Abstract
A small amount of information about the composition and functioning of prokaryotes and viruses in the Kara, Laptev and East Siberian seas makes it impossible to provide a quantitative assessment of all parameters of the carbon cycle which is necessary to analyze and predict the impact of climate change on the entire Arctic biota. Of particular interest are the areas of the inner and outer shelf of the Arctic seas which are, on the one hand, a zone of mixing of river and sea waters of the Ob, Yenisei, Lena, Indigirka and Kolyma rivers with a large amount of the smallest detrital particles; on the other hand, it is a zone of highly saline marine areas, which determined the task of our study. The modern methods of studying the structural and functional characteristics of microorganisms are used in the work: epifluorescence microscopy and transmission electron microscopy; the growth rate and production of plankton heterotrophic prokaryotes were determined by dilution method in situ and the method involving application of antibiotics, modified for natural habitats. On the shelf of the Siberian Arctic seas, the abundance of free viruses, frequency of visibly infected prokaryotic cells and viral-mediated mortality of prokaryotes values varied within 0.1–13.6 × 106 viruses mL−1, 0.2–5.6% abundance of prokaryotes, 1.4–64.4% production of heterotrophic prokaryotes, respectively. We suggest that the input of a large amount of the smallest organic particles on which a significant amount of viruses is absorbed into the coastal waters of the Siberian seas (including riverine–marine water mixing zone) leads to a decrease in viral infection and mortality of prokaryotes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The current state and changes in the Arctic Basin, both climatic and anthropogenic, largely depend on the processes occurring on the shelf of the marginal Arctic seas, mainly in areas of the strong continental runoff impact on the marine environment and biota (Lisitzin 1995; Gordeev et al. 1996; McClelland et al. 2012). Huge volumes of fresh water (2300–2500 km3 per year) containing large amounts of allochthonous nutrients and suspended and dissolved terrigenous material and pollutants (more than 150 million tons) enter the Arctic seas with Siberian river runoff (Flint et al. 2018). In the estuarine zone of the Ob and Yenisei rivers and in the zones adjacent to the Lena, Indigirka, and Kolyma estuaries, freshwater runoff interacts with marine water, forming unique high-gradient pelagic biotopes, where the matter entering with continental runoff is subjected to biogeochemical transformation (Flint et al. 2010).
In aquatic ecosystems, including the riverine and marine water mixing zone, heterotrophic prokaryotes play a key role in the decomposition and transformation of organic matter, both natural and anthropogenic, and are an important component of planktonic food webs through which the majority of the carbon flux passes (Cole et al. 1988; Wheeler et al. 1996). Protozoan grazing and viral lysis are the major cause of bacterioplankton mortality (Wommack and Colvell 2000; Anderson and Rivkin 2001; Weinbauer 2004; Vaque et al. 2008). When bacteria are consumed by protozoans, a significant amount of bacterial carbon enters higher levels of the food web. As a result of viral lysis, carbon of lysed cells does not pass to higher trophic levels but enters the environment (“viral shunt”) and is actively involved in metabolism of heterotrophic bacteria, i.e., captured in the microbial loop (Wommack and Colvell 2000; Middelboe et al. 2002).
Studies conducted in different Arctic regions have demonstrated that viruses are the most numerous component of the plankton community and play a significant role in the functioning of microbial communities in cold waters, as well as in temperate and tropical marine waters (Suttle 2007; Maranger et al. 2015; Sandaa et al. 2018). In the meantime, until recently, data on the ecological role of viruses in the Siberian Arctic seas were not available.
There are very few works focused on the study of planktonic microbial communities on the inner shelf of the Siberian Arctic seas that receive large river discharge, as well as factors that determine the structural and functional characteristics of heterotrophic microorganisms and viruses. Such studies are of interest, especially for predicting possible changes in the structural and functional organization of planktonic microbial communities due to an increase in the temperature of the surface waters of the Arctic seas and an increasing runoff of Siberian rivers (Gordeev 2000).
The aim of this work was to study the effects of runoff from large Siberian rivers on the structural and functional characteristics of virus communities on the inner and outer shelves of the Siberian Arctic seas. Based on the published (Kopylov et al. 2015, 2017, 2020, 2021a, b) and unpublished new data, this article is the first to briefly overview the features of the virioplankton structure, the level of viral infection, and virus-induced mortality of heterotrophic prokaryotes in the riverine and marine waters mixing zone versus those in the zones significantly less subjected to the effect of runoff from the Ob and Yenisei rivers in the Kara Sea; the Lena River in the Laptev Sea; and the Indigirka and Kolyma rivers in the East Siberian Sea.
Materials and methods
Study sites and sampling
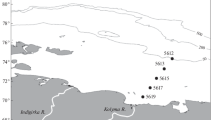
The studies were conducted in the riverine–marine water mixing zone (RMMZ): in the Kara Sea, in the northern part of the Yenisei estuary в September 17–22, 2011 (Kopylov et al. 2015), and the northern part of the Ob estuary in September 3–7, 2013 (Kopylov et al. 2017); in the Laptev Sea, in the area adjacent to the Lena Delta in September 8–14, 2015 (Kopylov et al. 2020); in the East Siberian Sea, in areas adjacent to the Kolyma estuaries in September 8–9, 2017 (Kopylov et al. 2021a) and Indigirka in September 5–7, 2017 (Kopylov et al. 2021b), and in outer shelf high-salinity marine water zones (MZ) (Table 1, Fig. 1). Water samples were collected during cruises 59, 63, and 69 of the R/V Akademik Mstislav Keldysh and cruise 125 of the R/V Professor Shtockman with 5-L Niskin bottles on a Rosette 32 sampler equipped with a CTD (SBE-911, Sea Bird Equipment, USA).
Ten water samples were taken from horizons of 0, 5, 6, 8, 10, 16, 20, 25 m at 3 stations in the RMMZ in the Kara Sea in the northern part of the Yenisei estuary (depths cтaнций 20–28 m). Twelve samples of water were taken from horizons of 0, 5, 6, 8, 12, 15, 16, 18, 20 m at 3 stations in the RMMZ in the Kara Sea in the northern part of the Ob estuary (depths of stations of 16–23 m). Eight water samples were taken from horizons of 0, 8, 10, 20 m at 3 stations in the RMMZ in the Laptev Sea, in the area adjacent to the Lena Delta (depths of stations of 10–23 m). Ten water samples were taken from horizons of 0, 6, 8, 10, 11, 17, 20 m at 2 stations in the RMMZ adjacent to the mouth of the Indigirka River (depths of stations of 13–21 m). Eleven water samples were taken from horizons of 0, 5, 8, 13, 16, 17, 20 m at 2 stations in the RMMZ adjacent to the mouth of the Kolyma River (depths of stations of 17–22 m). Five water samples were taken from horizons of 0, 9, 30, 60, 115 m on the outer shelf in the Kara Sea, at a station in MZ located to the north of the Yenisei estuary (station depth of 120 m). Eight water samples were taken from horizons of 0, 8, 15, 20, 23, 27, 35 m in the Kara Sea at 3 MZ stations located to the north of the Ob estuary (depths of 30–37 m). Eleven water samples were taken from horizons of 0, 8, 20, 26, 45, 60, 70, 83 m in the Laptev Sea at 3 stations in the MZ (depths of stations of 71–90 m). Twelve water samples were taken from horizons of 0, 15, 17, 20, 25, 27, 33, 42, 47, 54 m in the East Siberian Sea, at 3 stations in the MZ located north of the mouth of the Indigirka River (depths of stations of 45–59 m). Nine water samples were taken from horizons of 0, 15, 20, 26, 20, 33, 35, 47 m at 2 stations in the MZ located north of the mouth river Kolyma in the East Siberian Sea (depth of stations of 47 m).
