Abstract
Diatoms constitute an important and diverse component of terrestrial protist communities but remain poorly studied, especially in the Antarctic realm. Here, we investigated the diversity and community structure of the terrestrial diatom flora from the Ulu Peninsula, James Ross Island (Maritime Antarctic Region) using a morphology-based dataset and physico-chemical measurements. A total of 97 taxa belonging to 27 genera was identified in 59 samples from terrestrial environments, including soils and rock walls. The flora was dominated by the genera Hantzschia, Luticola, and Humidophila. Eight distinct diatom assemblages could be distinguished and were mainly structured by differences in environmental characteristics such as vegetation coverage, moisture, conductivity, pH, and nutrient concentrations. In general, James Ross Island harboured a unique diatom flora as evidenced by very low similarity values with other (sub-)Antarctic localities. Only 16% of the taxa have a typical cosmopolitan distribution, whereas 70% showed a restricted Antarctic distribution, supporting previous indications of high species-level endemism in environments characterized by harsh abiotic conditions. In addition, several of the cosmopolitan species uncovered in this study might harbour substantial levels of hidden diversity, including endemic taxa, as previously revealed for the Pinnularia borealis species complex on James Ross Island. Taken together, the present study improves our knowledge and understanding of the diversity, ecology, and community structure of the terrestrial diatom flora of Ulu Peninsula and highlights that soils and wet rock walls represent important terrestrial habitats in this transitional zone between Maritime and Continental Antarctica.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diatoms (Bacillariophyceae) are the most species-rich group of algae on Earth (Mann 1999) and one of the most abundant and diverse algal groups in the Antarctic Region (Jones 1996; Van de Vijver and Beyens 1999; Sabbe et al. 2003). They are powerful indicators used to explore and interpret a broad array of ecological conditions, issues, and questions, ranging from water quality monitoring to biogeography. Due to their specific ecological preferences and the long-term preservation of their silica valves in aquatic sediments, diatoms are also excellent indicators for stratigraphic correlations and paleoecological reconstructions (Smol and Stoermer 2010). Although the majority of diatom species are bound to aquatic habitats, a large number of diatom taxa are able to survive in non-submerged wet, moist to dry habitats such as mosses, litter, bark, lichens, rocks, and soils (Van de Vijver et al. 2002a; Pfister et al. 2017; Chattová 2018; Foets et al. 2020).
Terrestrial diatom communities in Polar Regions must overcome various extreme environmental conditions including habitat desiccation, heavy winds, freezing, lack of sunlight during winter, and continuous sunlight together with high levels of ultraviolet radiation during the summer (McKnight et al. 1999; Esposito et al. 2008; Pinseel et al. 2017a). Despite this, diatoms were able to successfully adapt to the polar environment and have developed specific strategies to survive harsh conditions. These include production of numerous extracellular macromolecular substances that act to lower the freezing point, or the formation of long-lasting and desiccation-resistant resting stages (Krembs et al. 2002; Hejduková et al. 2020), which have been the subject of recent controlled laboratory experiments (Souffreau et al. 2010, 2013a; Hejduková et al. 2019, 2020).
While freshwater diatom communities have been intensively studied from numerous (sub-)Antarctic localities (e.g. Van de Vijver et al. 2002b; Sabbe et al. 2003; Esposito et al. 2008; Kopalová and Van de Vijver 2013; Pla-Rabes et al. 2013; Chattová et al. 2014), recent studies on terrestrial diatom communities in Antarctica remain scarce and restricted to a handful of studies. Specifically, Fermani et al. (2007) examined soil microalgal communities from an active volcano on Deception Island (South Shetlands) and found 74 diatom taxa. In the sub-Antarctic, Van de Vijver et al. (2002a) reported a diverse soil diatom flora with 230 taxa from Ile de la Possession, Crozet archipelago, while Moravcová et al. (2010) later identified 164 taxa in soils sampled from wandering albatross (Diomedea exulans) colonies on the same island. Finally, a fourth study describing the diversity and distribution patterns of soil diatom communities on Ile Amsterdam (southern Indian Ocean) is currently in preparation (Cahová, unpublished results).
Diatom communities of Continental Antarctica have recently been studied in several regions: the Larsemann (Sabbe et al. 2003), Bunger (Gibson et al. 2006), and Vestfold (Bishop et al. 2020) Hills in Princess Elizabeth Land, the McMurdo Dry Valleys in Victoria Land (Stanish et al. 2012, 2013; Sakaeva et al. 2016), and the Skarvsnes ice-free area along the Sôya Coast (Ohtsuka et al. 2006). Nevertheless, these studies were focused mostly on sediments or benthic algal mats from continental Antarctic lakes and streams, which are predominantly composed of cyanobacteria, chlorophytes, and diatoms (Spaulding et al. 1997). Diatoms can be important phototrophs in these environments, particularly in stream microbial mats subjected to continuous streamflow, where diatoms comprise up to 70% of the biomass (Alger et al. 1997; Kohler et al. 2015). In Sabbe et al. (2003), a total of 31 taxa was found during their analysis of freshwater and saline lake sediments in the Larsemann Hills and Rauer Islands, and Gibson et al. (2006) identified 29 diatom taxa in microbial mats from lakes in the Bunger Hills. Using an updated fine-grained taxonomical approach, Bishop et al. (2020) revealed the presence of 183 diatom taxa, including 19 marine species, in saline lakes of the Vestfold Hills, in contrast to the original 67 taxa reported in Roberts and McMinn (1999). The McMurdo Dry Valleys in Victoria Land constitute the most well-studied Continental Antarctic area. Spaulding et al. (1997) found 50 diatom taxa in the sediment core of Lake Hoare, while Sakaeva et al. (2016) identified 43 diatom species in microbial mats of 25 ponds, Stanish et al. (2012) reported 41 species from meltwater streams, and Stanish et al. (2013) recognized 29 diatom taxa in cryoconite holes as a subset of the species found in streams. Finally, Ohtsuka et al. (2006) observed 21 species in freshwater lake algal mats from the Skarvsnes ice-free area. In the aforementioned studies, most of the reported taxa are currently considered aerophilic, especially cosmopolitan genera such as Hantzschia or Luticola, which are often recorded in benthic communities. However, there is currently a lack of data on the diversity and composition of true soil diatom communities.
Meanwhile, on the Antarctic Peninsula, several paleoecological studies on James Ross Island have applied diatoms as a proxy for reconstruction of past environmental conditions (Burckle and Wasell 1995; Björck et al. 1996; Håkansson et al. 1996). Yet, none of these studies presented a detailed diatom iconography, and the validity of the diatom identifications can, therefore, not be checked and compared with recent publications (Zidarova et al. 2016a). However, the non-marine diatom flora of the entire Antarctic Peninsula, including James Ross Island, has recently been under revision and led to the description of many new species (e.g. Kopalová et al. 2009, 2011; Van de Vijver et al. 2011, 2014, 2015, 2016; Zidarova et al. 2016b; Pinseel et al. 2017b; Bulínová et al. 2018). Several recent studies that followed this modern fine-grained taxonomical concept dealt with freshwater diatom communities (Kopalová et al. 2012, 2013; Skácelová et al. 2015), as well as diatoms inhabiting mosses (Kopalová et al. 2014) and lichens (Chattová 2018) on James Ross Island. Most recently, Kopalová et al. (2019) investigated habitat controls of diatom communities in the lake-wash zone from Clearwater Mesa (CWM, James Ross Island). This study revealed that different habitat types, including fine sediment, stones, terrestrial mosses, wetted marginal soils, and black cyanobacterial mats, host different diatom communities which are largely controlled by the physiographic characteristics of their habitats. Despite all these recent taxonomic and ecological efforts, our knowledge of the ecology and habitat preferences, community dynamics, and associations of soil diatom assemblages on James Ross Island remains limited.
This study provides a detailed ecological analysis of the terrestrial diatom flora of James Ross Island. We do so by investigating the diatom flora from the Ulu Peninsula, the largest ice-free area of the island. Specifically, we aimed to identify (i) the diversity and community composition of terrestrial diatoms present on the Ulu Peninsula, (ii) the controls on terrestrial diatom community structure in the area, and (iii) the degree of similarity between the terrestrial diatom flora of James Ross Island and diatom communities from other terrestrial habitats from across the Antarctic realm. At last, we discuss the potential prevalence of unrecognized (pseudo)cryptic diversity, by integrating molecular phylogenetic research on the cosmopolitan diatom species complex Pinnularia borealis (Pinseel et al. 2019, 2020), which is a common component of the terrestrial flora on James Ross Island, into our present study.
