Abstract
An invasive predator eradication campaign is planned for 2024 on Amsterdam Island, one of world’s top priority island for seabird conservation. In order to monitor the effects on seabird colonies post-eradication, a survey of burrow-nesting species and population monitoring of albatrosses, penguins, skuas and terns was organised pre-eradication. Several counting techniques and acoustic methods were used to infer presence/absence of burrow-nesting species and to estimate abundance of other species, as well as genetic methods for species identification. In total 14 breeding (or probably breeding) seabird species were detected on Amsterdam Island, among which eight burrowing petrels including two species never described on the island: the Juan Fernandez petrel Pterodroma externa and the sooty sherwater Ardenna grisea. Based on these new data, the introduced mammal eradication campaign on Amsterdam, if successful, will likely be extremely beneficial for seabird conservation, and may also favor the colonization of Amsterdam by new seabird species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Amsterdam and Saint-Paul Islands in the South Indian Ocean share zoogeographic similarities with the Tristan da Cunha group in the South Atlantic and to the Chatham and Juan Fernandez groups in the South Pacific (Bourne and David 1995; Jouventin et al. 2006; Masello et al. 2022). Since its discovery in the sixteenth century, Amsterdam has experienced several human-related ecological perturbations and damages (Jouventin 1994; Jaeger et al. 2018). At least 22 bird species are known or suspected to have bred on Amsterdam prior to first human contact (Jouanin and Paulian 1960; Worthy and Jouventin 1999), but several species are now locally extinct among which endemic species (Micol and Jouventin 1995; Olson and Jouventin 1996). Today, a total of 10 bird species breed on Amsterdam, including one introduced passerine (the common waxbill Estrilda astrild) and nine seabird species (Worthy and Jouventin 1999; Duriez and Delord 2012): northern rockhopper penguin Eudyptes moseleyi, Indian yellow-nosed albatross Thalassarche carteri, endemic Amsterdam albatross Diomedea amsterdamensis, sooty albatross Phoebetria fusca, soft-plumaged petrel Pterodroma mollis, grey petrel Procellaria cinerea, Macgillivray’s prion Pachyptila macgillivrayi, Antarctic tern Sterna vittata sanctipauli, and subtropical brown skua Catharacta antarctica hamiltoni.
Amsterdam is a critical breeding ground for four globally threatened seabird species: the Amsterdam albatross, Indian yellow-nosed albatross, sooty albatross and northern rockhopper penguin (Heerah et al. 2019). These last three species have worsened dramatically during the past decade, with extremely low reproductive success and declining populations (Weimerskirch et al. 2018; Barbraud et al. 2020). Declines are mainly caused by the occurrence of avian cholera (Pasteurella multocida) and possibly eysipelas (Erysipelothrix rhusiopathiae) that induces high chick mortality, and by introduced mammals that predate eggs and chicks (Micol and Jouventin 2002; Jaeger et al. 2018). Invasive house mice Mus musculus, brown rats Rattus norvegicus and feral cats Felis catus occur throughtout Amsterdam (Micol and Jouventin 1995). These constitute a major threat to seabird populations and their eradication is a priority conversation objective (Brooke et al. 2004; Holmes et al. 2019). On Amsterdam, an eradication campaign of all the three invasive mammal species is planned for 2024, as part of the RECI (Restauration des écosystèmes insulaire de l’océan Indien) project financed by European Union, French Development Agency (AFD) and French Southern and Antarctic Territories administration (TAAF). The eradication of those three invasive mammals’ species is included in the National Plan of Actions to improve the conservation status of the Amsterdam albatross (2018–2027). Recent studies showed that to assess the success of invasive predators’ management, research should provide a comprehensive feed-back, incorporating pre- and post-eradication dynamics of seabird populations (Brooke et al. 2018; Philippe-Lesaffre et al. 2022). Expected impacts of eradication campaigns on seabird populations are mainly due to (1) removal of predation due to invasive species, (2) diet changes in relation to elimination of substitute prey, and (3) potential negative effects of secondary poisoning for scavengers (Brooke et al. 2018). Therefore, on Amsterdam long-term seabird monitoring will be necessary to evaluate changes due to rodenticide application and due to the absence of invasive predators.
Penguins, albatrosses, and to a lower extend skuas, have been regularly monitored since the early 1970s on Amsterdam. The population trends and demographic processes are relatively well-known for some of these species (e.g. Barbraud et al. 2020; Delord et al. 2021; Weimerskirch et al. 2018). In contrast, knowledge of burrow-nesting species is insufficient and fragmentary. Species belonging to this group are notoriously difficult to detect and census as they spend daytime offshore to feed and come back to their breeding colonies at night (Warham 1990). Moreover, burrows are often difficult to detect, particularly in dense and tall vegetation such as on Amsterdam. From fossil remains, several species have been identified as originally breeding on Amsterdam: several species of genus Pterodroma, Pachyptila, and Puffinus, the common diving petrel Pelecanoides urinatrix, white-faced storm petrel Pelagodroma marina, white-bellied storm petrel Fregetta grallaria, and non-identifiable specimen of genus Oceanites (Worthy and Jouventin 1999). However, these petrel populations have declined dramatically or disappeared due to introducted predators. Only Macgillivray’s prion, grey petrel and soft-plumaged petrel still appear to breed on Amsterdam, but in very low numbers and breeding sites remain poorly known (Bretagnolle 1995; Duriez and Delord 2012).
As part of the planned eradication program, we conducted several surveys on Amsterdam between 2021 and 2022 during seasonal breeding activities of seabirds with the aim of inventoring breeding seabird species, especially the nocturnal burrow-nesting seabirds. Here, we report some of the first results from these surveys documenting the unexpected discovery of new breeding species on Amsterdam Island.