Temperature and salinity data were obtained by the SBE-911 via temperature and conductivity sensors. Water subsamples (150 mL) for microscopic studies were fixed with 25% glutaraldehyde (final concentration 1%) immediately after sampling.
Prokaryote abundances and sizes
The abundance and size of heterotrophic prokaryotes were estimated by epifluorescence microscopy. From 2 to 7 mL of fixed water subsample was poured onto a black Nuclepore filter (pore size 0.2 μm) and stained with DAPI (4′,6-diamidino-2-phenylindole) at a final concentration of 1 μg mL−1 (Porter and Feig 1980). Counts were done under an Olympus BX51 epifluorescent microscope (Olympus, Japan) using Cell F Image Analysis Software at 1000× magnification. On each filter, at least 400 prokaryotes were counted and the dimensions of at least 100 cells were measured. The carbon content in prokaryotic cells (C, fgC cell−1) was calculated using the allometric equation C = 120 × V0.72, where V is the mean volume of prokaryotic cells, µm3 (Norland 1993).
Growth and production of heterotrophic prokaryotes
The growth rate and production of plankton heterotrophic prokaryotes were determined by dilution method according to changes in their abundance in isolated water samples which were exposed to in situ temperature in a temperature-controlled chamber for 36–40 h. Samples of the sea water were diluted 1:10 with water filtered through a 0.2 µm pore size membrane filter to remove phagotrophic organisms (Ducklow and Hill 1985; Tremaine and Mills 1987). Sample volume to carry out the dilution method was 100 mL.
All experiments were conducted in three replicates. The prokaryotic specific growth rate (μ, h−1) was calculated according to the formula: μ = (lnNt − lnN0)/t, where N0 and Nt are initial and final abundances of prokaryotes, t is the incubation time, h. The production of prokaryotic plankton (PPR, mg C m−3 day−1) was calculated as the product of the prokaryotic specific growth rate and biomass in undiluted sea water.
The heterotrophic prokaryote production in the Kara Sea was estimated by a method involving application of antibiotics (Sherr et al. 1986), modified for natural habitats (Weisse 1989; Sazhin et al. 2010). In contrast to the original method, the water pre-filtration to remove the mesozooplankton was not applied, taking into account its low abundance in the study area, but the samples were controlled visually. Our reason for skipping the water filtration was to avoid increasing the extra organic matter, which may result after the destruction of the plankton organisms during the water’s sieving. Finally, additional slides were prepared to take into account the number of potential bacteria consumers and heterotrophic nano- and microplankton. The grazing on bacteria was estimated using the parallel exposition of the samples with and without antibiotics (benzylpenicillin, 1 mg L–1; vancomycin, 200 mg L–1), which stopped the bacterial growth but had no influence on the grazers (Sherr et al. 1986). In order to assess the antibiotics’ activity dynamics and their possible impact on the bacterial lysis, as well as to estimate the daily dynamics of the bacterial growth, experiments were performed simultaneously with bacterial production incubation As a result, the bacterial production was calculated for a 12 h time period for the further recalculation for a 24 h time period taking into account the daily dynamics of the bacterial abundance (Sazhin et al. 2010).
Particle abundances
Yellow pico-sized organic particles (0.25 to 3.0 µm in size), which were clearly distinguished from prokaryotic cells, were also counted, as well as prokaryotes, on DAPI-stained filters by epifluorescence microscopy (Porter and Feig 1980; Mostajir et al. 1995; Wells and Deming 2003). On each filter, at least 400 of smallest detrital particles were counted.
Viral abundance
The viral particles were counted under an epifluorescent microscope using SYBR Green I fluorochrome and Whatman Anodisc aluminum oxide membrane filters (pore size 0.02 μm) (Noble and Fuhrman 1999). Depending on the viral abundance, between 0.2 and 1.0 mL of water was poured onto the Anodisc filters. For each water sample, two filters were analyzed; counts yielded a minimum of 600 viruses. The carbon content in the viral particles was taken as 0.055 fg C virus−1 (Steward et al. 2007).
Concurrently, viruses were counted and their sizes were determined using transmission electron microscopy (Bettarel et al. 2000). Viruses were identified on the basis of morphology (round or hexagonal capsid structures, tailed and nontailed), size and staining characteristics. In addition, we measured the capsid size of viruses, determined the proportion of prokaryotic cells with attached viruses of the total prokaryotic abundance, the proportion of smallest detrital particles with attached viruses of the total abundance of smallest detrital particles, the abundance of viruses attached to one prokaryotic cell and to one detrital particle.
Viral activities
Transmission electron microscopy was used to estimate the frequency of visibly infected cells (FVIC, estimated as the share (%) of total prokaryotic abundance), the mean number of fully matured phages in prokaryotes [i.e., burst size (BS), viruses cell−1]. Samples (50 mL) were centrifuged at 100,000×g for 2 h on an OPTIMA L-90k ultracentrifuge (Beckman Coulter, USA) on Pioloform/carbon-coated 400-mesh nickel grids to obtain a bacteria and phage concentrate. Two grids were thus prepared for each water sample. The grids were then positively stained at room temperature with 1% aqueous solutions of uranyl acetate and lead citrate. The grids were further analyzed under a JEM 1011 electron microscope (Jeol, Japan) at 50,000×–150,000× magnification. No less than 800 prokaryotic cells were analyzed per grid.
To calculate the fraction of infected cells to the total heterotrophic prokaryotic abundance (i.e., FIC, %), the following equation was used (Binder 1999):
The viral-mediated mortality of prokaryotes (VMPR) (expressed as a percentage of the production of heterotrophic prokaryotes, PPR) was estimated as (Binder 1999):
The absolute values of both FVIC and FIC were used.
The rate of viral-induced mortality of prokaryotes (VIM, cell mL−1 day−1 or mgC m−3 day−1) was calculated by the equation VIM = VMPR × PPR. The production of virioplankton (PV, viruses mL−1 day−1) was calculated by the equation PV = BS × VIM, where VIM is cells mL−1 day−1. The viral turnover time (TV) was estimated as the viral abundance divided by viral production. The amount of labile organic matter released from lysed prokaryotic cells into the water was calculated as the difference between VIM and PV. The values obtained are overstated, since the calculations did not take into account energy consumption by viruses for capsid protein synthesis and nucleic acid replication. Such data are still unknown and unavailable in the literature.
Statistical analyses
Correlations between the parameters were analyzed using to the Pearson’s correlation coefficient calculated by Past 4.03 software (Hammer et al. 2001) and regarding the prerequisites for the data analyzed. The differences between the biological parameters in the described zones were detected using an independent sample t test for equality of the means.