Material and methods
Study site
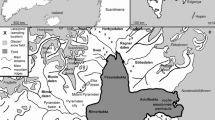
James Ross Island (64° 10′ S, 57° 45′ W) is a large island (total surface area = 2450 km2) located in the north-western part of the Weddell Sea, close to the northern tip of the Antarctic Peninsula (Fig. 1). This area represents a transitional zone between Maritime and Continental Antarctic regions (Øvstedal and Lewis-Smith 2001). The mean annual temperature is around − 6.3 °C (Ambrozova et al. 2019). The active layer of permafrost varies in thickness across the ice-free areas on James Ross Island (50–89 cm), with the thickest active layer (89 cm) observed by Hrbáček et al. (2019) on the Berry Hill slopes, one of the sampling sites in this study. More than 75% of the island is covered with ice (Rabassa et al. 1982), with the largest ice-free area, the Ulu Peninsula (310 km2), situated in the northern part of the island (Kavan et al. 2017). The latter also represents the largest ice-free area in the entire Antarctic Peninsula region (Hrbáček et al. 2017). Deglaciation along the northern coast of the Ulu Peninsula started 12.9 ± 1.2 ka ago and continued through the Holocene (Nývlt et al. 2014). The ice-free part of the Ulu Peninsula is composed of two main geological units: Cretaceous back-arc basin sediments, and mostly subglacial Neogene to Quaternary volcanic rocks, mainly composing of sub-aerial cap basalts, hyaloclastite breccias, and tuffs. (Olivero et al. 1986; Smellie et al. 2008; Svojtka et al. 2009). Although vascular plants are absent, with vegetation being limited to bryophytes and lichens, the exposed land on the island is rich in vegetation oases (i.e. areas covered by autotrophic organisms), comprising a great variety of terrestrial algae, cyanobacteria, lichens, and mosses (Barták et al. 2015). Those oases are located on specific sites with sufficient moisture availability, or near seal carcasses providing a nutrient source (Barták et al. 2015; Nývlt et al. 2016). The human presence on the island is limited to the Ulu Peninsula, where the Johann Gregor Mendel Czech Antarctic Station is located.
a Location of James Ross Island in the north-western part of the Weddell Sea (Antarctic Digital Database 2018) b Map of James Ross Island (Antarctic Digital Database 2018) with the study area highlighted c a modified map of James Ross Island-Northern part (Czech Geological Survey 2009) with indication of sampling sites according to the 8 groups as defined in this study and the main topographic indications used in the text
Sampling
Fifty-nine terrestrial samples (51 soil samples and 8 samples from wet rocks or wet rock walls) were collected during a field campaign in January–February 2015. The sampling sites (indicated on Fig. 1) were selected based on differences in geology, soil composition, moss cover, and animal influence in order to maximize variability in environmental characteristics. After removing possible vegetation cover, the upper two centimetres of soil were collected into 50 ml falcon tubes. Wet walls and rocks were sampled by scraping off the rock surface (approximately 10 g from an area of 5–10 cm2) with a knife and a spatula into 20 ml falcon tubes. Each sample was geographically localized using GPS, accompanied by a detailed site description. Light microscope observations were conducted at the Mendel station using an Olympus CX31 microscope, and samples were scanned for the presence of living cells under a microscope before fixing with 3% formaldehyde. For 43 soil samples (excluding the rocks and rock walls), an additional 50–60 g of soil was taken and deep-frozen. Upon arrival in the Trace Analytical Laboratory (Masaryk University, Czech Republic), soil samples were air dried, mixed thoroughly to ensure homogeneity, sieved, and weighed. For the extraction, 25 g of dry soil was suspended in 125 ml of extractant (Mehlich 1984), and after shaking for 20 min, the resulting suspension was filtered through a medium porosity filter, to be subsequently analysed for NO32−N, NH4+–N, PO43−–P, SO42−, Cl−, Ca2+, total organic carbon (TOC), total carbon (TC), and inorganic carbon (IC). pH and conductivity were measured in the laboratory, following dilution in distilled water and subsequent filtration, using a 3540 Bench combined pH and conductivity meter. In order to determine the moisture content of the samples, an additional amount of soil for 45 samples was collected in 15 ml falcon tubes and closed air tight to avoid loss of moisture during transport. Upon arrival in the laboratory, samples were weighed and dried in an oven at 60 °C for 4 weeks. Simultaneously, 40 empty falcon tubes of the same type were placed in the oven to assess the average weight loss of the falcon tubes. Using the mass of the filled and empty falcon tubes before and after drying, the original relative moisture content of the samples was calculated as a percentage.
Sample preparation and sample analysis
Samples were prepared for further analysis following the method described in van der Werff (1955): small parts of the samples were cleaned by adding 37% H2O2 and heating to 80 °C for about 1 h. The reaction was completed by addition of an excessive amount of saturated KMnO4. Following digestion and centrifugation (three times 10 min at 3700 rpm), cleaned material was diluted with distilled water to avoid excessive concentrations of diatom valves on the slides. Cleaned diatom valves were mounted in Naphrax®. Samples and slides are stored at the Department of Botany and Zoology, Masaryk University in Brno (Czech Republic). In each sample, 400 diatom valves were identified and enumerated on random transects by means of oil immersion at ×1000 magnification using an Olympus BX51 microscope, equipped with Differential Interference Contrast (Nomarski) optics and the Olympus Stream Motion Imaging System. Upon counting 400 valves, slides were scanned to detect rare species. Diatoms were identified to species level, primarily using Zidarova et al. (2016a) and references therein. For the identification of species of the genus Hantzschia, the publication by Bulínová et al. (2018) was additionally consulted.
Data analysis
Unless otherwise indicated, statistical analyses were performed in the R statistical environment (version 4.1.0; R Core Team 2021; RStudio Team 2021) using the packages vegan (Oksanen et al. 2020) and ggplot2 (Wickham 2016). To evaluate the extent to which our sampling effort represented the terrestrial diatom flora of Ulu Peninsula, the incidence-based species richness estimator (ICE, Chao et al. 2000) and the mean Chao2 richness estimator (Chao 1984) were calculated, using the EstimateS program version 9.1.0. (Colwell 2013).
To visualize the terrestrial diatom communities of the Ulu Peninsula, Non-metric Multidimensional Scaling (NMDS) was performed using two dimensions. NMDS was based on Bray–Curtis dissimilarity calculated on square-root-transformed species data. Furthermore, classical agglomerative clustering was employed to investigate whether terrestrial diatom communities form distinct groups as a function of their environmental preferences using Bray–Curtis distances and the complete linkage method. Resulting groups were projected onto the NMDS and subsequent ordinations. Indicator species characterizing each group were identified using the extended Indicator Species Analysis.
To visualize differences in physico-chemical variables among sampled sites, and how these related to the clustered groups, we performed a Principle Components Analysis (PCA) using the prcomp R function. As samples collected from rocks and wet walls did not have corresponding metadata, these analyses were restricted to soil samples. Variables were first log10 transformed to achieve approximately normal distributions, and a constant of 0.65 × the value of the smallest non-zero datum was applied to variables with zeros (i.e. per cent vegetation, per cent shelter, and Cl). Data were scaled and zero centred, and plotted with the ggplot function using the ggfortify and ggplot2 packages (Tang et al. 2016; Wickham 2016). Distance-based redundancy analysis (db-RDA) models were then created to investigate which combination of these physico-chemical variables best explain variability among soil diatom communities. To do this, Bray–Curtis distances were calculated for diatom communities, and candidate models were constructed by first eliminating all variables with variance inflation factors greater than 5 to reduce collinearity among parameters. As a result, the full model included PO4, SO42−, TN, TC, pH, conductivity, pct_moisture, pct_shelter, and pct_vegetation. The most parsimonious combination of variables was then determined via backward selection using the ordistep function in vegan (Oksanen et al. 2020) with p values as the selection criteria. At last, a pairwise comparison of the terrestrial diatom flora of James Ross Island with other (sub-)Antarctic islands where similar studies were conducted (Ile de la Possession, Ile Amsterdam, and Deception Island) was performed. The Community Coefficient of Sørensen (1948) was used with the following formula: 2c/(a + b + 2c), where a and b represent the number of species exclusively observed in each of two islands and c is the number of species shared between these islands. An additional similarity analysis was performed in the same way between the terrestrial diatom flora of Ulu Peninsula and other diatom communities inhabiting different habitats on the same island.
Results
Species composition and diversity
A total of 95 diatom taxa belonging to 27 genera was observed. Two additional taxa were observed outside the counts, bringing the total number of soil-inhabiting diatoms up to 97. All samples contained living diatom cells. The permanent slides of the samples JRI4, JRI6, JRI24, JRI26, and JRI44 did not contain sufficient diatom valves, even after counting an entire slide. An additional slide was made and counted for these samples. Finally, two samples (JRI6 and JRI44) yielded less than 400 diatom valves despite generating more slides and were subsequently removed from further analysis, together with samples JRI15 and JRI16 where mostly marine taxa prevailed, reducing the original set of 59 samples to 55.
Despite all recent taxonomic revisions of the James Ross diatom flora, four taxa (4% of all observed taxa) belonging to genera Achnanthes, Luticola, Nitzschia and Placoneis, could not be identified to species level. These unknown taxa were observed in low abundances in three samples. More in-depth morphological SEM investigations will be necessary to determine the correct taxonomic position of these taxa. The species richness estimators indicated the theoretical number of taxa in the study area approximately equal to 109 (Chao2) or 117 (ICE). Therefore, the sampling effort scored 87% (Chao2) or 81% (ICE) of all present taxa. The species richness of the samples varied between 5 (sample JRI26) and 42 (sample JRI27). The average number (and standard deviation) of taxa per sample is equal to 18 ± 6, with a median number of 17.5.
The five most abundant species accounted for 47.2% of all counted valves. The dominant species and species formae were Hantzschia amphioxys f. muelleri Ts. Kobayashi with more than 18% of all counted valves, followed by Hantzschia cf. abundans Lange-Bertalot (7.7%), Humidophila sceppacuerciae Kopalová (7.6%), Humidophila vojtajarosikii Kopalová, Zidarova & Van de Vijver (7.1%), and Luticola truncata Kopalová & Van de Vijver (6.1%). On the other side of the spectrum, 70 taxa (more than 73% of all counted taxa) together represented a total relative abundance of less than 1%. Figure 2 shows the distribution of the most abundant taxa in the eight diatom communities. Table 1 provides an alphabetical list of all observed taxa together with their most current biogeographical distributions and ecological preferences. Micrographs of selected taxa can be found in Fig. 3. The genera Luticola D.G.Mann (22 taxa), Pinnularia Ehrenberg and Nitzschia Hassall (9 taxa), Humidophila R.L.Lowe et al. (8 taxa), and Hantzschia Grunow and Muelleria (Frenguelli) Frenguelli (7 taxa) were the most species-rich genera. Other important genera included Achnanthes Bory and Stauroneis Ehrenberg (4 taxa). Based on abundance, the genera Hantzschia (29%), Luticola (23.8%) and Humidophila (18.9%) were dominant.
