Abstract
To understand the community structure and the functional dynamics of phytoplankton over the long term, it is essential to identify rapid changes in the properties of Antarctic phytoplankton communities in relation to ongoing changes in environmental factors due to climate change. This study investigated short-term variability in the phytoplankton biomass and its composition over the summer of 2010 when the sea surface temperature was lowest and chlorophyll-a (chl-a) concentrations were the highest, relative to a 15-year monitoring period (1996–2011). We assessed the intraseasonal variability of the phytoplankton assemblage structure and its synchrony with changes in the main environmental variables in Marian Cove of King George Island, Antarctica. Chlorophyll-a concentrations in summer 2010 (January–February) were significantly higher (up to 24 μg L−1) when the high phytoplankton carbon biomass (603 μg C L−1) was dominated by the sympagic diatom Navicula glaciei, the benthic diatoms Licmophora belgicae and Fragilaria striatula, the planktonic diatoms Thalassiosira antarctica and Thalassiosira spp. (cell size < 10 μm), and the Haptophyceae nanoplanktonic cells of Phaeocystis antarctica. Intraseasonal processes such as easterly winds direction on Maxwell Bay appeared to be the main factors affecting the advection of cold, nutrient-rich waters, and water stability that enhanced phytoplankton growth in Marian Cove.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The coastal ecosystem of Antarctica is vulnerable to the main drivers of climate change such as warming, ozone depletion, and anthropogenic pollution (Kang et al. 1997; Smith et al. 2007; Rückamp et al. 2011; Lee et al. 2015). Ongoing climate change is causing major changes to all levels of the Antarctic ecosystem, encompassing microbial communities (Piquet et al. 2011) and including phytoplankton function and structure (Deppeler and Davidson 2017; van Leeuwe et al. 2018). Particularly, the coastal areas of the Western Antarctic Peninsula (WAP) are among the most rapidly warming regions on Earth (Hansen et al. 1999; Vaughan et al. 2003). For example, during the past 50 years, the mean global atmospheric temperature has increased by 0.6 °C, whereas the atmospheric temperature of the WAP has risen by 1.5 °C during the same period (Cook et al. 2005, 2014, 2016; Clarke et al. 2007), which has resulted in substantial reduction of ice sheet and marine-terminating glaciers (Turner et al. 2013; Rignot et al. 2019), an increase in precipitation, and an increase in glacial discharge into the ocean. In addition to atmospheric warming, recent studies revealed that ocean warming has been attributed as a major driver to the glacier retreat in this region (Cook et al. 2016). As these drivers change the chemical properties and physical processes in Antarctic coastal water bays, it is likely that the prolonged and constant environmental changes at the WAP will change the structure and function of the phytoplankton communities and affect the complexity of the food web in this ecosystem (Massom et al. 2006; Meredith et al. 2017). Melting glaciers in the Antarctic Peninsula have increased the biomass of nanophytoplankton (< 20 μm; Moline et al. 2004; Montes-Hugo et al. 2009; Mendes et al. 2013, 2018). As micro-phytoplankton (> 20 μm cell size and chain-forming diatoms) are the main taxonomical contributor to the primary productivity of the Antarctic food web (Corbisier et al. 2004; Choy et al. 2011), changes in its species composition may drastically alter the entire ecosystem (Clarke et al. 2007). Thus, whole-community algal analyses (e.g., using size-class groups) are needed.
Several processes drive the temporal (intraseasonal, seasonal, and interannual) succession of phytoplankton assemblages. The main one is the development of phytoplankton blooms, and their interactions are complex. In coastal Antarctic waters, growth-limiting micronutrients (e.g., Fe) and wind-driven water stability have been suggested as the main drivers of phytoplankton blooms during the summer season (Brandini and Rebello 1994; Schloss et al. 2014; Höfer et al. 2019). During summer, coastal waters are characterized by the occurrence of large phytoplankton blooms (chlorophyll-a > 10 μg L−1; Schloss et al. 2014; Wasilowska et al. 2015; Egas et al. 2017; Höfer et al. 2019) mainly consisting of micro-phytoplankton taxa such as the chain-forming diatoms Thalassiosira, Fragilariopsis, Chaetoceros, and Pseudo-nitzschia (Kopczynska 2008). Nano- (Cryptophyceae, Prasinophyceae, and Prymnesiophyceae) and pico-phytoplankton may also bloom during the summer seasons (Kopczynska 2008; Schofield et al. 2017). These blooms cover a wide coastal region of Antarctica and may have ecological implications, including influencing biomass/nutrient ratios and impacting water primary and secondary production coupling. Phytoplankton composition in nearshore habitats (coves and, bays) are characterized by a dominance of benthic assemblages, whereas planktonic diatoms and flagellates are frequently occurring in open ocean areas of WAP (Lange et al. 2018). In Marian Cove, benthic diatoms appear to make a significant contribution to the biomass and diversity, playing a main role transferring energy to the upper trophic levels of benthic communities (Ahn et al. 2016; Ha et al. 2019; Bae et al. 2021). Blooms events of benthic diatoms in this rapidly deglaciated fjord (Ahn et al. 2016; Ha et al. 2019) may act as the major food source to the benthic food web and possible fueling adjacent nearshore Antarctic waters (Ha et al. 2019).
Marian Cove is a tributary inlet of Maxwell Bay at King George Island and its entrance is open to the southwest, allowing the water mass in the bay to be influenced by the Bransfield Strait (Chang et al. 1990). King George Island, on which the Korea Antarctic Research Program is stationed, belongs to the WAP where warming and glacier melting are proceeding fast over the several decades (Ducklow et al. 2006; Cook et al. 2014, 2016; Turner et al. 2016; Rignot et al. 2019). King Sejong Station is located in the nearshore area of Marian Cove and has been used as a base for monitoring the population dynamics of plankton over the long term (Kang et al. 1997; Lee et al. 2015). Kang et al. (2002) and Ahn et al. (1997) evaluated the relationship between environmental factors and phytoplankton assemblages and observed an increase in benthic diatoms due to the wind and tidal currents in the nearshore waters of Maxwell Bay during the summer months. Recently, Lee et al. (2015) reported that seawater temperature dynamics were positively related to monthly chl-a variability from 1996 to 2008 in Marian Cove, where small-cell phytoplankton were a significant contributor to the phytoplankton community.
Many studies have been conducted on phytoplankton composition in the coastal waters of the WAP during the summer season to evaluate the role of environmental variables on phytoplankton structure (Dayton et al. 1986; Perrin et al. 1987; Brandini and Rebello 1994; Ahn et al. 1997; Schloss et al. 2002, 2014; Kim et al. 2018; Lange et al. 2018; Van Leeuwe et al. 2020), and several have included year-round monitoring in the Antarctic coastal stations (Krebs 1983; Kang et al. 1997, 2002; Lee et al. 2015). Intraseasonal temporal variation in phytoplankton community structure can be used to identify and monitor marine ecosystem responses to unusual environmental changes. We investigated the dynamics of phytoplankton composition and biomass through the summer of 2010 in Marian Cove, an Antarctic nearshore area.