Results
Environmental parameters
Freshwater of Siberian Rivers with a lower density than seawater spread in the upper layer of coastal sea water with a thickness of 6–15 m. The salinity in the upper layer of the water column increased with distance from the river mouth due to horizontal and vertical mixing of riverine and sea water. In our research, the average salinity for the water column in RMMZ was 7–15 times lower than that in MZ; water temperature, conversely, was 3–6 times higher (Table 1) (Flint et al. 2018).
In areas of the inner shelf of Arctic seas adjacent to mouths of large Siberian rivers, coastal marine water, along with desalination, is enriched with dissolved and suspended organic matter, in particular, with the smallest organic particles from 0.25 to 3.0 µm stained with DAPI, which are pico-sized detritus (PPD) (Mostajir et al. 1995; Wells and Deming 2003). The maximum concentration (up to 35 × 105 particles mL−1) of such PPD was detected in RMMZ of the East Siberian Sea near the Kolyma estuary (Table 2). In MZ, the average abundances of the PPD (NPPD) were three to nine times lower than in RMMZ. The proportion of PPD with attached viruses in NPPD was on average 21.9 ± 8.7% (range: 10.7–31.7%) for RMMZ and 25.5 ± 4.7% (range: 21.3–31.0%) for MZ. The maximum number of viruses attached to one detrital particle was 15.
Abundance and production of prokaryotes
In our studies, the abundance of prokaryotes in the Kara and Laptev seas (NPR) was significantly higher in RMMZ than in MZ: by 2.5–6.0 times. The same tendency was observed in the East Siberian Sea where these values differed by 1.3–1.4 (Table 2). On the shelf of Siberian Artic seas, the average cell volume of prokaryotes was between 0.035 and 0.135 µm3. In the Kara and Laptev seas the biomass of prokaryotes in RMMZ was significantly higher than in MZ (t 19 = 2.13, p = 0.007; t 12 = 2.2, p = 0.003; t 15 = 2.36, p = 0.001 for Ob, Yenisei and Lena rivers respectively) (Fig. 2). The opposite situation was observed in the western part of the East Siberian Sea, where in the area of the inner shelf receiving Indigirka River runoff, the biomass of prokaryotes in RMMZ was 1.5 times lower than in MZ (t 13 = 2.16, p = 0.009) due to the higher average volume of prokaryotic cells in outer shelf waters and the small difference between the abundance of prokaryotes in waters of the inner and outer shelf (Fig. 2). The difference in prokaryotic production (PPR) between RMMZ and MZ was statistically significant (t 19 = 2.12, p = 0.05; t 16 = 2.45, p = 0.019 for Ob and Lena rivers, respectively) only in the Kara and Laptev seas (Fig. 2).
Means (± standard deviation) of cell volumes of prokaryotes, VPR, µm3 (a); biomass of prokaryotes, BPR, mgC m−3 (b); production of prokaryotes, PPR, 103 cell mL−1 day−1 (c) and production of prokaryotes, PPR, mgC m−3 day−1 (d) in RMMZ and MZ in the areas where Siberian rivers flow into the Arctic seas (O-KS Ob River-Kara Sea, Y-KS Yenisei River-Kara Sea, L-LS Lena River-Laptev Sea, I-ESS Indigirka River-East Siberian Sea, K-ESS Kolyma River-East Siberian Sea)
The NPR/NPPD ratio in RMMZ in the Kara and Laptev seas (3.0–4.2) was markedly higher than in the East Siberian Sea (1.3–2.3). The ratio was 1.2–10.2 times higher in MZ versus RMMZ. The proportion of prokaryotes with attached viruses in NPR was on average 17.1 ± 2.9% (range: 10.0–31.0%) for the surveyed RMMZ and 16.5 ± 3.9% (range: 8.8–38.9%) for MZ. In all samples studied, 1 to 7 viruses were most often attached to the surface of one prokaryotic cell; in rare cases, up to 30 viruses were attached.
Structure and abundance of virioplankton
Virioplankton included free viruses (NVF), viruses attached to prokaryotic cells (NVPR), and viruses attached to particles of pico-sized detritus (NVPPD) (Fig. 3). The abundance of free viruses (NVF) in the East Siberian Sea tended to be lower in RMMZ than in MZ, whereas in the Kara and Laptev seas, on the contrary, NVF was significantly higher in RMMZ than in MZ (t 20 = 2.1, p = 0.085; t 12 = 2.26, p = 0.032; t 15 = 2.36, p = 0.005 for Ob, Yenisei and Lena rivers respectively) (Table 2). The NVF/NPR ratio in shelf waters of the Siberian Arctic seas varied between 1.5 and 39.1, and was on average higher in MZ (4–11) than in RMMZ (2–6). The capsid diameter of free viruses (DV) ranged between 16 and 389 nm and was on average higher in RMMZ than in MZ. The proportion of viruses with DV less than 40 nm was higher in MZ, while the proportion of large viruses with DV larger than 100 nm was higher in RMMZ (Fig. 4).
Electron micrographs of viruses in shelf waters of the Siberian Arctic seas: a–c free virus particle; d prokaryotes with viruses on surface—black arrows indicate viruses on cell surface; e virus-infected prokaryotes—black arrows indicate viruses inside cell; f, g viruses attached to detrital particle, black arrows indicate viruses attached to detrital particle
Fraction (%) of free viruses with capsid diameters < 40 nm (1), 40–100 nm (2) and > 100 (3) in RMMZ and MZ in the areas where the Siberian rivers flow into the Arctic seas (O-KS Ob River-Kara Sea, Y-KS Yenisei River-Kara Sea, L-LS Lena River-Laptev Sea, I-ESS Indigirka River-East Siberian Sea, K-ESS Kolyma River-East Siberian Sea). Numerals indicate the average diameter of capsids of free viruses (mean ± SD, nm)
The abundance of viruses attached to prokaryotic cells (NVPR) and their proportion in the total amount of virioplankton (NVT) in RMMZ (on average 1.9–4.8 × 105 viruses mL−1 and 4–14%, respectively) tended to be slightly higher than in MZ (on average 0.3–4.4 × 105 viruses mL−1 and 3–10%, respectively). At the same time, the abundance of viruses attached to PPD (NVPPD, on average (1.7–4.9) × 105 viruses mL−1) (t 19 = 2.13, p = 0.001; t 12 = 2.18, p < 0.0001; t 16 = 2.45, p = 0.009; t 18 = 2.36, p = 0.002 for Ob, Yenisei, Lena and Indigirka rivers respectively) and their proportion in NVT in RMMZ (12–15%) (t 14 = 2.26, p = 0.014; t 12 = 2.2, p = 0.007; t 16 = 2.31, p = 0.092; t 10 = 2.23, p = 0.044 for Ob, Yenisei, Lena and Indigirka rivers respectively) were significantly higher than those in MZ (on average 0.2–1.6 × 105 viruses mL−1 and 2–5%, respectively) in the Laptev and Kara Seas and had the same tendency in the East Siberian Sea.
The NVPPD/NVT ratio reached 35–39% in the East Siberian Sea and 21–25% in the other two seas. As a result, the virioplankton structure in RMMZ differed from that in MZ by a higher proportion of viruses attached to PPD and a lower proportion of free viruses (Fig. 5).