Stacked bar chart of the total relative abundances of the dominant taxa in the eight different groups defined in this study. The figure shows the distribution of the seven most abundant species of James Ross Island in the eight groups determined by the NMDS analysis. Each column represents one group, and each colour stands for one of the dominant species
LM micrographs of selected taxa 1 Orthoseira roeseana valve view, 2 Orthoseira roeseana pleural view, 3 Achnanthes coarctata raphe valve, 4 Achnanthes coarctata rapheless valve, 5 Achnanthes muelleri raphe valve, 6 Achnanthes muelleri rapheless valve, 7 Achnanthes taylorensis raphe valve, 8 Achnanthes taylorensis rapheless valve, 9, 10 Luticola muticopsis, 11 Luticola truncata, 12, 13 Navicula romanedwardii, 14, 15 Chamaepinnularia krookiformis, 16 Humidophila australis, 17, 18 Pinnularia borealis, 19, 20 Humidophila keiliorum, 21, 22 Hantzschia amphioxys f. muelleri, 23, 24 Hantzschia hyperaustralis, 25, 26 Nitzschia homburgiensis, 27, 28 Nitzschia kleinteichiana, 29, 30 Pinnularia australomicrostauron. Scale bar represents 10 μm
Distribution and similarity analysis
Following the most recent taxonomic concepts (based on information provided by Zidarova et al. 2016a), 67 species (70% of all observed taxa) showed a restricted Antarctic distribution with a majority of species only confined to the Maritime Antarctic Region (MA) (58 species, representing 60% of all observed taxa). Only fifteen species (16%) had a typical cosmopolitan distribution (C). The results also showed that nine species (9%) also occur in Continental Antarctica (CA), but only two species (2%) were shared with South America (AM).
The similarity analysis based on presence/absence data indicated that the diatom flora of Ulu Peninsula (James Ross Island), showed only a limited affinity to soil samples from other (sub-)Antarctic islands, with Sørensen index values ranging from 0.09 to 0.27 (Table 2). The highest similarity was noted with nearby Deception Island (South Shetlands), whereas the lowest similarity was observed with Ile Amsterdam (Southern Indian Ocean). Table 3 shows the results of the similarity analysis between the terrestrial diatom flora of soils and wet rock walls and other diatom communities inhabiting different habitats on James Ross Island, with Sørensen index values equalling 0.36–0.61. For James Ross Island specifically, the highest similarity was noted with the lichen inhabiting diatom flora from Ulu Peninsula (0.61), followed by the freshwater diatom communities from Ulu Peninsula (0.52–0.54) and the epilithic, moss, and epipepelic diatom flora from CWM. The lowest similarity (0.36) was observed with the wet soil samples from the CWM lake-wash zone.
Community analysis
Based on a cluster analysis with Bray–Curtis similarity, the samples were divided into eight groups (Fig. 4), and an indicator species analysis was used to identify taxa characteristic of each individual group.
An overview of the principal characteristics of all eight groups is presented in Table 4.
Group 1 was visualized in the centre of the NMDS plot (Fig. 4) and represented the most widespread diatom assemblage in this study, as it contained 28 samples and covered a wide range of environmental parameters. The top indicator species included H. amphioxys f. muelleri, H. cf. abundans, and H. sceppacuerciae. Other rather abundant taxa were H. vojtajarosikii, Luticola truncata, and L. muticopsis (Van Heurck) D.G.Mann. Several presumed cosmopolitan taxa such as the P. borealis Ehrenberg complex Achnanthes coarctata (Brébisson) Grunow, and Nitzschia homburgiensis Lange-Bertalot reached relatively high abundances (5–8% of counted valves). The proportion of cosmopolitan taxa was generally quite high, reaching 25% of the relative abundance, although Maritime Antarctic taxa still dominated the counts with 43% of all counted valves (Fig. 5).
Group 2 was located on the bottom left of the NMDS plot (Fig. 4), and the top indicator species were H. vojtajarosikii and Nitzschia kleinteichiana Hamsher et al. Both samples were dominated by small-celled Maritime Antarctic taxa (91% of all counted valves) mainly of the genera Humidophila, Nitzschia, and Luticola. Sample JRI41 was dominated by Nitzschia annewillemsiana Hamsher et al., a species exclusively found in this sample.
Group 3 was visualized on the right part of the NMDS plot (Fig. 4) and was characterized by high abundances (32% of all counted valves) of its top indicator species Achnanthes muellerii G.W.F. Carlson. The co-dominant taxa included Hantzschia amphioxys f. muelleri, H. cf. abundans, and L. muticopsis. This group had the highest proportion of species bound to the Southern Hemisphere (42% of counted valves, Fig. 5), due to high abundances of A. muellerii (32% of all counted valves) and L. muticopsis (9% of all counted valves), whereas the Maritime Antarctic taxa reached only 12% of the relative abundance.
Group 4 was situated in the upper part of the NMDS plot (Fig. 4) and was characterized by high abundances of Achnanthes taylorensis D.E.Kellogg et al. and Nitzschia stelmachpessiana Hamsher et al. Sample JRI52, with high abundances of H. vojtajarosikii placing it in the middle of the NMDS plot, should also be included in this group. Meanwhile, both group 5 samples were dominated by several Luticola species (L. truncata, L. muticopsis, L. doliiformis Kopalová & Van de Vijver, and L. pusilla Van de Vijver et al.) and had high relative abundances of Maritime Antarctic taxa (76% of the counted valves, Fig. 5).
Group 6 samples grouped together in the upper left part of the diagram and were dominated by big-celled Hantzschia taxa, such as H. amphioxys f. muelleri, H. cf. abundans, and H. hyperaustralis Van de Vijver & Zidarova. Other important taxa include Navicula romanedwardii Zidarova, Kopalová & Van de Vijver, Luticola muticopsis, L. doliiformis, L. truncata, and A. coarctata. The dominant flora of group 6 resembled group 1, but differed by the absence of A. muellerii and H. sceppacuerciae. The proportion of cosmopolitan taxa (17% of the counted valves) was quite high, further underlying its similarity to group 1.
On the other hand, group 7 represented an entirely different diatom flora from the previous groups, with a high proportion of cosmopolitan taxa (exceeding 53% of all counted valves). The taxa Fragilaria cf. parva Tuji & D.M.Williams, Nitzschia gracilis Hantzsch, N. kleinteichiana, Pinnularia australomicrostauron Zidarova, Kopalová & Van de Vijver, and N. homburgiensis dominated the community. Several other taxa were entirely restricted to this group, including Geissleria gabrielae Van de Vijver & Zidarova, Mayamaea excelsa (Krasske) Lange-Bertalot, Navicula dobrinatemniskovae Zidarova & Van de Vijver, and Neidium nyvltii Hamilton et al. Finally, the group 8 diatom community was species poor and dominated by Humidophila sceppacuerciae, Hantzschia amphioxys f. muelleri, and Luticola truncata. Other typical taxa included small-celled species of the genus Humidophila, such as H. inconspicua (Kopalová & Van de Vijver) R.L.Lowe et al., H. australoshetlandica Kopalová, Zidarova & Van de Vijver, and H. keiliorum Kopalová. These taxa dominated the samples from the soils situated directly next to Whisky Glacier. The majority of the taxa from this diatom community was restricted to Maritime Antarctica and account together for 67% of all counted valves from the last group.
Terrestrial diatom communities and their environment
Data exploration by PCA (Fig. 6) revealed two strongly negatively correlated gradients: on one hand, moisture and vegetation cover, and on the other specific conductance, the latter being highly linked to Cl−, Ca2+ and SO42−. The biggest group, group 1, displayed a wide breadth of environmental conditions which almost covers the full breadth of the gradient. In general, group 1 sites mostly contained samples from wet soils with moss cover, and from wet rock walls. Group 1 was characterized by moderate ion (SO42−, Cl−, Ca2+) and nutrient values and higher organic content. The highest nitrogen (TN 48.3 mg l−1) and organic content (TOC 504.4 mg l−1) were detected in sample JRI10, a soil crust sample taken in the vicinity of a seal carcass. The lowest pH (6.7) from the whole dataset was detected in sample JRI32, a sandy sample taken from the Johnson Mesa plateau.
The results of a principal component analysis (PCA) showing differences in physico-chemical variables [pH, conductivity, SO42−, NO32–N, NH4+–N, PO43−–P, Cl−, Ca2+, total organic carbon (TOC), total carbon (TC), inorganic carbon (IC), per cent moisture (pct_moisture), per cent shelter (pct_shelter) and per cent vegetation (pct_vegetation)] among sampled sites and groups
Group 2 was composed of only two high altitude, exposed samples with low conductivity, nutrient, and ion values. Sample JRI41 was taken near Omega Lake and had the lowest conductivity from the entire data set (23 μS cm−1). Group 3 represented relatively wet, low, and medium altitude samples characterized by a well-developed vegetation cover and high moisture content. One sample (JRI23) was an exception, as it was taken from bare ground with salt precipitations near the stream flowing from Phormidium Lake. The samples in this group were characterized by a wide range of specific conductance, low nutrient values, and slightly elevated Cl− and SO42− values.
In contrast, some of the smaller groups (e.g. group 4 and group 5) plotted on the periphery, likely indicating more extreme environmental conditions. For example, group 4 sites were located in dry, oligotrophic, bare soils at low altitude, covered with salt precipitations which caused extremely high conductivity (range 122–18,000 μS cm−1), (median 13,500 μS cm−1) and Cl− (range 228–6400 mg l−1), (median 4000 mg l−1) values. Meanwhile, group 5 consisted of two relatively dry soil samples with salt precipitation and without any vegetation cover. The highest nitrogen level (TN 119.8 mg l−1) was detected in sample JRI5. The samples were further characterized by slightly elevated ions (Ca2+, Cl−) and higher conductivity values (425–1539 μS cm−1).
