Abstract
Comparing to the ostracod studies on the other islands in the world, studies on the ostracod fauna of the islands in Antarctica are scarce. During the sixth Turkish Antarctica Expedition (TAE 6) to the islands (Dismal, Horseshoe, Nansen, Livingstone) in Western Antarctica, sediment and water samples were collected from 32 different water bodies (lakes, ponds, creeks, springs, littoral zones of sea). Among several new reports of different taxonomic groups which are under investigation, a new marine ostracod species (Xestoleberis nansenensis n. sp.) was encountered from Nansen Island. This is the first report of an ostracod from this island below 60° S in Antarctica. The new species has several different features both in the carapace and soft body parts. Absence of ostracods from other islands sampled may be related to several a/biotic factors, such as water chemistry (e.g., relatively low calcium levels), extreme aquatic conditions (e.g., low-temperature values), improper habitat conditions (e.g., too little or absence of sediment in the water bodies), and isolation of the island(s) from the mainland. Although our new species is a marine form, comparative literature review indicated that there is no non-marine living ostracod species reported from the islands visited during the expedition. Possible reasons are discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Previous studies on marine ostracods [Kornicker 1970, 1975; Hartmann 1986, 1992; Whatley et al. 1998 (and references in there)]; Dingle 2000, 2003; Brandão and Dingle 2014; Brandão et al. 2019; De Broyer et al. 2021; Salvi et al. 2022) and on other taxonomic groups (e.g., Pugh et al. 2002; Hodgson et al. 2013; Melis and Salvi 2020) showed relatively high and unique (e.g., mostly endemic, rare) species diversity in Antarctica, the fifth largest continents of the world. Accordingly, there are between 571 and 515 marine ostracods (Brandão and Dingle 2014; Brandão et al. 2019) reported from the continent. Also, there are about 223 species of the genus Xestoleberis distributed worldwide (Brandão et al. 2023). In contrast to the marine ostracods, knowledge on the non-marine ostracods within 60° S is not well known from the area. Although studies (e.g., Pugh et al. 2002; Dartnall 2017) listed nine non-marine taxa, none of them seems to be found from the islands visited during the present study within 60° S. The aims of this study are to (1) describe a new ostracod species from Nansen Island, (2) provide information about physicochemical properties of water samples and discuss possible reasons of the species absence, and (3) inquire into the previous reports on non-marine ostracods from the continent Antarctica south of 60° S latitude.
Materials and methods
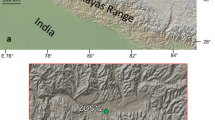
During the sixth Turkish Antarctica Expedition (TAE 6) to Western Antarctica including four islands, about 200-mg materials for the ostracods were collected in the plastic bottles and fixed with 70% ethanol in situ. Also, 100 mL of sediment and water samples were separately collected from 32 different water bodies (lakes, ponds, creeks, springs, marine, etc.) for the chemical analyses (Fig. 1). Plankton hand net (mesh size 150 µm) was used to collect samples from littoral zones and shallow water bodies, while core samplings were done from the middle of the lakes along with drilling core sampler. Samples were brought to the laboratory in the cooler where ostracod samples were kept in 70% of ethanol after filtering them under tap water through three standardized sieves with mesh size of 1:0.25:0.125 mm. Olympus BX-51 model stereo microscope was used to separate ostracods from the sediment. Fine needles were used to dissect specimens within lactophenol solution on slides. Specimens dissected were covered by a cover slip. Line drawings of the soft body parts were sketched with a camera lucida. All samples and slides are numbered and deposited in the Limnology Laboratory of the Biology Department. During the field studies, YSI multiprobe was used to measure the values of water variables (e.g., temperature, pH, salinity, electrical conductivity, redox potential, and dissolved oxygen), while water chemistry (i.e., major anions and cations) analyses were done at the Water Chemistry Laboratory, Hacettepe University (Türkiye. Standard Methods for the Examination of Water and Wastewater, Methods (APHA 1989) along with using DIONEX LC25, ICS-1000 Ion Chromatography system was run for the chemical analyses. Geographic information (e.g., ambient temperature, humidity and atmospheric pressure, latitude, longitude, elevation) was gathered with Kernel Anemometer before sampling at each site. Scanning Electron Microscope (SEM) photographs were taken at the Hacettepe University, Türkiye.
Results
Taxonomy
Description:
Superfamily Cytheroidea Baird, 1850
Family Xestoleberididae Sars, 1928
Genus Xestoleberis Sars, 1866
Genus: Xestoleberis Sars, 1866
The genus Xestoleberis is characterized as follows: Valves with crescent shaped scar so-called “Xestoleberis-eye spot” (Athersuch et al. 1989). Carapace surface is smooth and its shape strongly inflated from ovate, reniform to trigonal. Antennula (A1) has six segments, while Antenna (A2) has two segmented exopodite spinneret seta and three endopodite segments terminating into two stout claws. Males bear a pair of brush-shaped organs located between the first and second pair of thoracic legs. The thoracic appendages are known as walking legs.
Xestoleberis nansenensis n. sp.
Holotype: Adult female collected (February 28, 2022) from station 29, the type locality (64° 33.036′ S, 62° 05.142′ W; a water body (small puddle) about 50 m away from the sea on the coastal zone of the Nansen Island, Antarctica) mounted on a slide and covered with a cover slide (No: Ant-OK-01), valves were used in SEM photographing and kept (No: Ant-OK-02) in the laboratory.
Paratypes: 1 female collected from the type locality is kept in the micropaleontological slide.