Materials and methods
Study area
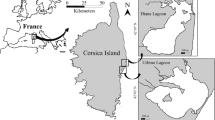
Maxwell Bay (MB) is surrounded by King George Island and Nelson Island, which belong to the South Shetland Islands (Fig. 1). Marian Cove (MC) is a typical glacial embayment (~ 4.5 km long and ~ 1.5 km wide) of MB and consists of 3 basins with maximum water depths of 100 –130 m. The MC is sheltered and its water exchange with the main bay is likely limited due to shallow submarine sill at a depth of 70 m at the entrance (Lee et al. 2015). Sea ice forms for 2 to 3 months during the winter time, but not every year. The inner cove is fringed with tidewater glaciers which have retreated about 1.9 km over the last six decades (Ha et al. 2019). During most of the summer months, these glaciers break up, bringing a substantial amount of glacier-melt water into the cove. Snow-melt water is also introduced from the snowfields (Moon et al. 2015 and literature therein). Water from melt-snow forms on the surface layer and floating ice is often observed as it flows into the bay, driven by the wind over the Maxwell Bay (Llanillo et al. 2019). The data presented in this article were obtained from fixed sampling points at the mouth of Marian Cove (62° 13′ S–58° 47′ W) from January 1 to February 29, 2010, as a part of the long-term monitoring program framework from 1996 to 2011. The distance from the entrance to the Marian Cove mouth at the sampling point is about 1 km, and the distance from the sampling point to the glacial wall inside the Marian Cove is about 4 km. The water depth of this sampling point was within an average of 2 m (and less < 3 m) and the water at the sampling point is well mixed by the wind and tidal currents (Llanillo et al. 2019).
Sampling and analyses of physical and chemical factors
Surface water was sampled from the pier at the station from 0.5 m depth with a PVC bottle (Nalgene). Water temperature and salinity were measured in situ using a conductivity sensor (YSI 30) to the first decimal unit (Lee et al. 2015). Moreover, the salinometer and temperature sensor were calibrated once every two weeks with a dedicated calibrator solution and double-checked with two identical machines (YSI 30). The seawater was filtered using a GF/F filter (47 mm, glass fiber filters, Whatman) and was kept in a bottle for nutrient analyses and stored at − 80 °C until further analyses. The samples were analyzed for SiO2, NO2 + NO3, and PO4 using an automatic nutrient analyzer (QUATRO-4ch, SEAL).
Meteorological data were obtained from the automatic meteorological observation system (AMOS-1) at King Sejong Station. The AMOS-1 comprises a wind vane and an anemometer installed at 10 m, a thermo-hygrometer at 2 m, and a rain gauge and a barometer at about 1.5 m (Park et al. 2013). The measurements were averaged over every 10 min.
Phytoplankton biomass and species composition
To analyze chl-a concentrations, seawater samples were collected at the site [avoiding low tide and sampling at high tide (ranged from − 21 to 223 cm)] and transferred to a laboratory; finally, a 500 mL aliquot sample was filtered through a GF/F filter (25 mm, glass fiber filters, Whatman). Then, 10 mL 90% acetone was added and each sample was stored in the dark for 12 h prior to analysis. Chl-a concentration was measured with a fluorometer (TD 700, Turner Design, USA). The fluorometer was calibrated twice a year. Moreover, the salinometer and temperature sensor were calibrated once every two weeks with a dedicated calibrator solution and double-checked with two identical machines (YSI 30). Size-fractionated chl-a procedure was conducted with 20 μm nylon mesh: cells that passed through the net were considered the pico- and nano-phytoplankton chl-a fraction (< 20 μm), and samples that were retained in the net were considered the micro-phytoplankton chl-a fraction (> 20 μm).
For microscopic examinations, HPMA (N-(2-hydroxypropyl methacrylamide)) slides were prepared using optical and fluorescence microscopes (Crumpto 1987; Kang et al. 2002). The HPMA slide is similar to the Utermöhl method (Utermöhl 1958). The number of phytoplanktons cells that are not precipitated is small, and sample observation is not restricted by the magnification of a microscope. Seawater samples (125 mL) were fixed and glutaraldehyde was added to a final concentration of 1%. Next, 100 mL was filtered through Gelman GN6 metrical filters (0.45 μm pore size, 25 mm diameter) and the filter was washed with 50–100 mL deionized water to remove any remained salt. The cleaned filter was placed upside down on a cover class and 3–5 drops of HPMA were applied, after which it was dried in a drying oven at 60 °C for 1 day. Subsequently, 3 to 4 drops of Norland optical adhesive (61, LOT 311) were applied to the dried cover class and it was hardened with Spectroline (15Watt Long Wave UV Lamp, XX15A) for 5 min. The HPMA slide was used for quantitative analyses of cell concentrations and biomass for a specific phytoplankton species. At least 10 fields or 300 cells were counted with a Zeiss Axiophot microscope through either a fluorescence or optical microscope. Micro-sized phytoplankton were counted at ×200 and ×400 magnification and nano- and pico-size phytoplankton were counted at ×1000 magnification. Diatoms were identified to the species or genus level when possible. Flagellates of size classes both larger than and less than 20 μm were counted to the level of Haptophyceae, Prasinophyceae, and Cryptophyceae. The following phytoplankton guides were used for the Phytoplankton of algae: Priddle and Fryxell (1985), Tomas (1997), Scott and Marchant (2005). To identify the taxonomical features, the phytoplankton specimens were observed using a scanning electron microscope (SEM; JMS6610LV, JEOL). The organic matter in the cells was removed to enable identification by SEM and the samples were treated according to the method of Kang et al. (2002).
The carbon biomass of phytoplankton (μg C L−1) was estimated by measuring the cell lengths of each taxon (at least 20 individuals per taxa) using an optical microscope (Axiophot, Zeiss). The mean cell bio-volumes were calculated using appropriate geometrical shapes (Sun and Liu 2003). The carbon biomass (pg C) of algae was estimated from the cell volume using modified Strathmann equations (Eqs. 7 and 8 in Smayda 1978): for diatoms, log10 C = 0.76 (log10 cell volume) − 0.352; and for autotrophic nanoflagellates and dinoflagellates, log10 C = 0.94 (log10 cell volume) − 0.60. The following conversion factor was used for the picophytoplankton group to transform the cell volume to carbon biomass: 220 fg C μm−3 (Borsheim and Bratbak 1987).
Statistical analyses
Principal component analysis (PCA) was used to detect environmental gradients in temperature, salinity, AT, NO3 + NO2, PO4, SiO2, and wind speed (n = 58) throughout the study area. All variables were previously centered and scaled by subtracting the mean and dividing by the standard deviation. The PCA was performed using the ‘vegan’ package (Oksanen et al. 2007) for R Software (R Core Team 2013). Non-metric multidimensional scaling (nMDS) was used to characterize associations of microphytoplankton species using the metaMDS function in the ‘vegan’ package based on the log-transformed [log10 (x + 1)] species abundance (n = 19). This analysis showed distinct phytoplankton assemblages between samples obtained in January and February (see “Results” section). We performed IndVal analysis (Dufrêne and Legendre 1997) to identify indicator species for both months based on log-transformed abundances (indval function in the labdsv R package). We applied this analysis to a set of 29 phytoplankton taxa carbon content: Gymnodinium spp. (> 20 µm), Gymnodinium spp. (< 20 µm), Coscinodiscus oculoides, Licmophora antarctica, Licmophora belgicae, Pleurosigma directum, Thalassiosira spp., Achnanthes bongrainii, Achnanthes brevipes, Cocconeis costata, Fragilaria striatula, Fragilariopsis kerguelensis, Fragilariopsis spp. (20–40 µm, single cell), Fragilariopsis spp. (20 µm, single cell), Licmophora gracilis, Navicula glaciei, Navicula perminuta, Pseudogomphonema sp., Thalassiosira spp. (20–40 µm), Thalassiosira spp. (< 20 µm), Thalassiosira spp. (> 40 µm), Fragilariopsis cylindrus (< 10 µm), Fragilariopsis pseudonana (< 10 µm), Fragilariopsis spp. (< 10 µm), Minidiscus spp., Thalassiosira spp. (< 10 µm), Cryptomonas spp., Phaeocystis antarctica, Pyramimonas spp..