Group 6 contained the wettest samples, mostly at lower altitudes, with elevated phosphorous values. This group had well-developed vegetation cover and high moisture and per cent shelter. Samples of this group were typically covered with well-developed moss vegetation, and Nostoc Vaucher ex Bornet & Flahault mats. Meanwhile, group 7 represented a series of wet, species-rich samples, apparent in the upper left part of the NMDS plot (Fig. 4). The samples were characterized by low conductivity values, and moderate organic content, and ion and nutrient values. Finally, group 8 comprised mostly higher altitude dry samples, without any vegetation coverage, and with low organic content and nitrogen values. The samples additionally showed the most alkaline pH of all localities (range 7.6–9.2).
To find the most influential set of environmental variables structuring soil diatom communities, a db-RDA was further performed. The best model (Fig. 7) explaining soil diatom communities included, SO42, TN, pH, pct_moisture, and pct_vegetation. All terms (all p < 0.03), as well as the full model (p = 0.001), were significant when tested with ANOVA. The adjusted R2 for the full model was 0.146, and the first three axes were significant, with adjusted R2 values of 0.055, 0.033, and 0.029, respectively. Among diatom species, Luticola muticopsis and P. borealis were most associated with TN, whereas Achnanthes spp. and Hantzchia abundans were associated with greater per cent vegetation and moisture. Finally, Hantzschia amphioxys f. muelleri was associated with greater pH values and lower TN.
Discussion
Species composition, indicator species, and general biogeography
The most abundant taxon in the terrestrial communities of Ulu Peninsula here investigated was Hantzschia amphioxys f. muelleri, an aerophilic species formae very commonly reported from Antarctica and South America. It is typically found in soils, seepage areas, wet moss vegetation, and within cyanobacterial colonies on soils (Zidarova et al. 2016a; Bulínová et al. 2018). Unfortunately, the distinction of H. amphioxys f. muelleri and H. amphioxys (Ehrenberg) Grunow remains questionable in the Antarctic Region (Bulínová et al. 2018), and it is difficult to reliably distinguish both taxa by means of LM only. Our specimens of H. amphioxys f. muelleri fitted into the species description sensu Bulínová et al. (2018). Although a small number of specimens with less capitate apices resembled H. amphioxys, we decided to include all individuals into the category of H. amphioxys f. muelleri, mainly due to the morphological overlap and the unclear ecological separation, since all the questionable specimens occurred in the samples together with H. amphioxys f. muelleri. All populations will need further detailed SEM analysis of the stria structure and the internal raphe endings to avoid confusion with the nominate variety, and to determine the exact ecological preferences of both taxa. Ideally, detailed SEM examinations of H. amphioxys populations on James Ross Island should be complemented with molecular work to further unravel species boundaries. A first study investigating the molecular phylogenetic diversity of the H. amphioxys-abundans complex on a global scale, including Antarctica, found the presence of distinct lineages within this species complex that could hardly be separated based on valve morphology alone (Souffreau et al. 2013b; Maltsev et al. 2021), suggesting additional species-level diversity might be hiding within established morphospecies on Ulu Peninsula as well.
Generally, within the five most abundant taxa, two can be considered to have a wider distribution, either cosmopolitan or in Antarctica and South America. In contrast, the other three most abundant taxa are restricted to Maritime Antarctica, being absent even from Continental Antarctica and the islands in the southern Indian Ocean. Several of the indicator species exhibit a strictly Antarctic distribution (A. taylorensis, H. hyperaustralis, P. australomicrostauron). Others are restricted to Maritime Antarctica, e.g. H. vojtajarosikii, H. sceppacuerciae, N. stelmachpessiana or N. kleinteichiana. Endemic Antarctic species showed a high indicator value for substrates and habitats characterized by more extreme environmental conditions. For example, A. taylorensis, a diatom typical for Continental Antarctica, was found together with N. stelmachpessiana. Both taxa were almost exclusively bound to dry, oligotrophic soils with high conductivity, covered with salt precipitations. In contrast, N. annewillemsiana, L. doliiformis, N. kleinteichiana and H. vojtajarosikii showed a high affinity to high altitude, dry and exposed habitats with low conductivity, organic content, nutrient and ion levels. Notably, several small-celled Maritime Antarctic Humidophila species (H. inconspicua, H. australoshetlandica and H. keiliorum) were found in high abundances (up to 40% of all counted valves) as pioneer microorganisms in the first stages of primary succession in the recently deglaciated area of Whisky Glacier. On the contrary, the big-celled cosmopolitan species (H. cf. abundans, N. homburgiensis, P. borealis) were more abundant in the communities with favourable environmental conditions, such as moss-covered, wet, sheltered habitats with higher organic content and nutrient values. These results indicate that endemic Antarctic species are more abundant in unfavourable habitats. Similar patterns were previously reported from Continental Antarctica (Esposito et al. 2006) and Livingston Island (Pla-Rabes et al. 2013).
The terrestrial diatom flora of soils and wet rock walls of Ulu Peninsula is more species rich (97 taxa) compared to most of the habitats on the same island. For example, Kopalová et al. (2012) recorded 69 taxa in 53 samples from seepages and streams on James Ross Island, Kopalová et al. (2019) reported 72 taxa from 175 limno-terrestrial samples from Clearwater Mesa, and Chattová (2018) found 56 taxa living on lichens (29 samples). The only dataset showing a higher species richness (123 taxa) is the combined freshwater species list of streams, seepage areas, and lakes from Ulu Peninsula presented by Kopalová et al. (2013). The result of a similarity analysis with the diatoms living in slightly wet to dry terrestrial lichens (Chattová 2018; Sørensen Index = 0.61) indicates a high affinity to the terrestrial diatom flora reported in our study, with only 9 species exclusively observed on lichens.
Interestingly, the lowest similarity values were found with samples from Clearwater Mesa, a prominent volcanic mesa located about 25 km to the southeast of the J.G. Mendel Station (Kopalová et al. 2019). The low similarity values with the Clearwater Mesa dataset are not surprising considering the spatial heterogeneity and differences in microhabitat diversity. The wet soil samples from Clearwater Mesa were taken in the immediate vicinity of the epipelon and epilithon samples in the lake splash zone, and their species composition reflected the adjacent benthic littoral communities, possibly as a result of past lake level changes (Roman et al. 2019). The Clearwater Mesa species list comprises more taxa bound to freshwater habitats, compared to our dataset from Ulu Peninsula, and therefore, the main differences are reflected in the proportion of terrestrial species. The Clearwater Mesa dataset contains only two Humidophila species: Humidophila australis (Van de Vijver & Sabbe) R.L.Lowe et al., and H. inconspicua, whereas our terrestrial dataset included eight different Humidophila taxa. The same pattern can be seen in other terrestrial genera, such as Luticola, Hantzschia and Muelleria. The latter genus is entirely missing from the freshwater samples of Clearwater Mesa and is known to be almost exclusively terrestrial (Spaulding and Stoermer 1997; Van de Vijver et al. 2010, 2014). On the contrary, the samples from Clearwater Mesa contained more Craticula Grunow and Psammothidium Bukhtiyarova & Round species. Moreover, in the samples from Clearwater Mesa, four different Achnanthidium Kützing species can be found, whereas the terrestrial samples from the Ulu Peninsula completely lack any Achnanthidium taxa. Most Achnanthidium species are typically found in lentic habitats (Van de Vijver and Kopalová 2014).
When comparing James Ross Island with other (sub-)Antarctic localities, the most similar diatom flora was found on the relatively nearby Deception Island (Sørensen Index = 0.26). The observed similarity is restricted to more common, often cosmopolitan species, such as N. homburgiensis, Chamaepinnularia krookiformis (Krammer) Lange-Bertalot & Krammer, and Microcostatus naumanii (Hustedt) Lange Bertalot, as well as taxa confined to the Southern Hemisphere such as Brachysira minor (Krasske) Lange-Bertalot. Typical Maritime Antarctic species shared between the islands include Stauroneis latistauros Van de Vijver & Lange-Bertalot, Stauroneis husvikensis Van de Vijver & Lange-Bertalot, and Muelleria algida S.A. Spaulding & Kociolek. The situation is similar with Ile de la Possession where the similarity is limited to cosmopolitan taxa (Orthoseira roeseana (Rabenhorst) O’Meara, Hantzschia abundans) or taxa typical for the Southern Hemisphere (A. muellerii, L. muticopsis).
The result of a similarity analysis with Ile Amsterdam (Sørensen Index = 0.09) indicates a very low affinity of both diatom floras, with only eleven shared species, including presumed cosmopolitan taxa such as A. coarctata, Nitzschia soratensis E. Morales & Vis, and P. borealis. No shared species were found for Humidophila and Luticola, despite both genera being abundantly present on both islands. Although Ile Amsterdam and James Ross Island share the basalt bedrock with similar physico-chemical conditions for community/habitat establishment, thousands of kilometres of open ocean separating these areas, combined with differences in the microhabitat diversity and climate, resulted in extremely low similarity in species diversity. In contrast to James Ross Island, Ile Amsterdam enjoys a temperate oceanic climate as recognized by the Köppen-Geiger climate classification (Peel et al. 2007), and these milder climate conditions allowed the development of a relatively diverse flora of mosses and higher plants. However, despite the apparent differences, a comparison of species diversity of these two remote islands yielded information on similar environmental requirements on the genera level.
