Abstract
Objectives
To evaluate the diagnostic performance of computed tomography (CT) in patients with hypertrophic cardiomyopathy (HCM) and suspected coexistent coronary artery diseases (CADs).
Methods
Sixty patients were enrolled in this study. Cardiac CT examination included CT coronary angiography (CTCA) and delayed enhancement CT. CT performance in evaluation of the coronary artery was assessed and compared with that of catheter-based coronary angiography (CA). The left ventricle (LV) wall thickness, functional indices and myocardial delayed enhancement (MDE) were measured via cardiac magnetic resonance (CMR) and CT images.
Results
Compared with catheter-based CA, CTCA produced a 100 % (24/24) sensitivity, a 94.4 % (34/36) specificity, a 92.3 % (24/26) positive predictive value and a 100 % (34/34) negative predictive value. CT-measured LV wall thickness and functional indices were correlated with those measured via CMR (P < 0.01), though the CT-measured values were smaller than the CMR-measured values. Bland-Altman analysis showed the volume of the focal MDE determined via CT was slightly smaller than that determined using CMR (mean difference: 0.3 cm3).
Conclusions
For patients with HCM and suspected coexistent CAD, this comprehensive cardiac CT protocol can be helpful in ruling out coronary stenosis and can provide information regarding morphology, function and tissue characterization of the LV myocardium.
Key Points
• Multislice computed tomography (MSCT) can evaluate coronary arteries in HCM patients with coexisting CAD
• Delayed enhancement CT can show the myocardial fibrosis in HCM patients
• MSCT may provide information regarding coronary stenosis and myocardial fibrosis
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The natural history of hypertrophic cardiomyopathy (HCM) varies. Apart from sudden premature death, most patients survive with the disease for many years [1]. HCM has been found with increasing frequency in elderly patients [1, 2]. The detection of concomitant epicardial coronary disease in HCM patients with a likelihood of coexisting coronary artery diseases (CAD) is important, as severe concomitant CAD is associated with an increased risk of death and identification of coexistent CAD will impact management strategies [3–5]. Cardiovascular magnetic resonance (CMR) has been used to assess the prognosis of the disease [6–8]. It provides information regarding tissue characteristics, especially the identification of myocardial fibrosis or scarring. However, CMR is not suitable for clinically evaluating coronary artery or for HCM patients after pacemaker or implantable cardioverter-defibrillator (ICD) implantation. Due to the recent development of multislice computed tomography (MSCT), cardiac CT examination has been expanded from evaluating coronary stenosis and ventricular function to imaging myocardial viability. Using delayed enhancement CT after contrast agent administration, myocardial viability can be assessed in a manner similar to that of CMR [9–13]. MSCT provides the ability to combine coronary and myocardial tissue imaging into one 15-minute examination [12].
Therefore, it seems realistic to establish a comprehensive CT protocol that can simultaneously provide information regarding coronary arteries, cardiac function and myocardial viability for HCM patients with specific requests to exclude CAD and for patients with contraindications for CMR. This approach differs from that in which the patients are exposed to multiple other tests (e.g., cardiac catheterization, nuclear study) that may involve substantial additional radiation exposure. In this study, we try to evaluate the diagnostic value of MSCT in patients with HCM and suspected coexistent CAD.
Methods
Ethics and study population
The study was approved by our institutional ethics committee, and informed written consent was obtained from each patient.
Between November 2009 and March 2013, there were 193 patients with HCM who underwent CMR at our institution. The diagnosis of HCM was made based on the echocardiographic demonstration of a hypertrophied, non-dilated left ventricle in the absence of another cardiac or systemic disease capable of producing a similar magnitude of hypertrophy [5]. This study first excluded those patients with contraindications for CMR (claustrophobia, metal implants, cardiac pacemakers, etc.) and MSCT (pregnancy, impaired renal function, contrast media allergy) examinations. Then, a pretest probability for CAD was estimated for the rest of the patients using the Duke Clinical Score that includes different types of chest discomfort, age, gender and traditional risk factors, according to the references [14, 15]. Patients were categorized into groups with a low (1 % to 30 %), intermediate (31 % to 70 %) or high (71 % to 99 %) estimated pretest probability of having CAD. This resulted in a total of sixty patients with an intermediate-to-high likelihood of CAD being referred for catheter-based coronary angiography (CA) and consequently enrolled in the study. All sixty patients received CMR, MSCT and catheter-based CA within one month. Based on the CMR findings, the prevalence of HCM phenotypes in our study was as follows: asymmetric HCM without left ventricular outflow tract (LVOT) obstruction for 20 cases; asymmetric HCM with LVOT obstruction for 27 cases; apical HCM for 8 cases; and symmetric HCM for 5 cases.
