Abstract
For top consumers in marine environments, trophic discrimination factors (∆13C and ∆15N) between food and consumers’ tissues are expected to be similar among related species. However, few studies conducted in the laboratory indicate a large variability among species, which should be potentially higher in free-ranging animals. Here, we test for differences in tissue-specific ∆13C and ∆15N values of two wild penguin species (Chinstrap Pygoscelis antarctica and Gentoo P. papua) breeding in sympatry at Livingston Island, Antarctica. A total of 41 adults and 28 chicks, and food items comprised exclusively by Antarctic krill (Euphausia superba, n = 22) in Chinstraps and almost exclusively in Gentoos, were sampled for stable isotope analyses. Overall, Δ13C values varied between -1.8 and 4.0 ‰ and Δ15N values ranged from 1.2 to 6.1 ‰, and these differed between species, tissues and age-classes. Δ13C in adult penguins differed between species for feather and blood. Species-specific differences in Δ13C and Δ15N were seen in chick nail and muscle, while only Δ13C values differed between species in feathers. Our results show that trophic discrimination factors can differ substantially between closely related species consuming similar prey, especially in Δ13C value. Variation in Δ13C was driven by species, tissue and age-class, while variation in Δ15N was mostly driven by tissue type. Trophic discrimination factors may be associated to physiological and/or stress factors which may fluctuate in the wild, and this was particularly evident on chicks. This study highlights the use of diet-specialised species for the determination of trophic discrimination factors in the wild.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Assessment of trophic ecology, through stable isotope analyses (SIA) relies on the principle that individual consumers acquire the isotopic composition of their prey (see Newsome et al. 2007 for a review). Thus, important ecological and trophic information on marine environments can be gathered with the use of stable isotopes of carbon (13C/12C, δ13C) and nitrogen (15N/14N, δ15N), the most assessed stable isotopes in marine organisms (Newsome et al. 2007). The use stable isotope data is now routinely applied for tracing food-web structure and function, e.g. species trophic positions can be estimated, and the relative dietary importance of food sources can be quantified (Phillips et al. 2014). However, basal information on trophic relationships between consumers and prey need to be assessed to investigate different aspects of trophic ecology and food-web structure (Hoeinghaus and Zeug 2008). Consequently, information on trophic discrimination factors between food and consumers’ tissues (also termed fractionation factors or enrichment factors, hereafter ΔX) are essential to reconstruct diets, assess variability in isotopic values, estimate trophic level, investigate food-web structures and understand how stable isotopes flow through food webs (Caut et al. 2009; Auerswald et al. 2010; Boecklen et al. 2011; Barton et al. 2019).
For practical reasons, ΔX values are often assumed to be constant (typically around + 1‰ for δ13C and + 3.5‰ for δ15N, but see Hussey et al. 2014) but, in reality, many variables affect trophic discrimination factors (Barnes et al. 2007; Newsome et al. 2007), which may vary considerably (i.e. from − 3 to + 3‰ in δ13C, from − 1 to + 10‰ in δ15N, Peterson and Fry 1987). It is known that trophic discrimination factors can vary depending on the taxon. Taxonomically similar organisms generally have similar physiological processes, and thus it is expected to show ΔX values related to taxonomic identity (Vanderklift and Ponsard 2003). However, inherent variation (i.e. isotopic deviations that arise from individual differences in physiology despite consuming the same diet) is not limited to taxon, and intra-specific variability in ΔX values might be affected by physiological stresses such as lack of proteins (Vanderklift and Ponsard 2003; Barnes et al. 2008; Vander Zanden et al. 2012). This should affect individuals in different forms and be particularly relevant considering for instance different age-classes due to differentiated physiological characteristics and individual mass-adjusted metabolic rate (Vanderklift and Ponsard 2003; Costantini 2008). Thus, the effect of growth (and respective metabolism) may induce variability in ΔX values in chicks versus adults. This may result into overestimated populations’ isotopic niches, which usually assumes the isotopic variation observed is purely due to differences in habitat and diet (Vander Zanden et al. 2012) due to movement between geographic locations with different baseline isotopic values (and their temporal variability) or shifts in dietary composition (Hinke et al. 2015). However, it is important to note that consumer stable isotope values can change over time due to shifts in dietary composition or movement between geographic location with differing isotopic baselines (Cherel and Hobson 2007; McMahon et al. 2013). There is also evidence the ΔX values can be dependent at the diet scale (Vanderklift and Ponsard 2003; Caut et al. 2009), for instance, the type of food (e.g. animal matter, plant matter, detritus), the diet protein quantity and quality, and the diet isotopic ratios (Vander Zanden and Rasmussen 2001; Vanderklift and Ponsard 2003; Robbins et al. 2005). Therefore, studies using SIA should adopt species-diet-specific values available in literature, when possible/appropriate (Cherel et al. 2005a). In the lack of such studies, average values from related species consuming similar prey should be adopted, acknowledging that a sensitivity analysis should be used in order to estimate how much error there might be (Cherel et al. 2005a). Additionally, trophic discrimination factors are tissue-specific due to differences in biochemical composition in lipid content and amino acids composition, also taking into account the variation in protein turnover leading to differences in isotopic incorporation rates among tissues (Wolf et al. 2009). In birds, for instance, feathers show typically higher Δ13C and Δ15N values than blood (Cherel et al. 2005a, 2014). As feathers are mainly composed of keratin and metabolically inert after synthesis, they reflect the diet during periods of growth, whereas blood retains information on diet up to the previous 3–4 weeks (Hobson and Clark 1992). Moreover, the catastrophic moult of adult penguins that renew their whole plumage while fasting ashore during a 2–4 weeks period leads to identical isotopic values in all the body feathers, thus contrasting with large inter-feathers δ13C and δ15N values induced by the protracted moult of other avian species (Carravieri et al. 2014). Thus, feather stable isotope values in penguins should reflect the diet prior to moult, since feather growth requires endogenous reserves to be synthetized, and this might result in enriched nitrogen values (Cherel et al. 2005b)(but see Polito et al. 2011c).