Shares (%) of free viruses (3); viruses attached to the cells of prokaryotes (2) and viruses attached to the particles of pico-sized detritus (1) in the total abundance of virioplankton in RMMZ and MZ in the areas where Siberian rivers flow into the Arctic seas (O-KS Ob River-Kara Sea, Y-KS Yenisei River-Kara Sea, L-LS Lena River-Laptev Sea, I-ESS Indigirka River-East Siberian Sea, K-ESS Kolyma River-East Siberian Sea)
Viral infection and virus-mediated mortality of prokaryotes
The frequency of visibly infected cells (FVIC) and the viral-mediated mortality of prokaryotes (VMPR) in shelf waters of the Siberian Arctic seas varied widely (0.2–5.6% NPR и 1.4–64.4% PPR, respectively (Table 3). The maximum FVIC and VMPR values, with the exception of the Indigirka River, an East Siberian Sea section, were found in the RMMZ. The average values of FVIC and VMPR in the RMMZ of all three seas did not differ significantly, while in the MZ of the East Siberian Sea they were 1.6–2.3 times higher than in the MZ of the Kara and Laptev seas.
A moderate negative correlation between NVPPD and FVIC (r = -0.45, p = 0.04, n = 21) and a moderate positive correlation between PPR and FVIC (r = 0.51, p = 0.03, n = 21) were found in RMMZ of the East Siberian Sea.
Rod-shaped prokaryotes predominated in NPR in all RMMZ, whereas on the outer shelf of seas, rod-shaped prokaryotes dominated in three MZ, and vibrio-shaped prokaryotes, in two MZ (Fig. 6). The proportion of coccus-shaped prokaryotes and vibrio-shaped prokaryotes in the total abundance of infected prokaryotes tended to be higher in MZ than in RMMZ. At the same time, infected filamentous prokaryotes were either not detected in MZ or their proportion in NPRVIC was significantly lower than that in RMMZ.
Shares (%) of rod-shaped prokaryotes (1), coccus-shaped prokaryotes (2), vibrio-shaped prokaryotes (3), filamentous prokaryotes (4) in the total number of infected prokaryotes in RMMZ and MZ in the areas where Siberian rivers flow into the Arctic seas (O-KS Ob River-Kara Sea, Y-KS Yenisei River-Kara Sea, L-LS Lena River-Laptev Sea, I-ESS Indigirka River-East Siberian Sea, K-ESS Kolyma River-East Siberian Sea)
The average number of viruses inside prokaryotic cells (BS) on the shelf of the Siberian Arctic seas varied within 5.7–17.4 viruses per cell, reaching maximum values in the RMMZ of the Kara Sea (Table 3). With the exception of the Yenisei River—the Kara Sea region, the differences in BS between RMMZ and MZ were not significant.
The minimum and maximum ratio of NPRVIC to the abundance of prokaryotes with attached viruses (NPRV) differed by an order of magnitude: from 5 to 56 cells with attached viruses per one cell with visible intracellular viruses. The average NPRVIC/NPRV ratios in the East Siberian Sea were close, whereas they differed by 1.6–2.0 times in the Kara Sea. In the entire data set, NPRVIC and NPRV were positively correlated (r = 0.92, p < 0.005, n = 83).
As a result, viruses lysed on average for RMMZ and MZ: in waters of the East Siberian Sea (0.3–0.5) × 105 and (0.3–0.4) × 105 cells mL−1 day−1, respectively; in waters of the Laptev Sea, 0.3 × 105 and 0.03 × 105 cells mL−1 day−1, respectively; and in waters of the Kara Sea (1.0–1.4) × 105 and 0.3 × 105 cells mL−1 day−1, respectively.
Viral production
The lytic viral production (PV) in RMMZ in the Kara Sea was essentially higher than in RMMZ in the Laptev and East Siberian seas (Table 3). At the same time, if the average PV values in the East Siberian Sea were similar in RMMZ and MZ or differed only by 1.6 times, in other seas they differed by 6.8–7.1 times. The average TV values in the Kara Sea area subjected to the effect of the Ob River runoff were markedly higher than in other studied areas of the Siberian Arctic seas. The minimum TV values were recorded in the East Siberian Sea (Table 4).
Discussion
Characteristics of particle-rich waters
Rivers flowing into Siberian Arctic seas are characterized by a high concentration of terrigenous suspended matter. Indigirka and Kolyma are the most turbid rivers among all the major rivers of the Russian Arctic. The average concentration of suspended matter in the river runoff in the East Siberian Sea is 134 g m−3, which is 3–7 times higher than for rivers that flow into all other seas of the Russian Arctic (Gordeev et al. 1996).
The maximum concentrations of particles found in the RMMZ in the Siberian Arctic seas (3.8–34.9 × 105 mL−1) were higher than the concentrations of particles found nearshore in the vicinity of the mouths of Canadian Arctic rivers. On the Beaufort Shelf, the concentration of particles in the surface waters (≤ 6 m) was maximum near the Mackenzie River Delta (1.7 × 105 mL−1), which then declined to the observed minimum of 2.75 × 103 mL−1 off the shelf (Wells et al. 2006). In Franklin Bay, surface particle concentrations at the station near the mouth of the Holton River (3.0 × 104 mL−1) were an order of magnitude higher than in surface waters of other marine stations (8.9 × 103 mL−1) (Wells et al. 2006).
Factors affecting virus dynamics and prokaryote-virus interactions
Analysis of data on the distribution of temperature, salinity, abundance of microorganisms and viruses in the Siberian Arctic seas (Kopylov et al. 2015, 2017, 2020, 2021a, b) showed that on the shelf of the Kara Sea and the Laptev Sea, between water temperature (T, °C) and NPR and between T and PPR strong positive correlations existed (respectively, r = 0.69, p < 0.005, n = 77 and r = 0.8, p < 0.005, n = 52). Negative correlations were observed between salinity (S) and NPR (r = − 0.74, p < 0.005, n = 77) and between S and PPR (r = − 0.84, p < 0.005, n = 52). At the same time, on the shelf of the East Siberian Sea, no relationship between the NPR, PPR and TºC. Only a negative correlation was detected between water salinity and PPR: r = − 0.5, p < 0.001, n = 37.
According to the results of other microbiological studies in the coastal waters of the Siberian Arctic seas, the maximum abundance and biomass of planktonic prokaryotes were recorded, as a rule, in the zones near deltas and estuaries of the rivers, and the prokaryotic abundance and biomass decreased beyond the effect of river runoff significantly (Sorokin and Sorokin 1996; Meon and Amon 2004; Mosharova et al. 2016; Bezzubova et al. 2020).
The results of NVF and NVF/NPR determination in the Siberian Arctic seas are within the range of values recorded in the central Arctic and other Arctic seas, namely (0.1–64.1) × 106 viruses mL−1 and 0.8–70.0 respectively (Steward et al. 1996, 2007; Howard-Jones et al. 2002; Hodges et al. 2005; Clasen et al. 2008; Venger et al. 2016). The FVIC and VMPR in shelf waters of the Siberian Arctic seas were in the range of values of these parameters recorded in the central Arctic and other Arctic seas: 0–5.2% and < 1.0–57.9% PPR respectively (Steward et al. 1996, 2007; Howard-Jones et al. 2002; Hodges et al. 2005; Clasen et al. 2008; Venger et al. 2016).