Etymology: The new species is named by adding the suffix -ensis to the type locality (Nansen Island). Gender feminine.
Description: Carapace whitish in color. In lateral view, carapace subovate; dorsal margin rounded, ventral margin slightly concave, posterior margin rounded, and anterior margin pointed (Fig. 2A–D). Left valve (LV) overlapping right valve (RV). Hinge merodont. Four long adductor muscle scars (Fig. 2E, H–G) vertically located closer to ventral margin, one oval dorsal (antennal) scar with a thin tubercule-like structure inside located upper right (anterodorsally) of the adductor scars (Fig. 2H).
Xestoleberis nansenensis n. sp. (Holotype). Female. A LV with pore openings external view, B LV internal view (attention to the postero-dorsal margin slightly bending down), C LV close external view of posterior end, D LV close external view of anterior end, E Adductor muscle scars external view, F LV close internal view of posterior margin, G LV close internal view of anterior margin, H Adductor muscle scars internal view, and I Close internal view of pore openings. Circled area covers Xestoleberis spot. Note to views of C–E which are reversed from A to show details on the carapace. Note to oblique views of F and G. Scale: A, B 100 mm; C, D 35 mm; E 10 mm; F, G 30 mm; H 20 mm; and I 10 mm
The area of “Xestoleberis spot” is not well seen externally but in internal view, it is inconspicuous, small in shape, and located anterodorsally above the adductor muscle scars (Fig. 2A). Carapace surface with thin, partially completed longitudinal striations and normal pore openings with short seta (Fig. 2E, I). Carapace moderate in size (L = 0.50 mm; H = 0.27 mm; n = 2). Internal view, both margins with two lists on the calcified region.
Antennula (A1) (Fig. 3A): Five segmented; first segment with a medium-size short smooth seta. Second segment long about size of last three segments and with a short anterior seta. Third segment short about half of previous segment with a short medial seta and antero-ventral seta. Penultimate segment long about 2/3 of second segment with three setae; a long smooth seta extending about half of terminal segment, two antero-ventral setae unequally long, a short seta about size of terminal segment, long seta extends tips of terminal setae. Terminal segment ending with three almost equal size smooth short setae about size of the segment.
Antenna (A2) (Fig. 3B): Five segmented. First segment subrectangular and long about size of next two segments. Second segment with a long stout two-joined exopod reaching tips of terminal claws, two unequally long ventro-apical setae present, short seta almost half of the third segment, and long seta reaching half of penultimate segment. Third segment subdivided, subrectangular narrowing to distal margin with one short anterior seta. Penultimate segment thin with one stout small claw-like ventral seta and one anterior seta. Terminal segment very small, ending with one well-developed claw.
Mandibula (Md) (Fig. 3C): Coxa without teeth (diagnostic character). Exopod with two almost equally long and smooth setae reaching about size of first endopodial segment. Endopod with three segments. First segment with one long antero-ventral seta, extending tips of terminal setae. Second segment very short with two anterior setae. Terminal segment with one long medial seta slightly plumose and four smooth terminal setae medium in size.
Maxillula (Mxl) (Fig. 3G): Vibratory plate with 12 hirsute setae (not shown). Exopod with two plumose long setae. Endopodite with 3 endites (masticatory lobes) and with two-segmented palp. First palp segment without seta and second palp segment very short with two unequally long setae and one claw-like smooth seta. Endites ending with 5, 4, and 4 short setae, respectively. First endite with one smooth antero-distal seta; second endite with two almost equally long distal setae (not shown in Fig. 3G).
First thoracic leg (T1) (Fig. 3D): Four segmented with a well-developed terminal claw. First segment without seta. Second segment with one smooth anterior apical seta about length of third segment. Third segment with very small postero-distal seta. Fourth segment smooth without seta.
Second thoracic leg (T2) (Fig. 3E): Three segmented with a well-developed terminal claw. First and third segments without seta. Second segment with one anteromedial smooth seta.
Third thoracic leg (T3) (Fig. 3F): Four segmented with a well-developed terminal claw. First, third, and fourth segments without seta. Second segment with one medial size antero-apical seta. Fourth segment anteriorly with bump-like structure.
Uropod (Fig. 3H): With two medium-size setae.
Genital organ rounded. Males not observed.
Color: Translucent to whitish.
Water chemistry
Tables 1 and 2 display measurements of physicochemical water properties. Among them, water temperature and electrical conductivity (referring to salinity) appear to be most effective on species occurrence. Accordingly, water characteristics of the type locality of the new species (Tables 1 and 2) can be characterized as cold, saline, highly alkaline, and well oxygenated. Among the variables, chloride and sodium were the dominant elements (Fig. 4), while magnesium level was higher than calcium. Except bromide ion, levels of some ions measured (e.g., NO−2, NO−3, PO−4, NH+4, F−, and Li−) were below the standard values and not significant.
Discussion
Distinguishing features of X. nansenensis n. sp.
Figures 2 and 3 obtain possibility of comparing several dis/similarities of the new species from its congenerics. Some of those distinguishing features are discussed below.