Currently, Bayesian models are widely used in ecological studies, including diet reconstruction through stable isotope mixing models (e.g. MixSIAR, Parnell et al. 2010). These models allow users to incorporate variability in trophic discrimination factors, but they are highly sensitive to variation in ΔX values (Bond and Diamond 2011; Healy et al. 2018). Therefore, the use of proxy trophic discrimination factors may not be appropriate for species or tissues where the specific value is unknown (Bond and Diamond 2011), leading to misused and misinterpreted results (Phillips et al. 2014). This assumption gains further relevance considering wild population studies where physiological and environmental factors may considerably influence variation in ΔX values among individuals (McCutchan et al. 2003; Barton et al. 2019).
Trophic discrimination factors are species-, tissue- and diet-specific, and although several experiments have been carried out in top predators (e.g. Lesage et al. 2002; Caut et al. 2011; Borrell et al. 2012; Giménez et al. 2016), these remain largely unknown for the great majority of upper-trophic-level marine organisms. Evidently, trophic discrimination factors cannot be predicted for all species in all environments (Barnes et al. 2007). The recently developed SIDER package for R (Healy et al. 2018) allows a greater degree of confidence on the choice of trophic discrimination factors, and it is especially recommended in cases where study-specific data from feeding trials is unavailable. However, the uncertainty of adopting precise ΔX values in food-web modelling is extremely high for most species and environments (Phillips et al. 2014), especially when there are no specific available studies (Barton et al. 2019). For instance, in the Southern Ocean, it is crucial to determine species-specific trophic discrimination factors between consumers and the Antarctic krill Euphausia superba for a better understanding of the food-web structure (Polito et al. 2013). Antarctic krill is a key species that constitutes the link between lower- and upper-trophic levels, and thus relevant in food-web modelling (Polito et al. 2013; Reiss et al. 2017). Moreover, and from the taxon point of view, there are very few studies publishing trophic discrimination factors for consumers from different taxa in general (Caut et al. 2009), and seabirds in particular (Bond and Jones 2009). Most studies are conducted on captive animals on controlled diets and are not often measured experimentally in field because a varied diet increases potential errors when estimating trophic discrimination factors (Wolf et al. 2009). However, trophic discrimination factors derived from captive studies might not be realistic in wild-caught animals (e.g. due to different prey ingestion), and there is a lack of experimental studies conducted in wild animals testing and comparing how much ΔX values vary within and among species (Wolf et al. 2009).
In this study, we estimated trophic discrimination factors (Δ13C and Δ15N values) in two closely related seabird species breeding in sympatry at Livingston Island, South Shetland Islands (Antarctica), the Chinstrap Pygoscelis antarctica and Gentoo P. papua penguins. We focussed on potential variability in three factors: species, tissue and age-class. To our knowledge, no information on Δ13C and Δ15N values from any tissue of Chinstrap penguins exists, but Polito et al. (2011b) estimated 1.3‰ for Δ13C and 3.5 ‰ for Δ15N between herring Clupea harengus and Gentoo penguins breast feathers measured in laboratory. However, such values may vary considerably between penguin species, e.g. in the blood and feathers of King penguins Aptenodytes patagonicus and Rockhopper penguins Eudyptes chrysocome (Cherel et al. 2005a), or between tissues (blood and claws) in African penguins Spheniscus demersus (Barquete et al. 2013). Thus, while reviewing available studies on trophic discrimination factors in penguins’ species, we foresee a large overall variation in Δ13C and Δ15N values in this study conducted in the wild. For each penguin species, we analysed different tissues in both adults (whole blood and feathers) and chicks (down feathers, nails and muscle) to determine species- and age-class-specific isotopic discrimination for each penguin’s tissue and Antarctic krill. Euphausiids (mainly Antarctic krill) represented almost the entirety of items found in stomachs of Chinstrap penguins (i.e. 99.9%) and Gentoo penguins (i.e. 99.4% krill; 0.5% fish) on King George Island, South Shetlands Islands, during the breeding season (Panasiuk et al. 2020). Likewise, both penguin species are known to feed predominantly on Antarctic krill at Livingston Island (Miller et al. 2010; Polito et al. 2015; Dimitrijević et al. 2018), although fish may be also a component of the Gentoo Penguin’s diet (Miller et al. 2010). This constitutes an excellent opportunity to study and determine for the first time precise trophic discrimination factors between the consumers and specific keystone prey (i.e. Antarctic krill) in two wild penguins’ populations, under similar environmental conditions. Specifically, the goals of this study were to: (1) evaluate any differences in Δ13C and Δ15N values between two related and sympatric penguin species consuming a similar diet in a natural environment, (2) test for differences in tissues with different biochemical composition, and (3) test the potential effect of growth (i.e. between age-classes) in the two penguin’s species by evaluating age-class-specific trophic discrimination factors in feathers of adults and chicks.
Materials and methods
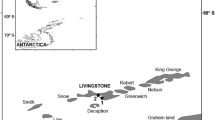
Fieldwork was carried out simultaneously on Chinstrap and Gentoo penguins during the chick-rearing period at Livingston Island, South Shetland Islands, between December 2011 and January 2012. Specifically, tissue samples of Chinstrap penguins were collected at the breeding colony of Miers Bluff point on Hurd Peninsula (62° 43′ S, 60° 25′ W; January 9th) and the shore close to the Bulgarian Base (62° 38′ S, 60° 21′ W; December 17th) and those of Gentoo penguins at the breeding colony of Hannah Point (62° 39′ S, 60° 36′ W; December 20th) and Bulgarian Base (January 13th and 14th). All these areas are located on the south part of the island distanced by c.a. 11 km from each other (Fig. 1).