Cardiac CT examination
The cardiac CT examination was performed using a dual-source CT (SOMATOM Definition; Siemens Medical Solutions, Forchheim, Germany). The imaging parameters included: detector collimation, 2 × 32 × 0.6 mm; rotation time, 330 ms; and temporal resolution, 83 ms. A retrospective ECG-gating protocol was used in order to assess coronary arteries and LV functional indices and wall thickness. Tube voltage (kV) and current (mAs) settings depended on the patient’s body habitus; 120 kV was used for patients (n = 10) with a body mass index (BMI) > =27 kg/m2, 100 kV was used for those with a BMI <27 kg/m2; and the tube current-time product ranged from 330 to 438 mAs. Tube current modulation and pitch adaptation were employed. After the scout images were obtained, a bolus tracking was used to determine the optimal enhancement time for the coronary artery and bilateral ventricles. A total amount of 85 to 95 ml of iodinated contrast agent (Ultravist 370, Schering, Germany) was administered intravenously at a rate of 4.0 to 4.5 ml/s, followed by a 30-ml saline solution flush administered at the same rate. The coronary artery assessment used the best diastolic phases that were automatically reconstructed by the scanner with additional reconstructions at other phases if needed. Phase data obtained from multiple slices (slice thickness: 0.75 mm; reconstruction increment: 0.5 mm) were reconstructed from 0 % to 90 % of the cardiac cycle in 10 % increments for the assessments of LV functional indices and maximal end-diastolic wall thickness.
Delayed enhancement acquisition with a prospective ECG-triggering at the 75 % of the R-R interval with no “padding” was performed seven minutes after the arterial phase following the contrast agent injection [13]. The tube voltage was chosen based on the patient’s BMI, 100 kV for the patients with a BMI > =27 kg/m2 and 80 kV for those with BMI <27 kg/m2. Serial LV slices 8 mm thick on the short-axis and 5 mm thick on the long-axis were reconstructed with an extra smooth filter (Siemens B10f).
CMR examination
All CMR examinations were performed on a 1.5-T MRI system (Sonata; Siemens Medical Solutions, Erlangen, Germany). Cine images were acquired with a true-fast steady-state free precession pulse sequence and breath-hold, and were acquired in the short-axis with two-, three- and four-chamber views encompassing the entire LV volume from apex to base. Diastolic phase myocardial delayed enhancement (MDE) images in the same orientations as the cine images were acquired ten minutes after the intravenous infusion of gadolinium chelate contrast agent (Magnevist, 0.2 mmol/kg) with a prospective ECG-gated phase-sensitive inversion recovery TurboFLASH sequence. Inversion times were optimized to null normal myocardium. Imaging parameters were as follows: repetition time/echo time, 6.7/3.1 ms; flip angle, 25°; image matrix, 256 × 156; inversion recovery time, 260 ~ 300 ms; section thickness, 8 mm (contiguous short axis) or 5 mm (long axis images) with no intersection gap.
Catheter-based coronary angiography
Catheter-based coronary angiography was performed and analyzed by a radiologist (four years of catheter-based coronary angiography experience) who was blinded to the results of the CT angiography. The standard 16 segment classification system from the American Heart Association (AHA) was used [16]. The vessel segments were evaluated using the quantitative coronary analysis method [17]. Lesions causing a reduction in lumen diameter of 50 % or more were considered to be significant lesions.
