Abstract
Fulmarine petrels are top predators in the Antarctic region preying mostly on squid, fish, and carrion. Their diets have been widely studied, but less is known about the role of skeletal structures in the processes they use to obtain food. Here, we comparatively describe the skulls of fulmarine petrels, namely, the Giant Petrels (Macronectes), the Southern Fulmar (Fulmarus glacialoides), and the Cape Petrel (Daption capense), emphasizing those structures associated with the muscles responsible for opening/closing the jaws. The skull is dorsoventrally flattened and the bill is hooked-tipped and elongated in the studied species, but we found significant differences for relative bill length and relative cranium depth among them. These characteristics can be related to surface seizing and streamlining for diving and pursuing/capturing prey underwater. Longer bills also indicate that the mandible muscles are more posteriorly positioned relative to the bill tip, an adaptation for a fast bite, which is more pronounced in Giant Petrels. Nevertheless, there are broad areas of origin for the mandible muscles in the fossa musculorum temporalium and in the Os palatinum, especially in Giant Petrels. We thus infer that those muscles are well developed and hypothesize that, despite the adaptation for fast movements, their jaws are still capable of a relatively powerful bite. The Giant Petrels and Cape Petrel present a similar pattern of dorsoventral flattening of the skull, an adaptation for diving in pursuit of prey. In Giant Petrels, a flattened skull with a hooked-tipped bill also facilitates their feeding behavior of inserting the bill and head into carcasses for tearing flesh. We conclude that fulmarine petrels present variable morphological characters adapted to the different feeding strategies they employ in the Antarctic and the Southern Ocean.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Procellariidae is a monophyletic group of seabirds that presently contains 96 species into 16 genera (Winkler et al. 2020). Within this group, the fulmarine petrels consist of seven small to large (250 g to 5 kg), variably plumaged species: the Northern and Southern Giant Petrels (Macronectes halli and M. giganteus), the Northern and Southern Fulmars (Fulmarus glacialis and F. glacialoides), the Cape Petrel (Daption capense), the Antarctic Petrel (Thalassoica antartica), and the Snow Petrel (Pagodroma nivea) (Onley and Scofield 2007; Winkler et al. 2020). All species but the Northern Fulmar are distributed in Antarctic and sub-Antarctic waters (Winkler et al. 2020), where they constitute an important portion of the top predator community (Creet et al. 1994). The diets of these seabirds have been extensively studied (e.g., Arnould and Whitehead 1991; Hunter and Brooke 1992; Creet et al. 1994; Hodum and Hobson 2000), but the role of their skeletal structures in the processes they use to obtain food had not been investigated.
The cranium and jaws are most intimately involved in feeding, hence anatomical adaptations for this purpose are more likely to be found there (Burton 1974; Burger 1978; Donatelli et al. 2014). In mammals, for example, skull morphology (especially of the teeth) has been used to infer information about behavior, environment, feeding preferences, and important life events (Evans and Pineda-Munoz 2018). Avian skeletons, in turn, were extensively studied from a taxonomic approach in the late 1800s and the first half of the 1900s (see Sibley and Ahlquist 1990; Livezey and Zusi 2006, 2007). Early osteology-based works on fulmarine petrels were conducted by Pycraft (1899: Macronectes, Thalassoica, and Daption), Shufeldt (1907: Fulmarus), Kuroda (1954: Macronectes, Fulmarus, and Pagodroma), and, more recently, by Piro and Acosta Hospitaleche (2018: Macronectes).
In more recent years, feeding system morphology of birds has been also investigated in the context of evolutionary biology (e.g., Bock and Morioka 1971; Genbrugge et al. 2011), especially in relation to feeding behavior, and strict functional morphological investigations have also been conducted (e.g., Richards and Bock 1973; Estrella and Masero 2007; Carlos et al. 2017; Ferreira et al. 2018). For example, a recent study showed that the skull of waterbirds (Aequornithes) that feed on zooplankton is morphologically different from those preying on fish and/or cephalopods, and pursuit divers also present a different skull morphology from the species that feed by a combination of surface seizing and plunging or dipping (Chávez-Hoffmeister 2020). Bone structures, such as the mandibulosphenoidal joint, have also been related to the trophic habits of procellariiforms and sphenisciforms, showing differences in shape and strength of the structures of more and less strictly piscivorous species (Acosta Hospitaleche et al. 2020).
Our aim here is to describe the skulls of representatives of extant species of fulmarine petrels, with a focus on the structures that are particularly associated with the muscles responsible for opening and closing the jaw (i.e., the Musculi mandibulae, sensu Vanden Berge and Zweers 1993). We also aim to discuss our results in the context of the feeding biology of the studied species. For convenience, we selected the species that are seasonal migrants within the Brazilian Exclusive Economic Zone (Carlos 2009) since an adequate series of specimens are available in national museums and collections.
Materials and methods
We studied 41 skulls of adult birds: 5 of Macronectes giganteus, 6 of M. halli, and 4 of unidentified specimens of Giant Petrels, 16 of Fulmarus glacialoides, and 10 of Daption capense (Online Resource 1). All material belongs to the following collections: Museu de Ciências Naturais, Centro de Estudos Costeiros, Limnológicos e Marinhos do Instituto de Biociências, Universidade Federal do Rio Grande do Sul (MUCIN), Imbé; Coleção de Aves do Departamento de Zoologia, Instituto de Biociências, Universidade Federal do Rio Grande do Sul (DZ-UFRGS), Porto Alegre; Museu de Ciências Naturais da Secretaria do Meio Ambiente e Infraestrutura do Rio Grande do Sul (MCN), Porto Alegre; and Museu de Ciências e Tecnologia da Pontifícia Universidade Católica do Rio Grande do Sul (MCP), Porto Alegre (Appendix).