Material and methods
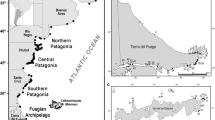
Amsterdam Island (37°50’ S; 77°31’E) is a small (58 km2) uninhabited volcanic island situated in the southern Indian Ocean, north of the Subtropical Front and 85 km north of Saint-Paul Island (38°43’S, 77°31’E). For conspicuous surface-nesting seabirds (northern rockhopper penguin, Amsterdam albatross, Indian yellow-nosed albatross, sooty albatross, subtropical brown skua, Antarctic tern) we censused the whole island population using entire sampling methods. For these species, surveys were performed during the incubation period: late September 2022 for northern rockhopper penguins, late October 2021 for Indian yellow-nosed, late October 2022 for sooty albatrosses, mid-November 2021 for skuas and Antarctic terns, and April 2022 for Amsterdam albatross for which the entire population is monitored annually (Rivalan et al. 2010). We recorded locations and counted all nests of Amsterdam albatrosses and skuas occupied by an incubating adult. For northern rockhopper penguins, Indian yellow-nosed albatrosses and sooty albatrosses, the number of occupied nests was determined from photographs taken from fixed points during the early incubation period. The presence of a bird on a nest was considered as indicative of a breeding bird sit on top of an egg (see Weimerskirch et al. 2018 and Barbraud et al. 2020 for further details). For Antarctic terns, which breed in coastal cliffs (Segonzac 1972), we censused most of the island coastline by visual surveys. Between late November and mid-December, an observer followed the coastal contour of the island and scanned for terns’ nests or displays of territorial (alarm calls against observer or skuas) or nesting behaviors (birds with prey in the beak, adults in cavities, fledglings). Shorelines that could not be accessed on foot were scanned using binoculars. Territorial pairs and occupied nests were counted.
For burrow-nesting seabirds we used a combination of various methods to detect breeding sites. Our aim here was to assess species presence and breeding status but not abundance. First, we used passive acoustic monitoring to determine species presence (Dufour et al. 2016). We used autonomous digital recording devices called song meters (Model SM4, Wildlife Acoustics, Inc.). Song meters were programmed to record from dusk to dawn, starting 1 h before sunset and stopping 1 h after sunrise with 15 min on/off cycles. They were deployed in suitable habitat for burrow-nesting seabirds, but also near tall grassy slopes potentially providing shelter from wind and wave noise. Song meters were deployed at 65 different sites on the island. Most (88%) deployment sites were situated near coastal cliffs and SM4 were deployed every 500 to 1000 m. We mainly targeted coastal sites as on nearby Saint-Paul Island most burrowing petrels breed near coastal cliffs (Barbraud et al. 2021). The island was then divided into a grid 500 × 500 m to report the presence of song meters and the presence of species detected (Fig. 1). At each deployement site, audio recordings were carried out for at least 20 nights in summer and/or in winter between January 2021 to December 2022 in order to cover the breeding period of all potential summer and winter breeding species (Fig. 1). Data were downloaded and sonagrams were compared by a human observer with publicly available recordings to identify species identity (eBird 2022; GBIF 2022). When vocalisations were detected from acoustic recordings, we searched for burrows in a 50 m radius circle around song meters. When one or more burrows were found, we deployed camera traps near the entrance of the burrows to determine occupancy and breeding status (Bird et al. 2021). Finally, we used video cameras with external displays (burrowscopes) to check burrow contents and to record breeding status (Lavers et al. 2019). In some cases, biometric measurements and blood samples were collected when acoustic criteria and photographs from camera traps were insufficient to identify species with certainty. Birds were captured by hand in or at the entrance of the burrows and were examined for: (1) presence/absence of brood patch, (2) morphometric measurements (bill length, bill width, bill depth and tarsus length with calipers ± 0.1 mm, wing length with a ruler ± 1 mm), (3) body mass (using a Pesola spring scale ± 1 g). A blood sample from the alar vein was taken after capture of the bird using a 25-gauge needle. Blood samples were stored on FTA cards until analysis. Birds were released at the entrance of the burrow immediately after processing. The entire process took around five minutes.
Opportunistic observations of birds not monitored in surveys described above were also recorded. For each opportunistic observation, number of individuals, date, time and GPS position were recorded.
For all species the breeding status was assigned according to the following criteria: a confirmed breeder was an indivdual seen with an egg or a chick or heard during all or most of the breeding season, a probable breeder was an individual seen in a burrow but without an egg or chick or heard during a part of breeding season, a probable nonbreeder was an individual heard on rare occasions.
DNA was extracted from a blood sample obtained from an individual of genus Pterodroma suspected to be Juan Fernandez petrel Pterodroma externa (shown in Fig. 3) using an E.Z.N.A. Tissue DNA Kit (Omega Bio-Tek, Norcross, GA, USA). For this specimen, A 649 bp fragment of cytochrome b was amplified by polymerase chain reaction (PCR) using primers aCytbPro5F-aCytbPro9R detailed in Pyle et al. (2011) in 15μL reaction volumes containing 7.5μL 2 × Taq PCR master mix (Qiagen, Hilden, Germany), 0.5 μM of each primer, 4.5μL dH2O, and 2μL of template DNA. Cycling conditions followed instructions for the Qiagen Taq PCR master mix. PCR products were cleaned using ExoSAP-IT (Applied Biosystems, Waltham, MA, USA), and sequenced in both directions using an Applied Biosystems 3730 DNA Analyzer. To confirm species identity, the obtained cytochrome b sequence was searched against the BLAST database (Altschul et al. 1990). Additionally, the sequence was aligned with sequences of other Pterodroma species and Aphrodroma brevirostris as an outgroup obtained from GenBank (Table S1). A phylogenetic tree was built under maximum likelihood using RAxML-NG 1.1.0 (Kozlov et al. 2019) with the GTR + I + G substitution model and 20 independent searches.
Results
Counts of occupied nest sites (and territorial pairs for terns) of surface-nesting species are shown in Table 1. The most abundant species was the Indian yellow-nosed albatross with ≈ 29 700 breeding pairs, followed by the Northern rockhopper penguin (≈ 4 100 breeding pairs) and the Sooty albatross with ≈ 500 breeding pairs. Other species were less abundant with < 100 breeding paris.