Data analysis
The image sets were transferred to two separate workstations for independent analysis of cardiac CT and CMR images, one workstation for analyzing cardiac CT images (software: Syngo, Siemens) and the other for CMR images (software: Argus, Siemens). CT and CMR data were assessed by two radiologists. One radiologist (five years of cardiac CT experience) assessed all of the CT data (including CTCA, functional indices and MDE) and the other radiologist (eight years of CMR experience) assessed all of the CMR data (functional indices and MDE) independently; each radiologist was blinded to each other’s result.
CT coronary angiography (CTCA) analysis
Similar to the catheter-based CA, a significant stenosis on CTCA images was defined as a 50 % or more narrowing of the coronary artery lumen. Each vessel was analyzed using the axial datasets with multiplanar reformations in at least two directions. Same as catheter-based CA, the analysis used the standard 16 segment classification system from the AHA [16].
LV functional indices and maximal end-diastolic wall thickness evaluation
The global LV functional indices and maximal end-diastolic wall thickness were analyzed by manually tracing the endocardial and epicardial contours. The papillary muscles and trabeculae were included in the LV cavity for delineation of the LV endocardial border. The LV functional indices and maximal end-diastolic wall thickness were assessed using the standard 17-segment model from the AHA [18]. The analysis was performed on the short and long axis images. The following indices were measured: LV mass; LV ejection fraction (EF); LV stroke volume (SV); LV end-systolic volume (ESV); and LV end-diastolic volume (EDV). The end-diastolic wall thickness was determined in 17 myocardial segments.
MDE images analysis
Qualitative analysis
A qualitative interpretation of the delayed enhancement CMR and CT images was independently performed by the two radiologists. The presence of LV MDE was assessed visually. An apparent MDE was defined as regions of hyper-enhanced myocardium on the delayed images, and the normal myocardium was defined visually as a myocardium region without any apparent delayed enhancement [13].
Quantitative analysis
For cases with MDE lesions in the same segment defined by both CT and CMR, the contrast-to-noise ratio (CNR) of the MDE was calculated from their corresponding CT and CMR images separately. The CT attenuation and CMR signal intensity (SI) were respectively sampled by placing two regions of interest (ROI) – one within the area of an apparent MDE and the other within a remote myocardium (If there were more than one hyper-enhanced region shown on one segment, all regions were included). The contrast difference was calculated as the difference in the ROI signal between the MDE and the remote myocardium, and the noise level was estimated with the standard deviation (SD) of the ROI signal of the remote myocardium [13]. The CNR was computed as the ratio of the former to the latter, resulting in the SI CNR for CMR and the attenuation CNR for CT, respectively.
Then, the volume of the MDE was measured from the corresponding CT and CMR images separately. Because it is difficult to manually delineate the border of a diffuse MDE with an intermediate-to-low intensity, we only selected those focal MDE that have well-defined borders with a bright intensity and surrounded by healthy myocardium [19]. All data analyses were blinded between the two radiologists except the lesion volume measurement. For lesion volume measurement, we reviewed the CMR and CT images together to include only focal MDE lesions with well-defined borders that could be found on both the CMR and CT images, and consequently excluded those diffuse MDE lesions with ill-defined borders. Then, one week later, the two radiologists measured the MDE volume from the CT and CMR images separately. The size of the focal MDE was visually traced on the consecutive CT and CMR images and the volume of the focal MDE in each segment was computed according to the equation: MDE volume = ∑MDE area × slice thickness.
Statistical analysis
All data were presented as mean ± SD. The diagnostic accuracy of CTCA for the assessment of coronary stenosis was calculated and compared with the catheter-based CA results. The diagnostic accuracy of CT in the detection of MDE was analyzed with the CMR results as the reference. Differences in CNR between CMR SI and CT attenuation were analyzed with the Student’s t-test. Pearson’s correlation coefficient and Bland-Altman analysis were performed to determine the correlation and agreement limits for LV functional indices, LV maximal end-diastolic wall thickness and focal MDE volume between CMR and CT. All statistical analyses were done with MedCalc 12.2 software, and a P value less than 0.05 was considered statistically significant.