-
(1)
Carapace shape, ornamentation, muscle scars, and Xestoleberis spot:
Xestoleberis nansenensis n. sp. carapace shape is very much identical to X. setouchiensis of Okubo (1979); however, the new species differs based on the following features: (i) anteriorly X. setouchiensis is more pointed than X. nansenensis n. sp., (ii) ventral margin in the new species is concave but it is convex in X. setouchiensis, and (iii) X. setouchiensis has both sieve-type and lip-type pores (Group A of Sato and Kamiya 2007), but a few lip-type pores and normal pores are dominant in the new species. Additional differences are also found in the soft body parts discussed below. At first glance, the members of the genus Xestoleberis can be separated from others by having a relatively smooth carapace and subovate shape (Luz and Coimbra 2015; Le and Tsukagoshi 2018). In contrast, carapace surface of X. nansenensis n. sp. bears fine longitudinal striations which are completed or incomplete along the valves in appearance and easy to observe in SEM photographs (Fig. 2A, E). This feature is comparatively different than most (if not all) other species of the genus with smooth surface but some species (e.g., cf. X. granulosa in Brady 1880; X. kerguelenensis, X. capensis in Müller 1908) are also known with ornamentations, such as fine to large pores and/or pits (e.g., see X. davidiana in Chapman 1915 and X. gracilariaii in Chand and Kamiya 2016).
Besides smooth surface, pore type(s) on the carapace is also used for taxonomic descriptions; for instance, species of the genus generally consists of two (or three) types of pore opening: a normal type (or simple pore), lip type and a sieve type of pores (Puri 1974; Hanai and Ikeya 1991; Sato and Kamiya 2007; Luz and Coimbra 2015). In some cases, multiple pore types are observed; for example, Sato and Kamiya (2007) grouped their 13 species into three groups as Group A (sieve and lip types), Group B (sieve type), and Group C (sieve and simple types). Xestoleberis nansenensis n. sp. has different combination of pore types as is the case in Chand and Kamiya (2016). Its carapace consists mostly of lip type scattered over the carapace and a few normal pore types mostly located closer to the ventral edge and a few through the anterior and posterior edges. In total, there can be about 53 (46 lip, 7 simple type) pores on each valve. Moreover, some species did only have one pore type. Sato and Kamiya (2007) pinpointed that females do have more pore numbers than males, but this is not applicable for our new species due to lack of males. Although numbers of pores are not commonly used during species description, this may be an important tool for future studies (Sato and Kamiya 2007).
In terms of muscle scars, at first glance, the new species resembles members of the genus by means of having four adductor muscle scars. The main difference is that these scars are longitudinally placed and relatively longer than scars reported in other species. However, comparing to other species (e.g., cf. X. munensis in Le and Tsukagoshi 2018, X. amazonica in Luz and Coimbra 2015) with a common type of U-shaped upper (frontal) muscle scar located vertically, this scar in the new species is almost oval in shape with a small spine-like part developed inside.
Among the differences, “Xestoleberis spot” is also used to separate the genus from other related genera Keyser (1988 p. 184) provided detailed information about the formation of this spot, indicating that the spot is probably formed due to “…an irregularity in the inner calcified layer of the shell. Apart from that it is just a place where two muscle scars are present.” Accordingly, one possible reason of the flexibility of the shape of the spot is considered as “…the different degree of filling of the reservoir of the spinning gland alters the direction of the pulling force of the muscles” (Keyser, opt. cit.). Based on this explanation, one may consider reasons for different occurrences of the shape of the spot. For example, in some species (X. brasilinsularis), the spot is very small and/or almost not visible (Luz and Coimbra 2014). In some other species, for example, see X. vietnamensis, it is divided into two pieces (Le and Tsukagoshi 2018). Therefore, this view may explain finding a small inconspicuous shape of the spot in the new species. Nevertheless, this character should be carefully used to identify the species from others.
In size comparison, X. nansenensis n. sp. most resembles two species (X. machadoae and X. brasilinsularis) in Luz and Coimbra (2014). However, the new species is relatively larger (length = 0.50 mm) than these two when the lengths of the type species, X. brasilinsularis and X. machadoae, are 0.42 and 0.34 mm, respectively. On the other hand, it should be underlined that the range among the species vary; therefore, such information should be used carefully.
-
(1)
Chaetotaxy and soft body parts:
Xestoleberis nansenensis n. sp. covers several unique and diagnostic characteristics in the soft body parts as discussed:
-
(i)
First antenna (A1) has five segments. Similar to X. petrosa (Chand and Kamiya 2016) and few more species listed in Sato and Kamiya (2007), the first segment is comparatively longer than that in the other species. Okubo (1980) stated X. setouchiensis has five segments (podomeres), but line drawings show six segments. Thus, this needs to be confirmed with further studies. Also, it should be noted that numbers of segments are not possible to compare with many of the other species because of lack of line drawings of many Xestoleberis species but see exception in Hartmann (1984). This is mostly due to the reports based on fossil and/or only carapace descriptions (see Kaesler and Waters 1972). However, a few resembling species with soft body parts can be compared to the new species. For example, Le and Tsukagoshi (2018) reported two species (X. vietnamensis and X. munensis) with six podomeres that the length ratios of these species ranged from 25:24:7:13:1:6 to 25:24:8:8:13:6, respectively. There is a gradual decrease from proximal to distal segment. This ratio in X. nansenensis n. sp. differs from others as 3.5:4.0:1.6:2.2:1.0. Similarly, Sato and Kamiya (2007) described 7 of 13 species with five segments from the coast of Japan. They indicated that numbers of segments on A1 can be used during taxonomic description. If this is true, the genus Xestoleberis may be separated into several subgenera or genera. Further studies may focus on this issue. Besides segmentation, other differences are seen in the location and numbers of setae. For example, unlike one short seta on the first segment of X. nansenensis n. sp., such seta is absent in several species (e.g., see X. vietnamensis, X. munensis, X. planuventer, X. kurosio, X. sagamiensis, X. notoensis, X. ryukyuensis, X. sesokoensis, X. magnoculus) (Sato and Kamiya 2007), while a small setal group was observed in several other species (e.g., cf. X. penna and other six species in Chand and Kamiya 2016). Comparing the locations of the setae, there are only a few setae located on the anterior edges of the new species compared to others; however, most of the setae on A1 are indeed seen on the anterior ends of the segments. Also, terminal segment ends with three smooth, short, and almost equal sized setae, while these setae are unequally long in those species (e.g., X. vietnamensis, X. munensis) compared here. It should be noted that the middle one of the terminal setae on A1 of the new species is most probably aesthetasc “Ya” but is very much seen like a seta.