A total of 4279 recording days were obtained from song meters, resulting in 21 395 h of recordings which were analysed. About 81% of sampled cells revealed acoustic contacts (34 revealed acoustic contacts out a total of 42 grid cells with song meters). This resulted in 2359 acoustic contacts corresponding to burrow-nesting seabird species (Table 2). Combining results obtained from all survey methods, a total of 11 burrow-nesting species were detected, of which six were breeding, two probably breeding and three likely not breeding (Table 3, Fig. 2). Eight of these burrow-nesting species were previously unrecorded from Amsterdam either as breeders or probable breeders or on land: the flesh-footed shearwater Ardenna carneipes, sooty shearwater A. grisea, Juan Fernandez petrel, black-winged petrel P. nigripennis, great-winged petrel P. macroptera, Spectacled petrel Procellaria conspiscillata (Appendix S8), black-bellied storm petrel Fregetta tropica, and common diving petrel Pelecanoides urinatrix. Only one individual identified as a Juan Fernandez petrel (Fig. 3) could be captured in February 2022 for measurements (Table 4) and blood sampling.
Searching the obtained cytochrome b sequence of this Pterodroma individual against the BLAST database identified the best match as Pterodroma externa (GenBank accession U74339) with one substitution and 99.85% sequence identity. The next closest matches were two Galapagos petrel P. phaeopygia individuals (GenBank accessions HQ420319 and HQ420332) with 12 substitutions and 98.15% sequence identity. In a phylogenetic tree the individual placed with the P. externa individual found by BLAST searching, and these two sequences were sister to individuals of P. phaeopygia and Hawaiian petrel P. sandwichensis (Fig. 4). These results confirmed the identity of this individual as Juan Fernandez petrel.
A phylogenetic tree of Pterodroma species using the obtained cytochrome b sequence of the individual of interest (marked with an asterisk) and other species obtained from GenBank (Table S1)
A total of seven burrows used by flesh-footed shearwaters and between three and five burrows used by sooty sherwaters were detected with camera traps. Data indicated the regular presence of birds at these burrows throughout the breeding season and territorial behaviour and matings were filmed. However, most of burrows were too deep to reach the nest chamber using the burrowscope. One breeder of sooty sherwater with an egg was observed in one burrow with the burrowscope in December 2021. In the same period, an adult flesh-footed shearwater was observed in a burrow. Later in the season (early February) two young downy chicks were seen in these same two burrows. However, breeding was unsuccessful as no chick was later observed, neither during burrowscope inspection nor on views obtained from camera traps placed in front of burrow. From camera traps, up to three adults morphologically similar to the identified Juan Fernandez petrel were observed on several occasions vocalizing and making courtships near a burrow, and one individual was observed digging another burrow nearby. However, the nest chamber was to deep for the burrowscope and could not be inspected. No chick or fledgling was detected on views taken by camera traps.
One burrow frequented by a great-winged petrel and four burrows frequented by grey petrels were detected in cliffs but were inaccessible to be inspected using a burrowscope. One spectacled petrel was observed in flight at dusk in late April 2022 but was not re-observed later.
Discussion
Before this study, nine seabird species were known to breed regularly on Amsterdam (Duriez and Delord 2012) all of which were detected during our survey. Although in 2012, one pair of northern giant petrels Macronectes halli was breeding on Amsterdam and one pair of australasian gannets Morus serrator was reported trying to breed (Demay et al. 2014; CEBC unpublished data), none of these species were observed breeding during our survey in 2021 and 2022.
We detected eight seabird species previously unrecorded from Amsterdam, all Procellariiformes and all burrow-nesting species. These included two Ardenna shearwaters, three Pterodroma, one Procellaria, one Pelecanoides, and one Fregetta species. Among these new recorded species, at least five were frequently detected during most of the breeding season with individuals regularly observed frequenting burrows. Therefore, it is extremely likely that these species were breeding on Amsterdam, although breeding could be confirmed only for the flesh-footed shearwater and sooty shearwater with individuals observed incubating an egg or a chick. We discuss below these new and confirmed records of burrowing species and changes in numbers of surface-nesting species.
Antarctic tern
This species breeds on islands throughout the Southern Ocean and the Antarctic Peninsula (Harrison et al. 2021). Although breeding colonies were reported since the early 1930s on Amsterdam (Aubert de la Rüe 1932) there is no accurate count of the island breeding population. During our survey all coastal areas were prospected except about half of the length of the western coast which remains inaccessible. Thus, our estimate of 92 territorial pairs and occupied nests was considered as a minimum of number of breeding pairs. The breeding population on nearby Saint-Paul with more that 500 pairs in 2018 have dramatically increased following the eradication of black rats (Rattus rattus) (Barbraud et al. 2021).
Subtropical brown skua
This subspecies breeds on Tristan and Gough archipelagos and on Amsterdam and Saint-Paul (Harrison et al. 2021; Barbraud et al. 2021). The breeding population at Amsterdam has been increasing since the early 1970s when less than 10 pairs were breeding on the island (Segonzac 1972). The species was persecuted until it was declared proctected in 1972 by the Terres Australes et Antarctiques Françaises administration. Since then, breeding numbers have increased from 15 pairs in 1983 to 78 in 2021 corresponding to a 1.04 (\(i.e. \frac{78}{15}^{\left(\frac{1}{38}\right)}\)) annual population growth rate. Several factors may explain this increase, such as the protected species status and the sharp increase of subantarctic fur seals Arctocephalus tropicalis (Guinet et al. 1994; Pacoureau et al. 2017) as skuas feed on seal placentas and carcasses.
Northern rockhopper penguin
In the southern Indian Ocean this species breeds only on Amsterdam and Saint-Paul (Harrison et al. 2021). Other breeding localities are situated in the southern Atlantic Ocean (Tristan da Cunha and Gough Islands). Results of the 2022 count indicate that the population decline in ongoing on Amsterdam, with a 66% decrease in the number of breeding pairs since 2015 (i.e. a 12% annual decline) when 12 161 breeding pairs were counted (Barbraud et al. 2020). The decline of northern rockhopper penguins at Amsterdam has probably occurred since the early 1950s. Segonzac (1972) estimated the entire Amsterdam population at 100 000 breeding pairs in the early 1970s, and there is evidence that one colony went extinct between the early 1950s and the early 1980s. The causes of decline were discussed in Barbraud et al. (2020) and include predation of eggs and chicks by introduced Norway rats and feral cats and by native subtropical brown skuas, and infectious agents (Pasteurella multocida and Erysipelothrix rhusiopathiae). However, the decline was relatively important (29%) between 2021 (5791 breeding pairs) and 2022 and may have partly resulted from an additional cause of mortality. Indeed, in February 2021, while adult northern rockhopper penguins were molting on Amsterdam, a fire occurred in Entrecasteaux cliffs where most penguins molt. Short visits to this site after the fire revealed the presence of burnt corpses of penguins on molting sites hit by the fire.