Results
Patient demographic data is shown in Table 1. Patient mean age was 59.0 ± 11.1 years. Forty percent of them had exertional chest pain. Many of them had one or more coronary risk factors. Using the Duke Clinical Score to estimate significant CAD, 35 of the 60 patients were categorized into the group having an intermediate likelihood of CAD, and the group mean of the estimated pretest probability was 50.3 %. The other 25 patients were categorized into the high likelihood group with a mean estimated pretest probability of 85.5 %.
The calculated mean radiation dose was 10.5 mSv (ranged from 8.5 to 13.9 mSv) for the CT examination, 9.7 mSv (ranged from 7.8 to 12.1 mSv) for the CTCA, and 1.0 mSv (ranged from 0.7 to 2.1 mSv) for the delayed enhancement CT, using the formulation from [20].
Prevalence of CAD
According to the catheter-based CA findings, 24 of the 60 patients had at least one coronary artery stenosis that exceeded 50 % reduction of the lumen. Based on the typical ECG changes, and the presence of an angiographically demonstrated partial or complete occlusion infarct-related artery, 7 patients were diagnosed with myocardial infarction. With catheter-based CA as the reference, CTCA yielded a 100 % (24/24) sensitivity, a 94.4 % (34/36) specificity, a 92.3 % (24/26) positive predictive value and a 100 % (34/34) negative predictive value on a per-patient basis. For the per-segment analysis, a total of 106 segments were excluded due to anatomically absent, severe calcifications or poor image quality, resulting in a total of 854 segments for comparison with catheter-based CA. The diagnostic performance of CTCA for detecting significant stenosis resulted in a sensitivity of 93.2 % (41/44), a specificity of 95.3 % (772/810), a positive predictive value of 51.9 % (41/79) and a negative predictive value of 99.6 % (772/775).
LV functional indices and maximal end-diastolic wall thickness
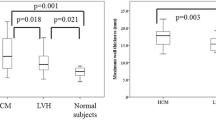
A total of 1,020 myocardial segments from the 60 patients was assessed with CMR and CT. The CT-measured LV maximal end-diastolic wall thickness and LV functional indices, which include LV mass, EF, SV, ESV and EDV, were found to significantly correlate with the CMR-measured results at the P < 0.01 level. The CT measure was also found to underestimate these parameters compared to the CMR measure mirrored by the Bland-Altman data, as shown in Table 2 and Figs. 1 and 2.
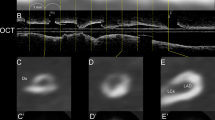
The patient is a 46-year-old male with a history of 16 years’ hypertrophic cardiomyopathy and 10 years’ systemic hypertension. His main complaint is chest pain. CTCA shows a more than 50 % stenosis in the proximal of the left anterior descending artery (LAD, A and B). The coronary angiography also demonstrates the coronary stenosis (C). Myocardial delayed enhancement is found in the insertion area of the right ventricle wall into the anterior and posterior ventricular septum (arrow) with both CT (D) and CMR (E)
MDE images
CMR detected MDE in 55 patients. The detection of MDE with CMR and CT was found to agree with each other in 54 patients and in 935 segments. But, compared with the CMR results, diffuse MDE was not detected on the CT images in six patients. The diagnostic performance of CT in the detection of MDE is shown in Table 3, and Fig. 1 shows a case in which the detection of MDE with CT agreed with CMR. Compared with CMR, CT detected MDE lesions in 194 segments. For these MDE lesions, although CMR exhibited a better CNR than CT (11.3 ± 3.9 % vs. 6.0 ± 0.9 %, P < 0.01), the mean CT value of a delayed enhanced myocardium showed a significant difference from that of a remote normal myocardium (122.4 ± 3.5HU vs. 82.1 ± 3.5HU, P < 0.01). The CT-measured volume of focal MDE lesions in 149 segments significantly correlated with that measured via CMR (r = 0.98, P < 0.01). The Bland-Altman plot showed that the mean difference in the volume of MDE measured by CMR (6.3 ± 9.1 cm3) vs. CT (6.0 ± 8.6 cm3) was 0.3 cm3, and the lower [−1.96 SD] and upper [+1.96 SD] limits of the agreement were −2.4 cm3 and 2.9 cm3, respectively (Fig. 3).
