinson PJ (2009) The effects of cancer chemotherapy on liver imaging. Eur Radiol 19:1752–1762
Pilgrim CH, Thomson BN, Banting S, Phillips WA, Michael M (2012) The developing clinical problem of chemotherapy-induced hepatic injury. ANZ J Surg 82:23–29
Carnaghi C, Tronconi MC, Rimassa L et al (2007) Utility of 18 F-FDG PET and contrast-enhanced CT scan in the assessment of residual liver metastasis from colorectal cancer following adjuvant chemotherapy. Nucl Med Rev Cent East Eur 10:12–15
Angliviel B, Benoist S, Penna C et al (2009) Impact of chemotherapy on the accuracy of computed tomography scan for the evaluation of colorectal liver metastases. Ann Surg Oncol 16:1247–1253
Spatz J, Holl G, Sciuk J, Anthuber M, Arnholdt HM, Markl B (2011) Neoadjuvant chemotherapy affects staging of colorectal liver metastasis–a comparison of PET, CT and intraoperative ultrasound. Int J Colorectal Dis 26:165–171
van Kessel CS, van Leeuwen MS, van den Bosch MA et al (2011) Accuracy of multislice liver CT and MRI for preoperative assessment of colorectal liver metastases after neoadjuvant chemotherapy. Dig Surg 28:36–43
van Kessel CS, Buckens CF, van den Bosch MA, van Leeuwen MS, van Hillegersberg R, Verkooijen HM (2012) Preoperative imaging of colorectal liver metastases after neoadjuvant chemotherapy: a meta-analysis. Ann Surg Oncol 19:2805–2813
Seo HJ, Kim MJ, Lee JD, Chung WS, Kim YE (2011) Gadoxetate disodium-enhanced magnetic resonance imaging versus contrast-enhanced 18 F-fluorodeoxyglucose positron emission tomography/computed tomography for the detection of colorectal liver metastases. Invest Radiol 46:548–555
Chen L, Zhang J, Zhang L et al (2012) Meta-analysis of gadoxetic acid disodium (Gd-EOB-DTPA)-enhanced magnetic resonance imaging for the detection of liver metastases. PLoS One 7:e48681
Lafaro KJ, Roumanis P, Demirjian AN, Lall C, Imagawa DK (2013) Gd-EOB-DTPA-enhanced MRI for detection of liver metastases from colorectal cancer: a surgeon’s perspective! Int J Hepatol 2013:572307
Chung WS, Kim MJ, Chung YE et al (2011) Comparison of gadoxetic acid-enhanced dynamic imaging and diffusion-weighted imaging for the preoperative evaluation of colorectal liver metastases. J Magn Reson Imaging 34:345–353
Koh DM, Collins DJ, Wallace T, Chau I, Riddell AM (2012) Combining diffusion-weighted MRI with Gd-EOB-DTPA-enhanced MRI improves the detection of colorectal liver metastases. Br J Radiol 85:980–989
Kim YK, Lee MW, Lee WJ et al (2012) Diagnostic accuracy and sensitivity of diffusion-weighted and of gadoxetic acid-enhanced 3-T MR imaging alone or in combination in the detection of small liver metastasis (</= 1.5 cm in diameter). Invest Radiol 47:159–166
Donati OF, Fischer MA, Chuck N, Hunziker R, Weishaupt D, Reiner CS (2013) Accuracy and confidence of Gd-EOB-DTPA enhanced MRI and diffusion-weighted imaging alone and in combination for the diagnosis of liver metastases. Eur J Radiol 82:822–828
Macera A, Lario C, Petracchini M et al (2013) Staging of colorectal liver metastases after preoperative chemotherapy. Diffusion-weighted imaging in combination with Gd-EOB-DTPA MRI sequences increases sensitivity and diagnostic accuracy. Eur Radiol 23:739–747
Ba-Ssalamah A, Uffmann M, Saini S, Bastati N, Herold C, Schima W (2009) Clinical value of MRI liver-specific contrast agents: a tailored examination for a confident non-invasive diagnosis of focal liver lesions. Eur Radiol 19:342–357
Chakraborty DP, Winter LH (1990) Free-response methodology: alternate analysis and a new observer-performance experiment. Radiology 174:873–881
Metz CE (1986) ROC methodology in radiologic imaging. Invest Radiol 21:720–733
Shin NY, Kim MJ, Lim JS et al (2012) Accuracy of gadoxetic acid-enhanced magnetic resonance imaging for the diagnosis of sinusoidal obstruction syndrome in patients with chemotherapy-treated colorectal liver metastases. Eur Radiol 22:864–871
Lubezky N, Metser U, Geva R et al (2007) The role and limitations of 18-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) scan and computerized tomography (CT) in restaging patients with hepatic colorectal metastases following neoadjuvant chemotherapy: comparison with operative and pathological findings. J Gastrointest Surg 11:472–478
Jeong HT, Kim MJ, Park MS et al (2012) Detection of liver metastases using gadoxetic-enhanced dynamic and 10- and 20-minute delayed phase MR imaging. J Magn Reson Imaging 35:635–643
Lowenthal D, Zeile M, Lim WY et al (2011) Detection and characterisation of focal liver lesions in colorectal carcinoma patients: comparison of diffusion-weighted and Gd-EOB-DTPA enhanced MR imaging. Eur Radiol 21:832–840
Han NY, Park BJ, Sung DJ et al (2014) Chemotherapy-induced focal hepatopathy in patients with gastrointestinal malignancy: gadoxetic acid-enhanced and diffusion-weighted MR imaging with clinical-pathologic correlation. Radiology 271:416–425
Tamada T, Ito K, Ueki A et al (2012) Peripheral low intensity sign in hepatic hemangioma: diagnostic pitfall in hepatobiliary phase of Gd-EOB-DTPA-enhanced MRI of the liver. J Magn Reson Imaging 35:852–858
Acknowledgments
The scientific guarantor of this publication is Prof. Jeong Min Lee. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. This work was supported by Research Settlement Fund for the new faculty of SNU (No 800-20140181). The Medical Research Collaboration Center (Seoul National University Hospital) kindly provided statistical advice for this manuscript. Institutional review board approval was obtained. Written informed consent was waived by the institutional review board. Methodology: retrospective, case-control study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, M.H., Lee, J.M., Hur, B.Y. et al. Gadoxetic acid-enhanced MRI and diffusion-weighted imaging for the detection of colorectal liver metastases after neoadjuvant chemotherapy. Eur Radiol 25, 2428–2436 (2015). https://doi.org/10.1007/s00330-015-3615-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-3615-5