Blood samples (c.a. 1 mL blood from the brachial vein using 25G hypodermic needles) and 6–8 breast feathers were collected from randomly captured adults (Chinstrap: n = 12, Gentoo: n = 29). Tissue samples of penguin chicks (i.e. down feathers, toenails and muscle tissue) were collected from dead birds (Chinstrap: n = 13, Gentoo: n = 15), which had recently died from unknown causes (carcasses were in a very good condition, with very well-preserved stomach contents), and found randomly in their colonies. Six to eight down feathers were collected from chicks; toenails from the middle toe of the left leg, and muscle tissue (i.e. from thigh muscle) of the left leg. Blood samples, toenails and muscle tissue were stored frozen until isotopic analyses and feathers were stored in dry plastic bags. In chicks, toenails and muscle tissues grow slowly but continuously, and they reflect the diet from the moment they were formed (i.e. in the egg, the diet of the mother in the pre-laying period) and throughout the growth of the chick after the egg hatching (i.e. the onset of the breeding season prior to their death) (Vasil et al. 2012). On the other hand, as down feathers are metabolically inert after synthesis (Hobson and Clark 1992), their isotope signatures reflect mother’s diet (during the pre-laying). Vasil et al. (2012) demonstrated that down feather and toenail isotope values from dead and living Pygoscelis penguin chicks displayed similar isotopic values (although muscle has not been tested), despite the cause of death, supporting the use of opportunistic sampling in stable isotope analyses.
Collection of faecal and stomach content samples to determine diet
Fresh feces (i.e. recently produced, still viscous, not dried) from adults (Chinstrap: n = 59, Gentoo: n = 73) were collected randomly from Miers Bluff (in December 28th, January 9th and 16th), Hannah Point (in December 20th, 29th and January 3rd) and the shore close to the Bulgarian Base (in December 15th, 24th and January 13th). Stomach contents were collected from 3 Chinstrap penguin chicks and 15 Gentoo penguin chicks (all dead chicks tissue samples were obtained from). All diet samples were stored frozen and analysed within 24 h in the laboratory of the Bulgarian base “St. Kliment Ohridski”. Stomach contents of recently dead chicks and feces of adults were then examined, and the frequency of occurrence (FO; %), number (N) and mass (M; %, for stomach contents only) were determined for all prey following Xavier et al. (2015) and Petta et al. (2020). Crustacean (through their carapaces) and fish (through their otoliths) species were identified using reference collections and identification guides (Reid 1996; Xavier et al. 2020).
Antarctic krill exclusively dominated stomach contents/faeces in Chinstrap penguins and nearly exclusively in Gentoo penguins (Table 1). Antarctic krill was highly abundant (i.e. a total of 1526 individuals) and present in 100% of samples collected from individuals of both species and age-classes. Other prey was extremely rare, and only found in the diet of Gentoo penguins. These included 11 small crustaceans (i.e. amphipods and Mysidacea) from three adults and four chicks, and one individual fish (i.e. one otolith of mackerel icefish Champsocephalus gunnari) from one adult.
Antarctic krill sampling for stable isotope analyses
A total of 327 Antarctic krill specimens (87 from Chinstrap chicks and 240 from Gentoo chicks) were measured (carapace and total length) to test for any differences in the size of specimens consumed between species. Intact specimens of Antarctic krill (i.e. complete items, still fresh) were collected from Chinstrap chick stomachs (n = 10 Antarctic krill individuals) and Gentoo chick stomachs (n = 12 Antarctic krill individuals) for stable isotope analyses (Cherel 2008), although a potential initial degradation due to bacterial activity might have occurred.
Stable isotope analyses (SIA)
δ13C (‰) and δ15N (‰) values were measured in penguin tissues and in Antarctic krill obtained from stomach contents of both species. Prior to SIA, feathers and toenails were cleaned of surface contaminants using successive rinses in a 2:1 chloroform–methanol solution. All tissues were dried in an oven at 60 °C for 24 h and then homogenised. Feathers were cut into small fragments (removing the base) and toenails and thigh muscle from penguins and Antarctic krill (removing the exoskeleton) were milled with a mortar and pestle. Lipids were extracted from muscle and Antarctic krill, using a 2:1 chloroform–methanol solution. The low lipid content of whole blood does not typically require lipid extraction (Cherel et al. 2005a).
Carbon and nitrogen isotope ratios were determined by a continuous-flow isotope ratio mass spectrometer (Delta V Advantage, Thermo Scientific, Bremen, Germany) coupled to an elemental analyser (Flash EA1112, Thermo Scientific) at Marine and Environmental Sciences Centre, University of Coimbra. Approximately 0.3 mg of each sample was combusted in a tin cup for simultaneous determination of carbon and nitrogen isotope values. Stable isotope values are present in the usual δ notation based on the Vienna Pee-Dee Belemnite (V-PDB) for carbon and atmospheric N2 (Air) for nitrogen and expressed as: δ13C (‰) or δ15N (‰) = [(Rsample/Rstandard) − 1] * 1000, where R = 13C/12C or 15N/14N, respectively. Replicate measurements of secondary isotopic reference material (acetanilide STD, Thermo scientific-PN 338 36700) in every batch, indicate precision < 0.2 ‰ for both δ13C and δ15N values.
Calculation of diet-tissue trophic discrimination
The Antarctic krill sampled from the stomachs of Chinstrap and Gentoo penguin’s chicks did not differ significantly in either stable isotopes (i.e. δ13C and δ15N) and biometry measurements (i.e. carapace and total length) (see Table A1, Online Resource 1). These results show that the Antarctic krill eaten at the time of sampling and consumed by both penguin species is isotopically identical and have similar sizes, suggesting no Antarctic krill partitioning during the chick-rearing period of Chinstrap and Gentoo penguins. Thus, Antarctic krill obtained from Gentoo and Chinstrap chicks were pooled, and the resulting mean isotopic values were used to estimate trophic discrimination factors. Diet-tissue trophic discrimination factors between tissues of consumers (i.e. penguin species and age-classes) and their diet (i.e. Antarctic krill) was calculated as:
where X is 13C or 15N. The notation ΔXconsumer-diet was calculated for each individual based on its specific δXtissue value and the overall mean δXkrill value of Antarctic krill (i.e. − 25.53‰ for δ13C and 5.61‰ for δ15N, n = 22), and was abbreviated to ΔX in this study.