Discussion
The present study evaluated CAD and cardiac morphology, function and tissue characterization in patients with HCM using cardiac CT. Cardiac CT had an excellent diagnostic performance for CAD compared to catheter-based CA and can be an alternative to CMR for the assessment of myocardial fibrosis. We consider that simultaneous evaluation of coronary stenosis and myocardial fibrosis by cardiac CT may be beneficial for improved prognosis of HCM patients.
HCM has been found with increasing frequency in elderly patients and the underlying epicardial CAD in those HCM patients with typical angina was suspected to increase with advanced age [21]. Assessing a coronary artery with catheter-based CA or CTCA was indicated for patients with HCM and chest discomfort who had a likelihood of CAD [5]. The reported prevalence of CAD in HCM varies in the literature. In a large sample study, Sorajja et al. found that 53 % of adult patients with HCM had CAD in differing degrees of severity, and patients with HCM who have concomitant severe CAD are at an increased risk of death [3]. The authors concluded that concomitant severe CAD can be used as an additional prognostic factor in the evaluation of patients with HCM. Another study demonstrated the feasibility of using MSCT to assess the coronary artery and myocardium in HCM [22]. Based on these studies, the present study enrolled a total of 60 patients with HCM and chest discomfort who had an intermediate-to-high likelihood of CAD, and found that 24 of them had significant catheter-based CA-proven coronary stenosis. CTCA showed excellent diagnostic performance in detecting CAD in comparison with catheter-based CA, except the positive predictive value on the per-segment analysis in which the small number of positive segments may partially account for the lower positive predictive value. Studies assessing the usefulness of CTCA for detecting CAD in patients with various estimated pretest probabilities of CAD also demonstrated similar results [14]. Furthermore, the MSCT measurements of LV maximal end-diastolic wall thickness and functional indices were found to correlate with that measured using CMR, but slightly underestimate these parameters measured with CMR. Therefore, one should be cautious when evaluating cardiac function in patients with HCM using CT data.
Twenty-four patients had catheter-based CA-proven coronary artery stenosis. All of them showed MDE on the CMR images (17 patients had focal MDE, 5 patients had diffuse MDE, and 2 patients had focal and diffuse MDE). The pattern of hyper-enhancement at delayed enhancement images in patients with HCM is theoretically different from that in patients with CAD. If the MDE is mainly caused by CAD, the typical sign of myocardial infarction would be subendocardial or transmural MDE distributed at certain coronary artery regions. In contrast, if the MDE is mainly caused by HCM, the typical sign would be mid-wall MDE in the hypertrophied myocardium that does not correspond to any particular coronary artery distribution. Nevertheless, for some extensive MDE that involve multi-myocardial segments, either on the delayed enhancement CMR or CT images, it is difficult to precisely interpret whether the underlying aetiology of these lesions is HCM or CAD or a combination of both. In some cases, if the broadly distributed ischemia is transmural and chronic, the myocardium will show wall thinning [23], as opposed to LV hypertrophy in HCM. Accordingly, we may use the different wall thickness to help to differentiate CAD from HCM. But this may not work for end-stage HCM presented as LV dilatation, myocardial thinning and hypokinesia. This dilated-hypokinetic evolution of HCM is subsequent to acute myocardial infarction or is developed gradually without a clinical infarction, resulting in LV dilatation and wall thinning [24].
For the performance of delayed enhancement CT in detecting non-ischemic MDE, recently published papers demonstrate a comparable diagnostic value between CMR and MSCT for qualitatively detecting MDE in patients with HCM [22, 25]. Our results are in agreement with these studies. However, it is difficult to conduct a quantitative analysis for diffuse MDE lesions on CT images alone, though it is possible to do so with co-registered CMR and CT images. Berliner et al. measured the CMR SI and the CT attenuation for diffuse MDE lesions [25]. When a diffuse MDE lesion was detected on the CMR, even though its corresponding area on the CT images did not show any visually detectable fibrosis, they measured the CT attenuation by placing the CMR-determined lesion ROI in the same area on the CT images, and found that the delayed CT images did detect a significant difference between the normal myocardium and the diffuse MDE. In clinical practice, however, if there is no CMR as reference (such as contraindication for CMR), it is difficult to determine where to measure the attenuation on the CT images for a patient with diffuse MDE because most diffuse MDE lesions are visually undetectable on CT images.