Results
Hydrography, meteorology, and inorganic nutrients
From 1996 to 2011, the mean annual surface water temperature at Marian Cove Station ranged from − 0.40 to 1.50 °C. The mean summer (January–February) surface water temperature in 2010 was the lowest recorded during this period (− 0.57 °C) (Fig. 2a). The mean annual surface salinity was 33.61 (SD = ± 0.96, n = 320), where the mean monthly value (Fig. 2b) was lowest in summer (January = 33.00) and highest in winter (July = 33.90). During the peak of summer (January), the low-salinity (30.1–34.8) surface water was likely due to the inflow of glacial and snow-melt runoff from the surrounding glaciers and snow field of Marian Cove (Fig. 2d). On the other hand, the high salinity value in winter peak (July) was mainly due to formation of sea ice. Meteorology data for the summer 2010 season showed that the prevailing wind was from the southeast (SE), with a mean velocity of 7.06 m s−1 (1.84–15.72 m s−1), which drove the surface water toward the inlet of Marian Cove (Fig. 3a). The area was ice-free during summer and in general, cold and saline water associated with the high-velocity SE winds were observed during the first 2 weeks of February.
During the 2 months period (Jan 1st to Feb 28th, 2010), nitrate, orthophosphate, and silicic acid concentrations averaged 19.97, 1.51, and 115.60 μM, respectively (Fig. 4). Although concentrations of inorganic nitrogen (nitrate + nitrite) and silicic acid varied greatly on a weekly basis during summer, their concentrations gradually decreased by the end of January and before starting to increase and then were maintained throughout February. Inorganic nitrogen decreased from 25 μM to 19 μM, while silicic acid decreased from 120 μM to 80 μM at the end of January.
Dynamics of carbon biomass and phytoplankton composition
The annual chl-a concentrations in 2010 ranged from 0.13 to 23.78 μg L−1 (mean = 2.01 ± 3.32 μg L−1), where the monthly mean was the highest in summer (January = 8.25 μg L−1) and the lowest in winter (July = 0.27 μg L−1) (Fig. 5a). During the 2 months period of summer of 2010, four major phytoplankton biomass pulses with high chl-a peak were detected in the surface waters of Marian Cove (Fig. 5b). The major contributors to the blooms were microphytoplankton (> 20 μm) (50–87%; Fig. 5a). The highest peak (23.78 μg L−1) occurred in the first week of February (Fig. 5a). The carbon biomass followed the temporal pattern of the chl-a concentration, ranging from 42.30 to 608.52 μg C L−1 (Fig. 5b). Centric and pennate diatoms comprised the majority of the phytoplankton biomass (Figs. 5b, 6, 7). During the first peak (second week of January), the major contributors to the carbon biomass were the diatoms Navicula glaciei, Licmophora belgicae, and Odontella litigiosa, while Thalassiosira antarctica and N. glaciei dominated in the third week (Fig. 6). In February, two additional carbon biomass pulses were observed when the sympagic pennate diatom N. glaciei and the benthic diatoms L. belgicae and Fragilaria striatula dominated. Phytoflagellate (< 20 μm) carbon biomass was also significant and showed a peak in January with values up to 59.12 μg C L−1 (mean = 35.92 μg C L−1) decreasing slightly in February (mean = 27.41 μg C L−1; Fig. 5c). Most of the carbon from nanoflagellates during this period was due to Cryptomonas spp., Pyramimonas spp., and Phaeocystis antarctica, which contributed on average 38% of the total phytoplankton carbon. Small athecate dinoflagellates cells belonging to Gymnodinium spp. (cell size ~ 20 μm) were also important contributors to the total carbon biomass during January 2010. Short peaks of picophytoplankton cells frequently occurred throughout the study period (Fig. 5c). In terms of carbon biomass, picophytoplankton increased before the main diatom blooms with values ranging between 0.013 and 0.300 μg C L−1.
Temporal variability of carbon biomass (μg C L−1) during summer 2010 of dominant phytoplankton species in surface waters of Marian Cove: Thalassiosira spp. (< 160 µm) (a), Fragilaria striatula (40 µm–60 µm) (b), Navicula glaciei (25 µm–40 µm) (c), Achnanthes bongrainii (40 µm–80 µm) (d), Licmophora belgicae (80 µm–130 µm) (e), Pseudogomphonema sp. (18 µm–69 µm) (f), Odontella litigiosa (60 µm–130 µm) (g), Pyramimonas spp. (< 10 µm) (h), Minidiscus spp. (< 10 µm) (i), Licmophora antarctica (80 µm–100 µm) (j)
Microphotographs of A External girdle view of Achnanthes bongrainii (A.Mann, 1937). B External view of Cocconeis costata (Gregory, 1855). C External girdle view of Licmophora belgicae (Peragallo, 1921). D Internal view of basal pole with rimoportulae. E External valve view of Navicula glaciei (Van Heurck). F Girdle view of Odontella litigiosa (Van Heurck, 1909) Haban. G Fluorescent image of Phaeocystis antarctica (Karsten, 1905). H Valve view of Thalassiosira antarctica (Comber, 1896). (Scale bars: A–D = 10 µm, E = 2 µm, F = 10 µm, G = 5 µm, H = 10 µm)
In terms of nano- and micro-phytoplankton abundance, Phaeocystis antarctica (up to 3.93 × 106 cells L−1) was the dominant species throughout the summer period. Both single-cell and colonial forms were observed and the motile cell of P. antarctica ranged in diameter from 3 to 9 μm. Despite picophytoplankton being numerically dominant, micro-phytoplankton diatoms and nano-phytoplankton constituted a significant portion of the total carbon biomass. During the summer blooms, diatoms comprised approximately 58% of the total micro-phytoplankton abundance, fluctuating between 1 and 2 orders of magnitude during this period. With the exception of Thalassiosira spp. (< 10 μm), the sympagic diatom N. glaciei, Minidiscus spp., Fragilariopsis spp. (< 10 μm), Fragilariopsis cylindrus (< 10 μm), and Fragilariopsis pseudonana (< 10 μm) were the main species at Marian Cove.