-
(ii)
Second antenna (A2) has differences clearly seen in chaetotaxy compared to the other species discussed here. First, the third segment is subdivided. In some ostracod species, for example, in the males of candonid [e.g., Neglecandona neglecta (Sars 1887)] species, A2 includes five segments (Meisch 2000). For example, the terminal segment of the new species ends with a well-developed hook-like claw but there are two claws in almost all the species (e.g., X. vietnamensis, X. munensis, X. gracilariaii, X. marcula, and see species listed in Sato and Kamiya 2007, Chand and Kamiya 2016). Another difference is seen in the exopod which includes a three-segmented spinneret seta located antero-distally. This seta is usually without division (e.g., see X. concava, X. gracilariaii in Chand and Kamiya 2016) or two segmented (e.g., X. marcula, X. setouchiensis).
-
(iii)
Mandibula (Md) has a very interesting, unique, and different characteristics. Unlike other ostracod species (see e.g., Okubo 1979, 1980; Sato and Kamiya 2007; Hirosaki 2013; Chand and Kamiya 2016), the teeth on the coxa are either reduced or absent. This is considered a diagnostic characteristic of the species; however, without further speculation, there can be two possible reasons for this unusually interesting feature; (1) this may be an anomaly in this individual. To support this view, there should be more individuals checked out for this character, but none are available at the moment, and (2) the part of the coxa might have been broken and lost during the dissection. This approach is unlikely since both mandibulae (Fig. 3C) are clearly seen in the slide. Future studies are needed to compare the character, and again, more individuals should be analyzed to clarify the issue. Additionally, there are only three segments (or so-called podomere) in the new species. In comparison, there are generally four segments in most of the ostracod species, but exceptions occur (cf. X. ryukyuensis in Sato and Kamiya 2007). Also, numbers of setae on exopod of the first segment (2 setae) is similar to X. ikeyai reported from Japan (Sato and Kamiya 2007) and six other species (X. becca, X. concava, X. gracilariaii, X. marcula, X. natuvuensis, X. penna) reported from Fiji Archipelago by Chand and Kamiya (2016). Differences in the setal group in the endopodial segments and length of the segments are clearly seen among the species. For example, unlike the smooth and almost equally long setae in the new species, there is one long and on short hirsute seta found in X. ikeyai and two hirsute setae in other species. First segment is not divided and is longer than the next two segments. Claws are not well developed (or not claw like) on the third (terminal) segment. Readers are suggested to compare mandibula with all other known species of the genus (e.g., see Chand and Kamiya 2016).
-
(iv)
Reporting 12 hirsute setae on the vibratory plate of the maxillula is another different character even though number varies among the species from 13 to 17 smooth and/or plumose or hirsute setae (Sato and Kamiya 2007; Chand and Kamiya 2016). For example, X. setouchiensis has 15 feather-like setae and first palp with four antero-distal setae (Okubo 1980) but two small setae occur in the first palp in the new species (Fig. 3).
-
(v)
Thoracic legs: They are all similar in shape ending with a claw. Thoracic legs (T1–T3) have four segments. Basal setae are not observed in the new species even though many species of the genus bear at least one or more basal setae. There may be one exception in X. ikeyai where the first thoracic leg seems to have three segments (see Fig. 12E in Sato and Kamiya 2007). However, these authors stated four segments (podomeres) in the text. This information needs to be confirmed.
-
(vi)
Uropod is reduced in all the species of the genus Xestoleberis. This is also the case in the new species where there are two smooth setae in the uropod. Comparison of the numbers of setae among the species, variations can be seen from one to three smooth or hirsute or both types of setae. There are at least three species (X. petrosa, X. gracilariaii, X. becca) (see Chand and Kamiya 2016) known with one short seta, while four (X. penna, X. concava, X. marcula, X. natuvuensis) and nine species (X. sagamiensis, X. notoensis, X. ryukyuensis, X. sesokoensis, X. kuroshio, X. magnoculus, X. ikeyai, X. vietnamensis, X. munensis) are described with two and three setae, respectively. Numbers of setae and shape of the uropod are commonly used in taxonomic descriptions of many ostracods, but this is not common in the genus Xestoleberis. However, this may be used to make groups of the species and can be more commonly used during the taxonomic description of the live species.
Female genital organ is rounded in the new species similar to the other species while male organs (e.g., hemipenis) cannot be discussed in here due to lack of males.