The volume of the focal MDE determined using CT and CMR showed good correlation between the two techniques. But there were some outliers on the Bland-Altman plot, and most of them were from the MDEs with a large volume. When a large lesion occupied more than one segment of the myocardium, the main part of the lesion could be assigned to one segment on the CT images by the CT reader but to an adjacent segment on the CMR images by the CMR reader because of our blinded analysis, resulting in an outlier. Unlike this per-segment analysis, the per-patient analysis calculates the overall MDE number/volume in one patient and, therefore, should reveal a better agreement, as shown in the previous study [25]. Our results showed that the MDE volume was slightly underestimated by CT, contrary to a previous study that showed a slightly overestimated volume of MDE lesions by CT [25]. The different analysis levels (per-segment by us, per-patient by reference [25]) and inter-observer variability may explain the differences between the two studies. Since the extent of MDE was an independent predictor of sudden death [26], further studies are necessary to determine the accuracy and precision of CT in detecting MDE. In addition, CMR-compatible pacemaker and ICDs are being developed, and the feasibility of CMR imaging in patients with pacemaker and ICDs at 1.5 T has been demonstrated [27]. A rational utilization of multi imaging modalities in patients with HCM and suspected coexistent CAD is important and may avoid under/overestimated prognoses.
Study limitations
There are several limitations to this study: (1) This is a small sample study for testing the diagnostic value of MSCT in patients with HCM and suspected coexistent CAD. The higher incidence of HCM with coexistant CAD in the present study does not reflect the prevalence of concomitant CAD in unselected patients with HCM, and therefore this cardiac CT protocol should only be used in patients with HCM suspected of having coexistent CAD or in patients with a contraindication of CMR; (2) In contrast to CMR, CT involves the use of iodinated contrast agents and radiation exposure to the patients. However, further reducing the radiation exposure to the patients is possible due to a variety of existing low dose radiation techniques. For the purpose of evaluating LV function, in this study we used a retrospective ECG-gating protocol in the arterial phase CT acquisition. If assessing LV function is not needed, using a perspective ECG-gating protocol could further reduce the radiation dose; (3) Due to the technique limitations, we only measured the focal lesions with well-defined borders and a bright intensity. Lesions with an intermediate-to-low intensity and ill-defined diffuse lesions were not measured, resulting in a selection bias and, thus, the observed good correlation of myocardial fibrosis volume between CMR and CT in this study was only established under this very strict condition.
Conclusions
The present study proposed a cardiac CT protocol for comprehensive assessment of concomitant CAD in patients with HCM. MDE CT is promising as an alternative method for MDE CMR in the assessment of myocardial delayed enhancement lesions in HCM. This comprehensive CT protocol could provide a comprehensive evaluation of HCM in patients with contraindications of CMR or in patients who are suspected of having coexistent CAD.