On the shelf of the Kara and Laptev seas, positive correlations were observed between NPR and NVF, as well as between NPR and NPRVIC, between PPR and NPRVIC, between T and NPRVIC: r = 0.74, p < 0.005, n = 77; r = 0.7, p < 0.005, n = 77; r = 0.58, p < 0.005, n = 52 and r = 0.67, p < 0.005, n = 77 respectively. The NVF and NPRVIC were negatively correlated with S: r = − 0.73, p < 0.005, n = 77 and r = − 0.72, p < 0.005, n = 77, respectively. At the same time, no correlations were found between NPR and NVF and between T and NPRVIC on the shelf of the East Siberian Sea, but positive correlations were found between NPR and NPRVIC (r = 0.74, p < 0.005, n = 41), as well as between PPR and NPRVIC (r = 0.59, p < 0.005, n = 41).
Whereas in the Kara and Laptev seas the average FVIC and VMPR were higher by 1.4–1.9 times in RMMZ than in MZ, the values of the parameters were similar in these zones of the East Siberian Sea (Table 3).
The close values of viral infection of prokaryotes in waters with positive (in RMMZ) and negative (in MZ) temperatures in the East Siberian Sea are apparently explained by a relatively small difference between the values of abundance and production of prokaryotes affecting the degree of their infection in RMMZ and MZ compared to other Siberian Arctic Seas.
Based on the experimentally obtained values of the specific growth rate of heterotrophic prokaryotes (µ) in RMMZ (range = 0.241–0.398 day−1, mean = 0.308 day−1; T = 0.2–6.7 °C) and in MZ (range = 0.211–0.344 day−1, mean = 0.278 day−1, T = − 0.5 to − 1.7 °C), the community of planktonic prokaryotes in the outer shelf of the East Siberian Sea is adapted to negative water temperature conditions. As a result, in the East Siberian Sea, the average prokaryote production in RMMZ and MZ differed only by 1.2 times, whereas in the Laptev and Kara Seas prokaryote production in RMMZ was higher than in MZ by 2–6 times.
As shown previously, planktonic bacteria in the North Water Polynya can grow and reproduce viruses at subzero temperatures at rates comparable to bacteria in temperate systems, if the rate of organic carbon supply is sufficiently high and even though their temperature growth optima are always much higher than in situ temperatures (Middelboe et al. 2002). During the study period, the organic carbon supply of planktonic prokaryotes inhabiting inner and outer shelf of the East Siberian Sea differed slightly. The concentrations of dissolved organic carbon (DOC) in the East Siberian Sea in September 2017 at stations adjacent to the Indigirka River estuary and on the outer shelf were comparable: 2.0–15.6 and 0.2–24.3 mg C L−1, respectively; at stations adjacent to the Kolyma River estuary and on the outer shelf, 2.0–13.9 and 2.0–10.6 mg C L−1, respectively. The DOC concentrations were determined at the same stations and in the same samples, where heterotrophic prokaryotes and viruses were examined. The maximum values were recorded in the near-bottom layers. The DOC distribution in the offshore area has no or an extremely weak correlation with river runoff (Flint et al. 2018). Furthermore, at the end of the growing season, mortality of planktonic organisms was observed in the shelf waters of the East Siberian Sea. The vertical flux of organic carbon of dead organisms (larvacean houses and carcasses of copepods) and fecal pellets, determined on the shelf in the western part of the East Siberian Sea using sediment traps, was 54–55 mg C m−2 day−1 in the plume zone of the Indigirka River and 32–45 m−2 day−1 in the marine area of the shelf (Drits et al. 2019).
At the same time, a high content of the smallest detrital particles in the water mixing zones can decrease the level of infection and mortality of prokaryotes. Contacts between viruses and the smallest suspended organic particles are more frequent at a higher NPPD in the East Siberian Sea compared to other Siberian Arctic seas; as a result, a significant amount of free viruses is adsorbed to PPD, which reduces the possibility of viral infection of new prokaryotic cells (Murray and Jackson 1992). Accordingly, the frequency of contacts between viruses and prokaryotes decreases in these waters, which apparently decreases the level of viral infection and virus-induced mortality of prokaryotes. This phenomenon was described earlier (Murray and Jackson 1992). The low abundance of viruses during the transitional period from spring to summer is explained by the adsorption of viruses to inorganic suspended particles entering the coastal waters of Arctic seas with runoff from adjacent areas and to organic (detrital) particles formed in large quantities after phytoplankton bloom (Schoemann et al. 2005). As a result of glacier melting in the Arctic, a large amount of small inorganic and organic particles, to which a significant amount of viruses are adsorbed, enter the coastal waters, which leads to a decrease in viral infection and mortality of algae and prokaryotes (Maat et al. 2019a). The researchers have suggested that during further global warming, viral losses will increase due to adsorption to suspended particles entering the marine environment from melting Arctic glaciers (De Corte et al. 2011; Maat et al. 2019b).
Warming of the Arctic causes increased river runoff, coastal erosion, and thawing of permafrost. Together, this leads to increasing washout of terrestrial dissolved and particulate organic matter into coastal Arctic ecosystems. This organic matter can be expected to affect both the carbon and nutrient flux through the microbial food web and composition of the microbial community (Müller et al. 2018). Permafrost degradation recorded in recent decades is causing an increase in Siberian river runoff into Arctic regions (Lisina et al. 2020). An increase in inflow to the Arctic seas of freshwater from Siberian rivers with an even higher content of suspended organic matter is probably leading to an increase in adsorption of viruses to suspended particles and a decrease in the role of viruses in the mortality of heterotrophic prokaryotes.
Comparison of viral production in the Siberian Arctic Seas and in other Arctic regions
The PV measured in the water column of the Siberian Arctic seas were either comparable with production rates previously reported for Arctic waters (1.1 × 105 to 13.2 × 105 viruses mL–1 day–1 in the North Water Polynya by Middelboe et al. (2002); 7.2 × 105 to 122.4 × 105 viruses mL–1 day–1 in the North Sea by Winter et al. (2004); 0.7 × 105 to 18.5 × 105 viruses mL–1 day–1 in Franklin Bay by Wells and Deming (2003); 0.2 × 105 to 140 × 105 viruses mL–1 day–1 in the Chukchi Sea by Steward et al. (1996) and 0.3 × 105 to 77 × 105 viruses mL–1 day–1 on Canadian Arctic Shelf (CAS) by Payet and Suttle (2013) or lower (14.4 to 84 × 105 viruses mL–1 day–1) at a depth of 1.5–3.0 m during the ice melting season in the Kongsfjorden by De Corte et al. (2011). The TV values on the shelf of the Siberian Arctic seas were comparable to those obtained in CAS (stations in the Mackenzie–Amundsen Gulf System, Franklin Bay): range: 0.01–0.52 day−1 (Payet and Suttle 2013).