Importance of reporting a new species
Brady (1907) using samples from the National Antarctic Expedition of Great Britain was the first to report nine ostracods from Winter Quarters and environs in Antarctica (see details in Brady 1907). Among them, X. reniformis was the only species of the genus described as a new species from Antarctica. Later, studies on the ostracods from the continent have been increased although the frequency seems to be relatively low. Following Brady’s reports, Müller (1908) (five species of the genus collected from the Indian Ocean sector of the Antarctic shelf) and Chapman (1916, 1919) (five species from McMurdo Sound and Recent muds of the Ross Sea) announced several other species, including members of Xestoleberis. Benson (1964) introducing an undescribed Xestoleberis sp. provided a list of early studies on ostracods reported from Antarctica. As it was underlined, these species are mostly marine benthonic and/or planktonic ostracods (and few were sub/fossils) collected from open sea and/or nearby the mainland. Similarly, Majewski and Olempska (2005) listed 29 marine podocopid ostracods around Admiralty Bay on King George Island, in which X. cf. rigusa was reported. The authors concluded that there was no clear relationship between ostracods and environmental variables used in their study (see discussion below). Most recently, Nazik et al. (2022) described six species belonging to six genera from two (Hovgaard and Horseshoe islands) of seven sites with 12 samplings collected around the islands, including King George, Horseshoe, Calmette, Videla, Hovgaard, Nansen, and Deception islands. The authors did not report the species of the genus Xestoleberis in their study. Also, ostracods were not reported from Nansen Island by the authors. In contrast, Xestoleberis nansenensis n. sp. is the first ostracod report from a water body (a puddle with about 100 cm2 of surface area and 30 cm of depth ca. 50 m away from the sea) on the Nansen Island even though there are some species already reported from the areas nearby (e.g., see Nazik et al. 2022). As stated above during the present study, ostracods were solely collected from this island and other 31 water samples did not cover a sign of ostracods. Absence of ostracods from other samples can be correlated with (i) improper aquatic conditions and water chemistry (i.e., low water temperature, levels of calcium or magnesium), (ii) low or absence of sediment levels, and (iii) isolation of the islands from the mainland. One may also consider biotic factors herein, but I did not observe fish or larger invertebrates during the sampling from the type locality. For example, no fish was seen in the lakes and other water bodies sampled on the islands. Eventually, one may consider biotic factors are less or no importance on the absence of ostracods.
In terms of improper aquatic conditions, as indicated above, bromide level (29.3 mg/L) of the waters collected during the present study was comparatively found higher (Table 2) than the freshwater systems (ca. 0.5 mg/L) (WHO 2009) but it was even low for the seawater where the range was generally known from 65 mg/L to well over 80 mg/L (Al-Mutaz 2000). Such a level of bromide can be expected since occurrence of bromide in natural aquatic sites coincides with NaCl due to similar physicochemical characteristics of 10 marine sampling sites. In contrast calcium and magnesium followed by Na and Cl were dominant in the 22 freshwater sites (Fig. 4). Meanwhile, we do not know if (or how) the bromide and ostracods are related. Thus, this part of the study is open for further discussion. On the other hand, finding the species from a very cold and saline water challenges our knowledge about ostracod tolerance level. For example, it is known that several species of freshwater ostracods can tolerate very high (up to 55 °C) (Külköylüoğlu et al. 2003) and very low (ca. 0.5–1.0 °C) temperature levels (Delorme 1991; Kiss 2007) but in seawater this seems to be much lower (0.3 °C this study). Now, we may not explain how ostracod tolerate such extreme conditions due to lack of studies. Further studies are needed.
Second possible explanation of the absence of ostracods from the sampling sites was inadequate levels of sediments. According to field observation by the author, except lake samples, all other water bodies showed very scarce and/or no sediment enough to support ostracods on the bottom of the water bodies, including the type locality. This also applies to samples taken from the littoral zone of the seawater. According to the previous studies (Adams et al. 1992; Boyd 2015), sediments as source of different chemicals play critical role in aquatic ecosystems. Considering most of the ostracods are bottom dependent, a few swimming species may prefer living in or around the macrophytes. They mostly inhabit parts in and/or just above the sediment where ostracods find possibilities to feed and survive on several different organisms and organic matter (Puri 1966). Implication of this approach is that lack of sediments reduces the chance of finding suitable habitat for ostracods, which may be the case in the present study.
Isolation of the islands may be another explanation of the absence of ostracods from many sites sampled during the present study. Antarctica has a long geological history going back to about Precambrian age. It has mainly two parts East and West Antarctica. The sampling sites (Table 1) were located on the western part of the continent. According to Nazik et al. (2022), species found from the sites closer to the present sampling location are known from about Oligocene to Recent. Indeed, Williams et al. (2008) reported well-preserved non-marine ostracods from the Miocene, indicating a lake setting (Palaeolake Boreas) probably suitable for the ostracods. Implication of this information is that the separation of the continent from the mainland is a long story. Hence, such a long-term isolation may cause absence (or low) freshwater ostracod species living on the islands.