Data analyses
All data were tested for normality (Kolmogorov–Smirnov test) and homogenous variances (Levene’s test). Groups (i.e. Antarctic krill sampled from the stomach of Chinstrap and Gentoo penguin chicks, and stable isotope values of adults and chicks) were compared using Mann–Whitney U tests (for non-parametric data, i.e. biometric measurements of Antarctic krill) and t-tests (for parametric data, i.e. stable isotope values). Table A2 (Online Resource 1) shows stable isotope values for the different tissues in adult and chicks of Chinstrap and Gentoo penguins, as well as species comparisons in δ13C and δ15N values (and respective Δ13C and Δ15N values), and Table A3 (Online Resource 1) details the individual isotopic data for both Antarctic krill and consumers. We tested for differences in Δ13C and Δ15N values between the two penguin species and among the different tissues with different biochemical composition in the adults and chicks separately using linear mixed-effects models. The potential effect of growth (i.e. between age-classes) was tested in the two penguin’s species using feathers as it was the only tissue sampled for both adults and chicks. In these analyses the individual was treated as a random effect. Values are presented as mean ± SD. All statistical tests were performed with Statistica 10.0.
Results
Species-, tissue- and age-class comparisons in Δ13C and Δ15N values of Pygoscelis penguins
In adults, δ13C values measured in the tissues (i.e. blood and feathers) of Gentoo penguins were higher than in Chinstrap penguins. However, similar δ15N values between adults of the two species were detected (Fig. A1 and Table A2, Online Resource 1). We found statistically significant differences in adults’ Δ13C values between species (Chinstraps vs. Gentoos) and tissues (blood vs. feathers), with no tissue*species interaction (Table 2). There were also statistically significant differences in the Δ15N values of adults between tissues, but not between species and tissue*species interaction (Table 2).
In chicks, statistically significant differences were found in Δ13C values between species (Chinstraps vs. Gentoos) and tissues (feathers vs. nails vs. muscle), with no tissue*species interaction (Table 2). Also, statistically significant differences were found in chicks’ Δ15N values between species (Chinstraps vs. Gentoos), tissues (feathers vs. nails vs. muscle), and in tissue*species interaction (Table 2).
Considering age-classes, statistically significant differences in Δ13C values of feathers were found either in Chinstraps (adults vs. chicks: F1,23 = 35.04, p < 0.0001) or Gentoos (adults vs. chicks: F1,42 = 25.52, p < 0.0001). Adults of both species showed higher Δ13C values in feathers than chicks (Table 3). However, no differences were found in Δ15N values between adults and chicks of both Chinstrap (F1,23 = 1.27, p = 0.2715) and Gentoo penguins (F1,42 = 1.44, p = 0.2367).
Trophic discrimination factors between penguins’ tissues and Antarctic krill
A variation in trophic discrimination factors was detected among and within groups (Fig. 2). Overall, Δ13C values varied between − 1.8 and 4.0‰ (i.e. 5.8‰) and Δ15N values ranged from 1.2 to 6.1‰ (i.e. 4.9‰) and, in general, feathers showed the highest enrichment in both isotopes, and blood and muscle the lowest. We found negative Δ13C values between Antarctic krill and the blood of both adult Chinstrap (− 0.4‰) and Gentoo penguins (− 0.1‰), and an increment of 2.0 and 2.7‰ in feathers, respectively (Table 3, Fig. 2). The two adult penguin species were similar in Δ15N values for both blood (Chinstrap: 2.51 ± 0.37; Gentoo: 2.34 ± 0.30) and feathers (Chinstrap: 3.10 ± 0.78; Gentoo: 3.11 ± 0.81) (Fig. 2 and Table 2, and Fig. A1 and Table A2, Online Resource 1). However, in relation to penguins’ chicks, our results show very distinct Δ13C and Δ15N values between both species (in general higher ΔX values in Gentoo chicks), with the only exception of Δ15N values in feathers (Table 3, Fig. 2).
Variability in trophic discrimination factors a ∆13C (‰) and b ∆15N (‰) between Antarctic krill Euphausia superba and tissues of Chinstrap (Pygoscelis antarctica) and Gentoo (Pygoscelis papua) penguins. Circles are mean values for each group, boxplots show 95% confidence intervals and lines are minimum and maximum values
Discussion
This study highlights that trophic discrimination factors can differ substantially from typical values often assumed for free-ranging species, and especially in Δ13C (overall Δ13C values varied between − 1.8 and 4.0 ‰ and Δ15N values between 1.2 to 6.1‰). This variation was species-, tissue-, and age-class-specific in free-ranging Pygoscelis penguins, possibly associated to biochemical and/or metabolic processes during tissue synthesis and physiological and/or stress factors which can fluctuate in the wild.
We assumed that sampled Chinstrap and Gentoo penguins fed exclusively upon Antarctic krill to estimate ΔX values, as previous studies demonstrated that both species are specialised on this item at South Shetland Islands (Miller et al. 2010; Panasiuk et al. 2020), and particularly at our study site (i.e. Livingston Island) (this study; Miller et al. 2010; Polito et al. 2015; Dimitrijević et al. 2018). Since this study was conducted in wild animals, our results on potential consumption of fish and other items could be underestimated, especially in Gentoo penguins, and we cannot be 100% assured that individuals did not eventually prey on other items. Indeed, an underestimated fish contribution to the diet might have occurred when evaluating diet of both penguin species (Polito et al. 2015). Our results based on representative faecal and stomach content samples showed that Chinstrap penguins were extremely specialised in Antarctic krill (at least during the short term no other prey was found), while some Gentoo penguins exhibited slightly broader dietary niche consuming a few other items. In fact, despite the high predominance of Antarctic krill, there is evidence of fish consumption by Gentoo penguins (up to 29% of diet composition by mass) at South Shetland Islands in the past (i.e. between 1997 and 2004) (Miller et al. 2010). Polito et al. (2015), found that niche partitioning between Chinstrap and Gentoo penguins is a function of the higher krill consumption and a greater use of pelagic and meso-pelagic foraging habitats by the former. It is known that Gentoo penguins feed more in benthic, inshore habitats than Chinstrap penguins (Polito et al. 2011c, 2015), leading to a more diversified diet which might influence isotopic values observed. However, other items in the diet of Gentoo penguins only comprised 1.2% of total food items consumed in 2011/2012, from which the majority (i.e. 1.1%, see Table 1) were small crustaceans (amphipods and Mysidacea) related to Antarctic krill. Additionally, Antarctic krill collected from both penguin species were isotopically similar. Although we acknowledge there is a small temporal mismatch between temporal integration of feces/stomach contents (snapshot) and the stable isotope method (tissue-specific isotopic turnover), this should not substantially drive the variation (and differences) in isotopic values between species. This is particularly true considering the time of sampling (i.e. in the short term), reflected by tissues with high turnover rates such as blood, and the recently synthesised tissues of chicks (Hobson and Clark 1992; Vasil et al. 2012; Vander Zanden et al. 2015). However, since the isotopic composition of feathers reflects the diet of Gentoo and Chinstrap adult penguins during moult (Cherel et al. 2000), these are indicators of diet at the end of the previous breeding season (i.e. from late March 2011). The growth of new feathers occurs once during this period, near their breeding colonies (Trivelpiece et al. 1987), and we assumed that both species fed on Antarctic krill during such period with no isotopic changes over different time-scales (Polito et al. 2015; Dimitrijević et al. 2018). Still, just as for blood, δ15N values of feathers did not differ between adults of the two species, suggesting that similar prey was consumed during both short- and long-term timescale. However, we acknowledge some variability in feathers could potentially derive from different prey and/or isotopic values of Antarctic krill during the previous breeding season and drive potential differences in feathers between adults and chicks.