To compare osteological features and proportions among species, we initially studied a skull of a specimen of M. halli (DZ-UFRGS 001) and compared it with other specimens of the same species. We firstly compared them with skulls of M. giganteus (and with those of 4 unidentified Giant Petrel specimens) and then with skulls of F. glacialoides and D. capense (following the procedures of Dénes and Silveira 2007). We noted no significant pattern of anatomical distinction between M. halli and M. giganteus; therefore, we pooled the two species as “Giant Petrels” in our descriptions and discussion. We used their skulls as a reference for comparisons, given that the genus exhibits relatively significant differences in size and development of osteological features.
We examined specimens under an 8 × illuminated magnifying glass and photographed them with a Nikon D7000 digital camera with a 60-mm 2.8 Nikon macro lens. We took the following measurements (after Burger 1978) on specimens: cranium length and depth and upper jaw length (Online Resource 1). All measurements were taken to the nearest 0.1 mm with digital calipers. The cranium length seems to be less affected by adaptive modifications (Goodman and Fisher 1962: 179). Therefore, we used cranium length to calculate proportions (upper jaw length/cranium length and cranium depth/cranium length) to minimize the effects of size among species and sexual size dimorphism within each species. We used data from the literature (van Franeker and Braak 1993; Weidinger and van Franeker 1993; González-Solís 2004) to compare upper jaw length/cranium length between males and females of each species. The proportions were similar (range of differences: 0.01–0.08), suggesting that males and females present isometric scaling in the relation of upper jaw length to cranium length and this presumably applies to the relation of cranium depth to cranium length.
The mandible of birds has been hypothesized to function as a lever that pivots around the postorbital ligament (Zusi 1962), which runs from the apex of the processus postorbitalis to the processus lateralis mandibulae (Baumel and Raikow 1993). Therefore, we used the upper jaw length/cranium length ratio as a way to represent the mechanical advantage of the mandible. The mechanical advantage of any bone–muscle lever can be expressed as the ratio of the force arm to the resistance arm. A high mechanical advantage indicates a relatively more forceful bite; low mechanical advantage indicates a relatively less forceful, but faster bite (Hildebrand and Goslow 2006). We tested for differences in proportions of measures among species using a one-way analysis of variance (ANOVA). We then performed Tukey’s post hoc tests, given the sufficiently low p-value obtained from the ANOVA. Statistical analyses were performed at a significance level of 0.05 using the software RStudio v. 1.2.5033 (R Core Team 2021). For the cranium depth/length ratio, we did not include three specimens of Macronectes spp., since their quadrate bones were affixed (in this case, n = 12).
We mostly followed the anatomical nomenclature of the Nomina Anatomica Avium (Baumel and Witmer 1993; Baumel and Raikow 1993; Vanden Berge and Zweers 1993). The main exceptions included following Cracraft (1968) for the partes ossis lacrimalis and Zusi and Livezey (2006) for terms pertaining to the palatum osseum.
Results
The angulus craniofascialis is obtuse (ca. 150°–160°) in all studied species, especially in the Giant Petrels. Viewed from the side, the frons rises gradually and smoothly from the zona flexoria craniofascialis to the parietal region, forming a gentle concave curve in the Giant Petrels and Cape Petrel and a slightly convex profile in the Southern Fulmar (zfc—Fig. 1). In the Giant Petrels, the dorsal surface of the frontal region (f—Fig. 2) exhibits an elongate, narrow depressio frontalis (df—Fig. 2), which extends from just where the two fossae glandularum nasales (fgn—Fig. 2) approach each other at the rostral part of the parietal region (p—Fig. 2). The depression is shorter due to juxtaposition of the fossae glandularum nasales in the Southern Fulmar and is less marked in the Cape Petrel.
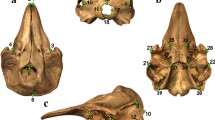
Lateral view of the skull of Northern Giant Petrel (Macronectes halli) (a), Southern Fulmar (Fulmarus glacialoides) (b), and Cape Petrel (Daption capense) (c). Apertura nasi ossea (ano), caput ossis lacrimalis (cl), crista nuchalis transversa (cnt), crista temporalis dorsalis (ctd), fossa musculorum temporalium (ft), fossa subtemporalis (fs), hamulus rostri maxillae (hrm), lamella dorsalis (ld), lamella ventralis (lv), margo tomialis rostri maxillae (mtrm), pes ossis lacrimalis (pol), processus descendens ossis lacrimalis (pdol), processus maxillaris ossis nasalis (pmon), processus paraocciptalis (pp), processus postorbitalis (ppo), rostrum maxillae (rm), tuberculum lacrimale arcus jugalis (tlaj), and zona flexoria craniofascialis (zfc). In blue, the respective muscles originating from each structure: Musculus adductor mandibulae externus (mame), Musculus pterygoideus (mp), and Musculus depressor mandibulae (md)
Dorsal view of the skull of Northern Giant Petrel (Macronectes halli) (a), Southern Fulmar (Fulmarus glacialoides) (b), and Cape Petrel (Daption capense) (c). Apertura nasi ossea (ano), caput ossis lacrimalis (cl), crista nuchalis transversa (cnt), crista temporalis dorsalis (ctd), frontal region (f), depressio frontalis (df), fossa glandulae nasalis (fgn), fossa musculorum temporalium (ft), Os nasale (on), Os premaxillare (op), processus frontalis ossis premaxillaris (pfop), processus frontalis ossis nasalis (pfon), parietal region (p), and zona flexoria craniofascialis (zfc). In blue, the respective muscle originating from each structure: Musculus adductor mandibulae externus (mame)
The avian maxilla, or upper jaw, is a compound structure made of the Os premaxillare, Os maxillare, and Os nasale (Baumel and Witmer 1993). Zusi in Baumel and Witmer (1993: 73, annot. 40) restrictively refers to the “rostrum maxillae” as the rostral end of the upper jaw formed by the fusion of the left and right bodies of the Ossa premaxillaris; however, Livezey and Zusi (2006) apply the term in a broader sense as a synonym for the whole maxilla, a usage we followed herein. In Giant Petrels (cf. Piro and Acosta Hospitaleche 2018) and in the other studied species, the processus frontalis ossis nasalis (pfon—Fig. 2) appears to overlie the Os frontale and along with the caudal end of the processus frontalis ossis premaxillaris (pfop—Fig. 2), form the zona flexoria craniofacialis, which in dorsal view appears as a flat, rostrocaudally narrow, transverse band (zfc—Fig. 2).