In addition to these possibilities, the lack of species may be due to the lack of studies on the islands. Although several different species of different taxonomic groups have been reported from the waters on the islands (Majewski and Olempska 2005; Dartnall 2017), scarcity of ostracods may not be well explained. Hence, this may be challenging for future studies. Herein, one may argue about earlier ostracod species reported alive from the islands. For example, Dartnall (2017), listing several different aquatic species, reported nine freshwater ostracods (Candona sp. from South Georgia islands (Dartnall 2017); Eucypris pestai [recently accepted name Amphicypris pestai (Graf 1931)] from South Georgia islands (Graf 1931); Cypretta sp. (recently Cypretta cf. seurati) from Macquarie Island (Lofthouse 1967); Eucypris corpulenta [recently known as Ramotha corpulenta (Sars 1895)] from Îles Kerguelen and the Poincaré peninsula (Lofthouse 1967); Eucypris fontana [recently known as Argentocypris sarsi see Díaz and Martens 2014 (Graf 1931)] from South Orkney and South Georgia islands (Graf 1931; De Deckker 1981a); Eucypris virens (Jurine 1820) from Macquarie Island and Îles Kerguelen (Dartnall 2017); Ilyodromus kerguelensis Müller 1906 from Possession Island, Îles Kerguelen, Îles Crozet, and Prince Edward Islands (Lofthouse 1967; Dartnall 2017); Cypridopsis frigogena (recently known as Neocypridopsis frigogena, see Martens and Behen 1994) (Graf 1931) from South Orkney and South Georgia islands (Graf 1931, De Deckker 1981b; and Tanycypris sp., Dartnall 2017 from South Georgia) from the Antarctic islands. However, from a careful detailed search on the locations listed in Dartnall (2017), I found out that except two species some of these ostracods previously reported did not belong to the area within the 60° S of Antarctica. For example, Graf (1931) listed A. fontana and N. frigogena from the samples provided by Dr. W. König in 1926 but König’s samples were collected from Grytviken (S. Georgia Island) which is located north of 60° S. On the other hand, De Deckker (1981a, b) identified two species (N. frigogena, A. sarsi) from the lakes on Singy Island (S. Orkney Islands) located at the border of 60° S latitude. Therefore, at the moment, except these two species, there is no other freshwater ostracods from the islands as far as the present study shows.
Finally, it should be stated that the new species includes several characters different from the other species of the genus Xestoleberis. Comparing the characters provided in Athersuch et al. (1989), it may also be a new genus of the family. However, due to lack of specimens, description of the species is tentatively done in here. Thus, its taxonomic status can possibly be verified with providing more specimens.
Conclusion
Based on the differences in soft body parts and carapace introduced above, Xestoleberis nansenensis n. sp. is proposed as a new species from the Nansen Island, Antarctica. This is the first ostracod species found from this island. Absence of ostracods from other islands sampled during the study are discussed based on improper aquatic conditions (e.g., low-temperature values) and historical background of the area. Although marine and brackish water ostracods are known from the continent, the known literature reveals that there appears to be only two non-marine living ostracod species from the freshwater habitats within the 60° S in Antarctica. This result suggests that non-marine ostracods can be found in all six continents, but future studies are thought to clarify the issue.
Data availability
All data on the measured environmental variables used in this study are included within this paper and they can be available upon request.
References
Adams WJ, Kimerle RA, Barnett JW Jr (1992) Sediment quality and aquatic life assessment. Environ Sci Technol 26:1864–1875
Al-Mutaz I (2000) Water desalination in the Arabian Gulf region. Water Purif Technol 2:245–265
APHA (1989) Standard methods committee of the American Public Health Association, American Water Works Association, and Water Environment Federation. Preface. In: Lipps WC, Baxter TE, Braun-Howland E (eds) Standard methods for the examination of water and wastewater. APHA Press, Washington DC
Athersuch, J, Horne DJ, Whittaker JE (1989) Marine and Brackish Water Ostracods (superfamilies Cypridacea and Cytheracea): keys and notes for the identification of the species. Synopses of the British Fauna, Brill, E.J., Leiden
Benson RH (1964) Recent Cytheracean Ostracodes from McMurdo Sound and the Ross Sea, Antarctica. Univ Kans Paleontol Contrib 6:1–36
Boyd C (2015) Water quality: an introduction. Springer, Cham. https://doi.org/10.1007/978-3-319-17446-4
Brady GS (1880) Report on the Ostracoda dredged by the H.M.S. Challenger during the years 1873–1876. Report of the Voyage of H.M.S. Challenger. Zoology 1:1–184
Brady GS (1907) Crustacea. V-Ostracoda. Nat Hist Rep Br Antarct Discov Exped 3:1–9
Brandão SN, Dingle RV (2014) Benthic Ostracoda. In: De Broyer C, Koubbi P, Griffiths HJ, Raymond B et al (eds) Biogeographic atlas of the Southern Ocean. Scientific Committee on Antarctic Research, Cambridge, pp 142–148