To our knowledge, this is the first study determining trophic discrimination factors in Chinstrap penguins and in penguins’ chicks, and the first aimed at free-ranging penguins in the wild (Table 3). There is only one study addressing trophic discrimination factors in feathers of adult Gentoo penguins, based on herring in captivity, which estimated 1.3‰ for Δ13C and 3.5‰ for Δ15N (Polito et al. 2011a). While Δ15N values were similar, the Δ13C values contrasted considerably with our results for the same tissue (i.e. 2.7‰ for Δ13C and 3.1‰ for Δ15N). Following a similar pattern, our results showed more pronounced differences between the two studied species in Δ13C (in both adults and chicks) than in Δ15N (only on chicks). In a broad sense, such differences may thus arise from different environmental conditions (i.e. captivity vs. wild) and/or specific diet (i.e. herring vs. Antarctic krill), although we must highlight that small differences in the diet and/or foraging habitat may have driven differences between the two sympatric studied species. Interestingly, we found negative Δ13C values in the whole blood of both Chinstrap and Gentoo adult penguins (and in muscle of Chinstrap chicks), indicating a depletion in 13C regarding food consumed (i.e. Antarctic krill). Similarly, Cherel et al. (2005b) also found negative Δ13C values (− 0.81‰) between blood of King penguins and food (i.e. herring), but in captivity. This is somewhat unexpected because normally there are both a 13C and 15N enrichment in consumers’ tissues relative to foods, but these can vary depending on physiological and environmental factors (Newsome et al. 2007), especially in the wild where environmental conditions are not stable and stress responses of birds may differ considerably (Costantini 2008).
Differences were found in trophic discrimination factors among tissues. Information on tissue-specific trophic discrimination factors in Pygoscelis penguins is only available for captive Gentoo penguins. Polito et al. (2009) found a large variation in trophic discrimination factors across different egg components of Gentoo penguins, especially in Δ13C values, suggesting that such variation likely reflects differences in biochemical and metabolic processes during tissue synthesis. Although we did not perform analyses on egg components, we agree with Polito et al. (2009), as both Δ13C (in adults and chicks) and Δ15N (in chicks) differed significantly between the tissues analysed in our study. In general, and independently on the species, Δ13C and Δ15N values are higher in feathers than in blood of adults (Table 3, see also Cherel et al. 2014), and our results also corroborate higher values of down feathers in comparison to muscle and nails (from chicks). Such differences in diet-tissue trophic discrimination factors are supposedly explained by tissue-specific biochemical composition (Wolf et al. 2009); accordingly, lipid content and amino acid composition in different tissues are the main sources of variation in stable isotopes, and particularly in δ13C (Cherel et al. 2014). However, the low C:N mass ratios in our samples (up to 3.5, Tables A1 and A2), which is a good proxy of lipid content in animal tissues (Post et al. 2007), suggest that amino acid composition was the main source of tissue’s variation during this study. Still, the slightly higher C:N mass ratios (and respective lipid content) in blood of both adult penguins’ species, especially in relation to feathers, may explain some of the variation detected between tissues (Cherel et al. 2014). A similar approach to ours was performed by Borrell et al. (2012) who estimate diet-tissue trophic discrimination factors (Δ13C and Δ15N) between a wild population of fin whales Balaenoptera physalus feeding on the euphausiid krill Meganyctiphanes norvegica. They found tissue-specific ΔX values (in bone and brain) ranging from 1.3 to 3.1‰ in carbon and from 2.0 to 4.3‰ in nitrogen. This variation was not as large as in penguins, but they attributed variation to tissue composition, which is consistent with our results.
To the best of our knowledge, no studies addressed trophic discrimination factors on penguins’ chicks. Our results point to age-class-specific trophic discrimination factors, at least in feathers. However, potential underlying physiological or biochemical differences between adult feathers and chick down feathers that could contribute to differences in the stable isotope values and resulting discriminant factors might influence these outputs. Adults and chicks of both species presented distinct Δ13C values (lower in chicks), but similar Δ15N values, resulting thus into an apparent effect of growth (i.e. between chicks and adults) in the variability of Δ13C values. The stress in wild birds associated with age-class-specific traits that are continually exposed to changing environments might affect metabolic rates of individuals (Costantini 2008). Moreover, the effect of anabolism/catabolism influencing in different ways organisms in a steady state or in those that decrease in body mass/fasting, may affect individual variation in trophic discrimination factors, especially between different age-classes (Vanderklift and Ponsard 2003; Aguilar et al. 2014).
While reviewing published studies on trophic discrimination factors estimated from penguins (Table 3), we found that, overall, mean ΔX values ranged from − 0.81 to 2.9‰ (7.2‰ if considering eggshell carbonate) in carbon and from 1.8 to 4.8‰ in nitrogen. These are mean values that do not take into consideration individual variability; still, it is evident that trophic discrimination values may vary considerable among species, tissues and age-classes (i.e. between adults and chicks, this study), and diet also should affect ΔX values (Vanderklift and Ponsard 2003). Potential differences between wild-caught and captive animals should be also taken into consideration, as our results displayed considerable differences in comparison with the bulk of Δ13C values estimates from penguins in captivity. We draw attention to the potential high variability in trophic discrimination factors in birds, even among related species, because the current widely used mixing models are highly sensitive to variation in ΔX values (Bond and Diamond 2011). Limiting the potential bias to a minimum is currently required, and highly specialised species/populations are of great advantage to determine species-specific trophic discrimination factors in the wild, acknowledging the impossibility of conducting studies in every species and environments.