The first two components of the PCA analysis explained 37.3% (PC1) and 20.8% (PC2) of the total variance (Fig. 8a), indicating that all inorganic nutrients and salinity were negatively correlated with time and decreased from January to February (Fig. 8a, PC1). The associations between salinity and nutrients were consistent with the observed temporal gradients of high salinity (> 33.0) and wind direction change (to SE), coinciding with the post-melt month (February). The NMDS analysis based on the most frequent phytoplankton taxa indicated that some diatom species are indicators of this environmental gradient (Fig. 8b). The IndVal analysis identified a group characterized by the biomass dominance of diatoms species, classified by month (p < 0.05). In January, the most important biomass contributors were centric diatoms such as Thalassiosira spp. and Minidiscus spp., and the flagellate Pyramimonas spp., while in February, they were the pennate diatoms Pseudogomphonema sp., Licmophora antarctica, and Fragilaria striatula.
Principal Component Analysis (PCA) plot showing the temporal distribution of the samples in the multivariate ordination space (a). Vectors indicate the direction and contribution of the different descriptors to the first two PCA axes: Air temperature (AT; °C), water temperature (Temp; °C), salinity (Sal), Wind speed (WS; m s−1), Silicic acid (Si, µM), Orthophosphate (PO4, µM), Nitrate (NO3, µM). All data were previously centered and scaled by subtracting the mean and dividing by the standard deviation. Colors of symbols indicate month (i.e., groups of sampling days at January and February 2010) recognized after hierachical cluster analysis performed with the sample scores of the two first PCA axes. Dispersion NMDS ordinal distribution displaying the logarithmically transformed cell carbon biomass [ln (x + 1)] of the most frequent occurring phytoplankton species (< 10 of total samples); In bold case, significant “indicators species” during January (Thalassiosira spp., Minidiscus spp., Pyramimonas spp.) and February (Pseudogomphonema sp., Licmophora antarctica, Fragilaria striatula) (IndVal analysis and significance p > 0.05) (b). Gymnodinium spp. (> 20 µm) = Gym1, Gymnodinium spp. (< 20 µm) = Gym2, Coscinodiscus oculoides = Cos, Licmophora antarctica = Lant, Licmophora belgicae = Lbel, Pleurosigma directum = Pdir, Thalassiosira spp. = Tspp, Achnanthes bongrainii = Abon, Achnanthes brevipes = Abre, Cocconeis costata = Coc, Fragilaria striatula = Fstr, Fragilariopsis kerguelensis = Fker, Fragilariopsis spp. (20 µm–40 µm, single cell) = Fra1, Fragilariopsis spp. (< 20 µm, single cell) = Fra2, Licmophora gracilis = Lgra, Navicula glaciei = Ngla, Navicula perminuta = Nper, Pseudogomphonema sp. = Pseu, Thalassiosira spp.(20 µm–40 µm) = Thal1, Thalassiosira spp. (< 20 µm) = Thal2, Thalassiosira spp. (> 40 µm) = Thal3, Fragilariopsis cylindrus (< 10 µm) = Fcyl, Fragilariopsis pseudonana (< 10 µm) = Fpse, Fragilariopsis spp. (< 10 µm) = Fspp, Minidiscus spp. = Mini, Thalassiosira spp. (< 10 µm) = Thal4, Cryptomonas spp. = Cyrp, Phaeocystis antarctica = Phae, Pyramimonas spp. = Pyra
Discussion
The importance of wind
In the summer of 2010, high chl-a concentrations (mean = 6.80 μg L−1 and up to 23.78 μg L−1) were observed in the nearshore waters of Marian Cove; these were much higher than the mean value of 1.55 μg L−1 from 1996 to 2011. During the study, the phytoplankton community varied markedly in terms of carbon biomass, cell abundance, and taxonomic composition. Solitary and colonial P. antarctica cells dominated small diatoms (sympagic, benthic, and planktonic) (30.28% of total cell abundance). In the last decade, P. antarctica events have been more frequent, especially during the early stages of spring phytoplankton blooms, when haptophyte populations dominated the phytoplankton assemblages in both shelf and non-shelf waters of the WAP region (Arrigo et al. 2017).The mean chl-a peak during the summer 2010 season (January–February) was higher than during the summers in other years (1996–2011), and some environmental factors were associated with this particular bloom event. Phytoplankton biomass changes dramatically depending on water temperature (Kang et al. 2002; Behrenfeld 2005) and the prevailing wind direction (Schloss et al. 2014; Höfer et al. 2019). The summer blooms of 2010 occurred due to changes in the dominant wind direction (easterly) and speed, and its effect on water column stability, accounting for the increased phytoplankton biomass near the surface (Brandini and Rebello 1994; Schloss et al. 2014).
Specifically, in Marian Cove, additional frequent changes in wind direction were observed during 2010 compared to the entire study period. Here, the dominant wind direction is usually westerly, but in the summer of 2010, diatom blooms were concomitant with long-lasting prevailing (> 6 m s−1) easterly wind, which may have caused an inflow of deep, nutrient-rich waters from Maxwell Bay. The bloom in 2010 was triggered by wind and consequently induced upwelling in Marian Cove (Brandini and Rebello 1994); this fits the mechanistic explanation given for the diatom bloom observed in Maxwell Bay in summer 2017 (Höfer et al. 2019). In this study, a large phytoplankton bloom (chl-a > 15 μg L−1) was observed at Maxwell Bay and South Bay, which consisted almost exclusively of benthic (Fragilaria and Licmophora) and planktonic diatoms (Pseudo-nitzschia, Thalassiosira, and Chaetoceros). During the summer diatom peak (January), the relatively high contribution of a few heterotrophic Gymnodiniales species to the total carbon biomass during the study period was associated with low-salinity (30–33) surface water, supporting the idea of a mixed assemblage of centric and pennate diatoms and flagellates under the influence of meltwater and low salinity (Lima et al. 2019).
Studies have indicated that climate change will lead to anomalous climatological events including changes in the dominant wind direction (Vaughan et al. 2003; Massom and Stammerjohn 2010). In the eastern coastal bays of King George Island, low wind speeds and easterly winds have favored phytoplankton blooms, where benthic diatoms appear to dominate the summer community (Lange et al. 2018). Schloss et al. (2002, 2014) reported that the dominant easterly wind promoted the surface water circulation in the Bransfield Strait, which resulted in inflowing seawater from the open sea remaining in the photic zone for a longer period of time. The fact that similar climatological processes have occurred at Admiralty Bay and Potter Cove since 2010 suggests that remote climatic signals are now more applicable to oceanographic–cryospheric dynamics. According to Costa and Agosta (2012), an anomalous stationary cyclone to the northwest of the Antarctic Peninsula and an anticyclone over the Southeast Pacific Ocean persisted throughout the summer of 2010. This can be related to positive surface temperature anomalies in the South Pacific, over the source region of the cyclone, through the generation of local consistent mean-flow baroclinicity. For example, strong northerly winds have been reported to create unusually compact coastal ice and snow cover during summer, which contributes to the formation of major phytoplankton blooms in WAP (Massom et al. 2006). However, according to Costa and Agosta (2012), an anomalous stationary cyclone to the northwest of the Antarctic Peninsula and an anticyclone over the Southeast Pacific Ocean persisted throughout the summer of 2010. Costa and Agosta (2012) also described an anomalous easterly wind component during the winter season, which could further favor cold temperature anomalies over the study area on a regional scale. In addition, changes in chl-a may reflect the dynamics of the nutrient supply induced by the Antarctic seawater circulation (Pollard et al. 2002; Sokolov and Rintoul 2007; Thompson and Youngs 2013). Therefore, the synergetic regional climatological anomalies (e.g., wind field and air temperature) occurring during 2010 may have decreased the sea surface temperature and weakened the stratification, creating the physical conditions that could advect nutrients to the upper layer, enhancing native phytoplankton assemblages (Schloss et al. 2014).