In all studied taxa, the apex rostri maxillae is downcurved in a hook shape, forming the hamulus rostri (sensu Livezey and Zusi 2006) (hrm—Fig. 1). In Giant Petrels, the curvature of the apex rostri is even more pronounced when the rhamphotheca is preserved (e.g., MCP 1486; 1518). The margo tomialis rostri maxillae (excluding the apex; mgrm—Fig. 1) is slightly curved in lateral view. Ventrally, the processus palatinus ossis premaxillaris is separated medially from its counterpart by a large fenestra ventromedialis (sensu Livezey and Zusi 2006), which is continuous with the fossa choanalis palatini (fcp—Fig. 3). The apertura nasi ossea (ano—Fig. 3) are longer than high, with a characteristic holorhinal type.
Ventral view of the skull of Northern Giant Petrel (Macronectes halli) (a), Southern Fulmar (Fulmarus glacialoides) (b), and Cape Petrel (Daption capense) (c). Angulus rostrolateralis of pars lateralis palatini (arpl), apertura nasi ossea (ano), crista lateralis of pars lateralis palatini (clplp), fossa choanalis palatini (fcp), fossa ventralis of pars lateralis palatini (fvp), lamella ventralis of pars choanalis palatini (lv), Os maxillare (om), Os premaxillare (op), processus rostralis palatini (prp), and zona flexoria palatina (zfp). In blue, the respective muscle originating from each structure: Musculus pterygoideus (mp)
In all studied taxa, the caput ossis lacrimalis (sensu Cracraft 1968; cl—Fig. 1) is enlarged and fused with the frontal region just posterior to the zona flexoria craniofacialis. It is noteworthy that the caput ossis lacrimalis also extends farthest rostrad beneath the processus maxillaris ossis nasalis (pmon—Fig. 1). The processus descendens ossis lacrimalis (sensu Cracraft 1968) is short and exhibits the distinct curvatura semicircularis ducti nasolacrimalis (sensu Livezey and Zusi 2006). This process terminates into a small pes lacrimalis (sensu Cracraft 1968; pol—Fig. 1) that ventrally approaches the tuberculum lacrimale arcus jugalis (tlaj—Fig. 1). The Os ectethmoidale is a square-shaped, relatively well-developed lamina that laterally contacts the processus descendens and pes ossis lacrimalis.
In all studied taxa, the fossa musculorum temporalium (sensu Zusi and Livezey 2000; ft—Fig. 1) occupies most of the area of the squamosal region (sensu Posso and Donatelli 2005) and extends rostrally on the processus postorbitalis. In Giant Petrels, the processus postorbitalis is lateroventrally projected and terminates into a sharp, pointed end; in the Southern Fulmar, this process is almost square shaped, with two ends forming angles close to 90°, similar to the Cape Petrel, in which the upper tip is more pronounced, forming a more acute angle (ppo—Fig. 1). The fossa musculorum temporalium is dorsally delimited by the crista temporalis dorsalis (ctd—Fig. 1) and approaches its counterpart in midline, especially in Giant Petrels. The caudal margin of the fossa musculorum temporalium is bounded by a laminar crista nuchalis transversa (cnt—Fig. 2), which runs caudolaterad to the processus paraoccipitalis (pp—Fig. 1). The crest is more prominent in Giant Petrels, so that the fossa musculorum temporalium is deeper in these taxa than in the Southern Fulmar and Cape Petrel. The fossa subtemporalis (fs—Fig. 1), delimited rostrally by the caudal margin of the fossa musculorum temporalium and caudally by the lateral part of the crista nuchalis transversa, is not developed in all studied species due to the convexity of the Os squamosum.
The pars maxillaris palatini (sensu Zusi and Livezey 2006) in all studied taxa consists of a dorsoventrally flattened processus rostralis palatini (prp—Fig. 3) that extends rostrad to about the caudal margin of the apertura nasi ossea where it is interposed between, and joined to, the Os maxillare and Os premaxillare. Immediately caudal to its juncture with the Os premaxillare, the processus rostralis exhibits an inconspicuous transverse bending zone, the zona flexoria palatina. The length of the processus rostralis, estimated from the zona flexoria palatina to the rostral margin of the pars choanalis palatini, slightly exceeds that of the Os palatinum proper (Fig. 3).
The pars choanalis palatini (sensu Zusi and Livezey 2006) is continuous with the processus rostralis and comprises the paired lamellae dorsales and lamellae ventrales (sensu Zusi and Livezey 2006). In all studied taxa, the lamellae dorsales are separated from each other and convoluted into a high, funnel-like cornu nasale (sensu Zusi and Livezey 2006). The lamellae ventrales (lv—Fig. 1, 3) are also separated from each other and ventrally projected, forming a triangular-like structure when viewed from the side.
As the name implies, the pars lateralis palatini (sensu Zusi and Livezey 2006) represents a lateral-to-ventrolateral expanse separating the lamellae dorsales and lamellae ventrales and provides the main surface area for the origin of the Musculus pterygoideus, which connects the Os palatinum and Os pterygoideum to the caudomedial surface of the mandible and the processus medialis mandibulae (Vanden Berge and Zweers 1993; Zusi and Livezey 2006). The fossa ventralis of pars lateralis palatini (fvp—Fig. 3) is rostrocaudally elongated and deep in all studied species, especially in Giant Petrels (Fig. 6). Laterally, the fossa is bounded by a distinct but not thick crista lateralis (sensu Zusi and Livezey 2006; clplp—Fig. 3). The angulus rostrolateralis of pars lateralis palatini (arpl—Fig. 3) demarcates the rostrolateral limit of the Musculus pterygoideus (Zusi and Livezey 2006) and, although not prominent, is distinctly present in all studied taxa.