Terrestrial diatom communities
It is widely accepted that marine birds and mammals exert a large impact on the nearby environment under the form of trampling, manuring, salt input, and physical disturbances (Moravcová et al. 2010). Although the animal activity on Ulu Peninsula is quite limited, as no penguin rookeries or colonies of marine mammals are present, most nutrient-enriched samples with the higher organic matter content come from areas where the influence of animals, especially birds such as the brown skua (Stercorarius antarcticus), gentoo penguin (Pygoscelis papua), and Adélie penguin (Pygoscelis adeliae), were clearly visible. In our dataset, those sites are represented by a few samples in groups 1, 3, and 6. The highest nitrogen level (TN 119.8 mg l−1) was detected in sample JRI5 (group 5), a dry soil sample taken between 2 seal mummies, 300 m far from the Mendel station. Similarly, the highest organic content (TOC 504.4 mg l−1) was detected in sample JRI10 (group 1), a soil crust sample taken in the close vicinity (5–10 cm) of a seal carcass. This sample was dominated by P. borealis and L. muticopsis. Decaying seal carcasses represent one of the few spots of nutrient release in the generally nutrient deficient polar environment of the northern ice-free area of Ulu Peninsula making them an excellent site for colonization by algae, cyanobacteria, lichens, and mosses (Nývlt et al. 2016; Zvěřina et al. 2017). In addition, sample JRI3 (group 3) was taken from a wet soil between the Lachman lakes, two shallow coastal lakes located in the proximity of the sea at very low altitude (Nedbalová et al. 2013), with a few skua nests nearby. This sample has higher values for organic matter and nutrient concentrations, likely due to the visible manuring and trampling by birds, and is dominated by A. muelleri and L. muticopsis. These taxa are typically reported from coastal areas with elevated salinity levels and higher nutrient input (Zidarova et al. 2016a). Our results (Fig. 7) showed a prominent relationship between the TN content and the abundances of L. muticopsis, suggesting that the taxon can be particularly suited to inhabit sites with increased nitrogen availability. Similar results were obtained by Bishop et al. (2021), showing that an increase in nitrogen may preferentially favour L. muticopsis.
Apart from animal influence, some sites were clearly influenced by human activity. For example, sample JRI30 (group 1), which showed elevated nitrogen and TOC values, was collected on a leeward site of a hyaloclastite breccia near Monolith Lake. Seasonal Argentinean and Czech field camps are regularly established on this location (Nedbalová et al. 2013). The sample was dominated by P. borealis and H. sceppacuerciae, whereas L. muticopsis was completely lacking.
In general, the diatom communities from animal influenced sites on Ulu Peninsula differed considerably from similar sites at other (sub-)Antarctic localities. On Ile de la Possession, a different diatom flora was observed in soils influenced by elephant seals and penguins, with high frequencies of Pinnularia subantarctica var. elongata (Manguin) Van de Vijver & Le Cohu, Humidophila crozetikerguelensis (Le Cohu & Van de Vijver) R.L. Lowe et al., and Pinnularia australoshetlandica G.W.F.Carlson (Van de Vijver et al. 2002a). Similarly, samples influenced by the wandering albatross on Ile de la Possession had a distinct diatom flora dominated by Humidophila ingeae (Van de Vijver) R.L.Lowe et al., Sellaphora cf. seminulum (Grunow) D.G.Mann, and Eunotia paludosa Grunow (Moravcová et al. 2010). The nutrient-enriched soils influenced by albatrosses on Ile Amsterdam harboured a completely different diatom composition, with Chamaepinnularia gracilistriata Van de Vijver & Beyens, and Tryblionella debilis Arnott as dominant taxa (Cahová et al., unpublished results). A more similar diatom flora of animal involved soils was reported from Deception Island, with communities being dominated by Psammothidium germainii (Manguin) Sabbe, Mayamaea atomus (Kützing) Lange-Bertalot, Chamaepinnularia krookiformis, L. muticopsis, and P. borealis (Fermani et al. 2007). The latter three were also present in relatively large numbers on James Ross Island.
Most of the examined deglaciated mineral soils of Ulu Peninsula have low nutrient and organic contents (Nývlt et al. 2016; Cannone et al. 2008), suggesting that the nutrient loading is more stochastic with trophic top-down recycling of carbon and nutrients through carcasses and active food waste reprocessing. On Ulu Peninsula, dry soil samples without any vegetation cover are dominated by A. taylorensis, N. stelmachpessiana, H. inconspicua, H. australoshetlandica, H. keiliorum, and several Luticola taxa (Fig. 7). Those diatom communities differ considerably from bare soils on Île de la Possession, where the diatom flora is composed of P. borealis, H. ingeae, and Psammothidium germainii (Van de Vijver et al. 2002a). On Deception Island, P. germainii, together with several Luticola taxa, also dominate the soil communities (Fermani et al. 2007), whereas on James Ross Island, only one broken valve of P. germainii was found when scanning the slides for rare taxa. P. germainii generally seems to prefer slightly acidic soils (Van de Vijver et al. 2002b), and such environments are missing from James Ross Island.
As indicated by the results of the PCA (Fig. 6), environmental variables related to salinity (conductivity, SO42−, Cl−, Ca2+) measured in marine-based soils of groups 3, 4, and 5 have a significant impact on the diatom species composition. Overall, the results suggest that the ion gradient away from the coastline in combination with drier higher elevation habitats inland, is deterministic in the development of stable soil diatom communities.
One of the richest diatom communities was associated with stable sites with persistent vegetation cover and good seasonal liquid water availability and was represented by groups 1 and 6. This community was observed in the area of Cape Lachman and from a seepage area located on north-facing slopes of Berry Hill, supplied by meltwater from annual snow deposition and frozen ground (Skácelová et al. 2015). The diatom communities of both groups show some clear similarities, with high frequencies of various Hantzschia taxa, L. muticopsis, L. truncata, and N. romanedwardii. The main differences between the two groups include extremely low abundances of H. sceppacuerciae and N. homburgiensis, and the complete absence of A. muelleri, in the samples of group 6. The samples in group 6 show generally lower nitrogen and Cl− values, which are a possible explanation for the absence of A. muelleri, a taxon that prefers higher nutrient and salinity levels (Zidarova et al. 2016a). The relatively high degree of stability, moisture, and nutrient availability resulted in a diatom flora lacking typical pioneer species. Thirty-five diatom taxa were found in three soil samples taken from this seepage on a foothill of Berry Hill Mesa. The diversity of soils is comparable to microbiological mats from the bottom of three streams passing through this particular area, where Skácelová et al. (2015) reported 33 diatom taxa. This generally high diversity is clearly linked to moisture availability, which depends on the access of meltwater, the permeability of the bedrock surfaces, total water retention in the landscape, and moss coverage. The area of Berry Hill slopes is wet during the entire austral summer due to the availability of meltwater from short-term snowfields accumulated during the preceding winter season and runoff from the active layer of permafrost appearing on the surface on warm days (Hrbáček et al. 2020; Skácelová et al. 2015). The wet soil diatom communities of the other (sub-)Antarctic islands are completely different. The wet soils in valleys on Ile de la Possession and higher altitudes on Ile Amsterdam are dominated by Eunotia paludosa (Van de Vijver et al. 2002a; Cahová et al. unpublished results) due to acidic pH (< 5.5) and peat formation. In contrast, the pH gradient on Ulu Peninsula is not very strong, mainly driven by the parent material composition (the Cretaceous marine sediments together with subalkaline volcanism) combined with the input of soluble salts, resulting in a rather uniform, circumneutral to slightly alkaline soil pH (Smellie et al. 2008; Košler et al. 2009; Svojtka et al. 2009), favouring more alcalophilus terrestrial diatom communities.
Cryptic diversity: P. borealis as an example
The current study was entirely based on morphological data. As a result, the true species-level diversity might have been underestimated in several widely distributed species complexes that are known to harbour (pseudo)cryptic diversity. Examples include H. amphioxys and P. borealis (Souffreau et al. 2013b; Pinseel et al. 2019; Maltsev et al. 2021). More specifically, a recent analysis of the global species-level diversity of P. borealis using molecular phylogenetic methods included several of the samples collected for this study (Pinseel et al. 2020), and now allows to shed a more detailed light on the diversity and biogeography of this species complex on James Ross Island.
Pinseel et al. (2020) isolated P. borealis cells from seventeen aquatic and terrestrial samples of James Ross Island, nine of which were collected and analysed in the framework of this study. Using automated molecular species delimitation methods, Pinseel et al. (2020) identified a total of six species that belong to the P. borealis complex on James Ross Island. Five of these are present in the samples collected in this study, whereas the sixth is known from aquatic habitats only (P. catenaborealis, Pinseel et al. 2017b). Analysis of four aquatic and terrestrial samples from nearby Vega Island revealed the presence of another three species of the P. borealis complex in close vicinity to James Ross Island (Pinseel et al. 2020). Three of the species found on James Ross Island were included in a morphometric analysis, involving LM and SEM data. This revealed that one of them was morphologically distinct from the other included taxa from James Ross Island: it showed subtle differences in size and shape that can be revealed via morphometric analyses (Fig. 4 in Pinseel et al. 2019, lineage L). However, the other two species showed almost complete overlap in their morphological features and could not be confidently distinguished from one another using morphological data (Fig. 4 in Pinseel et al. 2019, lineages C and D). Furthermore, the analyses by Pinseel et al. (2020) indicated that within the samples investigated in this study, up to three species of the P. borealis complex co-occurred within the same sample, which further complicates morphological identifications within environmental samples.