Brandão SN, Angel MV, Karanovic I, Perrier V, Meidla T (2019) WoRMS Ostracoda: world Ostracoda database (version 2018-03-05). In: Roskov Y, Ower G, Orrell T, Nicolson D, Bailly N, Kirk PM, Bourgoin T, DeWalt RE, Decock W, Nieukerken E van, Zarucchi J, Penev L, (eds) Species 2000 & ITIS Catalogue of Life, 2019 annual checklist. Species 2000: Naturalis, Leiden, the Netherlands. Digital resource at www.catalogueoflife.org/annual-checklist/2019
Brandão SN, Antonietto LS, Nery DG, Santos SG, Karanovic I (2023) World Ostracoda Database. https://doi.org/10.14284/364.https://www.marinespecies.org/ostracoda. Accessed 6 Feb 2023
Chand P, Kamiya T (2016) Seven new species of the genus Xestoleberis (Ostracoda: Podocopida: Cytheroidea) from the Fiji Archipelago. Zootaxa 4208:325–348. https://doi.org/10.11646/zootaxa.4208.4.2
Chapman F (1915) Report on the Foraminifera and Ostracoda: Zool. Results of fishing experiments carried out by F.I.S. Endeavor, 1911, 3:1–51
Chapman F (1916) Report on the Foraminifera and Ostracoda from elevated deposits on the shores of the Ross Sea: Rept. Sel. Invest. British Antarctic Exped. 1907–09, Geology, vol. II, Contrib. Paleontology Petrology South Victoria Land, Ostracoda, pp 37–40
Chapman F (1919) Ostracoda. Australasian Antarctic Expedition, 1911–1914. Scientific Reports, Series C. Zool Bot 5:5–45
Dartnall HJG (2017) The freshwater fauna of the South Polar Region: a 140-year review. Pap Proc R Soc Tasman 151:19–57. https://doi.org/10.26749/rstpp.151.19
De Broyer C, Clarke A, Koubbi P, Pakhomov E, Scott F, Vanden Berghe E, Danis B (2021) Register of Antarctic Marine Species. http://www.marinespecies.org/rams. Accessed 22 Sept 2021
De Deckker P (1981a) On Eucypris fontana (Graf). Stereo-Atlas of Ostracod Shells 8:87–92
De Deckker P (1981b) On Notiocypridopsis frigonena (Graf). Stereo-Atlas of Ostracod Shells 8:101–106
Delorme LD (1991) Ostracoda. In: Thorpe JH, Covich AP (eds) Ecology and classification of North American invertebrates. Academic Press, New York, pp 691–722
Díaz AR, Martens K (2014) On Argentocypris sara gen. nov., sp. nov. (Ostracoda) from the Patagonian wetlands of Argentina. Crustaceana 87:513–530. https://doi.org/10.1163/15685403-00003300
Dingle RV (2000) Ostracoda from CRP−1 and CRP−2/2A Victoria Land Basin, Antarctica. Terra Antarct 7:479–492
Dingle RV (2003) Recent subantarctic benthic ostracod faunas from the Marion and Prince Edward Is− lands Archipelago, Southern Ocean. Rev Esp Micropaleontol 35:119–155
Graf H (1931) Süsswasser-Ostracoden aus Südgeorgien. Zool Anz 93:185–191
Hanai T, Ikeya N (1991) Two new genera from the Omma-Manganji ostracode fauna (Plio-Pleistocene) of Japan-with a discussion of theoretical versus purely descriptive Ostracoda nomenclature. Trans Proc Palaeont Soc Jpn New Ser 163:861–878
Hartmann G (1984) Zur Kenntnis der Ostracoden der polynesischen Inseln Huahine (Gesellschaftsinseln) und Rangiroa (Tuamotu-Inseln). Mitt Hamb Zool Mus Inst 81:117–169
Hartmann G (1986) Antarktische benthische Ostracoden I (mit einer Tabelle der bislang aus der Antarktis bekannten Ostracoden). Auswertung der Fahrten der "Polarstern" Ant. III/2 (SibexSchnitte) und der Reise 68/1 der "Walther Herwig" (T. 1: Elephant Island) in die Antarktis.—Mitt Hamb Zool Mus Inst 83:147–221
Hartmann G (1992) Antarktische benthische Ostracoden. VIII. Auswertung der Reise der “Meteor“ (Ant. 11/4) in die Gewässer um Elephant Island und der Antarktischen Halbinsel. Helgol Meeresunters 46:405–424
Hirosaki M (2013) Taxonomy of two new species of ostracod gene Xestoleberis and study about the stages of an interstitial adaptation. Unpublished Master’s thesis, Shizuoka University, p 17. (in Japanese with English abstract)
Hodgson DA, Roberts SJ, Smith JA, Verleyen E, Sterken M, Labarque M, Sabbe K, Vyverman W, Allen CS, Leng MJ, Bryant C (2013) Late Quaternary environmental changes in Marguerite Bay, Antarctic Peninsula, inferred from lake sediments and raised beaches. Quat Sci Rev 68:216–236
Jurine L (1820) Histoire des Monocles, qui se trouvent aux environs de Genéve. I–XVI:1–260
Kaesler RL, Waters JA (1972) Census of Holocene species of Xestoleberis (Ostracoda, Podocopida) from the southern oceans. Paleont Contr 60:1–35
Keyser D (1988). The origin of the Xestoleberis-spot. In: Hanai T, Ikeya N, Ishizaki K (eds) Evolutionary biology on Ostracoda. Proceedings of the ninth ınternational symposium on Ostracoda, Tokyo, pp 177–186
Kiss A (2007) Factors affecting spatial and temporal distribution of Ostracoda assemblages in different macrophyte habitats of a shallow lake (Lake Fehér, Hungary). Hydrobiologia 585:89–98
Kornicker LS (1970) Ostracoda (Myodocopina) from the Peru-Chile Trench and the Antarctic Ocean. Smithson Contrib Zool 32:1–42
Kornicker LS (1975) Antarctic Ostracoda (Myodocopina). Smithson Contrib Zool 163:1–720
Külköylüoğlu O, Meisch C, Rust RW (2003) Thermopsis thermophila n. gen. n. sp. from hot springs in Nevada, USA (Crustacea, Ostracoda). Hydrobiologia 499:113–123
Le DD, Tsukagoshi A (2018) Three new species of the genera Loxoconcha and Xestoleberis (Crustacea, Ostracoda, Podocopida) from central and southern Vietnam. Zootaxa 4472:111–126. https://doi.org/10.11646/zootaxa.4472.1.5