Three partes rami mandibulae are often recognized: symphisialis, intermedia, and caudalis (Baumel and Witmer 1993). The pars symphisialis includes the rostral, often pointed segment where the two opposite rami mandibularum fuse with each other, forming the rostrum mandibulae (rm—Fig. 4). In all studied taxa, the rostrum is short (< 10% of the total length of the ramus mandibulae), curved downward relative to the margo tomialis mandibulae and its dorsal surface is concave or cup shaped. The pars intermedia extends from the caudal limit of the rostrum mandibulae to the angulus mandibulae dorsalis (sensu Livezey and Zusi 2006; amd—Fig. 4). The angulus mandibulae dorsalis is evident and downcurved; therefore, the margo dorsalis of pars intermedia mandibulae (mdpi—Fig. 4) is inclined relative to the margo dorsalis of pars caudalis mandibulae (mdpcm—Fig. 4). Dorsally, the pars intermedia bears a distinct and thick crista tomialis (Fig. 4).
Dorsolateral views of the mandible (mandibula) of Northern Giant Petrel (Macronectes halli) (a), Southern Fulmar (Fulmarus glacialoides) (b), and Cape Petrel (Daption capense) (c). Angulus mandibulae dorsalis (amd), fossa articularis quadratica (faq), fossa aditus canalis neurovascularis (fac), margo dorsalis of pars caudalis mandibulae (mdpcm), margo dorsalis of pars intermedia mandibulae (mdpi), processus lateralis mandibulae (plm), processus medialis mandibulae (pmm), processus pseudocoronoidei mandibulae (p1, p2), processus retroarticularis mandibulae (prm), rostrum mandibulae (rm), and tuberculum pseudotemporales (tp)
In birds, the pars caudalis mandibulae provides areas of attachment for musculi mandibulae and facets for articulation with the Os quadratum (Baumel and Witmer 1993). In all studied taxa, there are two tuberculum-like proccessus pseudocoronoidei mandibulae (sensu Donatelli 1996; p1, p2—Fig. 4), both located on the margo dorsalis of pars caudalis. These processes serve as a point of insertion for the aponeurosis of the Musculus adductor mandibulae externus and pars rostralis (Baumel and Witmer 1993). A distinct, strong tuberculum pseudotemporales (tp—Fig. 4) is positioned caudal to the fossa aditus canalis neurovascularis (fac—Fig. 4) and rostral to the fossa articularis quadratica (faq—Fig. 4) in the Giant Petrels and Southern Fulmar; the tuberculum is inconspicuous in the Cape Petrel.
In all studied taxa, the cotyla medialis of the fossa articularis quadratica is the largest of the three facets for articulation with the Os quadratum. The cotyla medialis is separated from the cotyla lateralis by a distinct ridge-like crista intercotylaris. The cotyla lateralis and cotyla caudalis merge into a single surface, placed dorsally to the cotyla medialis.
The pars caudalis mandibulae also bears the processus medialis (pmm—Fig. 4) and the processus retroarticularis (prm—Fig. 4), the former being the strongest of the two. The fossa caudalis of processus medialis is deeper and well circumscribed in all studied taxa, especially in Giant Petrels. The processus lateralis (plm—Fig. 4) and the processus retroarticularis have a tubercular shape.
The parameters for the proportions of measures in each taxa are in Table 1. We found significant statistical differences for both ratios among taxa (cranium depth/cranium length: F = 4.62, df = 2, p-value = 0.01; upper jaw length/skull length: F = 160.4, df = 2, p-value < 0.02 × 10–14). The last p-value is presented here as an inequality due to the accuracy of the aov function in R. The p-values of Tukey’s post hoc tests for pairwise comparisons are in Table 2. The bill is relatively longer in Giant Petrels, followed by Southern Fulmar and Cape Petrel; and the cranium is more dorsoventrally flattened in Giant Petrels than in Southern Fulmar, with no statistical differences among the other taxa.
Discussion
The studied taxa have dorsoventrally flattened skulls with rostrocaudally elongated, hooked-tipped bills. These characteristics indicate streamlining for diving, pursuing, and capturing prey underwater, foraging techniques frequently employed by fulmarine petrels. The elongated bill of the studied species means that their mandible muscles are more posteriorly positioned relative to the bill tip: bill is relatively longer in Giant Petrels, followed by Southern Fulmar and then by Cape Petrel. In the studied taxa, the lever resistance arm, represented by most of the bill length, is longer than the force arm (Fig. 5); therefore, the mechanical advantage of the mandible opening-closing lever is low. This suggests an adaptation that favors speed over strength (Zusi 1962; Raikow 1970; Burger 1978; Morales-García et al. 2021), which is probably more pronounced in Giant Petrels. Their long, fast-moving mandibles are likely more advantageous for capturing and handling prey with the bill, as already proposed for other long-billed seabirds, e.g., frigatebirds, boobies, and cormorants (Burger 1978; Carlos et al. 2017). On the contrary, a relative decrease in jaw length reduces the resistance arm, thus improving the mechanical advantage (Hildebrand and Goslow 2006). Therefore, birds with proportionally shorter bills, like peppershrikes (Vireonidae), tend to have strong bites (Previatto and Posso 2015a, b).

Adapted from Bühler (1981)
Biomechanical models of the Musculus depressor mandibulae, Musculus adductor mandibulae externus, Musculi protractor pterygoidei et quadrati, and Musculus pterygoideus, showing their approximate attachment area and the respective movements for which they are responsible. The upward arrow at the mandibula and the downward arrow at the maxilla represent abduction; the downward arrow at the mandibula and the upward arrow at the maxilla represent adduction.
A further aspect should be considered with regard to jaw movements: the development of the muscles. Long bills are less efficient for handling prey (Ashmole 1968) or tearing flesh, unless they possess strong muscles. Birds mainly open the mandible with the Musculus depressor mandibulae and close the mandible with the Musculus adductor mandibulae externus, Musculus pterygoideus, and Musculus pseudotemporalis profundus; the last two named muscles simultaneously assist in retracting the upper jaw (rostrum maxillae) against the mandible (Vanden Berge and Zweers 1993) (Fig. 5). The size of these muscles has been often associated with the area available for their origins and insertions (e.g., Bock 1964; Owre 1967; Burger 1978; Donatelli 1996; Previatto and Posso 2015b).