Amsterdam albatross
This endemic species from Amsterdam Island has been increasing since its discovery in the early 1980s (Jaeger et al. 2018), and our count data indicate that the population is still growing with a record number of 65 breeding pairs in 2022. Although the at-sea distribution of this species largely overlaps with long-line fisheries (Thiebot et al. 2014), individuals are not or very poorly susceptible to bycatch (Rivalan et al. 2010; Corbeau et al. 2021). Moreover, this species does not seem to be impacted by infectious diseases present on Amsterdam or by introduced predators (Jaeger et al. 2018).
Sooty albatross
This species breeds on several islands of the southern Indian and Atlantic oceans (Harrison et al. 2021). Only three counts of the entire breeding population are available for Amsterdam (2003: 474 pairs, 2012: 394 pairs; this study: 515 pairs). Thus, although a previous study based on counts of the entire population suggested a decrease over the past 20 years (Weimerskirch et al. 2018), our recent population count and data from demographic study plots (Jaeger et al. 2018) rather suggest a stable population on Amsterdam.
Indian yellow-nosed albatross
The species breeds on several islands of the Crozet, Kerguelen, Marion and Prince Edward archipelagos in addition to Amsterdam and Saint-Paul (Harrison et al. 2021). The population on Amsterdam Island has been decreasing over the last 34 years (Weimerskirch et al. 2018) mainly due to infectious disease and predation by introduced mammals (Jaeger et al. 2018). Yet, compared to previous counts of the entire population in 2006 (≈ 27 000 pairs) and 2015 (≈ 22 800 pairs), our count in september 2021 suggests that the population trend is relatively stable during the past 15 years despite these persisting threats. Contrary to northern rockhopper penguins, adult Indian yellow-nosed albatrosses were poorly affected by the fire at Entrecasteaux cliffs in February 2021, where only chicks died from fire resulting is a very low breeding success (2%, CEBC unpublished data). Thus, the relatively high number of breeding pairs in 2021 may be partly due to the high breeding propensity of individuals that failed earlier during that year.
Black-bellied storm petrel
The species breeds on several Antarctic and subantarctic islands as well as on Tristan da Cunha and Gough Islands (Harrison et al. 2021). The taxonomy of Fregetta storm petrels is highly debated, as F. tropica and F. grallaria co-occur at some localities and because of plumage polymorphism in F. grallaria (Robertson et al. 2016). However, both species can be easily differentiated from sonagrams, and the sonagram from Amsterdam matches well with a black-bellied storm petrel. There is no fossil remain corresponding to black-bellied storm petrel on Amsterdam (Worthy and Jouventin 1999), and the species is only rarely observed at-sea nearby the island, generally in April (Roux and Martinez 1987; CEBC unpublished data). However, an individual with an incubation patch was captured on Saint-Paul in December 2018 (Appendix S1; C. Barbraud and K. Delord unpublished data) and another was also captured in December 2017 (H. Weimerskirch pers. comm.). This, combined with the acoustic contacts detected during our survey in May, October, and December, although they were few, indicate that individuals visit Amsterdam and Saint-Paul, and eventually suggest the possibility that the species breeds in few numbers on these islands.
Macgillivray’s prion
Macgillivray’s prion was known from a single population on Saint-Paul but recent genetic and morphological studies found that Gough Island medium-billed prions belong to the same evolutionary lineage as macgillivrayi, representing a new population of Macgillivray’s prion that originated through a colonization event from Saint-Paul (Masello et al. 2022). Macgillivray’s prions breed in high numbers (several 100,000 s pairs) on Gough Island in the South Atlantic where thery were recently discovered (Masello et al. 2022). On Amsterdam, we only recorded this species in January and October with very few contacts. Macgillivray’s prions were breeding in high numbers on Amsterdam as evidenced by the abundant fossil record (Worthy and Jouventin 1999), and are still known to breed as a relict population due to the presence of introduced mammalian predators (Jouventin 1994). Our few observations of this species suggest that the detected individuals were likely not breeding or were prospectors visiting the island. Indeed, breeding numbers on the nearby Saint-Paul Island have largely increased over the past 20 years following the eradication of black rats (Barbraud et al. 2021). Moreover, remains of Macgillivray’s prions have been found in pellets of brown skuas on Amsterdam Island in 2019 and 2020 (CEBC unpublished data).
Black-winged petrel
The species breeds on several islands of the Southwest Pacific where it is abundant (Harrison et al. 2021) and seems to be expanding into the Indian Ocean with birds seen breeding on Round Island off Mauritius (Brooke 2004), several individuals observed on Saint-Paul (Thiebot et al. 2010; Bigonneau 2013), and two sightings made at-sea ≈300 km northwest of Amsterdam and Saint-Paul in November 2018 (C. Barbraud unpublished data). Our single acoustic record in January suggests that the species is likely not breeding on Amsterdam but shows that indiviuals are visiting the island. Yet, we cannot entirely exclude that a breeding population of black-winged petrels could have been overlooked on Amsterdam or Saint-Paul (Thiebot et al. 2010).