Conclusions
Contrary to the general assumption that trophic discrimination factors are relatively constant between taxonomically related species, we found statistically significant differences in Δ13C and Δ15N values between two sympatric Pygoscelis penguin species preying on the same food source (i.e. Antarctic krill). Differences in Δ13C and Δ15N values were particularly pronounced between Gentoo and Chinstrap penguins’ chicks. In adults, however, differences in Δ13C (but not in Δ15N) were meaningful across tissues analysed. While differences in Δ13C were detected overall between species, tissues and age-classes (i.e. between adults and chicks), differences in Δ15N were detected among tissues in both adults and chicks and between species only in chicks. This highlights that particular attention on the selection of Δ13C values in trophic ecology models should be adopted.
This study provides the first data from penguins in the wild and also for chinstrap penguins and for penguins’ chicks. Overall, our results show that ΔX values may differ substantially from typical ones often assumed (i.e. around + 1‰ for δ13C value and + 3.5‰ for δ15N value) in free-ranging individuals. Among other potential factors (e.g. prey, sex and population), this variation can be tissue-, species- and age-class-specific. Moreover, this study highlights inherent variation possibly associated to physiological and/or stress factors. While expanding substantially the range of species for which trophic discrimination factors are known, we strongly contribute to minimising potential bias in trophic ecology models by providing specific ΔX values in wild bird species. This gains further relevance considering that many species are dependent of Antarctic krill as a primary resource.
References
Aguilar A, Giménez J, Gómez-Campos E, Cardona L, Borrell A (2014) δ15N Value does not reflect fasting in mysticetes. PLoS ONE 9:e92288. https://doi.org/10.1371/journal.pone.0092288
Auerswald K, Wittmer MHOM, Zazzo A, Scha R, Schnyder H (2010) Biases in the analysis of stable isotope discrimination in food webs. J Appl Ecol 47:936–941. https://doi.org/10.1111/j.1365-2664.2009.01764.x
Barnes C, Sweeting CJ, Jennings S, Barry JT, Polunin NVC (2007) Effect of temperature and ration size on carbon and nitrogen stable isotope trophic fractionation. Funct Ecol 21:356–362. https://doi.org/10.1111/j.1365-2435.2006.01224.x
Barnes C, Jennings S, Polunin NVC, Lancaster JE (2008) The importance of quantifying inherent variability when interpreting stable isotope field data. Oecologia 155:227–235. https://doi.org/10.1007/s00442-007-0904-y
Barquete V, Strauss V, Ryan PG (2013) Stable isotope turnover in blood and claws: A case study in captive African Penguins. J Exp Mar Bio Ecol 448:121–127. https://doi.org/10.1016/j.jembe.2013.06.021
Barton MB, Litvin SY, Vollenweider JJ, Heintz RA, Norcross BL, Boswell KM (2019) Implications of trophic discrimination factor selection for stable isotope food web models of low trophic levels in the Arctic nearshore. Mar Ecol Prog Ser 613:211–216
Boecklen WJ, Yarnes CT, Cook BA, James AC (2011) On the use of stable isotopes in trophic ecology. Annu Rev Ecol Evol Syst 42:411–440. https://doi.org/10.1146/annurev-ecolsys-102209-144726
Bond A, Diamond A (2011) Recent Bayesian stable-isotope mixing models are highly sensitive to variation in discrimination factors. Ecol Appl 21:1017–1023
Bond AL, Jones IL (2009) A practical introduction to stable-isotope analysis for seabird biologists: approaches, cautions and caveats. Mar Ornithol 37:183–188
Borrell A, Abad-Oliva N, Gómez-Campos E, Giménez J, Aguilar A (2012) Discrimination of stable isotopes in fin whale tissues and application to diet assessment in cetaceans. Rapid Commun Mass Spectrom 26:1596–1602. https://doi.org/10.1002/rcm.6267
Carravieri A, Bustamante P, Churlaud C, Fromant A, Cherel Y (2014) Moulting patterns drive within-individual variations of stable isotopes and mercury in seabird body feathers: implications for monitoring of the marine environment. Mar Biol 161:963–968. https://doi.org/10.1007/s00227-014-2394-x
Caut S, Angulo E, Courchamp F (2009) Variation in discrimination factors (Δ15N and Δ13C): the effect of diet isotopic values and applications for diet reconstruction. J Appl Ecol 46:443–453. https://doi.org/10.1111/j.1365-2664.2009.01620.x
Caut S, Laran S, Garcia-Hartmann E, Das K (2011) Stable isotopes of captive cetaceans (killer whales and bottlenose dolphins). J Exp Biol 214:538–545. https://doi.org/10.1242/jeb.045104
Cherel Y (2008) Isotopic niches of emperor and Adélie penguins in Adélie Land, Antarctica. Mar Biol 154:813–821. https://doi.org/10.1007/s00227-008-0974-3
Cherel Y, Hobson K (2007) Geographical variation in carbon stable isotope signatures of marine predators: a tool to investigate their foraging areas in the Southern Ocean. Mar Ecol Prog Ser 329:281–287. https://doi.org/10.3354/meps329281
Cherel Y, Hobson KA, Weimerskirch H (2000) Using stable-isotope analysis of feathers to distinguish moulting and breeding origins of seabirds. Oecologia 122:155–162. https://doi.org/10.1007/PL00008843
Cherel Y, Hobson KA, Hassani S (2005a) Isotopic discrimination between food and blood and feathers of captive penguins: implications for dietary studies in the wild. Physiol Biochem Zool 78:106–115. https://doi.org/10.1086/425202