The Musculus adductor mandibulae externus mainly originates from the fossa musculorum temporalium (Bühler 1981), which is broad and deep in the studied taxa, especially in Giant Petrels (Fig. 6). The Musculus pterygoideus mainly originates from the fossa ventralis of pars lateralis palatini (Vanden Berge and Zweers 1993). Nevertheless, Zusi and Livezey (2006) noted that in procellariiforms (e.g., Laysan Albatross [Phoebastria immutabilis]) the Musculus pterygoideus also occupies the lateral surface of the cornu nasale and the processus rostralis palatini; this also likely occurs in the studied taxa. It has been estimated that a third of the “biting force” (i.e., adduction of the mandible and retraction of the upper jaw) is exerted by the Musculus pterygoideus alone (Burger 1978). Therefore, we infer that in the studied species, particularly in Giant Petrels, the jaw adductor muscles are well developed and hypothesize that, despite the low mechanical advantage, their jaws are still capable of a relatively powerful bite. A relatively strong bite is particularly important for Giant Petrels, which kill large birds. For example, on Gough Island, in the South Atlantic, Giant Petrels kill penguins by grasping their hindneck and holding them underwater until they stop struggling (Ryan et al. 2008). In contrast, albatrosses have a broad though shallow fossa musculorum temporalium (cf. Dénes and Silveira 2007) and supposedly do not have jaws with high closing strength. These birds do not take strong muscular prey and often consume dead or moribund squid floating on the surface or indirectly from fish viscera discarded during fish processing (Prince and Morgan 1987; Vaske Júnior 2011).
Fossa musculorum temporalium (top) and fossa ventralis of pars lateralis palatini (bottom). Approximate attachment area of the Musculus adductor mandibulae externus and Musculus pterygoideus, respectively. From left to right: Northern Giant Petrel (Macronectes halli), Southern Fulmar (Fulmarus glacialoides), and Cape Petrel (Daption capense)
The Musculus depressor mandibulae also assists in elevating the upper jaw. It originates from the fossa subtemporalis and inserts into the fossa caudalis mandibulae and processus retroarticularis mandibulae (Zusi 1967; Vanden Berge and Zweers 1993), which are all inconspicuous structures in the studied taxa. As noted by Bock (1964), the size of this muscle is associated with the size and shape of the processus retroarticularis; nevertheless, the elongation of the process does not solely imply that the mandible is depressed more powerfully. Instead, a large Musculus depressor mandibulae will also assist in elevating the upper jaw (Bock 1964; Zusi 1967). Thus, we suggest that, in fulmarine petrels, the elevation of the upper jaw is mostly performed by the Musculus protractor pterygoidei et quadrati and the Musculus pterygoideus (Zusi 1967).
While foraging, jaws of birds are susceptible to forces other than that from the Musculus pterygoideus. These forces may cause abnormal retraction of the upper jaw. Cracraft (1968) hypothesized that the action of these “outside forces” has resulted in the evolution of other retractor stops to avoid the breakage of the zona flexoria craniofacialis and probably other bending zones, like the zona flexoria palatina. Cranial kinesis, which is the ability to move the upper beak relative to the braincase (Bout and Zweers 2001), maintains the primary axis of orientation of the bill and absorbs shocks (Zusi 1967). This mechanism prevents the jaws from disarticulation and/or damage when petrels are grasping small and/or fast-moving prey (Zusi 1967). In the studied taxa, at least one other mechanism helps to protect the jaws from disarticulation and damage: the projection of the caput ossis lacrimalis beneath the processus maxillaris ossis nasalis. This mechanism acts as a stop to prevent the excessive retraction of the upper jaw (Cracraft 1968).
Most procellariforms “surface-seize” planktonic crustaceans, squid, fish, or floating carrion while sitting on the water (Harper 1987; Shealer 2002). Nevertheless, the fulmarine petrels are a group with other feeding strategies that seem to be important: Giant Petrels and Cape Petrels dive in pursuit of prey/food using their wings and feet for propulsion (e.g., Harper 1987, Prince and Morgan 1987, van den Hoff and Newbery 2006). The similar cranium depth/cranium length ratio between Giant Petrels and Cape Petrel indicates that these taxa present a similar pattern of dorsoventral flattening of the skull, which could be related to the aforementioned feeding behavior. Besides being streamlined, their longer bills also allow rapid movements of the bill tip, thus facilitating the capture of prey (Ashmole 1968). For example, Harper (1987) observed Cape Petrels catching prey with rapid pigeon-like up-and-down head movements, dipping the bill or even the head.
Giant Petrels have some particularities. Firstly, their strongly hooked bills also serve for tearing flesh (Croxall and Prince 1980). They are unique among the procellarids in being able to efficiently walk on land, which allows them to exploit terrestrial prey and carrion, such as seal carcasses and small burrowing petrels (Prince and Morgan 1987; Hunter 1983; Hunter and Brooke 1992); recent records on Gough Island show that Southern Giant Petrels even depredated breeding Atlantic Yellow-nosed Albatrosses (Thalassarche chlororhynchos) (Risi et al. 2021) and conspecifics on Nelson Island (Grohmann Finger et al. 2021). At sea, Giant Petrels have been seen surface diving for carrion (van den Hoff and Newbery 2006) and preying on flesh of live sperm whales (Physeter macrocephalus) in aggregations around longlining operations (Towers and Gasco 2020). Secondly, the pronounced dorsoventral flattening of the skull of Giant Petrels likely favors their necrophagous feeding behavior, since they often insert the bill and head into carcasses to reach internal organs.