Juan Fernandez petrel
This endemic species breeds at Juan Fernandez Island (Southeast Pacific) in high numbers (Brooke 2004) and the population appears stable (Hodum 2009). The species is one of the most commonly sighted seabirds in the subtropical southeast Pacific Ocean (Ballance et al. 1997), and during breeding individuals undertake northward trans-equatorial migration in the North Pacific (Carboneras et al. 2020). Thus, our record at Amsterdam is well outside the expected range of the species. Nevertheless, recent records in French Polynesia (Flood et al. 2021), and in Chatham Islands and Tristan da Cunha (Imber et al. 1991; Miskelly 2013; Harrison et al. 2021) provide evidence that it very occasionally ranges westward at mid latitudes. Our data show the regular presence of the species on Amsterdam from December to May, thus covering most of the known breeding season at Juan Fernandez (September to May). Observations of several individuals in burrows strongly suggest that the species breeds on Amsterdam. Given that the species was never observed at-sea offshore Amsterdam and Saint-Paul, it is likely that this constitutes a relatively recent colonization event. Interestingly, Worthy and Jouventin (1999) indicated the former presence of one large Pterodroma species on Amsterdam based on fossil remains, potentially corresponding to the size of P. externa. Worthy and Jouventin (1999) attributed these fossil bones to P. macroptera, but our record suggests that some may belong to P. externa. Genetic analyses of fossil bones are needed to confirm this suspicion. Yet, we cannot exclude that a breeding population of Juan Fernandez petrel could have been overlooked as Amsterdam surveys have been incomplete. Observations at dusk of a large gadfly petrel with whitish underparts on Amsterdam in January and February 2003 (T. Deville pers. comm.) may correspond to a Juan Fernandez petrel, and similar observations made on Saint-Paul in April 2013 with an individual observed landing in a cliff, which may suggest that the species is also present on Saint-Paul (Bigonneau 2013). Moreover, one sighting at-sea ≈800 km north of Crozet in January 2003 and 2 sightings ≈1400 km and ≈1700 km northwest of Kerguelen in February 2004 indicate the presence of the species in the Indian Ocean (CEBC unpublished data).
Soft-plumaged petrel
This species breeds in several islands of the Southern Ocean, i.e. Gough, Tristan da Cunha, Antipodes and Maatsuyker (Tasmania) islands (Harrison et al. 2021). Soft-plumaged petrel is known to persist as a breeding species on Amsterdam (Roux and Martinez 1987; Bretagnolle 1995) but suffers from predation by rats and cats on adults, eggs and chicks (Roux and Martinez 1987). Fossil remains suggest that the species was abundant on Amsterdam (Worthy and Jouventin 1999). Our data show the regular presence of the species almost all year round, from July to April, with a peak of contact records from October to December. Observations of several individuals in burrows with camera traps from our survey, regular visual and auditory records, and remains of skulls predated by cats or rats (CEBC unpublished data) indicate that the species still breeds on Amsterdam. An adult soft-plumaged petrel was captured on Saint-Paul after calls of the species were heard (Thiebot et al. 2010), but breeding has not been confirmed (Barbraud et al. 2021).
Great-winged petrel
This species breeds on several subantarctic islands, Southwest Australia, Amsterdam and Saint-Paul, Tristan da Cunha and Gough (Harrison et al. 2021). Abundant fossil remains suggest that great-winged petrels were formerly abundant on Amsterdam (Worthy and Jouventin 1999). The species is regularly seen offshore Amsterdam from February to April and between July and September (Roux and Martinez 1987; CEBC unpublished data) and has been regularly recorded during the austral winter on Amsterdam since the 1980s (Thiebot 2011; CEBC unpublished data). Our data show the regular presence of the species on Amsterdam from February to July. Observation of an occupied burrow and of individuals with camera traps from our survey indicates that species still breeds on Amsterdam. Great-winged petrels breed on Saint-Paul with 130–150 occupied burrows (Barbraud et al. 2021).
Grey petrel
The species breeds on many islands of the Southern Ocean and South Atlantic as well as Antipodes and Campbell Islands in the Pacific (Harrison et al. 2021). Our data confirm that the species breeds on Amsterdam as it was frequently recorded and observed entering burrows from February to September. The species has regularly been observed at dusk flying on land or entering burrows (Daycard and Furet 1986; Roux and Martinez 1987; Duriez et al. 2005; Thiebot 2011; CEBC unpublished data) where it breeds in small numbers (Jouventin 1994), a remnant of what may once have been a much larger population (Worthy and Jouventin 1999). However, the species frequent areas in steep slopes and cliffs making the access to burrows extremely difficult. It is also a probable breeder in small numbers on Saint-Paul (Barbraud et al. 2021).
Spectacled petrel
Only one visual observation on land was made in April, thus outside the breeding season, and no acoustic or camera-trap record was obtained for this species. Therefore, it is likely not breeding on Amsterdam and no fossil remain was found (Worthy and Jouventin 1999) suggesting it has never bred or perhaps in extremely small numbers. The species is endemic from Inaccessible Island (Tristan da Cunha) in the South Atlantic, where its breeding numbers are increasing following the eradication of feral pigs Sus scrofa (Ryan et al. 2019). Tracking studies suggest that the species stays in the southern Atlantic Ocean during both the breeding and non-breeding periods (Reid et al. 2014; Bugoni et al. 2009), albeit sample sizes of tracked individuals were low. Nevertheless, spectacled petrels appear to frequent occasionnaly the Indian Ocean as individuals are very occasionnally observed offshore Amsterdam (Shirihai 2008, N. Gasco pers. comm.) and Kerguelen (Gasco et al. 2019).
Flesh-footed shearwater
The main breeding populations are situated on islands off South and Western Australia and north New Zealand to Cook Strait where they have been declining (Waugh et al. 2013; Lavers 2015). Although the species breeds on Saint-Paul, there were very few observational records on Amsterdam, apart from fossil records suggesting that the species formerly bred (Worthy and Jouventin 1999). One adult was captured on Amsterdam in 1953 (Segonzac 1972), and another was found on the ground nearby the Martin de Vivies research station in October 2016 (CEBC unpublished data), but their status was unknown. The discovery of a chick is proof that the species reproduces in Amsterdam. The species may have gone locally extinct and has been recolonizing the island relatively recently. Indeed, the population of flesh-footed shearwaters has been increasing on Saint-Paul since the early 1980s where at least 2000 pairs were breeding in 2018 (Barbraud et al. 2021).