Viral lysis of prokaryotes as source of organic carbon for noninfected prokaryotic populations
Viral lysis causes the death of a host cell but also releases additional nutrients to the microbial food web. The release of major nutrients (C, N and P) by viral lysis can relieve nutrient limitation and encourage the growth of noninfected bacterial populations (Noble and Fuhrman 1999; Wilhelm and Suttle 1999). In the Siberian Arctic Seas, the amount of organic carbon entering the aquatic environment as a result of viral lysis of prokaryotes (VIM − PV) ranged between 0.1 and 47.2 mg C m−3 day−1 (on average 0.7–6.9 mg C m−3 day−1) in RMMZ and between 0.04 and 2.4 mg C m−3 day−1 (on average 0.1–1.3 mg C m−3 day−1) in MZ (Fig. 7). As a result, the (VIM − PV)/PPR ratio varied between 2.2 and 64.4% (on average 7.0–25.6%) in RMMZ and between 1.1 and 22.2% (on average 4.8–10.7%) in MZ. Assuming that an average prokaryotic growth efficiency is 27% (Meon and Amon 2004), we calculated the daily organic carbon requirements of prokaryotic plankton (CPR) (Fig. 6). As a result, the (VIM − PV)/CPR ratio varied between 0.6 and 17.4% (on average 1.9–6.9%) in RMMZ and between 0.3 and 6.0% (on average 1.3–2.9%) in MZ (Fig. 7). The release of dissolved organic matter by viral lysis can lead to an increase in the prokaryotic production (by ~ 30%) (Fuhrman and Suttle 1993). Fischer and Velimirov (2002) showed that on average 15.2 μg C L−1 day−1 could potentially be released from viral lysis of bacterial cells, which corresponded to 46% of bacterial production in the water column. Thus, in general, the labile organic matter released by viral lysis of prokaryotes was a small source of carbon for noninfected heterotrophic prokaryotes on the shelf of Siberian Arctic Seas at the end of the growing season. However, in some RMMZs in the Kara Sea, the viral lysates were an important nutrient source for the noninfected prokaryotes.
The mean (± standard deviation) quantity of organic matter released by viral lysis of prokaryotes, VIM – PV, mgCm-3 × day−1 (a); daily organic carbon requirements of prokaryotic plankton, CPR, mgCm-3 × day−1 (b); (VIM − PV)/CPR ratio, % (c) in RMMZ and MZ in areas where Siberian rivers flow into Arctic seas (O-KS Ob River-Kara Sea, Y-KS Yenisei River-Kara Sea, L-LS Lena River-Laptev Sea, I-ESS Indigirka River-East Siberian Sea, K-ESS Kolyma River-East Siberian Sea)
Conclusions
The structure of virioplankton in the area of the inner shelf in zones affected by river runoff differs from that in the area of the outer shelf by a higher proportion of viruses attached to the smallest detrital particles and a higher proportion of viruses with a capsid diameter greater than 100 nm. At the same time, adsorption of large amounts of viruses to detrital particles translates into lower contact rates between host and viruses, reducing mortality of heterotrophic prokaryotes. In the Kara and Laptev Seas, the mortality of prokaryotes due to viral lysis in the autumn period was on average significantly higher in the inner shelf area in RMMZ (in conditions of higher water temperature and higher abundance and production of prokaryotes) than in the outer shelf area in zones with higher water salinity. Among the areas studied in the shelf of the Siberian Arctic Seas, the highest values of virus-induced mortality of prokaryotes were recorded in the Ob River—Kara Sea mixing zone. In the East Siberian Sea, where the difference in the abundance and production of prokaryotes in the studied zones of the inner and outer shelf was less significant than in other seas and the content of the smallest detrital particles in RMMZ was maximum, the average values of virus-induced mortality of prokaryotes did not differ.
In general, according to our data, in autumn, planktonic viruses play a minor role in controlling the abundance and production of heterotrophic prokaryotes in shelf waters of the Siberian Arctic seas, but in some water layers, both on the inner and outer shelf, the role of viruses in the mortality of prokaryotes is significant.
Abbreviations
- RMMZ:
-
Riverine and marine water mixing zone
- MZ:
-
Marine water zone
- PPD:
-
Particles of pico-sized detritus
- N PPD :
-
Abundance of particles of pico-sized detritus
- N PPDV :
-
Abundance of particles of pico-sized detritus with attached viruses
- N PR :
-
Abundance of prokaryotes
- N PRV :
-
Abundance of prokaryotes with attached viruses
- N PRVIC :
-
Abundance of visibly infected prokaryotic cells
- V PR :
-
Volume of prokaryotic cell
- B PR :
-
Biomass of prokaryotes
- P PR :
-
Production of heterotrophic prokaryotes
- N VF :
-
Abundance of free viruses
- N VPR :
-
Abundance of viruses attached to prokaryotic cells
- N VPPD :
-
Abundance of viruses attached to particles of pico-sized detritus
- D VF :
-
Capsid diameter of free viruses
- F VIC :
-
Frequency of visibly infected prokaryotic cells
- N PRVIC :
-
Abundance of infected prokaryotic cells
- BS:
-
Burst size
- FIC:
-
Frequency of infected prokaryotic cells
- VMPR:
-
Viral-mediated mortality of prokaryotes
- VIM:
-
Viral-induced mortality of prokaryotes
- P V :
-
Virioplankton production
- T V :
-
Virioplankton turnover time
References
Anderson MR, Rivkin RB (2001) Seasonal patterns in grazing mortality of bacterioplankton in polar oceans: a bipolar comparison. Aquat Microb Ecol 23:195–206. https://doi.org/10.3354/ame025195
Bettarel Y, Sime-Ngando T, Amblard C, Laveran H (2000) A comparison of methods for counting viruses in aquatic systems. Appl Environ Microbiol 66(6):2283–2289. https://doi.org/10.1128/aem.66.6.2283-2289.2000
Bezzubova EM, Seliverstova AM, Zamyatin IA, Romanova ND (2020) Heterotrophic bacterioplankton of the Laptev and East Siberian Sea shelf affected by freshwater inflow areas. Oceanology 60(1):62–73. https://doi.org/10.1134/S0001437020010026
Binder B (1999) Reconsidering the relationship between virally induced bacterial mortality and frequency of infected cells. Aquat Microb Ecol 18:207–215. https://doi.org/10.3354/ame018207
Clasen JL, Brigden SM, Payet JP, Suttle CA (2008) Evidence that viral abundance across oceans and lakes is driven by different biological factors. Freshw Biol 53:1090–1100. https://doi.org/10.1111/j.1365-2427.2008.01992.x
Cole JJ, Findley S, Pace ML (1988) Bacterial production in fresh and saltwater ecosystems: a cross-system overview. Mar Ecol Prog Ser 43:1–10. https://doi.org/10.3354/meps043001