Lofthouse P (1967) Cladocera, Ostracoda, and freshwater Copepoda. British Australian and New Zealand Antarctic Research Expedition. 1929–1931. Reports. Ser B Zool Bot 8:141–144
Luz NC, Coimbra JC (2014) New species of Xestoleberididae (Crustacea, Ostracoda) from Archipelago of São Pedro and São Paulo, Equatorial Atlantic. Iheringia Sér Zool 104:470–477. https://doi.org/10.1590/1678-476620141044470477
Luz NC, Coimbra JC (2015) The genus Xestoleberis (Ostracoda: Xestoleberididae) in the Northern, Northeastern and Eastern regions of the Brazilian continental shelf. Zootaxa 3974:177–195. https://doi.org/10.11646/zootaxa.3974.2.3
Majewski W, Olempska E (2005) Recent ostracods from Admiralty Bay, King George Island, West Antarctica. Pol Polar Res 26:13–36
Martens K, Behen F (1994) A checklist of the non-marine ostracods (Crustacea, Ostracoda) from South-American inland waters and adjacent islands. Bull Soc Nat Luxemb 22:1–81
Meisch C (2000) Freshwater Ostracoda of Western and Central Europe. Spektrum Akademischer Verlag, Süßwasserfauna von Mitteleuropa, Heidelberg, pp 522
Melis R, Salvi G (2020) Foraminifer and ostracod occurrence in a cool-water carbonate factory of the Cape Adare (Ross Sea, Antarctica): a key lecture for the climatic and oceanographic variations in the last 30,000 years. Geosciences 10:413
Müller GW (1906) Ostracoda. Wissenschaftliche Ergebnisse der Deutschen Tiefsee-Expedition‚ Valdivia’ 1989–1899 8:29–154
Müller GW (1908) Die Ostracoden der Deutschen Südpolar−expedition 1901–1903. In: von Drygalski E (ed) Deutsche Südpolar−expedition 10. . Zoologie 2. Verlag von Georg Reimer, Berlin, pp 51–181
Nazik A, Büyükmeriç Y, Kılıç AM, Yümün ZÜ (2022) Recent marine ostracods (Crustacea) around Hovgaard and Horseshoe Islands (Antarctica Peninsula). Geol Bull Turkey 64:43–52. https://doi.org/10.25288/tjb.1002087
Okubo I (1979) Three species of Xestoleberis (Ostracoda) from the Inland Sea of Japan. Proc Japn Soc Syst Zool 16:9–16
Okubo I (1980) Taxonomic studies on recent marine podocopid Ostracoda from the Inland Sea of Seto. Publ Seto Mar Biol Lab 25:389–443
Pugh PJA, Dartnall HJG, McInnes SJ (2002) The non-marine Crustacea of Antarctica and the Islands of the Southern Ocean: biodiversity and biogeography. J Nat Hist 36:1047–1103. https://doi.org/10.1080/00222930110039602
Puri HS (1966) Ecology and distribution of recent Ostracoda. In: Proceedings of symposium on crustacea, Part I, Marine Biological Association of India, Mandapam, pp 457–495
Puri HS (1974) Normal pores and the phylogeny of Ostracoda. Geosci Man 6:137–151
Salvi G, Anderson JB, Bertoli M, Castagno P, Falco P, Fernetti M, Montagna P, Taviani M (2022) Recent Ostracod Fauna of the Western Ross Sea (Antarctica): a poorly known ıngredient of polar carbonate factories. Minerals 12:937. https://doi.org/10.3390/min12080937
Sars GO (1895) On some South African Entomostraca raised from dried mud. Vidensk Selsk Skrift Math Nat 8:1–56
Sato T, Kamiya T (2007) Taxonomy and geographical distribution of recent Xestoleberis species (Cytheroidea, Ostracoda, Crustacea) from Japan. Paleontol Res 11:183–227
Whatley RC, Moguilevsky A, Chadwick J, Toy N, Ramos MIF (1998) Ostracoda from the South West Atlantic. Part III: the Argentinian, Uruguayan and Southern Brazilian Continental Shelf. Rev Esp Micropaleontol 30:89–116
Williams M, Siveter DJ, Ashworth AC, Wilby PR, Horne DJ, Lewis AR, Marchant DR (2008) Exceptionally preserved lacustrine ostracods from the Middle Miocene of Antarctica: implications for high-latitude palaeoenvironment at 778 south. Proc R Soc B 275:2449–2454. https://doi.org/10.1098/rspb.2008.0396
World Health Organization (2009) Bromide in drinking-water. Background document for development of WHO guidelines for drinking-water quality
Acknowledgements
I thank to Dr. Takahiro Kamiya (Kanazawa University) for comments on the species and sending me published materials. I thank Dr. Randy Gibson (San Marcos Aquatic Resources Center, U.S. Fish and Wildlife Service) for his help on the English. Also, Yohanna Klanten (Université Laval, Québec) is thanked for her assistance during core samplings of the lakes. Dr. Alaettin Tuncer, Alper Ataman, and Miraç Aksu are thanked for their help on digitizing line drawings and providing laboratory assistance and SEM photographing. This study was carried under the auspices of the Presidency of the Republic of Türkiye, supported by the Ministry of Industry and Technology, and coordinated by TÜBİTAK MAM Polar Research Institute. This work was supported by TÜBİTAK (Project No: 121Y372).
Author information
Authors and Affiliations
Contributions
The corresponding author O.K. collected the samples from each location, identified the species, and wrote the main manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Külköylüoğlu, O. A new ostracod species of the genus Xestoleberis from Nansen Island, Wilhelmina Bay, Southern Ocean, Antarctica. Polar Biol 46, 1123–1136 (2023). https://doi.org/10.1007/s00300-023-03189-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-023-03189-7