Sooty shearwater
This species occurs in all oceans and breeds in South Chile, Falkland Islands, Tristan da Cunha, Southeast Australia and New Zealand (Harrison et al. 2021). Although this is an extremely numerous species there are persistent signs of current decline (Brooke et al. 2004; Waugh et al. 2013). This new breeding species for Amsterdam was only known from extremely few fossils remains (Worthy and Jouventin 1999) and occasional observations at-sea offshore Amsterdam and Saint-Paul (Roux and Martinez 1987; Duriez et al. 2005; CEBC unpublished data). Therefore, as for the flesh-footed shearwater, it is possible that the species was breeding on Amsterdam but in very few numbers and remained undetected or that it went locally extinct and has been recolonizing the island relatively recently.
Common diving petrel
This species breeds in high numbers on subantarctic and subtropical islands of the Southern Hemisphere (Harrison et al. 2021). Fossil remains were found at several places, suggesting the species formerly bred at Amsterdam (Worthy and Jouventin 1999). A female was captured in November 1955 (Segonzac 1972), a dead individual was found in December 1984 (Roux and Martinez 1987) and Micol and Jouventin (1995) suspected that the species breeds at Amsterdam. Acoustic records from our survey indicate the regular presence common diving petrels during the breeding season from August to December, which suggests that a small population still breeds on Amsterdam. Surprisingly, there is only one at-sea observation of the species ≈300 km south of Amsterdam, and the species has never been observed on or nearby Saint-Paul (CEBC unpublished data). Indeed, the species breeds at Tristan da Cunha, where it is regularly observed at-sea (Ryan et al. 2022), but breeding numbers are relatively high at this locality (Brooke 2004). During breeding, the foraging range of common diving petrel can reach more that 200 km (Fromant et al. 2021) and individuals come back to colonies at night to avoid predators. This and the likely very small breeding numbers on Amsterdam might explain the quasi-absence of at-sea observations.
To conclude, this pre-eradication survey on Amsterdam revealed the presence of five unrecorded species and suggests the breeding of four additional species compared to Duriez and Delord (2012), the island totalizing at least 14 breeding seabird species. Acoustic recording devices were extremely useful to identify and locate burrow-nesting and nocturnal species, offering several advantages such as being easy to deploy and retrieve and recording simultaneously at multiples sites (Buxton and Jones 2012). Other surveys are needed to better evaluate the breeding status of some of these species and to obtain tissue samples to assess the phylogenetic status of their populations. Surprisingly, we did not detect the presence of species for which relatively abundant fossil bones were found, as the little shearwater (Puffinus assimilis), white-faced storm petrel (Pelagodroma marina), and white-bellied storm petrel (Fregetta grallaria) (Worthy and Jouventin 1999). It is also surprising to note that some species breeding on Saint-Paul (85 km away) were not identified on Amsterdam: the white-bellied petrels (Fregetta grallaria), fairy prion (Pachyptila turtur), and especially the subantarctic shearwater (Puffinus elegans) that is increasing on Saint-Paul, (Barbraud et al. 2021). Individuals assumed to belong to Puffinus assimilis were observed in flight on Amsterdam in 1956 (Segonzac 1972), but there is no recent record on any of the two islands. Fresh remains of white-faced storm petrels are regularly found in pellets of brown skuas on Amsterdam (CEBC unpublished data) suggesting the presence of the species on the island, yet to be discovered. Finally, based on these new records and on seabird population trends observed on Saint-Paul following eradication of invasive mammals (Barbraud et al. 2021), the introduced mammal eradication campaign on Amsterdam will be extremely beneficial for seabird conservation, if successful, and may also favor the colonization of Amsterdam by new seabird species in the future.
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Aubert de la Rüe E (1932) La faune des îles Saint-Paul et Amsterdam. Terre Et Vie 11:642–662
Ballance LT, Pitman RL, Reilly SB (1997) Seabird community structure along a productivity gradient: importance of competition and energetic constraint. Ecology 78:1502–1518
Barbraud C, Delord K, Bost CA, Chaigne A, Marteau C, Weimerskirch H (2020) Population trends of penguins in the French Southern Territories. Polar Biol 43:835–850
Barbraud C, Delord K, Le Bouard F, Harivel R, Demay J, Chaigne A, Micol A (2021) Seabird population changes following mammal eradication at oceanic Saint-Paul Island. Indian Ocean J Nat Conserv 63:126049
Bird JP, Fuller RA, Pascoe PP, Shaw JDS (2021) Trialling camera traps to determine occupancy and breeding in burrowing seabirds. Remote Sens Ecol Conserv 8:180–190
Bourne WRP, David ACF (1995) The early history and ornithology of St Paul and Amsterdam Islands, southern Indian Ocean. Gerfaut 85:19–36
Bretagnolle V (1995) Systematics of the soft-plumaged petrel Pterodroma mollis (Procellariidae): new insights from the study of vocalizations. Ibis 137:207–218
Brooke MDL (2004) Albatrosses and Petrels across the World. Oxford University Press, Oxford
Brooke MD, Bonnaud E, Dilley BJ, Flint EN, Holmes ND, Jones HP, Provost P, Rocamora G, Ryan PG, Surman C, Buxton RT (2018) Seabird population changes following mammal eradications on islands. Anim Conserv 21(1):3–12