Abbreviations
- AHA:
-
American Heart Association
- BMI:
-
body mass index
- CA:
-
coronary angiography
- CAD:
-
coronary artery disease
- CMR:
-
cardiovascular magnetic resonance
- CNR:
-
contrast-to-noise ratio
- CT:
-
computed tomography
- CTCA:
-
computed tomography coronary angiography
- DSCT:
-
dual source computed tomography
- EDV:
-
end-diastolic volume
- EF:
-
ejection fraction
- ESV:
-
end-systolic volume
- HCM:
-
hypertrophic cardiomyopathy
- ICD:
-
implantable cardioverter-defibrillator
- LVOT:
-
left ventricular outflow tract
- LV:
-
left ventricle
- MDE:
-
myocardial delayed enhancement
- MI:
-
myocardial infarction
- MSCT:
-
multislice computed tomography
- SV:
-
stroke volume
References
Kubo T, Kitaoka H, Okawa M, Nishinaga M, Doi YL (2010) Hypertrophic cardiomyopathy in the elderly. Geriatr Gerontol Int 10:9–16
Maron BJ, Niiura H, Casey SA, Soper MK, Wright GB, Seidman JG et al (2001) Development of left ventricular hypertrophy in adults in hypertrophic cardiomyopathy caused by cardiac myosin-binding protein C gene mutations. J Am Coll Cardiol 38:315–321
Sorajja P, Ommen SR, Nishimura RA, Gersh BJ, Berger PB, Tajik AJ (2003) Adverse prognosis of patients with hypertrophic cardiomyopathy who have epicardial coronary artery disease. Circulation 108:2342–2348
Okayama S, Soeda T, Kawakami R, Takami Y, Somekawa S, Ueda T et al (2013) Evaluation of coronary artery disease and cardiac morphology and function in patients with hypertrophic cardiomyopathy, using cardiac computed tomography. Heart Vessels 2013 Dec 11. [Epub ahead of print]
Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS et al (2011) 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American college of cardiology foundation/american heart association task force on practice guidelines. Developed in collaboration with the American association for thoracic surgery, American society of echocardiography, American society of nuclear cardiology, heart failure society of America, heart rhythm society, society for cardiovascular angiography and interventions, and society of thoracic surgeons. J Am Coll Cardiol 58:e212–e260
Pennell DJ, Sechtem UP, Higgins CB, Manning WJ, Pohost GM, Rademarkers FE et al (2004) Clinical indications for cardiovascular magnetic resonance (CMR): Consensus Panel report. Eur Heart J 25:1940–1965
Lima JA, Desai MY (2004) Cardiovascular magnetic resonance imaging: current and emerging applications. J Am Coll Cardiol 44:1164–1171
Maron MS, Maron BJ, Harrigan C, Buros J, Gibson CM, Olivotto I et al (2009) Hypertrophic cardiomyopathy phenotype revisited after 50 years with cardiovascular magnetic resonance. J Am Coll Cardiol 54:220–228
Mahnken AH, Koos R, Katoh M, Widberger JE, Spuentrup E, Buecker A et al (2005) Assessment of myocardial viability in reperfused acute myocardial infarction using 16-slice computed tomography in comparison to magnetic resonance imaging. J Am Coll Cardiol 45:2042–2047
Baks T, Cademartiri MAD, van der Giessen WJ, Krestin GP, Duncker DJ et al (2007) Assessment of acute reperfused myocardial infarction with delayed enhancement 64-MDCT. AJR 188:w135–w137
Lardo AC, Cordeiro MA, Silva C, Amado LC, George RT, Saliaris AP et al (2006) Contrast-enhanced multidetector computed tomography viability imaging after myocardial infarction characterization of myocyte death, microvascular obstruction, and chronic scar. Circulation 113:394–404
Polain L, de Waroux JB, Pouleur AC, Goffinet C, Pasquet A, Vanoverschelde JL, Gerber BL (2008) Combined coronary and late-enhanced multidetector-computed tomography for delineation of the etiology of left ventricular dysfunction: comparison with coronary angiography and contrast-enhanced cardiac magnetic resonance imaging. Eur Heart J 29:2544–2551
Nieman K, Shapiro MD, Ferencik M, Nomura CH, Abbara S, Hoffmann U et al (2008) Reperfused myocardial infarction: contrast-enhanced 64-section CT in comparison to MR imaging. Radiology 247:49–56
Meijboom WB, van Mieghem CA, Mollet NR, Pugliese F, Weustink AC, van Pelt N et al (2007) 64-slice computed tomography coronary angiography in patients with high, intermediate, or low pretest probability of significant coronary artery disease. J Am Coll Cardiol 50:1469–1475