Importance of melting of ice
To understand the influence of the melting of sea ice/glaciers on the observed blooms, qualitative analyses of some phytoplankton and environmental variables were conducted in the bloom study area. Seasonal melting of sea ice is considered a significant environmental driver that modulates phytoplankton production (Smith and Comiso 2009; Sabu et al. 2014). A previous study documented high chl-a concentrations (> 15 μg L−1) in Maxwell Bay during summer 2017, suggesting that Fe release from glacier runoff could be an important factor driving intense primary production in Antarctic bays (Höfer et al. 2019). Other studies have shown that the prolonged winter ice season results in high chl-a concentrations in the following summer in the WAP (Rozema et al. 2017), and sea-ice dynamics are a significant factor governing both photosynthetic biomass and phytoplankton community composition (Rozema et al. 2017; Mangoni et al. 2017). Marian Cove was frozen for 102 days in the winter of 2009 (Fig. 3c). There is marked interannual variability in fast ice due to changes in the direction, persistence, and strength of the prevailing winds, with more easterly winds leading to less frequent fast-ice breakout (Massom and Stammerjohn 2010). The main indicator of the influence of ice melt during the following summer of 2010 was the high abundance and dominance of Navicula glaciei at the sea surface in the bloom area.
Sea ice/glacier melting has both positive and negative effects on phytoplankton photosynthesis and biomass accumulation. First, the freshwater released during sea ice melting forms a buoyant barrier layer that limits vertical advection of new inorganic nutrients and decreases light penetration due to the accumulation of particles throughout the shallow mixed layer. Second, wind speeds and tidal cycles associated with water stability (low-speed haline stratification) and mixing (high speed) of the water column in Marian Cove (Llanillo et al. 2019) would explain the high (cell accumulation) and low (cell dispersal) chl-a concentrations, respectively. Third, the melting sea ice/glacier could also be a significant source of additional inorganic nutrients and Fe (Kim et al. 2015) fueling phytoplankton growth. Thus, it is plausible that the extended period of ice recorded previously could also be an extra source of macro- and micro-nutrients during the melting period in the following summer of 2010. Despite a significant reduction in macro- and micro-nutrients at the end of January, phytoplankton growth in Marian Cove was unlikely affected by nutrient availability (“non-limiting factor”) given their high concentrations throughout the year (Marian Cove: Fe = 38 nM; Kim et al. 2015). Here, we suggest that high abundance and carbon biomass of the sympagic diatom Navicula glaciei during the summer of 2010 may be considered the signature of its fate following the 2009–2010 ice-melting events (mostly low salinity values due to sea-ice melt and glacial runoff), and that environmental conditions were favorable for growth throughout the water column. Sympagic diatoms may contribute to pelagic blooms in eastern Antarctica (Riaux-Gobin et al. 2011; van Leeuwe et al. 2018). Hence, we hypothesize that the spring–summer melting process preceded by a previous winter with a long freeze might also play a significant role in shaping the phytoplankton composition (sympagic versus ice-free or planktonic species), more than typical air-ocean dynamics alone (i.e., wind-driven, tides, water stability, and bloom development).
Occurrence of nearshore phytoplankton blooms
Microscopic analyses of the species composition were conducted every 3 days and the results provided high temporal resolution of the data. During the main 2010 phytoplankton bloom, N. glaciei was the dominant sympagic species. Kang et al. (2002) reported that N. glaciei and N. perminuta, two sympagic diatoms, were abundant as the water temperature decreased and the amount of sea ice increased, even during the winter. Moreover, Kang et al. (2002) argued that the formation of frazil ice and the inflow of colder water formed near the glacier cliffs were main factors in the autumn, whereas wind-driven resuspension was the major mechanism increasing diatom biomass in the water column during the austral summer (Ahn et al. 1997). N. glaciei grows better when salinity is lower, increasing its abundance in low-salinity coastal water (Kang et al. 1999; Hernando et al. 2015). Moreover, it is mainly found on pack and permanent ice (Fernandes and Procopiak 2003). Our results reflect this and suggest that N. glaciei and other pennate diatom species (Pseudogomphonema sp., L. antarctica, and F. striatula) could be indicator species (as carbon biomass) to indicate the inflow of colder water and meltwater from glaciers during the spring and summer. We also saw that the increase in diatom cell abundance and the decrease in the summer water temperature were caused by the influx of colder water from glacier cliffs, and this influx was affected by strong southeasterly winds to the inner cove. Associated with the lower water temperature in 2010, N. glaciei was one of the most abundant species during summer and it accounted for the major portion of the total phytoplankton biomass. Smith and Nelson (1985) reported that a lower water temperature increased phytoplankton biomass in King George Island, which is in agreement with the general notion that coastal Antarctic phytoplankton can grow maximally in low in situ temperatures (Mura and Agusti 1996).
Although solitary cells and colonial forms of P. antarctica did not contribute significantly to the total carbon biomass (7.40%), they were important in terms of abundance (30.28%) and formed a constant biomass background accompanied by early diatom blooms during the summer of 2010. Our results are consistent with those of other studies that have indicated that P. antarctica is a dominant species in Antarctic coastal waters (Gibson et al. 1997; Rodriguez et al. 2002; Mendes et al. 2012). The mass proliferation and frequent occurrence of P. antarctica have been reported in many ecosystems, including areas high in nutrients and with good mixing near glaciers (Davidson and Marchant 1992). It was interesting to observe the co-occurrence of benthic, planktonic, and sympagic diatoms, Pyramimonas spp. and P. antarctica, suggesting good adaptation to low light conditions and the ability to uptake nutrients in the study area. In the middle of the study period (beginning of February), the advection of cold waters (close to 0 °C) caused by the wind dynamics (southeasterly wind direction) and/or by the adjacent sea-ice/glacier melting process might have facilitated the episodic injection of nutrient-rich waters to the top layer. The presence of sporadic mixing/stability events and their effects on phytoplankton can be seen in the reduced phytoplankton growth rates (based on changes in chl-a) at Marian Cove (Fig. 5d). It was noted that the high biomass of phytoplankton and growth of the dominant species were positively affected by the cold, nutrient-rich water conditions in Marian Cove. Although all nutrient concentrations were higher this season, the concentrations of inorganic nitrogen, silicic acid, and orthophosphate decreased rapidly due to the higher uptake of phytoplankton in the summer of 2010 (end of January) (Fig. 8a). Considering the geographical location of the study area, the abundant nutrients originating from the penguin colony at Narevsky Point could have also been a source of nutrients to the study area, brought there by the wind, as has been suggested for other coastal areas (Nedzarek 2008). Summer blooms differ from year to year, with glacial melting and wind-driven advection of nutrients favoring the dominance of benthic–sympagic diatoms (N. glaciei, Licmophora) and planktonic diatoms (Thalassiosira, Pseudo-nitzschia, Chaetoceros) prevailing in lighter coastal conditions. We are not discounting the potential role of tides (including the tidal cycle and internal tides), which can be a significant factor in changes of the hydrographic structure (and therefore physical–chemical properties) in Maxwell Bay and its tributary fjords, like Marian Cove (Llanillo et al. 2019). Specifically, we expect that a flood tide would induce the accumulation of nutrient-enriched waters and phytoplankton biomass, as well as phytoplankton cell resuspension and changes in species composition (from pelagic to benthic forms) in the upper water column toward the head of Marian Cove.