De Corte DS, Sintes E, Yokokawa T, Herndl GJ (2011) Changes in viral and bacterial communities during the ice-melting season in the coastal Arctic (Kongsfjorden, Ny-Ålesund). Environ Microb 13(7):1827–1841. https://doi.org/10.1111/j.1462-2920.2011.02497.x
Drits AV, Pasternak AF, Kravchishina MD, Arashkevich EG, Flint MV (2019) The role of plankton in the vertical flux in the East Siberian Sea shelf. Oceanology 59(5):669–677. https://doi.org/10.1134/S0001437019050059
Ducklow HW, Hill SM (1985) The growth of heterotrophic bacteria in the surface waters of warm core rings. Limnol Oceanogr 30(2):239–259. https://doi.org/10.4319/lo.1985.30.2.0239
Fischer UR, Velimirov B (2002) High control of bacterial production by viruses in eutrophic oxbow lake. Aquat Microb Ecol 27:1–12. https://doi.org/10.3354/AME027001
Flint MV, Semenova TN, Arashkevich EG, Sukhanova IN, Gagarin VI, Kremenetskiy VV, Pivovarov MA, Soloviev KA (2010) Structure of the zooplankton communities in the region of the Ob River’s estuarine frontal zone. Oceanology 50(5):766–779. https://doi.org/10.1134/S0001437010050139
Flint MV, Arashkevich EG, Artemiev VA et al (2018) Ecosystems of the Siberian Arctic seas. Materials of expeditions in 2015 and 2017. IO RAS, Moscow
Fuhrman JA, Suttle CA (1993) Viruses in marine planktonic systems. Oceanography 6:51–63
Gordeev VV, Martin JM, Sidorov JS, Sidorova MV (1996) A reassessment of the Eurasian river input of water, sediment, major elements, and nutrients to the Arctic Ocean. Am J Sci 296:664–691. https://doi.org/10.2475/ajs.296.6.664
Gordeev VV (2000) River input of water, sediment, major ions, nutrients and trace metals from Russian territory to the Arctic Ocean. In: Lewis EL, Jones EP, Lemke P, Prowse TD, Wadhams P (eds) The freshwater budget of the Arctic Ocean. NATO science series, vol 70. Springer, Dordrecht. https://doi.org/10.1007/978-94-011-4132-1_14
Hammer Ø, Harper DA, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron 4(1):9
Hodges LR, Bano N, Hollibaugh JT, Yager P (2005) Illustraing the importance of particulate organic matter to pelagic microbial abundance and community structure—an Arctic case study. Aquat Microb Ecol 40:217–227. https://doi.org/10.3354/ame040217
Howard-Jones MH, Ballard VD, Allen AE, Frischer ME, Verity PG (2002) Distribution of bacterial biomass and activity in the marginal ice zone of the central Barents Sea during summer. J Mar Syst 38:77–91. https://doi.org/10.1016/S0924-7963(02)00170-7
Kopylov AI, Zabotkina EA, Sazhin AF, Romanova ND (2015) Virioplankton in the Kara Sea: the impact of viruses on mortality of heterotrophic bacteria. Oceanology 55(4):561–572. https://doi.org/10.1134/S0001437015040104
Kopylov AI, Zabotkina EA, Romanenko AV, Sazhin AF, Romanova ND (2017) Virio- and bacterioplankton in the estuary zone of the Ob River and adjacent regions of the Kara Sea shelf. Oceanology 57(1):105–113. https://doi.org/10.1134/S0001437017010052
Kopylov AI, Zabotkina EA, Romanenko AV, Kosolapov DB, Sazhin AF (2020) Viruses in the water column and the sediment of the eastern part of the Laptev Sea. Estuarine Coast Shelf Sci 242: Art. 106836. https://doi.org/10.1016/j.ecss.2020.106836
Kopylov AI, Kosolapov DB, Zabotkina EA, Romanenko AV, Sazhin AF (2021a) Distribution and relationship between heterotrophic organisms and viruses on the East Siberian Sea shelf. Oceanology 61(2):220–232. https://doi.org/10.1134/S0001437021020089
Kopylov AI, Zabotkina EA, Kosolapov DB, Romanenko AV, Sazhin AF (2021b) Viruses and viral infection of heterotrophic prokaryotes in shelf waters of the western part of the East Siberian Sea. J Mar Syst 218: art. 103544. https://doi.org/10.1016/j.jmarsys.202.103544
Lisina AA, Grigoriev VYu, Frolova NL (2020) Features of the river runoff formation in the cryolithozone in modern climatic conditions. In: The IVth Vinogradov readings. Hydrology from cognition to worldview. Collected reports of the Int. Sci. Conf. in memory of the outstanding Russian Scientist Yu. B. Vinogradov, pp 1098–1101. BBM Publishing House, SPb
Lisitzin AP (1995) The marginal filter of the ocean. Oceanology 34:583–590
Maat DS, Visser RJW, Brussaard CPD (2019a) Virus removal by gracier-derived suspended fine sediment in the Arctic. J Exp Mar Biol Ecol 521:151–227. https://doi.org/10.1016/j.jembe.2019.151227
Maat DS, Prins MA, Brussaard CPD (2019b) Sediments from Arctic tide-water glaciers remove coastal marine viruses and delay host infection. Viruses 11(2):123. https://doi.org/10.3390/v11020123
Maranger R, Vaqué D, Nguyen D, Hebert M-P, Lara E (2015) Pan-Arctic patterns of planktonic heterotrophic microbial abundance and processes: controlling factors and potential impacts of warming. Progr Oceanogr 139:221–232. https://doi.org/10.1016/j.pocean.2015.07.006
McClelland JW, Holmes RM, Dunton KH, Macdonald RW (2012) The Arctic ocean estuary. Estuarine Coasts 35:353–368. https://doi.org/10.1007/s12237-010-9357-3
Meon B, Amon RMW (2004) Heterotrophic bacterial activity and fluxes of dissolved free amino acids and glucose in the Arctic rivers Ob, Yenisei and the adjacent Kara Sea. Aquat Microb Ecol 37:121–135. https://doi.org/10.3354/ame037121
Middelboe M, Nielsen TG, Bjørsen PK (2002) Viral and bacterial production in the North Water in situ measurements batch-culture experiments and characterization of a viral–host system. Deep Sea Res Part II Top Stud Oceanogr 49:5063–5079. https://doi.org/10.1016/S0967-0645(02)00178-9
Mosharova IV, Il’inskii VV, Mosharov SA (2016) State of heterotrophic bacterioplankton of Yenisei estuary and the zone of Ob-Yenisei discharge in autumn in relation to environmental factors. Water Res 43(2):341–352. https://doi.org/10.1134/S0097807816020093
Mostajir B, Dolan J, Rassoulzadegan F (1995) A simple method for the quantification of a class a labile marine pico- and nano-sized detritus: DAPI Yellow Particles (DYP). Aquat Microb Ecol 9:259–266. https://doi.org/10.3354/ame009259
Müller O, Seuthe L, Bratbak G, Paulsen ML (2018) Bacterial response to permafrost derived organic matter input in an Arctic Fjord. Front Mar Sci 5:1–12. https://doi.org/10.3389/fmars.2018.00263
Murray AG, Jackson GA (1992) Viral dynamics: a model of the effects of size, shape, motion and abundance of single-celled planktonic organisms and other particles. Mar Ecol Prog Ser 89(2/3):103–116. https://doi.org/10.3354/meps089103
Noble RT, Fuhrman JA (1999) Breakdown and microbial uptake of marine viruses and other lysis products. Aquat Microb Ecol 20:1–11. https://doi.org/10.3354/ame020001