Most of the species from the P. borealis complex that inhabit James Ross and Vega Island are not closely related but instead diverged tens of millions of years ago from their most common recent ancestor (Pinseel et al. 2020). The overall majority of these species seems to be restricted to Maritime Antarctica and possibly even James Ross and Vega Island (Pinseel et al. 2020, Supplementary Fig. 5). In fact, five of the recorded species on James Ross and Vega Island were not found elsewhere. Two additional species were restricted to Maritime Antarctica in general, including remote Bouvet Island, and a third was restricted to the Antarctic Peninsula, the South Shetland Islands and Continental Antarctica (Pinseel et al. 2020). At last, one species was shown to have a cosmopolitan distribution, being retrieved from the Maritime and sub-Antarctic regions, as well as South America, the Arctic and temperate regions in the Northern Hemisphere (Pinseel et al. 2020, Supplementary Fig. 5). Within the dataset of Pinseel et al. (2020), this cosmopolitan taxon seemed to be the most common and widespread P. borealis species in Maritime Antarctica, including James Ross Island. Undersampling might have skewed the species distributions of members of the P. borealis complex reported by Pinseel et al. (2020), and wider species distributions for several recorded species can, thus, not be excluded. Nevertheless, the data show high levels of hidden species-level diversity of P. borealis in James Ross Island and suggest a mix of endemic and more widely distributed species occur together in James Ross Island specifically, and Maritime Antarctica in general.
It is not yet clear whether the patterns observed for P. borealis can be generalized to other presumed cosmopolitan diatom species that inhabit James Ross Island. Further work, including molecular analyses, will be necessary to unravel the true nature of such cosmopolitan morphospecies. However, a previous study on another common diatom species complex in the area, H. amphioxys, also indicated potentially high levels of hidden diversity on a global scale (Souffreau et al. 2013b), and many other diatom species complexes around the world have been shown to harbour (pseudo)cryptic diversity (Amato et al. 2019). Taken together, this could suggest that the terrestrial diatom flora of James Ross Island, and by expansion the entire Maritime Antarctic region, might be even more diverse and unique than the here presented morphology-based dataset suggests.
Conclusion
Although further morphological, and preferably also molecular, analyses will be necessary to clarify the taxonomic position of some presented taxa, a diverse and unique diatom flora was found in this study. This indicates that soils and wet rock walls represent a valuable set of terrestrial habitats supporting rich diatom communities in the transitional zone between Maritime and Continental Antarctica. The terrestrial diatom flora of James Ross Island showed relatively little resemblance with the terrestrial flora from several other (sub-)Antarctic islands and was characterized by many species found to be endemic for Antarctica. This underscores the presence of clear biogeographical patterns in the area and highlights the unique nature of the terrestrial diatom flora inhabiting the northern tip of the Antarctic Peninsula. These findings are in line with previous research that found high levels of endemism also in Antarctica’s freshwater diatom flora which is, in fact, dominated by terrestrial genera in both Maritime and Continental Antarctica (Verleyen et al. 2021; Pinseel et al. 2021). This is thought to result from the near complete decimation of Antarctica’s true freshwater diatom flora in response to Neogene and Quaternary cooling, combined with evolution-in-isolation of ancient elements and cold-adapted colonizers (Verleyen et al. 2021; Pinseel et al. 2021).
Taken together, our results provide useful information for further investigations on Antarctica’s terrestrial diatom flora, including, but not limited to, colonization of seal mummies, application of terrestrial diatoms as proxy in paleoecological research, biogeographical meta-analyses of the Antarctic diatom flora, or studies on colonization processes of young soil communities in new environments arisen after the glacial retreats.
Data availability
All data generated or analysed during this study are included in this published article.
Code availability
Not applicable.
References
Alger AS, McKnight DM, Spaulding SA, Tate CM, Shupe GH, Welch KA, Edwards R, Andrews ED, House HR (1997) Ecological processes in a cold desert ecosystem: the abundance and species distribution of algal mats in glacial meltwater streams in Taylor Valley, Antarctica. INSTAAR Occasional Paper 51
Amato A, Kooistra WHCF, Montresor M (2019) Cryptic diversity: a long-lasting issue for diatomologists. Protist 170(1):1–7. https://doi.org/10.1016/j.protis.2018.09.005
Ambrozova K, Laska K, Hrbacek F, Kavan J, Ondruch J (2019) Air temperature and lapse rate variation in the ice-free and glaciated areas of northern James Ross Island, Antarctic Peninsula, during 2013–2016. Int J Climatol 39(2):643–657. https://doi.org/10.1002/joc.5832
Barták M, Váczi P, Stachoň Z, Kubešová S (2015) Vegetation mapping of moss-dominated areas of northern part of James Ross Island (Antarctica) and a suggestion of protective measures. Czech Polar Rep 5(1):75–87. https://doi.org/10.5817/CPR2015-1-8
Bishop J, Kopalová K, Kohler TJ, Van de Vijver B, Roberts D, McMinn A, Gibson J (2020) A re-investigation of lake sediment diatoms from the Vestfold Hills, Antarctica, using an updated, fine-grained taxonomy. Diatom Res 35(3):1–24. https://doi.org/10.1080/0269249X.2020.1794982
Bishop J, Wasley J, Waterman M, Kohler T, Van de Vijver B, Robinson S, Kopalová K (2021) Diatom communities differ among Antarctic moss and lichen vegetation types. Antarct Sci 33(2):118–132. https://doi.org/10.1017/S0954102020000620
Björck S, Olsson S, Ellis-Evans C, Håkansson H, Humlum O, de Lirio JM (1996) Late holocene palaeoclimatic records from lake sediments on James Ross Island, Antarctica. Palaeogeogr Palaeoclimatol Palaeoecol 121(3–4):220. https://doi.org/10.1016/0031-0182(95)00086-0
Bulínová M, Kochman-Kędziora N, Kopalová K, Van De Vijver B (2018) Three new Hantzschia species (Bacillariophyta) from the Maritime Antarctic Region. Phytotaxa 371(3):168–184. https://doi.org/10.11646/phytotaxa.371.3.2
Burckle LH, Wasell A (1995) Marine and freshwater diatoms in sediment pockets in igneous rocks from James Ross Island, Antarctica. Antarct J US 305:8–9
Cannone N, Wagner D, Hubberten HW, Guglielmin M (2008) Biotic and abiotic factors influencing soil properties across a latitudinal gradient in Victoria Land, Antarctica. Geoderma 144:50–65. https://doi.org/10.1016/j.geoderma.2007.10.008
Colwell RK (2013) EstimateS 9.1. 0. Statistical estimation of species richness and shared species from samples. Version, 9.1. User’s guide and application published at http://viceroy.eeb.uconn.edu/EstimateS
Chao A (1984) Non–parametric estimation of the number of classes in a population. Scand J Stat 11:265–270
Chao A, Hwang WH, Chen YC, Kuo CY (2000) Estimating the number of shared species in two communities. Stat Sin 10:227–246
Chattová B (2018) Diatoms (Bacillariophyta) associated with lichens from Ulu Peninsula (James Ross Island, NE Antarctic Peninsula). Czech Polar Rep 8:151–161. https://doi.org/10.5817/CPR2018-2-12
Chattová B, Lebouvier M, Van de Vijver B (2014) Freshwater diatom communities from Île Amsterdam (TAAF, southern Indian Ocean). Fottea 14:101–119. https://doi.org/10.5507/fot.2014.008
Esposito RMM, Horn SL, McKnight DM, Cox MJ, Grant MC, Spaulding SA, Doran PT, Cozzetto KD (2006) Antarctic climate cooling and response of diatoms in glacial meltwater streams. Geophys Res Lett. https://doi.org/10.1029/2006GL025903
Esposito RMM, Spaulding SA, McKnight DM, Van de Vijver B, Kopalová K, Lubinski D, Hall B, Whittaker T (2008) Inland diatoms from the McMurdo dry valleys and James Ross Island, Antarctica. Botany 86:1378–1392. https://doi.org/10.1139/B08-100
Fermani P, Mataloni G, Van de Vijver B (2007) Soil microalgal communities on an Antarctic active volcano (Deception Island, South Shetlands). Polar Biol 30(11):1381–1393. https://doi.org/10.1007/s00300-007-0299-6
Foets J, Wetzel CE, Teuling AJ, Pfister L (2020) Temporal and spatial variability of terrestrial diatoms at the catchment scale: controls on productivity and comparison with other soil algae. PeerJ 8:e9198. https://doi.org/10.7717/peerj.9198
Gibson JAE, Roberts D, Van de Vijver B (2006) Salinity control of the distribution of diatoms in lakes of the Bunger Hills, East Antarctica. Polar Biol 29:697–704. https://doi.org/10.1007/s00300-006-0107-8
Håkansson H, Olsson S, Björck S (1996) Diatom record and sediment chemistry of a shallow, glaciolacustrine basin on James Ross Island, Antarctica. PACT (rixensart) 50:417–430