Based on our analysis, we conclude that elongated hooked-tipped bills play an important role in the feeding behavior of the studied taxa, especially surface seizing, a widespread technique used among procellarids (Prince and Morgan 1987; Shealer 2002). We also propose that the studied taxa are capable of rapid yet strong jaw movements, which are particularly important for Giant Petrels that tear up carrion and secure large, living prey. The Southern Fulmar and Cape Petrel, in turn, do not feed on strong, muscular prey. For example, the Southern Fulmar feeds extensively on Histioteuthidae (Fonseca and Petry 2007), a family that contains mostly weakly muscled squids (Voss et al. 1998). Nevertheless, the feeding action of the jaws is also affected by the points and angles of origin and insertion of muscles, as well as the type of muscle fibers (Bock 1964; Burger 1978; Donatelli et al. 2014; Previatto and Posso 2015a). Thus, a more detailed hypothesis for the jaw movements and strength in fulmarine petrels and their relations with feeding habits should incorporate data on the jaw and anterior neck musculatures (Owre 1967; Burger 1978) and in loco observations of feeding behavior.
The Cape Petrel and Southern Fulmar present similar diets, foraging techniques and are more morphologically similar to each other than to the Giant Petrels, with previous studies suggesting that the two species share resources and occupy only slightly different trophic positions (Arnould and Whitehead 1991; Hodum and Hobson 2000). Furthermore, the trophic structure in Antarctic marine ecosystems has been considered simple at intermediate and upper trophic levels (Rau et al. 1992; Hodum and Hobson 2000), with species sharing common food resources. Nevertheless, the results presented here indicate that the fulmarine petrels are a morphologically diverse group of top predators in Antarctica, with dissimilarities in size and proportions of the skull. To our knowledge, this is the first study to discuss data on feeding biology of fulmarine petrels based on osteological characters, which should be a more widespread method to support the use of stable isotopes and stomach content analysis for a comprehensive approach.
Data availability
Raw data were generated at UFRGS and are available as Electronic Supplementary Material.
Code availability
Not applicable.
References
Acosta Hospitaleche C, Piro A, Sosa A (2020) The mandibulosphenoidal joint in penguins, albatrosses, and petrels: comparative anatomy and functional implications. Vertebr Zool 70(3):263–274. https://doi.org/10.26049/VZ70-3-2020-01
Arnould JPY, Whitehead MD (1991) The diet of Antarctic petrels, cape petrels and southern fulmars rearing chicks in Prydz Bay. Antarc Sci 3:19–27. https://doi.org/10.1017/S0954102091000056
Ashmole NP (1968) Body size, prey size and ecological segregation in five sympatric tropical terns. Syst Zool 17:292–304. https://doi.org/10.2307/2412008
Baumel JJ, Raikow RJ (1993) Arthrologia. In: King AS, Breazile JE, Evans HE, Vanden Berge JC (eds) Handbook of avian anatomy: nomina anatomica avium. Nuttall Ornithological Club, Cambridge, pp 133–187
Baumel JJ, Witmer LM (1993) Osteologia. In: King AS, Breazile JE, Evans HE, Vanden Berge JC (eds) Handbook of avian anatomy: nomina anatomica avium. Nuttall Ornithological Club, Cambridge, pp 45–132
Bock WJ (1964) Kinetics of the avian skull. J Morphol 114:1–42. https://doi.org/10.1002/jmor.1051140102
Bock WJ, Morioka H (1971) Morphology and evolution of the ectethmoid-mandibular articulation in the meliphagidae (Aves). J Morphol 135:13–50. https://doi.org/10.1002/jmor.1051350103
Bout RG, Zweers GA (2001) The role of cranial kinesis in birds. Comp Biochem Physiol A Mol Integr Physiol 131(1):197–205. https://doi.org/10.1016/s1095-6433(01)00470-6
Burger AE (1978) Functional anatomy of the feeding apparatus of four South African cormorants. Zool Afr 13:81–102. https://doi.org/10.1080/00445096.1978.11447608
Burton PJK (1974) Feeding and the feeding apparatus in waders: a study of anatomy and adaptations in the Charadrii. Trustees of the British Museum (Natural History), London
Bühler P (1981) The functional anatomy of the avian jaw apparatus. In: King AS, McLelland J (eds) Form and function in birds, vol 2. Academic Press, London, pp 439–468
Carlos CJ (2009) Seabird diversity in Brazil: a review. Sea Swallow 58:17–46
Carlos CJ, Alvarenga JG, Mazzochi MS (2017) Osteology of the feeding apparatus of magnificent Frigatebird Fregata magnificens and Brown Booby Sula leucogaster (Aves: Suliformes). Pap Avulsos Zool 57:265–274. https://doi.org/10.11606/0031-1049.2017.57.20
Chávez-Hoffmeister M (2020) Bill disparity and feeding strategies among fossil and modern penguins. Paleobiology 46(2):176–192. https://doi.org/10.1017/pab.2020.10
Cracraft J (1968) The lacrimal-ectethmoid bone complex in birds: a single character analysis. Am Midl Nat 80:316–359. https://doi.org/10.2307/2423530
Creet S, Van Franeker JA, Van Spanje TM, Wolff WJ (1994) Diet of the Pintado Petrel Daption capense at King George Island, Antarctica, 1990/91. Mar Ornithol 22:221–229
Croxall JP, Prince PA (1980) Food, feeding ecology and ecological segregation of seabirds at South Georgia. Biol J Linn Soc 14:103–131
Dénes FV, Silveira LF (2007) Cranial osteology and taxonomy of albatrosses of genus Diomedea Linneaus, 1758 and Thalassarche Reichenbach, 1853 (Procellariformes: Diomedeidae). Pap Avulsos Zool 47:43–61. https://doi.org/10.1590/S0031-10492007000300001