Gibbons RJ, Balady GJ, Bricker JT, Chaitman BR, Fletcher GF, Froelicher VF et al (2002) ACC/AHA 2002 guideline update for exercise testing: summary article. A report of the American college of cardiology/ American heart association task force on practice guidelines (committee to update the 1997 exercise testing guidelines). J Am Coll Cardiol 40:1531–1540
Scanlon PJ, Faxon DP, Audet AM, Carabello B, Dehmr GJ, Eagle KA et al (1999) ACC/AHA guidelines for coronary angiography. A report of the American college of cardiology/American heart association task force on practice guidelines (committee on coronary angiography). Developed in collaboration with the society for cardiac angiography and interventions. J Am Coll Cardiol 33:1756–1824
Leber AW, Knez A, von Ziegler F, Becker A, Nikolaou K, Paul S et al (2005) Quantification of obstructive and nonobstructive coronary lesions by 64-slice computed tomography: a comparative study with quantitative coronary angiography and intravascular ultrasound. J Am Coll Cardiol 46:147–152
Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK et al (2002) Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 105:539–542
Moon JC, McKenna WJ, McCrohon JA, Elliott PM, Smith GC, Pennell DJ (2003) Toward clinical risk assessment in hypertrophic cardiomyopathy with gadolinium cardiovascular magnetic resonance. J Am Coll Cardiol 41:1561–1567
Christner JA, Kofler JM, McCollough CH (2010) Estimating effective dose for CT using dose-length product compared with using organ doses: consequences of adopting international commission on radiological protection publication 103 or dual-energy scanning. AJR Am J Roentgenol 194:881–889
Maron MS, Olivotto I, Maron BJ, Prasad SK, Cecchi F, Udelson JE et al (2009) The case for myocardial ischemia in hypertrophic cardiomyopathy. J Am Coll Cardiol 54:866–875
Zhao L, Ma X, DeLano MC, Jiang T, Zhang C, Liu Y et al (2013) Assessment of myocardial fibrosis and coronary arteries in hypertrophic cardiomyopathy using combined arterial and delayed enhanced CT: comparison with MR and coronary angiography. Eur Radiol 23:1034–1043
Ordovas KG, Higgins CB (2011) Delayed contrast enhancement on MR images of myocardium: past, present, future. Radiology 261:358–374
Chun EJ, Choi SI, Jin KN, Kwag HJ, Kim YJ, Choi BW et al (2010) Hypertrophic cardiomyopathy: assessment with MR imaging and multidetector CT. Radiographics 30:1309–1328
Berliner JI, Kino A, Carr JC, Bonow RO, Choudhury L (2013) Cardiac computed tomographic imaging to evaluate myocardial scarring/fibrosis in patients with hypertrophic cardiomyopathy: a comparison with cardiac magnetic resonance imaging. Int J Cardiovasc Imaging 29:191–197
Chan RH, Maron B, Olivotto I, Assenza G, Hong MS, Lesser J et al (2012) Prognostic utility of contrast-enhanced cardiovascular magnetic resonance in hypertrophic cardiomyopathy: an international multicenter study. J Am Coll Cardiol 59(13s1):E1570
Naehle CP, Kreuz J, Strach K, Schwab JO, Pingel S, Luechinger R et al (2011) Safety, feasibility, and diagnostic value of cardiac magnetic resonance imaging in patients with cardiac pace makers and implantable cardioverters/defibrillators at 1.5 T. Am Heart J 16:1096–1105
Acknowledgments
The scientific guarantor of this publication is Zhanming Fan. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. This study has received funding by the China National Natural Science Fund Grant (81101173). No complex statistical methods were necessary for this paper. Institutional Review Board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. Some study subjects have been previously reported in “Assessment of myocardial fibrosis and coronary arteries in hypertrophic cardiomyopathy using combined arterial and delayed enhanced CT: comparison with MR and coronary angiography” published in European Radiology 2013 23:1034–1043). In the present study, the data of 2 patients overlapped the above mentioned study. Methodology: prospective, diagnostic or prognostic study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, L., Ma, X., Ge, H. et al. Diagnostic performance of computed tomography for detection of concomitant coronary disease in hypertrophic cardiomyopathy. Eur Radiol 25, 767–775 (2015). https://doi.org/10.1007/s00330-014-3465-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-014-3465-6