As Deppeler and Davidson (2017) suggested, if the community structure and functions of phytoplankton change due to climate change (e.g., decreased light and thawing), these changes will greatly affect the biogeochemical cycle of the Southern Ocean and this will amplify climate change. Therefore, changes in the dominant wind direction and melting of the sea ice/glaciers due to remote climatological forcing may alter the dominance of the main functional phytoplankton groups (cell size classes), biomass, and species composition of the phytoplankton community in glacier-influenced coastal shallow areas. It is essential to detect environmental changes and predict their potential effects by studying the community structure and functional dynamics of phytoplankton on a long-term basis in coastal areas of WAP.
Conclusion
This study reports intense phytoplankton blooms occurred during an austral summer (January and February 2010) in an Antarctic glacial fjord as compared with those over the 15-year period in the same area. The blooms were dominated by a mixture of several sympagic (N. glaciei), benthic (L. belgicae), and planktonic (T. antarctica) diatoms. The analysis showed that the major driving forces for the blooms were strong/weak SE winds, high levels of inorganic nutrients from oceanic waters, and possibly the inflow of lithogenic elements such as Fe laden in the glacier runoff. Given the large interannual variability of the environmental factors in the WAP, future work on the effects of glacier retreat, sea-ice melting, and glacier runoff on phytoplankton biomass and species composition is crucial for ecosystem modeling to predict the responses of marine microorganisms to climate change.
References
Ahn IY, Chung H, Kang JS, Kang SH (1997) Diatom composition and biomass variability in nearshore waters of Maxwell Bay, Antarctica, during the 1992/1993 austral summer. Polar Biol 17:123–130
Ahn IY, Moon HW, Jeon MS, Kang SH (2016) First record of massive blooming of benthic diatoms and their association with megabenthic filter feeders on the shallow seafloor of an Antarctic Fjord: does glacier melting fuel the bloom? Ocean Sci 51:273–279
Arrigo KR, van Dijken GL, Algerkamp A-C, Erickson ZK, Lewis KM, Lowy KE, Joy-Warren HL, Middag R, Nash-Arrigo J, Selz V, van de Poll W (2017) Early spring phytoplankton dynamics in the western Antarctic Peninsula. J Geophys Res Ocean 122:9350–9369. https://doi.org/10.1002/2017JC013281
Bae H, Ahn IY, Park J, Song SJ, Noh J, Kim H, Khim JS (2021) Shift in polar benthic community structure in a fast retreating glacial area of Marian Cove. West Antarct Sci Rep 11:241. https://doi.org/10.1038/s41598-020-80636-z
Behrenfeld MJ, Boss E, Siegel DA, Shea DM (2005) Carbon-based ocean productivity and phytoplankton physiology from space. Glob Biogeochem Cycles. https://doi.org/10.1029/2004GB002299
Borsheim KY, Bratbak G (1987) Cell volume to cell carbon conversion factors for a bacterivorous Monas sp. enriched from seawater. Mar Ecol Prog Ser 36:171–175
Brandini FP, Rebello J (1994) Wind field effect on hydrography and chlorophyll dynamics in the coastal pelagial of Admiralty Bay, King George Island, Antarctica. Antarct Sci 6:433–442
Chang KI, Jun HK, Park GT, Eo YS (1990) Oceanographic conditions of Maxwell Bay, King George Island, Antarctica (austral summer 1989). Ocean Polar Res 1:27–46
Choy EJ, Park H, Kim JH, Ahn IY, Kang CK (2011) Isotopic shift for defining habitat exploitation by the Antarctic limpet Nacella concinna from rocky coastal habitats (Marian Cove, King Grorge Islang). Estuar Coast Shelf Sci 92:339–346
Clarke A, Murphy EJ, Meredith MP, King JC, Peck LS, Barnes DKA, Smith RC (2007) Climate change and the marine ecosystem of the western Antarctic Peninsula. Philos Trans R Soc Lond B Biol Sci 362:149–166
Cook AJ, Fox AJ, Vaughan DG, Ferrigno JG (2005) Retreating glacier fronts on the Antarctic Peninsula over the past half-century. Science 308:541–544
Cook AJ, Vaughan DG, Luckman AJ, Murray T (2014) Anew Antarctic Peninsula glacier basin inventory and observed area changes since the 1940s. Antarct Sci 26:614–624
Cook AJ, Holland PR, Meredith MP, Murray LA, Vaughan DG (2016) Ocean forcing of glacier retreat in the western Antarctic Peninsula. Science 353:283–286
Corbisier TN, Petti MAV, Skowronski RSP, Brito TAS (2004) Trophic relationships in the nearshore zone of Martel Inlet (King George Island, Antarctica): δ13C stable-isotope analysis. Polar Biol 27:75–82
Costa AJ, Agosta EA (2012) South Pacific quasi-stationary waves and anomalously cold summers in the northernmost Antarctic Peninsula. Geoacta 37:73–82
Crumpto WG (1987) A simple and reliable method for making permanent mounts of phytoplankton for light and fluorescence microscopy. Limnol Oceanogr 32:1154–1159
Davidson AT, Marchant HJ (1992) Protist abundance and carbon concentration during a Phaeocystis-dominated bloom at an Antarctic coastal site. Polar Biol 12:387–395
Dayton PK, Watson D, Palmisano A, Barry JP, Oliver JS, Rivera D (1986) Distribution patterns of benthic microalgal standing stock at McMurdo Sound, Antarctica. Polar Biol 6:207–213
Deppeler SL, Davidson AT (2017) Southern Ocean phytoplankton in a changing climate. Front Mar Sci. https://doi.org/10.3389/fmars.2017.00040
Ducklow HW, Baker K, Martinson DG, Quetin LB, Ross RM, Smith RC, Stammerjohn SE, Vernet M, Fraser W (2006) Marine pelagic ecosystems: the west Antarctic Peninsula. Philos Trans R Soc Lond B Biol Sci 362:67–94
Dufrêne M, Legendre P (1997) Species assemblages and indicator spe-cies: the need for a flexible asymmetrical approach. Ecol Monogr 67:345–366. https://doi.org/10.2307/2963459
Egas C, Castillo CH, Delherbe N, Molina E, Santos ALD, Lavin P, Iglesia RDL, Vaulot D, Trefault N (2017) Short timescale dynamics of phytoplankton in Fildes Bay, Antarctica. Antarct Sci 29:217–228
Fernandes LF, Procopiak LK (2003) Observations on valve structures of Navicula directa (Wm. Smith) Ralfs in Pritchard and Navicula glaciei V. Heurck from rocky substrates in Antarctic Peninsula. Hoehnea 30:1–10
Gibson JA, Swadling KM, Burton HR (1997) Interannual variation in dominant phytoplankton species and biomass near Davis Station, East Antarctica. Proc NIPR Symp Polar Biol 10:77–89