Bugoni L, d’Alba L, Furness RW (2009) Marine habitat use of wintering spectacled petrels Procellaria conspicillata, and overlap with longline fishery. Mar Ecol Progr Ser 374:273–285
Buxton RT, Jones IL (2012) Measuring nocturnal seabird activity and status using acoustic recording devices : applications for island restoration : acoustic mmonitoring of nocturnal seabirds. J Field Ornithol 83:47–60
Carboneras C, Jutglar F, Kirwan GM (2020) Juan Fernandez petrel (Pterodroma externa), version 1.0. In: Del Hoyo J, Elliott A, Sargatal J, Christie DA, De Juana E (eds) Birds of the world. Cornell Lab of Ornithology, Ithaca
Corbeau A, Collet J, Orgeret F, Pistorius P, Weimerskirch H (2021) Fine-scale interactions between boats and large albatrosses indicate variable susceptibility to bycatch risk according to species and populations. Anim Conserv 24:689–699
Croll DA, Newton KM, McKown M, Holmes N, Williams JC, Young HS, Buckelew S, Wolf CA, Howald G, Bock MF (2016) Passive recovery of an island bird community after rodent eradication. Biol Invasions 18:703–715
Delord K, Cotté C, Terray P, Bost CA, Weimerskirch H, Barbraud C (2021) Factors affecting adult body condition in the endangered northern rockhopper penguin. Marine Biol 168:27. https://doi.org/10.1007/s00227-021-03832-z
Demay J, Delord K, Thiebot JB, Barbraud C (2014) First breeding record of the northern giant petrel Macronectes halli at Ile Amsterdam. Antarct Sci 26:369–370
Dufour O, Gineste B, Bas Y, Le Corre M, Artières T (2016) First automatic passive acoustic tool for monitoring two species of procellarides (Pterodroma baraui and Puffinus bailloni) on Réunion Island, Indian Ocean. Ecol Inf 3:55–60
Duriez O, Delord K (2012) Manchots, pétrels et albatros : Oiseaux des Terres australes et antarctiques françaises (TAAF). Ornithos 19:162–183
Duriez O, Jornvall H, Shirihai H (2005) Birds and wildlife of the french subantarctic islands : Crozet, Kerguelen and Amsterdam & Saint-Paul. Dutch Bird 27:87–115
eBird (2022) eBird: An online database of bird distribution and abundance. eBird, Cornell Lab of Ornithology, Ithaca, New York. http://www.ebird.org. Accessed: February 15, 2022
Flood RL, Zufelt K, Bretagnolle V, Shirihai H (2021) Pelagic birds around Rapa and Marotiri, French Polynesia, october–december 2019, with notes on rapa shearwater Puffinus myrtae and titan storm petrel Fregetta [grallaria] titan. Bull Br Ornith Club. https://doi.org/10.25226/bboc.v141i4.2021.a3
Fromant A, Delord K, Bost CA, Eizenberg Y, Botha J, Cherel Y, Bustamante P, Gardner BR, Brault-Favrou M, Lec’hvien A, Arnould JPY (2021) Impact of extrement environmental conditions: foraging behaviour and trophic ecology responses of a diving seabird, the common diving petrel. Progr Oceanogr 198:102676
GBIF (2022) GBIF Home Page. https://www.gbif.org. Accessed Feb 15 2022
Guinet C, Jouventin P, Georges JY (1994) Long term population changes of fur seals Arctocephalus gazella and Arctocephalus tropicalis on subantarctic (Crozet) and subtropical (St. Paul and Amsterdam) islands and their possible relationship to El Niño Southern Oscillation. Antarct Sci 6:473–478
Harrison P, Perrow MR, Larsson H (2021) Seabirds. The new identification guide. Lynx Edicions, Barcelona
Heerah K, Dias MP, Delord K, Oppel S, Barbraud C, Weimerskirch H, Bost CA (2019) Important areas and conservation sites for a community of globally threatened marine predators of the southern Indian Ocean. Biol Cons 234:192–201
Holmes ND, Spatz DR, Oppel S, Tershy B, Croll DA, Keitt B, Genovesi P, Burfield IJ, Will DJ, Bond AL, Wegmann A (2019) Globally important islands where eradicating invasive mammals will benefit highly threatened vertebrates. PLoS ONE 14(3):e0212128
Imber MJ, Merton DV, West JA, Tennyson AJD (1991) Juan Fernandez Petrels prospecting at the Chatham Islands. Notornis 38:60–62
Jaeger A, Lebarbenchon C, Bourret V, Bastien M, Lagadec E, Thiebot JB, Boulinier T, Delord K, Barbraud C, Marteau C, Dellagi K, Tortosa P, Weimerskirch H (2018) Avian cholera outbreaks threaten seabird species on Amsterdam Island. PLoS ONE 13:e0197291
Jouanin C, Paulian P (1960) Recherches sur des ossements d’oisaux provenant de l’Ile Nouvelle-Amsterdam (Océan Indien). Proc XIII Int Ornith Congr 1:368–372
Jouventin P (1994) Past, present and future of Amsterfdam Island, Indian Ocean. In: Nettleship DN, Burger J, Gochfeld M (eds) Seabirds on islands—threats, case studies and action plans. Birdlife Conservation Series, Cambridge
Jouventin P, Cuthbert RJ, Ottvall R (2006) Genetic isolation and divergence in sexual traits: evidence for the northern rockhopper penguin Eudyptes moseleyi being a sibling species. Mol Ecol 15:3413–3423
Kozlov AM, Darriba D, Flouri T, Morel B, Stamatakis A (2019) RAxML-NG: a fast, scalable and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics 35:4453–4455
Lavers JL (2015) Population status and threats to Flesh-footed shearwaters (Puffinus carneipes) in South and Western Australia. ICES J Mar Sci 72:316–327
Lavers JL, Hutton I, Bond AL (2019) Changes in technology and imperfect detection of nest contents impedes reliable estimates of population trends in burrowing seabirds. Global Ecol Conserv 17:e00579
Masello JF, Ryan PG, Shepherd LD, Quillfeldt P, Cherel Y, Tennyson AJD, Alderman R, Calderon L, Cole TL, Cuthbert RJ, Dilley BJ, Massaro M, Miskelly CM, Navarro J, Phillips RA, Weimerskirch H, Moodley Y (2022) Independent evolution of intermediate bill widths in a seabird clade. Mol Genet Genom 297:183–198
Micol T, Jouventin P (1995) Restoration of Amsterdam Island, South Indian Ocean, following control of feral cattle. Biol Cons 73:199–206