Hejduková E, Pinseel E, Vanormelingen P, Nedbalová L, Elster J, Vyverman W, Sabbe K (2019) Tolerance of pennate diatoms (Bacillariophyceae) to experimental freezing: comparison of polar and temperate strains. Phycologia 58:1–11. https://doi.org/10.1080/00318884.2019.1591835
Hejduková E, Elster J, Nedbalová L (2020) Annual cycle of freshwater diatoms in the high arctic revealed by multiparameter fluorescent staining. Microb Ecol 80:559–572. https://doi.org/10.1007/s00248-020-01521-w
Hrbáček F, Kňažková M, Nývlt D, Láska K, Mueller CW, Ondruch J (2017) Active layer monitoring at CALM-S site near JG Mendel Station, James Ross Island, eastern Antarctic Peninsula. Sci Total Environ 601:987–997. https://doi.org/10.1016/j.scitotenv.2017.05.266
Hrbáček F, Nývlt D, Láska K, Kňažková M, Kampová B, Engel Z, Oliva M, Mueller CW (2019) Permafrost and active layer research on James Ross Island: an overview. Czech Polar Rep 9(1):20–36. https://doi.org/10.5817/CPR2019-1-3
Hrbáček F, Cannone N, Kňažková M, Malfasi F, Convey P, Guglielmin M (2020) Effect of climate and moss vegetation on ground surface temperature and the active layer among different biogeographical regions in Antarctica. CATENA 190:104562. https://doi.org/10.1016/j.catena.2020.104562
Jones VJ (1996) The diversity, distribution and ecology of diatoms from Antarctic inland waters. Biodivers Conserv 5:1433–1449. https://doi.org/10.1007/BF00051986
Kavan J, Ondruch J, Nývlt D, Hrbáček F, Carrivick JL, Láska K (2017) Seasonal hydrological and suspended sediment transport dynamics in proglacial streams, James Ross Island, Antarctica. Geogr Anna A 97(1):38–55. https://doi.org/10.1080/04353676.2016.1257914
Kohler TJ, Stanish LF, Crisp SW, Koch JC, Liptzin D, Baeseman JL, McKnight DM (2015) Life in the main channel: long-term hydrologic control of microbial mat abundance in McMurdo Dry Valley Streams, Antarctica. Ecosystems 18:310–327. https://doi.org/10.1007/s10021-014-9829-6
Kopalová K, Van de Vijver B (2013) Structure and ecology of freshwater benthic diatom communities from Byers Peninsula, Livingston Island, South Shetland Island. Antarct Sci 25:239–253. https://doi.org/10.1017/S0954102012000764
Kopalová K, Elster J, Nedbalová L, Van de Vijver B (2009) Three new terrestrial diatom species from seepage areas on James Ross Island (Antarctic Peninsula Region). Diatom Res 24:113–122. https://doi.org/10.1080/0269249X.2009.9705786
Kopalová K, Nedbalová L, de Haan M, Van de Vijver B (2011) Description of five new species of the diatom genus Luticola (Bacillariophyta, Diadesmidaceae) found in lakes of James Ross Island (Maritime Antarctic Region). Phytotaxa 27:44–60. https://doi.org/10.11646/phytotaxa.27.1.5
Kopalová K, Veselá J, Elster J, Nedbalová L, Komárek J, Van de Vijver B (2012) Benthic diatoms (Bacillariophyta) from seepages and streams on James Ross Island (NW Weddell Sea, Antarctica). Plant Ecol Evol 145:190–208. https://doi.org/10.5091/plecevo.2012.639
Kopalová K, Nedbalová L, Nývlt D, Elster J, Van de Vijver B (2013) Diversity, ecology and biogeography of the freshwater diatom communities from Ulu Peninsula (James Ross Island, NE Antarctic Peninsula). Polar Biol 36:933–948. https://doi.org/10.1007/s00300-013-1317-5
Kopalová K, Ochyra R, Nedbalová L, Van de Vijver B (2014) Moss–inhabiting diatoms from two contrasting Maritime Antarctic islands. Plant Ecol Evol 147:67–84. https://doi.org/10.5091/plecevo.2014.896
Kopalová K, Soukup J, Kohler TJ, Roman M, Coria SH, Vignoni PA, Lecomte KL, Nedbalová L, Nývlt D, Lirio JM (2019) Habitat controls on limno-terrestrial diatom communities of Clearwater Mesa, James Ross Island, Maritime Antarctica. Polar Biol 42(8):1595–1613. https://doi.org/10.1007/s00300-019-02547-8
Košler J, Magna T, Mlčoch B, Mixa P, Nývlt D, Holub FV (2009) Combined Sr, Nd, Pb and Li isotope geochemistry of alkaline lavas from northern James Ross Island (Antarctic Penninsula) and implication for back-arc magma formation. Chem Geol 258:207–218. https://doi.org/10.1016/j.chemgeo.2008.10.006
Krembs C, Eicken H, Junge K, Deming JW (2002) High concentrations of exopolymeric substances in Arctic winter sea ice: implications for the polar ocean carbon cycle and cryoprotection of diatoms. Deep-Sea Res Pt I 49(12):2163–2181. https://doi.org/10.1016/S0967-0637(02)00122-X
Maltsev Y, Maltseva S, Kociolek JP, Jahn R, Kulikovskiy M (2021) Biogeography of the cosmopolitan terrestrial diatom Hantzschia amphioxys sensu lato based on molecular and morphological data. Sci Rep 11(1):1–19. https://doi.org/10.1038/s41598-021-89955-1
Mann DG (1999) The species concept in diatoms. Phycologia 38:437–495. https://doi.org/10.2216/i0031-8884-38-6-437.1
McKnight DA, Niyogi DK, Alger AS, Bomblies A, Conovitz PA, Tate CM (1999) Dry valley streams in Antarctica: ecosystems waiting for water. Bioscience 49(12):985–995. https://doi.org/10.1525/bisi.1999.49.12.985
Mehlich A (1984) Mehlich 3 soil test extractant: a modification of Mehlich 2 extractant. Commun Soil Sci Plan 15(12):1409–1416. https://doi.org/10.1080/00103628409367568
Moravcová A, Beyens L, Van de Vijver B (2010) Diatom communities in soils influenced by the wandering albatross (Diomedea exulans). Polar Biol 33(2):241–255. https://doi.org/10.1007/s00300-009-0700-8
Nedbalová L, Nývlt D, Kopáček J, Šobr M, Elster J (2013) Freshwater lakes of Ulu Peninsula, James Ross Island, north-east Antarctic Peninsula: origin, geomorphology and physical and chemical limnology. Antarct Sci 25:358–372. https://doi.org/10.1017/S0954102012000934
Nývlt D, Braucher R, Engel Z, Mlčoch B, ASTER team (2014) Timing of the Northern Prince Gustav Ice Stream retreat and the deglaciation of northern James Ross Island, Antarctic Peninsula during the last glacial-interglacial transition. Quaternary Res 82(2):441–449. https://doi.org/10.1016/j.yqres.2014.05.003
Nývlt D, Fišáková MN, Barták M, Stachoň Z, Pavel V, Mlčoch B, Láska K (2016) Death age, seasonality, taphonomy and colonization of seal carcasses from Ulu Peninsula, James Ross Island, Antarctic Peninsula. Antarct Sci 28(1):3–16. https://doi.org/10.1017/S095410201500036X
Ohtsuka T, Kudoh S, Imura S, Ohtani S (2006) Diatoms composing benthic microbial mats in freshwater lakes of Skarvsnes ice-free area, East Antarctica. Polar Biosci 20:113–130
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H (2020) vegan: community ecology package. R package version 2.5–7. https://CRAN.R-project.org/package=vegan
Olivero E, Scasso RA, Rinaldi CA (1986) Revision of the Marambio Group, James Ross Island, Antarctica. IAA, Contribución 331:1–28
Øvstedal DO, Lewis-Smith RL (2001) Lichens of Antarctica and South Georgia. A guide to their identification and ecology. Cambridge University Press, Cambridge
Peel MC, Finlayson BL, McMahon TA (2007) Updated world map of the Köppen-Geiger climate classification. Hydrol Earth Syst Sci 11(5):1633–1644
Pfister L, Wetzel CE, Klaus J, Martínez-Carreras N, Antonelli M, Teuling AJ, McDonnell JJ (2017) Terrestrial diatoms as tracers in catchment hydrology: a review. Wiley Interdiscip Rev Water 4(6):e1241. https://doi.org/10.1002/wat2.1241
Pinseel E, Van de Vijver B, Kavan J, Verleyen E, Kopalová K (2017a) Diversity, ecology and community structure of the freshwater littoral diatom flora from Petuniabukta (Spitsbergen). Polar Biol 40(3):533–551. https://doi.org/10.1007/s00300-016-1976-0
Pinseel E, Hejduková E, Vanormelingen P, Kopalová K, Vyverman W, Van de Vijver B (2017b) Pinnularia catenaborealis sp. Nov. (Bacillariophyceae), a unique chain-forming diatom species from James Ross Island and Vega Island (Maritime Antarctica). Phycologia 56(1):94–107. https://doi.org/10.2216/16-18.1
Pinseel E, Kulichová J, Scharfen V, Urbánková P, Van de Vijver B, Vyverman W (2019) Extensive cryptic diversity in the terrestrial diatom Pinnularia borealis (Bacillariophyceae). Protist 170(2):121–140. https://doi.org/10.1016/j.protis.2018.10.001
Pinseel E, Janssens SB, Verleyen E, Vanormelingen P, Kohler TJ, Biersma EM, Sabbe K, Van de Vijver B, Vyverman W (2020) Global radiation in a rare biosphere soil diatom. Nat Commun 11(1):1–12. https://doi.org/10.1038/s41467-020-16181-0
Pinseel E, Van de Vijver B, Wolfe AP, Harper M, Antoniades D, Ashworth AC, Ector L, Lewis AR, Perren B, Hodgson DA, Sabbe K, Verleyen E, Vyverman W (2021) Extinction of austral diatoms in response to large-scale climate dynamics in Antarctica. Sci Adv 7(38):eabh3233. https://doi.org/10.1126/sciadv.abh3233
Pla-Rabes S, Toro M, Van De Vijver B, Rocher C, Villaescusa JA, Camacho A, Quesada A (2013) Stability and endemicity of benthic diatom assemblages from different substrates in a maritime stream on Byers Peninsula, Livingston Island, Antarctica: the role of climate variability. Antarct Sci 25:254–266. https://doi.org/10.1017/S0954102012000922
Rabassa J, Skvarca P, Bertani L, Mazzoni E (1982) Glacier inventory of James Ross and Vega Islands, Antarctic Peninsula. Ann Glaciol 3:260–264
R Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. Accessed 26 Nov 2021
Roberts D, McMinn A (1999) Diatoms of the saline lakes of the Vestfold Hills, Antarctica. Bibl Diatomol 44:1–82