Donatelli RJ (1996) The jaw apparatus of the neotropical and of the afrotropical woodpeckers (Aves: Piciformes). Arq Zool 33:1–70. https://doi.org/10.11606/issn.2176-7793.v33i1p1-70
Donatelli RJ, Höfling E, Catalano AL (2014) Relationship between jaw apparatus, feeding habit, and food source in oriental woodpeckers. Zool Sci 31:223–227. https://doi.org/10.2108/zs130146
Estrella SM, Masero JA (2007) The use of distal rhynchokinesis by birds feeding in water. J Exp Biol 210(21):3757–3762. https://doi.org/10.2108/zs130146
Evans AR, Pineda-Munoz S (2018) Inferring mammal dietary ecology from dental morphology. In: Croft DA, Su DF, Simpson SW (eds) Methods in paleoecology: reconstructing cenozoic terrestrial environments and ecological communities. Springer, Cham, pp 37–51
Ferreira MMD, Tambussi CP, Degrange FJ, Pestoni S, Tirao GA (2018) The cranio-mandibular complex of the nightjar Systellura longirostris (Aves, Caprimulgiformes): functional relationship between osteology, myology and feeding. Zoology 132:6–16. https://doi.org/10.1016/j.zool.2018.11.001
Fonseca VSS, Petry MV (2007) Evidence of food items used by Fulmarus glacialoides (Smith, 1840) (Procellariiformes: Procellariidae) in Southern Brazil. Polar Biol 30:317–320. https://doi.org/10.1007/s00300-006-0185-7
van Franeker JA, ter Braak CJF (1993) A generalized discriminant for sexing fulmarine petrels from external measurements. Auk 110(3):492–502. https://doi.org/10.2307/4088413
Genbrugge A, Herrel A, Boone M, Van Hoorebeke L, Podos J, Dirckx J, Aerts P, Dominique A (2011) The head of the finch: the anatomy of the feeding system in two species of finches (Geospiza fortis and Padda oryzivora). J Anat 219:676–695. https://doi.org/10.1111/j.1469-7580.2011.01437.x
González-Solís J (2004) Sexual size dimorphism in northern giant petrels: ecological correlates and scaling. Oikos 105:247–254. https://doi.org/10.1111/j.0030-1299.2004.12997.x
Goodman HI, Fisher DC (1962) Functional anatomy of the feeding apparatus in waterfowl (Aves: Anatidae). Illinois University Press, Carbondale
Grohmann Finger JV, Corá DH, Petry MV, Krüger L (2021) Cannibalism in southern giant petrels (Macronectes giganteus) at Nelson Island, Maritime Antarctic Peninsula. Polar Biol 44:1219–1222. https://doi.org/10.1007/s00300-021-02859-8
Harper PC (1987) Feeding behaviour and other notes on 20 species of Procellariiformes at sea. Notornis 34:169–192
Hildebrand M, Goslow GE (2006) Análise da Estrutura dos Vertebrados. Atheneu, Rio de Janeiro
Hodum PJ, Hobson KA (2000) Trophic relationships among Antarctic fulmarine petrels: insights into dietary overlap and chick provisioning strategies inferred from stable-isotope (δ15N and δ13C) analyses. Mar Ecol Prog Ser 198:273–281. https://doi.org/10.3354/meps198273
Hunter S (1983) The food and feeding ecology of the Giant Petrels Macronectes halli and M. giganteus at South Georgia. J Zool 200(4):521–538. https://doi.org/10.1111/j.1469-7998.1983.tb02813.x
Hunter S, Brooke ML (1992) The diet of giant Petrels Macronectes spp. at Marion Island, Southern Indian Ocean. Colon Waterbirds 15:56–65. https://doi.org/10.2307/1521354
Kuroda N (1954) On the classification and phylogeny of the order Tubinares, particularly the shearwaters (Puffinus), with special considarations (sic) on their osteology and habit differentiation. The author, Tokyo
Livezey BC, Zusi RL (2006) Higher-order phylogeny of modern birds (Theropoda, Aves: Neornithes) based on comparative anatomy: I.—Methods and characters. Bulletin of Carnegie Museum of Natural History, Pittsburgh
Livezey BC, Zusi RL (2007) Higher-order phylogeny of modern birds (Theropoda, Aves: Neornithes) based on comparative anatomy. II. Analysis and discussion. Zool J Linn Soc 149:1–95. https://doi.org/10.1111/j.1096-3642.2006.00293.x
Morales-García NM, Gill PG, Janis CM, Rayfield EJ (2021) Jaw shape and mechanical advantage are indicative of diet in Mesozoic mammals. Commun Biol 4:242. https://doi.org/10.1038/s42003-021-01757-3
Onley D, Scofield P (2007) Albatrosses, petrels and shearwaters of the world. Bloomsbury Publishing, London
Owre OT (1967) Adaptations for locomotion and feeding in the anhinga and the double-crested cormorants. Ornithol Monogr 6:1–138. https://doi.org/10.2307/40166666
Piro A, Acosta Hospitaleche C (2018) Skull morphology and ontogenetic variation of the Southern Giant Petrel Macronectes giganteus (Aves: Procellariiformes). Polar Biol 42:27–45. https://doi.org/10.1007/s00300-018-2397-z
Posso SR, Donatelli RJ (2005) Skull and mandible formation in the cuckoo (Aves, Cuculidae): contributions to the nomenclature in avian osteology and systematics. Eur J Morphol 4(5):163–172. https://doi.org/10.1080/09243860500315507
Previatto DM, Posso SR (2015a) Jaw musculature of Cyclarhis gujanensis (Aves: Vireonidae). Braz J Biol 75:655–661. https://doi.org/10.1590/1519-6984.20113
Previatto DM, Posso SR (2015b) Cranial osteology of Cyclarhis gujanensis (Aves: Vireonidae). Pap Avulsos Zool 55:255–260. https://doi.org/10.1590/0031-1049.2015.55.18
Prince PA, Morgan RA (1987) Diet and feeding ecology of Procellariiformes. In: Croxall JP (ed) Seabirds: feeding biology and role in marine ecosystems. Cambridge University Press, Cambridge, pp 135–172
Pycraft WP (1899) Contributions to the Osteology of Birds. Part III. Turbinares. Proc Zool Soc Lond 1899:381–411