Cherel Y, Hobson KA, Bailleul F, Groscolas R (2005b) Nutrition, physiology, and stable isotopes: New information from fasting and molting penguins. Ecology 86:2881–2888. https://doi.org/10.1890/05-0562
Cherel Y, Jaquemet S, Maglio A, Jaeger A (2014) Differences in δ13C and δ15N values between feathers and blood of seabird chicks: implications for non-invasive isotopic investigations. Mar Biol 161:229–237. https://doi.org/10.1007/s00227-013-2314-5
Ciancio JE, Righi C, Faiella A, Frere E (2016) Blood-specific isotopic discrimination factors in the Magellanic penguin (Spheniscus magellanicus). Rapid Commun Mass Spectrom 30:1865–1869. https://doi.org/10.1002/rcm.7661
Costantini D (2008) Oxidative stress in ecology and evolution: lessons from avian studies. Ecol Lett 11:1238–1251. https://doi.org/10.1111/j.1461-0248.2008.01246.x
Dimitrijević D, Paiva VH, Ramos JA, Seco J, Ceia FR, Chipev N, Valente T, Barbosa A, Xavier JC (2018) Isotopic niches of sympatric Gentoo and Chinstrap Penguins: evidence of competition for Antarctic krill? Polar Biol 41:1655–1669. https://doi.org/10.1007/s00300-018-2306-5
Giménez J, Ramírez F, Almunia J, G. Forero M, de Stephanis R, (2016) From the pool to the sea: Applicable isotope turnover rates and diet to skin discrimination factors for bottlenose dolphins (Tursiops truncatus). J Exp Mar Bio Ecol 475:54–61. https://doi.org/10.1016/j.jembe.2015.11.001
Healy K, Guillerme T, Kelly SBA, Inger R, Bearhop S, Jackson AL (2018) SIDER: an R package for predicting trophic discrimination factors of consumers based on their ecology and phylogenetic relatedness. Ecography (cop) 41:1393–1400. https://doi.org/10.1111/ecog.03371
Hinke JT, Polito MJ, Goebel ME, Jarvis S, Reiss CS, Thorrold SR, Trivelpiece WZ, Watters GM (2015) Spatial and isotopic niche partitioning during winter in chinstrap and Adélie penguins from the South Shetland Islands. Ecosphere 6:1–32
Hobson KA, Clark RG (1992) Assessing avian diets using stable isotopes I: turnover of 13C in tissues. Condor 94:181–188
Hoeinghaus DJ, Zeug SC (2008) Can stable isotope ratios provide for community-wide measures of trophic structure? Comment. Ecology 89:2353–2357
Hussey NE, Macneil MA, Mcmeans BC, Olin JA, Dudley SFJ, Cliff G, Wintner SP, Fennessy ST, Fisk AT (2014) Rescaling the trophic structure of marine food webs. Ecol Lett 17:239–250. https://doi.org/10.1111/ele.12226
Lesage V, Hammill MO, Kovacs KM (2002) Diet-tissue fractionation of stable carbon and nitrogen isotopes in phocid seals. Mar Mammal Sci 18:182–193. https://doi.org/10.1111/j.1748-7692.2002.tb01027.x
McCutchan JH, Lewis WM, Kendall C, Mcgrath CC (2003) Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos 102:378–390
McKenzie KFR (2011) The foraging ecology of little penguin (Eudyptula minor) on Tiritiri Matangi Island. MSc Thesis, Massey University, Auckland
McMahon KW, Hamady LL, Thorrold SR (2013) A review of ecogeochemistry approaches to estimating movements of marine animals. Limnol Oceanogr 58:697–714. https://doi.org/10.4319/lo.2013.58.2.0697
Miller AK, Kappes MA, Trivelpiece SG, Trivelpiece WZ (2010) Foraging-niche separation of breeding Gentoo and Chinstrap Penguins, South Shetland Islands, Antarctica. Condor 112:683–695. https://doi.org/10.1525/cond.2010.090221
Mizutani H, Fukuda M, Kabaya Y (1992) 13C and 15N enrichment factors of feathers of 11 species of adult birds. Ecology 73:1391–1395
Newsome SD, Rio CM, Bearhop S, Phillips DL (2007) A niche for isotopic ecology. Front Ecol Environ 5:429–436. https://doi.org/10.1890/060150.01
Panasiuk A, Wawrzynek-Borejko J, Musiał A, Korczak-Abshire M (2020) Pygoscelis penguin diets on King George Island, South Shetland Islands, with a special focus on the krill Euphausia superba. Antarct Sci 32:21–28. https://doi.org/10.1017/S0954102019000543
Parnell AC, Inger R, Bearhop S, Jackson AL (2010) Source partitioning using stable isotopes: coping with too much variation. PLoS ONE 5:e9672. https://doi.org/10.1371/journal.pone.0009672
Peterson BJ, Fry B (1987) Stable isotopes in ecosystem studies. Annu Rev Ecol Syst 18:293–320. https://doi.org/10.1146/annurev.es.18.110187.001453
Petta JC, Shipley ON, Wintner SP, Cliff G, Dicken ML, Hussey NE (2020) Are you really what you eat? Stomach content analysis and stable isotope ratios do not uniformly estimate dietary niche characteristics in three marine predators. Oecologia 192:1111–1126. https://doi.org/10.1007/s00442-020-04628-6
Phillips DL, Inger R, Bearhop S, Jackson AL, Moore JW, Parnell AC, Semmens BX, Ward EJ (2014) Best practices for use of stable isotope mixing models in food-web studies. Can J Zool 92:823–835. https://doi.org/10.1139/cjz-2014-0127
Polito MJ, Fisher S, Tobias CR, Emslie SD (2009) Tissue-specific isotopic discrimination factors in gentoo penguin (Pygoscelis papua) egg components: Implications for dietary reconstruction using stable isotopes. J Exp Mar Bio Ecol 372:106–112. https://doi.org/10.1016/j.jembe.2009.02.014
Polito MJ, Abel S, Tobias CR, Emslie SD (2011a) Dietary isotopic discrimination in gentoo penguin (Pygoscelis papua) feathers. Polar Biol 34:1057–1063. https://doi.org/10.1007/s00300-011-0966-5
Polito MJ, Trivelpiece WZ, Karnovsky NJ, Ng E, Patterson WP, Emslie SD (2011b) Integrating stomach content and stable isotope analyses to quantify the diets of Pygoscelid penguins. PLoS ONE 6:e26642. https://doi.org/10.1371/journal.pone.0026642