Norland S (1993) The relationship between biomass and volume of bacteria. In: Kemp PF, Sherr BF, Sherr EB, Cole JJ (eds) Handbook of methods in aquatic microbial ecology. Lewis Publisher, Boca Raton, pp 303–308
Payet JP, Suttle CA (2013) The balance between lytic and lysogenic viral infection is driven by trophic status. Limnol Oceanogr 58:465–474. https://doi.org/10.4319/lo.2013.58.2.0465
Porter KG, Feig YS (1980) The use DAPI for identifying and counting of aquatic microflora. Limnol Oceanogr 25(5):943–948. https://doi.org/10.4319/lo.1980.25.5.0943
Sandaa R-A, Storesund JE, Olesin E, Paulsen ML, Larsen A, Bratbak G, Ray JL (2018) Seasonality drives microbial community structure, shaping both eukaryotic and prokaryotic host–viral relationships in an arctic marine ecosystem. Viruses 10:715. https://doi.org/10.3390/v10120715
Sazhin AF, Romanova ND, Mosharov SA (2010) Bacterial and primary production in the pelagic zone of the Kara Sea. Oceanology 50(5):759–765. https://doi.org/10.1134/S0001437010050127
Schoemann V, Becquevort S, Stefels J, Rousseau W, Lancelot C (2005) Paeocystis blooms in the global ocean and their controlling mechanisms: a review. J Sea Res 53:43–66. https://doi.org/10.1016/j.seares.2004.01.008
Sherr BF, Sherr EB, Andrew TL, Fallon RD, Newell SY (1986) Trophic interactions between heterotrophic Protozoa and bacterioplankton in estuarine water analyzed with selective metabolic inhibitors. Mar Ecol Prog Ser 32(2–3):169–179. https://doi.org/10.3354/meps032169
Sorokin YuI, Sorokin PYu (1996) Plankton and primary production in the Lena River Estuary and in the Southeastern Laptev Sea. Estuarine Coast Shelf Sci 43:399–418. https://doi.org/10.1006/ecss.1996.0078
Steward GF, Smith DC, Azam F (1996) Abundance and production of bacteria and viruses in the Bering and Chukchi Seas. Mar Ecol Prog Ser 131:287–300. https://doi.org/10.3354/meps131287
Steward GF, Fandino LB, Hollibaugh JT, Whitledge TE, Azam F (2007) Microbial biomass and viral infections of heterotrophic prokaryotes in the sub-surface layer of the central Arctic Ocean. Deep Sea Res Part I Oceanogr Res Pap 54:1744–1757. https://doi.org/10.1016/j.dsr.2007.04.019.
Suttle CA (2007) Marine viruses—major players in the global ecosystem. Nat Rev Microb 5:801–812. https://doi.org/10.1038/nrmicro1750
Tremaine SC, Mills AL (1987) Tests of the critical assumptions of the dilution method for estimating bacterivory by microeucaryotes. Appl Environ Microbiol 53(12):2914–2921. https://doi.org/10.1128/aem.53.12.2914-2921.1987
Vaque D, Guadavol O, Peters F, Felipe J, Angel L, Terrado R, Lovejoy C, Pedrós-Alió C (2008) Seasonal changes in planktonic bacterivory rates under the ice-covered coastal Arctic Ocean. Limnol Oceanograph 53(6):2427–2438. https://doi.org/10.4319/lo.2008.53.6.2427
Venger MP, Kopylov AI, Zabotkina EA, Makarevich PR (2016) The influence of viruses on bacterioplankton of the offshore and coastal parts of the Barents Sea. Rus J Mar Biol 42(1):26–35. https://doi.org/10.1134/S106307401601017X
Weinbauer MG (2004) Ecology of prokaryotic viruses. FEMS Microb Rev 28(2):127–181. https://doi.org/10.1016/j.femsre.2003.08.001
Weisse T (1989) The microbial loop in the Red sea: dynamics of pelagic bacteria and heterotrophic nanoflagellates. Mar Ecol Prog Ser 55:241–250. https://doi.org/10.3354/meps055241
Wells LE, Deming JW (2003) Abundance of bacteria, the Cytophaga–Flavobacterium cluster and Archaea in cold oligotrophic waters and nepheloid layers of the Northwest Passage, Canadian Archipelago. Aquat Microb Ecol 31:19–31. https://doi.org/10.3354/ame031019
Wells LE, Cordray M, Bowerman S, Miller L, Vincent WF, Deming JW (2006) Archaea in particles-rich waters of the Beaufort Shelf and Franklin Bay, Canadian Arctic; Clues to an allochthonous origin? Limnol Oceanogr 51:47–59. https://doi.org/10.4319/lo.2006.51.1.0047
Wheeler PA, Gosselin M, Sherr E, Thibaultc D, Kirchman DL, Benner R, Whitledge TE (1996) Active cycling of organic carbon in the central Arctic Ocean. Nature 380:697–699. https://doi.org/10.1038/380697a0
Wilhelm SW, Suttle CA (1999) Viruses and nutrient cycles in the sea. Bioscience 49(10):781–788. https://doi.org/10.2307/1313569
Winter C, Herndl CJ, Weinbauer MG (2004) Diel cycles in viral infection of bacterioplankton in the North Sea. Aquat Microb Ecol 35:207–216. https://doi.org/10.3354/ame035207
Wommack KE, Colvell RR (2000) Virioplankton: viruses in aquatic ecosystems. Microb Mol Biol Rev 64:69–114. https://doi.org/10.1128/mmbr.64.1.69-114.2000
Acknowledgements
The authors are deeply grateful to Aaron Carpenter for correcting the English translation. We also thank Dolors Vaque and Janina Rahlff for valuable comments which helped to improve the manuscript. The authors would like to thank the employees of the Center for Electron Microscopy of the Papanin Institute for Biology of Inland Waters of the Russian Academy of Sciences S.I. Metelev, G. Bykov, and Z. Bykova for their help in preparing the material for electron microscopy.
Funding
Expedition research carried out within the framework of the long-term program “Ecosystems of the Seas of the Siberian Arctic” and supported by Russian Science Foundation Project No. 22-17-00011, htpps://rscf.ru/project/22-17-00011.
Author information
Authors and Affiliations
Contributions
KAI had the idea for the article, he and ZEA performed the literature search, all coauthors performed data analysis and AK drafted and critically revised the work. All authors provided comments on the manuscript and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare lack of any potential competing interests.
Ethical Approval
Not applicable.
Consent to participate
All authors have agreed to participate in the study.
Consent for publication
Publication has been approved by all coauthors and the organization where the work was performed (Shirshov Institute of Oceanology RAS and Papanin Institute for Biology of Inland Waters RAS). Approval from the responsible authorities is not required. All authors have approved the manuscript for submission to “Polar Biology”.
Research involving human and animal rights
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kopylov, A.I., Zabotkina, E.A., Sazhin, A.F. et al. Structure of virioplankton and viral lysis of prokaryotes on the shelf of Siberian Arctic seas: impact of large river runoff. Polar Biol 45, 1581–1596 (2022). https://doi.org/10.1007/s00300-022-03087-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-022-03087-4