R Core Team (2021) R: a language and environment for statistical computing. Austria, Vienna
Raikow J (1970) Evolution of diving adaptations in the stifftail ducks. University of California Press, Berkeley
Rau GH, Ainley DG, Bengston JL, Torres JJ, Hopkins TL (1992) 15N/14N and 13C/12C in Weddell Sea birds, seals, and fish: implications for diet and trophic structure. Mar Ecol Prog Ser 84:1–8. https://doi.org/10.3354/meps084001
Richards LP, Bock W (1973) Functional anatomy and adaptive evolution of the feeding apparatus in the Hawaiian Honeycreeper genus Loxops (Drepanididae). Ornithol Monogr 15:1–173. https://doi.org/10.2307/40166695
Risi MM, Jones CW, Osborne AM, Steinfurth A, Oppel S (2021) Southern Giant Petrels Macronectes giganteus depredating breeding Atlantic Yellow-nosed Albatrosses Thalassarche chlororhynchos on Gough Island. Polar Biol 44:593–599. https://doi.org/10.1007/s00300-021-02810-x
Ryan PG, Sommer E, Breytenbach E (2008) Giant Petrels Macronectes hunting Northern Rockhopper Penguins Eudyptes moseleyi at Sea. Ardea 96:129–134. https://doi.org/10.5253/078.096.0116
Shealer DA (2002) Foraging behavior and food of seabirds. In: Schreiber EA, Burger J (eds) Biology of marine birds. CRC Press, Boca Raton, pp 137–177
Shufeldt RW (1907) On the osteology of the Tubinares. Am Nat XLI:109–124
Sibley CG, Ahlquist JE (1990) Phylogeny and classification of birds: a study in molecular evolution. Yale University Press, New Haven
Towers JR, Gasco N (2020) Giant petrels (Macronectes spp.) prey on depredating sperm whales (Physeter macrocephalus). Polar Biol 43:919–924. https://doi.org/10.1007/s00300-020-02687-2
van den Hoff J, Newbery K (2006) Southern Giant Petrels Macronectes giganteus diving on submerged carrion. Mar Ornithol 34(1):61–64
Vanden Berge JC, Zweers GA (1993) Myologia. In: King AS, Breazile JE, Evans HE, Vanden Berge JC (eds) Handbook of avian anatomy: nomina anatomica avium. Nuttall Ornithological Club, Cambridge, pp 189–247
Vaske Júnior T (2011) Are deep-sea cephalopods really common preys (sic) for oceanic seabirds? Biota Neotrop 11:177–180. https://doi.org/10.1590/S1676-06032011000100018
Voss NA, Nesis KN, Rodhouse PG (1998) The cephalopod family Histioteuthidae (Oegopsida): systematics, biology, and biogeography. Smithson Contrib Zool 586:293–372
Weidinger K, van Franeker JA (1993) Applicability of external measures to sexing of the Cape Petrel Daption capense at within-pair, within-population and between-population scales. J Zool Lond 245:473–482. https://doi.org/10.1111/j.1469-7998.1998.tb00122.x
Winkler DW, Billerman SM, Lovette IJ (2020) Shearwaters and Petrels (Procellariidae), version 1.0. In: Billerman SM, Keeney BK, Rodewald PG, Schulenberg TS (eds) Birds of the World. Cornell Lab of Ornithology, Ithaca. https://doi.org/10.2173/bow.procel3.01
Zusi RL (1962) Structural adaptations of the head and neck in the black skimmer. Nuttall Ornithological Club, Cambridge
Zusi RL (1967) The role of the depressor mandibulae muscle in kinesis of the avian skull. Proc US Natl Mus 123:1–28. https://doi.org/10.5479/si.00963801.123-3607.1
Zusi RL, Livezey BC (2000) Homology and phylogenetic implications of some enigmatic cranial features in galliform and anseriform birds. Ann Carnegie Mus 69:157–193
Zusi RL, Livezey BC (2006) Variation in the os palatinum and its structural relation to the Palatum osseum of birds (Aves). Ann Carnegie Mus 75:137–180. https://doi.org/10.2992/0097-4463(2006)75[137:VITOPA]2.0.CO;2
Acknowledgements
We thank Janaína C. Wickert and Maurício Tavares (MUCIN), Glayson A. Bencke (MCN), and Carla S. Fontana (MCP) for allowing us to study the specimens under their care. We are also grateful to Gabriela de Souza Pinto Arnoso (UFRGS) who elaborated Fig. 5. The manuscript benefited from reviews by Carolina Acosta Hospitaleche and three anonymous reviewers and the editorial advice of Dieter Piepenburg. MSM received an undergraduate fellowship from the Programa de Iniciação Científica BIC/UFRGS. CJC is supported by a Postdoctoral fellowship from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Funding
This research received support from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Author information
Authors and Affiliations
Contributions
MSM and CJC conceived and designed research. MSM and CJC examined and described specimens. MSM photographed and measured specimens and conducted the statistical analysis. MSM and CJC wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All studied specimens are deposited in scientific collections and museums; therefore, no ethical approval is required.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Appendix
Appendix
Specimens examined: Macronectes halli—DZ-UFRGS 001, 003, 004, 005; MUCIN 048, 050; Macronectes giganteus—DZ-UFRGS 002, 006; MCP 1622; MUCIN 047, 049; Macronectes sp.—MCN 622, 423; MCP 1518, 1486; Fulmarus glacialoides—MUCIN 0800, 0830, 0866, 0929; MCN 24, 381, 382, 464, 576; MCP 0851, 1661, 1666, 1680, 1681, 2280, 2677; and Daption capense—MUCIN 0367, 0540, 0602, 0691, 0755, 0973; MCN 384, 572; MCP 0575, 0585.
Rights and permissions
About this article
Cite this article
Mazzochi, M.S., Carlos, C.J. Skull morphology of four Antarctic fulmarine petrels (Aves: Procellariiformes): insights into their feeding biology. Polar Biol 45, 191–201 (2022). https://doi.org/10.1007/s00300-021-02983-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-021-02983-5