Polito MJ, Lynch H, Naveen R, Emslie S (2011c) Stable isotopes reveal regional heterogeneity in the pre-breeding distribution and diets of sympatrically breeding Pygoscelis spp. penguins. Mar Ecol Prog Ser 421:265–277. https://doi.org/10.3354/meps08863
Polito MJ, Reiss CS, Trivelpiece WZ, Patterson WP, Emslie SD (2013) Stable isotopes identify an ontogenetic niche expansion in Antarctic krill (Euphausia superba) from the South Shetland Islands, Antarctica. Mar Biol 160:1311–1323. https://doi.org/10.1007/s00227-013-2182-z
Polito MJ, Trivelpiece W, Patterson W, Karnovsky N, Reiss C, Emslie S (2015) Contrasting specialist and generalist patterns facilitate foraging niche partitioning in sympatric populations of Pygoscelis penguins. Mar Ecol Prog Ser 519:221–237. https://doi.org/10.3354/meps11095
Post DM, Layman CA, Arrington DA, Takimoto G, Quattrochi J, Montaña CG (2007) Getting to the fat of the matter: models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 152:179–189. https://doi.org/10.1007/s00442-006-0630-x
Reid K (1996) A guide to the use of otoliths in the study of predators at South Georgia. British Antarctic Survey, Cambridge
Reiss C, Cossio A, Santora J, Dietrich K, Murray A, Mitchell B, Walsh J, Weiss E, Gimpel C, Jones C, Watters G (2017) Overwinter habitat selection by Antarctic krill under varying sea-ice conditions: implications for top predators and fishery management. Mar Ecol Prog Ser 568:1–16. https://doi.org/10.3354/meps12099
Robbins CT, Felicetti LA, Sponheimer M (2005) The effect of dietary protein quality on nitrogen isotope discrimination in mammals and birds. Oecologia 144:534–540. https://doi.org/10.1007/s00442-005-0021-8
Trivelpiece WZ, Trivelpiece SG, Volkman NJ (1987) Ecological segregation of Adelie, gentoo and chinstrap penguins at King George Island, Antarctica. Ecology 68:351–361. https://doi.org/10.2307/1939266
Vander Zanden HB, Bjorndal KA, Mustin W, Ponciano JM, Bolten AB (2012) Inherent variation in stable isotope values and discrimination factors in two life stages of green turtles. Physiol Biochem Zool 85:431–441. https://doi.org/10.1086/666902
Vander Zanden MJ, Rasmussen JB (2001) Variation in δ15N and δ13C trophic fractionation: implications for aquatic food web studies. Limnol Oceanogr 46:2061–2066. https://doi.org/10.4319/lo.2001.46.8.2061
Vander Zanden MJ, Clayton MK, Moody EK, Solomon CT, Weidel BC (2015) Stable isotope turnover and half-life in animal tissues: a literature synthesis. PLoS ONE 10:e0116182. https://doi.org/10.1371/journal.pone.0116182
Vanderklift MA, Ponsard S (2003) Sources of variation in consumer-diet δ15N enrichment: a meta-analysis. Oecologia 136:169–182. https://doi.org/10.1007/s00442-003-1270-z
Vasil CA, Polito MJ, Patterson WP, Emslie SD (2012) Wanted: dead or alive? Isotopic analysis (δ13C and δ15N) of Pygoscelis penguin chick tissues supports opportunistic sampling. Rapid Commun Mass Spectrom 26:487–493. https://doi.org/10.1002/rcm.5340
Wolf N, Carleton SA, Del RCM (2009) Ten years of experimental animal isotopic ecology. Funct Ecol 23:17–26. https://doi.org/10.1111/j.1365-2435.2008.01529.x
Xavier JC, Hill SL, Belchier M, Bracegirdle TJ, Murphy EJ, Dias JL (2015) From ice to penguins: the role of mathematics in Antarctic research. In: Bourguignon JP, Jeltsch R, Pinto AVM (eds) Mathematics of energy and climate change, CIM series. Springer-Verlag, Switzerland, pp 389–414
Xavier JC, Cherel Y, Boxshall G, Brandt A, Coffer T, Forman J, Havermans C, Jażdżewska AM, Kouwenberg K, Schiaparelli S, Schnabel K, Siegel V, Tarling GA, Thatje S, Ward P, Gutt J (2020) Crustacean guide for predator studies in the Southern Ocean. Scientific Committee on Antarctic Research, Cambridge, UK
Acknowledgements
This work is part of SCAR AnT-ERA, ICED and SCAR-EGBAMM programs. This study benefitted from the strategic programme of MARE, financed by FCT (MARE—UID/MAR/04292/2020). FRC acknowledges the transitory norm contract (DL57/2016/CP1370/CT90) and JS the PhD Grant (SRFH/PD/BD/113487/2015) financed by the Foundation for Science and Technology (FCT; Portugal) and the European Social Fund (POPH, EU). Part of this data has been presented previously at the SCAR Open Science Conference 2020, Hobart, Australia. We would also like to thank Keith M. Hernandez, Arnaud Tarroux and four anonymous reviewers for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
Study design: FRC, YC and JCX. Fieldwork and collection of the samples: JS, AB, NC and JCX. Data analysis and processing: FRC, YC and JCX. FRC led the writing of the manuscript. All authors edited and revised the manuscript, contributed critically to revisions and gave final approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to declare.
Research involving animal rights
The fieldwork was conducted under a research permit from the project PENGUIN of the Portuguese Polar Programme PROPOLAR. The sampling methods used for this research were in accordance with recommendations from the Scientific Committee for Antarctic Research (SCAR).
Ethical approval
All applicable international, national and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ceia, F.R., Cherel, Y., Seco, J. et al. Variability in tissue-specific trophic discrimination factors (∆13C and ∆15N) between Antarctic krill Euphausia superba and free-ranging Pygoscelis penguins. Polar Biol 44, 1541–1551 (2021). https://doi.org/10.1007/s00300-021-02889-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-021-02889-2