Micol T, Jouventin P (2002) Eradication of rats and rabbits from Saint-Paul Island, French Southern territories. In: Veitch CR, Clout MN (eds) Turning the tide: the eradication of invasive species. IUCN SSC Invasive Species Specialist Group, Gland
Olson SL, Jouventin P (1996) A new species of small flightless duck from Amsterdam Island, southern Indian Ocean (Anatidae: Anas marecula). Condor 98:1–9
Pacoureau N, Authier M, Delord K, Guinet C, Barbraud C (2017) Early-life density-dependence effects on growth and survival in subantarctic fur seals. Pop Ecol 59:139–155
Philippe-Lesaffre M, Thibault M, Caut S, Bourgeois M, Berr T, Ravache A, Vidal E, Courchamp F, Bonnaud E (2022) Recovery of insular seabird populations years after rodent eradication. Cons Biol 37:e14042. https://doi.org/10.1111/cobi.14042
Pyle P, Welch AJ, Fleischer RC (2011) A new species of shearwater (Puffinus) recorded from Midway Atoll, Northwestern Hawaiian Islands. Condor 113:518–527
Reid TA, Ronconi RA, Cuthbert RJ, Ryan PG (2014) The summer foraging ranges of adult spectacled petrels Procellaria conspicillata. Antarct Sci 26:23–32
Rivalan P, Barbraud C, Inchausti P, Weimerskirch H (2010) Combined impacts of longline fisheries and climate on the persistence of the Amsterdam albatross Diomedea amsterdamensis. Ibis 152:6–18
Roux JP, Martinez J (1987) Rare, vagrant and introduced birds at Amsterdam and Saint-Paul Islands, Southern Indian Ocean. Cormorant 14:3–19
Robertson BC, Stephenson BM, Ronconi RA, Goldstein SJ, Sheperd L, Tennyson A, Carlile N, Ryand PG (2016) Phylogenetic affinities of the Fregetta storm-petrels are not black and white. Mol Phylogenet Evol 97:170–176
Ryan PG, Dilley BJ, Ronconi RA (2019) Population trends of Spectacled petrels Procellaria conspicillata and other seabirds at Inaccessible Island. Mar Ornithol 47:257–265
Ryan PG, Ward VL, Miller SM (2022) First record of a Common Diving Petrel Pelecanoides urinatrix from continental Africa, and a summary of diving petrel distribution in the southern ocean. Mar Ornithol 50:211–214
Segonzac M (1972) Données récentes sur la faune des Iles Saint-Paul et Nouvelle Amsterdam. Oiseau RFO 42:3–68
Shirihai H (2008) The Complete Guide to Antarctic Wildlife: Birds and Marine Mammals of the Antarctic Continent and the Southern Ocean. Princeton University Press, Princeton
Thiebot JB, Barbraud C, Scofield RP, Cherel Y, Bretagnolle V (2010) New petel record on Île Saint-Paul, southern Indian Ocean. Notornis 57:50–53
Thiebot JB, Delord K, Marteau C, Weimerskirch H (2014) Stage-dependent distribution of the critically endangered Amsterdam albatross in relation to Economic Exclusive Zones. Endanger Species Res 23:263–276
Warham J (1990) The petrels: their ecology and breeding systems. Academic Press, London
Waugh SM, Tennyson AJD, Taylor GA, Wilson KJ (2013) Population sizes of shearwaters (Puffinus spp.) breeding in New Zealand, with recommandations for monitoring. Tuhinga 24:159–204
Weimerskirch H, Delord K, Barbraud C, Le Bouard F, Ryan PG, Fretwell P, Marteau C (2018) Status and trends of albatrosses in the French Southern Territories, Western Indian Ocean. Polar Biol 41:1963–1972
Worthy TH, Jouventin P (1999) The fossif avifauna of Amsterdam Island, Indian Ocean. In: Olson SL, Wellnhofer P, Mourer-Chauviré C, Steadman DW, Marin LD (eds) Avian paleontology at the close of the 20th century. Proc 4th Int Meet Soc Avian Paleontol Evol. Smithsonian Institution Press, Washington
Bigonneau R (2013) Compte-rendu de la mission effectuée sur l’île Saint-Paul du 8 au 13 avril 2013. Programme 109 Ornitho-Eco IPEV, 17
Daycard L, Furet L (1986) Rapport de fin de mission. Zoologie. TAAF. 37ème mission (décembre 1985-décembre 1986)
Gasco N, Tixier P, Delord K, Guinet C, Duhamel G (2019) Scientific observation program around Kerguelen Island, a ship-of-opportunity for bird and mammal data collection. In: Welsford D, Dell J, Duhamel G (eds). The Kerguelen Plateau: marine ecosystem and fisheries. Proceedings of the Second Symposium:365–379. Australian Antarctic Division, Kingston, Tasmania, Australia. https://www.antarctica.gov.au/antarctic-operations/stations/other-locations/heard-island/research/symposium/
Hodum P (2009) Evaluación del estado de conservación de la fardela blanca de Juan Fernández (Pterodroma externa) y la fardela blanca de Másafuera (Pterodroma longirostris). Technical report for the Corporación Nacional Forestal (CONAF)
Miskelly CM (2013) Juan Fernandez petrel. In: Miskelly CM (ed) New Zealand Birds online. www.nzbirdsonline.org.nz
Thiebot JB (2011) Compte-rendu de fin de mission. Campagne d’hiver 2011 Amsterdam. RNN TAF
Acknowledgements
We thank, Florent Bastianelli, Clément Legeay, Colombe Lefort and Anthony Buttet for their help in the field. The Réserve Naturelle Nationale des Terres Australes Françaises and French Polar Institute (project IPEV n°109, PI C. Barbraud) logistically and financially supported the study. The Comité de l’Environnement Polaire and the Ministry of Research Ethics Committee approved procotols and activities undertaken in this study.
Author information
Authors and Affiliations
Contributions
CB, CL, YC and KD conceived the idea and designed the study. CL and CB wrote the first draft of the manuscript. QO, MF performed the field work. KD, CL, QO and MF led the curation of the data and prepared the data. ML and AW performed the genetic analysis. All authors contributed to manuscript revision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lesage, C., Cherel, Y., Delord, K. et al. Pre-eradication updated seabird survey including new records on Amsterdam Island, southern Indian Ocean. Polar Biol 47, 1093–1105 (2024). https://doi.org/10.1007/s00300-024-03282-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-024-03282-5