Ha SY, Ahn IY, Moon HW, Choi BH, Shin KH (2019) Tight trophic association between benthic diatom blooms and shallow-water megabenthic communities in a rapidly deglaciated Antarctic fjord. Estuar Coast Shelf Sci 218:258–267
Hansen J, Ruedy R, Sato M (1999) GISS analysis of surface temperature change. J Geophys Res Atmos 104:30997–31022
Hernando M, Schloss IR, Malanga G, Almandoz GO, Ferreyra GA, Aguilar MB, Puntarulo S (2015) Effects of salinity changes on coastal Antarctic phytoplankton physiology and assemblage composition. J Exp Mar Biol Ecol 466:110–119
Höfer J, Giesecke R, Hopwood MJ, Carrera V, Alarcon E, Gonzalez HE (2019) The role of water column stability and wind mixing in the production/export dynamics of two bays in the Western Antarctic Peninsula. Prog Oceanogr. https://doi.org/10.1016/j.pocean.2019.01.005
Kang SH, Kang JS, Chung KH, Lee MY, Lee BY, Chung HS, Kim YD, Kim DY (1997) Seasonal variation of nearshore Antarctic microalgae and environmental factors in Marian Cove, King George Island, 1996. Ocean Polar Res 8:9–27
Kang JS, Kang SH, Lee JH (1999) Cryophilic diatom Navicula glaciei and N. perminuta in anterctic coastal environment I. Morphol Ecol Algae 14:169–179
Kang JS, Kang SH, Lee JH, Lee SH (2002) Seasonal variation of microalgal assemblages at a fixed station in King George Island, Antarctica, 1996. Mar Ecol Prog Ser 229:19–32
Kim I, Kim G, Choy EJ (2015) The significant inputs of trace elements and rare earth elements from melting glaciers in Antarctic coastal waters. Polar Res. https://doi.org/10.3402/polar.v34.24289
Kim H, Ducklow HW, Abele D, Barlett EMR, Buma AGJ, Meredith MP, Rozema PD, Schofield OM, Venables HJ, Schloss IR (2018) Inter-decadal variability of phytoplankton biomass along the coastal West Antarctic Peninsula. Philos Trans R Soc A 376:20170174
Kopczynska EE (2008) Phytoplankton variability in Admiralty Bay, King George Island, South Shetland Islands: six years of monitoring. Pol Polar Res 29:117–139
Krebs WN (1983) Ecology of neritic marine diatoms, Arthur Harbor, Antarctica. Mar Micropaleontol 29:267–297
Lange PK, Ligowski R, Tenenbaum DR (2018) Phytoplankton in the embayments of King George Island (Antarctic Peninsula): a review with emphasis on diatoms. Polar Rec 54:158–175
Lee SH, Joo HM, Joo HT, Kim BK, Song HJ, Jeon MS, Kang SH (2015) Large contribution of small phytoplankton at Marian Cove, King George Island, Antarctica, based on long-term monitoring from 1996 to 2008. Polar Biol 38:207–220
Lima DT, Moser GA, Piedras FR, Da Cunha LC, Tenenbaum DR, Tenório MMB, Campos MVPB, Cornejo TO, Barrera-Alba JJ (2019) Abiotic changes driving microphytoplankton functional diversity in Admiralty Bay, King George Island (Antarctica). Front Mar Sci 6:1–17. https://doi.org/10.3389/fmars.2019.00638
Llanillo PJ, Aiken CM, Cordero RR, Damiani A, Sepúlveda E, Fernández-Gómez B (2019) Oceanographic variability induced by tides, the intraseasonal cycle and warm subsurface water intrusions in Maxwell Bay, King George Island (West-Antarctica). Sci Rep 9:1–17. https://doi.org/10.1038/s41598-019-54875-8
Mangoni O, Saggiomo V, Bolinesi F, Margiotta F, Budillon G, Cotroneo Y, Misic C, Rivaro P, Saggiomo M (2017) Phytoplankton blooms during austral summer in the Ross Sea, Antarctica: driving factors and trophic implications. PLoS ONE 12:e0176033
Massom RA, Stammerjohn SE (2010) Antarctic sea ice change and variability—physical and ecological implications. Polar Sci 4:149–186
Massom RA, Stammerjohn SE, Smith RC, Pook MJ, Iannuzzi RA, Adams N, Martinson DG, Vernet M, Fraser WR, Quetin LB, Ross RM, Massom Y, Krouse HR (2006) Extreme anomalous atmospheric circulation in the West Antarctica Peninsula region in austral spring and summer 2001/2002, and its profound impact on sea ice and biota. J Clim 19:3544–3571
Mendes CRB, Souza MSD, Garcia VMT, Leal MC, Brotas V, Garcia CAE (2012) Dynamics of phytoplankton communities during late summer around the tip of the Antarctic Peninsula. Deep Sea Res I 65:1–14
Mendes CRB, Tavano VM, Leal MC et al (2013) Shifts in the dominance between diatoms and cryptophytes during three late summers in the Bransfield Strait (Antarctic Peninsula). Polar Biol 36:537–547
Mendes CRB, Tavano VM, Dotto TS et al (2018) New insights on the dominance of cryptophytes in Antarctic coastal waters: a case study in Gerlache Strait. Deep Sea Res Part II 149:161–170
Meredith MP, Stammerjohn SE, Venables HJ, Ducklow HW, Martinson DG, Iannuzzi RA, Wessem JMV, Reijmer CH, Barrand NE (2017) Changing distributions of sea ice melt and meteoric water west of the Antarctic Peninsula. Deep Sea Res II 139:40–57
Moline MA, Claustre H, Frazer TK, Schofield O, Vernet M (2004) Alteration of the food web along the Antarctic Peninsula in response to a regional warming trend. Glob Change Biol 10:1973–1980
Montes-Hugo M, Doney SC, Ducklow HW, Fraser W, Martinson D, Stammerjohn SE, Schofield O (2009) Recent changes in phytoplankton communities associated with rapid regional climate change along the western Antarctic Peninsula. Science 323:1470–1473
Moon HW, Wan Hussin MRW, Kim HC (2015) The impacts of climate change on Antarctic nearshore mega-epifaunal benthic assemblages in a glacial fjord on King George Island: responses and implications. Ecol Indic 57:280–292
Mura MP, Agusti S (1996) Growth rates of diatoms from coastal Antarctic waters estimated by in situ dialysis incubation. Mar Ecol Prog Ser 144:237–245
Nedzarek A (2008) Sources, diversity and circulation of biogenic compounds in Admiralty Bay, King George Island, Antarctica. Antarct Sci 20:135–145
Oksanen J, Kindt R, Legendre P, O’Hara B, Gavin L, Stevens MHH, Oksanen MJ, Suggests M (2007) The Vegan package. Commun Ecol Package 10:631–637
Park SJ, Choi TJ, Kim SJ (2013) Heat flux variations over sea ice observed at the coastal area of the Sejong Station, Antarctica. Asia Pac J Atmos Sci 49:443–450
Perrin RA, Lu P, Marchant HJ (1987) Seasonal variation in marine phytoplankton and ice algae at a shallow Antarctic coastal site. Hydrobiologia 146:33–46
Piquet AMT, Bolhuis H, Meredith MP, Buma AGJ (2011) Shifts in coastal Antarctic marine microbial communities during and after melt water-related surface stratification. FEMS Microbiol Ecol 76:413–427