Roman M, Nedbalová L, Kohler TJ, Lirio JM, Coria SH, Kopáček J, Vignoni PA, Kopalová K, Lecomte KL, Elster J, Nývlt D (2019) Lacustrine systems of Clearwater Mesa (James Ross Island, north-eastern Antarctic Peninsula): geomorphological setting and limnological characterization. Antarct Sci 31(4):169–188. https://doi.org/10.1017/S0954102019000178
RStudio Team (2021) RStudio: integrated development environment for R. RStudio, PBC, Boston, MA. http://www.rstudio.com/. Accessed 26 Nov 2021
Sabbe K, Verleyen E, Hodgson DA, Vanhoutte K, Vyverman W (2003) Benthic diatom flora of freshwater and saline lakes in the Larsemann Hills and Rauer Islands, East Antarctica. Antarct Sci 15:227–248. https://doi.org/10.1017/S095410200300124X
Sakaeva A, Sokol ER, Kohler TJ, Stanish LF, Spaulding SA, Howkins A, Welch KA, Lyons WB, Barett JE, McKnight DM (2016) Evidence for dispersal and habitat controls on pond diatom communities from the McMurdo Sound Region of Antarctica. Polar Biol 39:2441–2456. https://doi.org/10.1007/s00300-016-1901-6
Skácelová K, Hrbáček F, Chattová B, Láska K, Barták M (2015) Biodiversity of freshwater autotrophs in selected wet places in northern coastal ecosystems of James Ross Island. Czech Polar Rep 5(1):12–26. https://doi.org/10.5817/CPR2015-1-2
Smellie JL, Johnson JS, McIntosh WC, Esser R, Gudmundsson MT, Hambrey MJ, van Wyk de Vries B (2008) Six million years of glacial history recorded in the James Ross Island Volcanic Group, Antarctic Peninsula. Palaeogeogr Palaeoclimatol Palaeoevol 260:122–148. https://doi.org/10.1029/201OSP001047
Smol JP, Stoermer EF (2010) The diatoms: applications for the environmental and earth sciences. Cambridge University Press, New York
Sørensen T (1948) A method of establishing groups of equal amplitude in plant sociology based on similarity of species content. K Dan Vidensk Selsk Skr 54:1–34
Souffreau C, Vanormelingen P, Verleyen E, Sabbe K, Vyverman W (2010) Tolerance of benthic diatoms from temperate aquatic and terrestrial habitats to experimental desiccation and temperature stress. Phycologia 49(4):309–324. https://doi.org/10.2216/09-30.1
Souffreau C, Vanormelingen P, Sabbe K, Vyverman W (2013a) Tolerance of resting cells of freshwater and terrestrial benthic diatoms to experimental desiccation and freezing is habitat-dependent. Phycologia 52(3):246–255. https://doi.org/10.2216/12-087.1
Souffreau C, Vanormelingen P, Van de Vijver B, Isheva T, Verleyen E, Sabbe K, Vyverman W (2013b) Molecular evidence for distinct Antarctic lineages in the cosmopolitan terrestrial diatoms Pinnularia borealis and Hantzschia amphioxys. Protist 164(1):101–115. https://doi.org/10.1016/j.protis.2012.04.001
Spaulding SA, Stoermer EF (1997) Taxonomy and distribution of the genus Muelleria Frenguelli. Diatom Res 12(1):95–113. https://doi.org/10.1080/0269249X.1997.9705405
Spaulding SA, McKnight DM, Stoermer EF, Doran PT (1997) Diatoms in sediments of perennially ice-covered Lake Hoare, and implications for interpreting lake history in the McMurdo Dry Valleys of Antarctica. J Paleolimnol 17:403–420
Stanish LF, Kohler TJ, Esposito RMM, Simmons BL, Nielsen UN, Wall DH, Nemergut DR, McKnight DM (2012) Extreme streams: flow intermittency as a control on diatom communities in meltwater streams in the McMurdo Dry Valleys, Antarctica. Can J Fish Aquat Sci 69:1405–1419. https://doi.org/10.1139/f2012-022
Stanish LF, Bagshaw EA, McKnight DM, Fountain AG, Tranter M (2013) Environmental factors influencing diatom communities in Antarctic cryoconite holes. Environ Res Lett. https://doi.org/10.1088/1748-9326/8/4/045006
Svojtka M, Nývlt D, Murakami M, Vávrová J, Filip J, Mixa P (2009) Provenance and post-depositional low-temperature evolution of the James Ross Basin sedimentary rocks (Antarctic Peninsula) based on fission track analysis. Antarct Sci 21(6):593. https://doi.org/10.1017/S0954102009990241
Tang Y, Horikoshi M, Li W (2016) ggfortify: unified interface to visualize statistical result of popular R packages. R J 8(2):478–489
Van de Vijver B, Beyens L (1999) Biogeography and ecology of freshwater diatoms in Subantarctica: a review. J Biogeogr 26:993–1000. https://doi.org/10.1046/j.1365-2699.1999.00358.x
Van de Vijver B, Kopalová K (2014) Four Achnanthidium species (Bacillariophyta) formerly identified as Achnanthidium minutissimum from the Antarctic Region. Eur J Taxon 79:1–19. https://doi.org/10.5852/ejt.2014.79
Van de Vijver B, Ledeganck P, Beyens L (2002a) Soil diatom communities from Île de la Possession (Crozet, sub-Antarctica). Polar Biol 25(10):721–729. https://doi.org/10.1007/s00300-002-0392-9
Van de Vijver B, Frenot Y, Beyens L (2002b) Freshwater diatoms from Île de la Possession (Crozet Archipelago, Subantarctica). Bibl Diatomol 46:1–412
Van de Vijver B, Mataloni G, Stanish L, Spaulding SA (2010) New and interesting species of the genus Muelleria (Bacillariophyta) from the Antarctic region and South Africa. Phycologia 49(1):22–41. https://doi.org/10.2216/09-27.1
Van de Vijver B, Zidarova R, de Haan M (2011) Four new Luticola taxa (Bacillariophyta) from the South Shetland Islands and James Ross Island (Maritime Antarctic Region). Nova Hedwigia 92:137–158. https://doi.org/10.1127/0029-5035/2011/0092-0137
Van de Vijver B, Zidarova R, Kopalová K (2014) New species in the genus Muelleria (Bacillariophyta) from the Maritime Antarctic Region. Fottea 14:77–90. https://doi.org/10.5507/fot.2014.006
Van de Vijver B, Kopalová K, Kociolek JP, Ector L (2015) Denticula jamesrossensis, a new freshwater diatom (Bacillariophyta) species from the Maritime Antarctic Region. Fottea 15:105–111. https://doi.org/10.5507/fot.2015.009
Van de Vijver B, Kopalová K, Zidarova R, Kociolek JP (2016) Two new Gomphonema species (Bacillariophyta) from the Maritime Antarctic Region. Phytotaxa 269:209–220. https://doi.org/10.11646/phytotaxa.269.3.4
Van der Werff A (1955) A new method for cleaning and concentrating diatoms and other organisms. Verh Internat Verein Theor Angew Limnol 12:276–277
Verleyen E, Van de Vijver B, Tytgat B, Pinseel E, Hodgson DA, Kopalová K, Chown SL, Van Ranst E, Imura S, Kudoh S, Van Nieuwenhuyze W, ANTDIAT consortium, Sabbe K, Vyverman W (2021) Diatoms define a novel freshwater biogeography of the Antarctic. Ecography 44(4):548–560. https://doi.org/10.1111/ecog.05374
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer, New York. https://ggplot2.tidyverse.org
Zidarova R, Kopalová K, Van de Vijver B (2016a) Diatoms from the Antarctic Region. I: Maritime Antarctica. Iconogr Diatomol 24:1–504
Zidarova R, Kopalová K, Van de Vijver B (2016b) Ten new bacillariophyta species from James Ross Island and the South Shetland Islands (Maritime Antarctic Region). Phytotaxa 272(1):37–62. https://doi.org/10.11646/phytotaxa.272.1.2
Zvěřina O, Coufalík P, Brat K, Červenka R, Kuta J, Mikeš O, Komárek J (2017) Leaching of mercury from seal carcasses into Antarctic soils. Environ Sci Pollut R 24:1424–1431
Acknowledgements
The authors wish to thank Dr. Jan Kavan, Dr. Luděk Sehnal, Dr. Peter Váczi and prof. Miloš Barták for their help during the sampling campaign. Dr. Jordan Bishop, P.B. Hamilton and one anonymous reviewer are thanked for critically reading the manuscript and suggesting substantial improvements. The authors would also like to thank to the scientific infrastructure of the Czech Antarctic Station “J.G. Mendel” and its crew for their support. E.P. is a research fellow of the Simons Foundation (Postdoc Fellow in Marine Microbial Ecology) and received funding from the Belgian American Education Foundation (B.A.E.F.), Fulbright Belgium, and the Fund for Scientific Research—Flanders (FWO) through aspirant grants 1104315N and 1104317N. This study was also supported by long-term research development project No. RVO 67985939 of the Czech Academy of Sciences.
Funding
E.P. is supported by a grant from the Simons Foundation (award 725407), and previously benefited from grants the Belgian American Education Foundation (B.A.E.F.), Fulbright Belgium, and the Fund for Scientific Research-Flanders (FWO) through aspirant Grants 1104315N and 1104317N. This study was also supported by long-term research development project No. RVO 67985939 of the Czech Academy of Sciences.
Author information
Authors and Affiliations
Contributions
BCH, DN, and BVDV conceived the ideas. BCH, TK, and TC performed the analyses and interpreted data. EP, TK, and TC are co-authors of the text. BCH performed the field sampling, did the microscopical observations, and analysed the diatom flora with a significant help of KK and BVDV. FH prepared Fig. 1 and commented on the manuscript. DN led the study and commented on the manuscript. BCH led the writing with significant input and editing from all authors.
Corresponding author
Ethics declarations
Conflict of interest
No conflicts of interest declared.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chattová, B., Cahová, T., Pinseel, E. et al. Diversity, ecology, and community structure of the terrestrial diatom flora from Ulu Peninsula (James Ross Island, NE Antarctic Peninsula). Polar Biol 45, 873–894 (2022). https://doi.org/10.1007/s00300-022-03038-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-022-03038-z