Pollard RT, Lucas MI, Read JF (2002) Physical controls on biogeochemical zonation in the Southern Ocean. Deep Sea Res II 49:3289–3305
Priddle J, Fryxell G (1985) Handbook of the common plankton diatoms of the Southern Ocean. British Antarctic Survey, Cambridge
R Core Team (2013) R: a language and environmental for statistical com-putting. R Foundation for Statistical Computing, Vienna, Austria. https://R-project.org
Riaux-Gobin C, Poulin M, Dieckmann G, Labrune C, Vetion G (2011) Spring phytoplankton onset after the ice break-up and sea-ice signature (Adélie Land, East Antarctica). Polar Res. https://doi.org/10.3402/polar.v30i0.5910
Rignot E, Mouginot J, Scheuchl B, Broeke M, Wessem MJ, Morlighem M (2019) Four decades of Antarctic Ice Sheet mass balance from 1979–2017. Proc Natl Acad Sci 116:1095–1103
Rodriguez F, Varela M, Zapata M (2002) Phytoplankton assemblages in the Gerlache and Bransfield Straits (Antarctic Peninsula) determined by light microscopy and CHEMTAX analysis of HPLC pigment data. Deep Sea Res II 49:723–747
Rozema PD, Venables HJ, Poll WH, Clarke A, Meredith MP, Buma AGJ (2017) Interannual variability in phytoplankton biomass and species composition in northern Marguerite Bay (West Antarctic Peninsula) is governed by both winter sea ice cover and summer stratification. Limnol Oceanogr 62:235–252
Rückamp M, Braun M, Suckro S, Blindow N (2011) Observed glacial changes on the King George Island ice cap, Antarctica, in the last decade. Glob Planet Change 79:99–109
Sabu P, Anilkumar N, George JV, Chacko R, Tripathy SC, Achuthankutty CT (2014) The influence of air–sea–ice interactions on an anomalous phytoplankton bloom in the Indian Ocean sector of the Antarctic Zone of the Southern Ocean during the austral summer, 2011. Polar Sci 8:370–384
Schloss IR, Ferreyra GA, Ruiz D (2002) Phytoplankton biomass in Antarctic shelf zones: a conceptual model based on Potter Cove, King George Island. J Mar Syst 36:129–143
Schloss IR, Wasilowska A, Dumont D, Almandoz GO, Hernando MP, Michaud-Tremblay CA, Saravia L, Rzepecki M, Monien P, Monien D, Kopczynska EE, Bers AV, Ferreyra GA (2014) On the phytoplankton bloom in coastal waters of southern King George Island (Antarctica) in January 2010: an exceptional feature? Limnol Oceanogr 59:195–210
Schofield O, Saba G, Coleman K, Carvalho F, Couto N, Ducklow H, Finkel Z, Irwin A, Kahl A, Montes-Hugo M, Stammerjohn S, Waite N (2017) Decadal variability in coastal phytoplankton community composition in a changing West Antarctic Peninsula. Deep Sea Res I 124:42–54
Scott FJ, Marchant HJ (2005) Antarctic marine protists. Australian Biological Resources Study, Canberra and Australian Antarctic Division, Hobart, p 563
Smayda TJ (1978) From phytoplankton to biomass. In: Sournia A (ed) Phytoplankton manual, monographs on oceanographic 6. UNESCO, Paris, pp 273–279
Smith WO, Comiso JC (2009) Southern ocean primary productivity: variability and a view to the future. Smithsonian at the Poles, Washington, DC
Smith WO, Nelson DM (1985) Phytoplankton bloom produced by a receding ice edge in the Ross Sea: spatial coherence with the density field. Science 227:163–166
Smith DM, Cusack S, Colman AW, Folland CK, Harris GR, Murphy JM (2007) Improved surface temperature prediction for the coming decade from a global climate model. Science 317:796–799
Sokolov S, Rintoul SR (2007) On the relationship between fronts of the Antarctic Circumpolar Current and surface chlorophyll concentrations in the Southern Ocean. J Geophys Res Oceans. https://doi.org/10.1029/2006JC004072
Sun J, Liu D (2003) Geometric models for calculating cell biovolume and surface area for phytoplankton. J Plank Res 25:1331–1346
Thompson AF, Youngs MK (2013) Surface exchange between the Weddell and Scotia Seas. Geophys Res Lett 40:5920–5925
Tomas CR (1997) Identifying marine phytoplankton. Academic Press, New York
Turner J, Maksym T, Phillips T, Marshall GJ, Meredith MP (2013) The impact of changes in sea ice advance on the large winter warming on the western Antarctic Peninsula. Int J Climatol 33:852–861
Turner J, Lu H, White I, King JC, Phillips T, Hosking JS, Bracegirdle TJ, Marshall GJ, Mulvaney R, Deb P (2016) Absence of 21st century warming on Antarctic Peninsula consistent with natural variability. Nature 535:411–415
Utermöhl H (1958) Zur vervollkommnung der quantitativen Phytoplankton-Methodik. Mitt Int Ver Theor Angew Limnol 9:1–38
Van Leeuwe MA, Tedesco L, Arrigo KR, Assmy P, Campbell K, Meiners KM, Rintala JM, Selz V, Thomas DN, Stefels J (2018) Microalgal community structure and primary production in Arctic and Antarctic sea ice: a synthesis. Elem Sci Anth. https://doi.org/10.1525/elementa.267
Van Leeuwe MA, Webb AL, Venables HJ, Visser RJW, Meredith MP, Elzenga JTM, Stefels J (2020) Annual patterns in phytoplankton phenology in Antarctic coastal waters explained by environmental drivers. Limnol Oceanogr 65:1651–1668
Vaughan DG, Marshall GJ, Connolley WM, Parkinson C, Mulvaney R (2003) Recent rapid regional climate warming on the Antarctic Peninsula. Clim Change 60:243–274
Wasilowska A, Kopczynska EE, Rzepecki M (2015) Temporal and spatial variation of phytoplankton in Admiralty Bay, South Shetlands: the dynamics of summer blooms shown by pigment and light microscopy analysis. Polar Biol 38:1249–1265
Acknowledgements
This work was supported by the Korea Polar Research Institute poject (KOPRI; PE 21110 & PE21170). J.L. Iriarte was partially supported by program FONDAP-IDEAL 15150003. We thank to Dr. María Vernet and two anonymous reviewers for providing constructive comments and suggestions that improved substantially the original version of the manuscript.
Author information
Authors and Affiliations
Contributions
MJ, EJY, SHK, and JLI wrote the manuscript. MJ, EJY, SHK, IYA, GSM, and SJP participated in discussions about the manuscript. MJ, EJY, and JP revised the manuscript. MJ, YL, HMJ, and JLI analyzed the data sets. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jeon, M., Iriarte, J.L., Yang, E.J. et al. Phytoplankton succession during a massive coastal diatom bloom at Marian Cove, King George Island, Antarctica. Polar Biol 44, 1993–2010 (2021). https://doi.org/10.1007/s00300-021-02933-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-021-02933-1