Abstract
Recent Arctic warming has major influences on biological communities, especially in freshwater environments. There is substantial evidence that lake ecosystems in the Canadian Arctic and Fennoscandia are undergoing changes that have been linked to human-induced climate warming during the past 150–100 years. However, only few data linking recent climatic changes with the changes in biological communities are available from the Russian Arctic. We investigated a short sediment core (bottom of the core dates to 1830 CE) from Lake Bolshoy Kharbey, the biggest lake of the Bol`shezemelskaya Tundra, western Russian Arctic, using chironomid, cladocera, diatom and palynological analyses. Variations in biological proxy were linked to regional meteorological data and compared with the available sub-recent palaeoecological and hydrobiological studies from the region. The overall change in species composition was the smallest for terrestrial vegetation (0.485 SD) followed by cladoceran communities (0.966 SD). Chironomid taxonomic turnover was 1.331 SD, and the greatest rate of change was observed in diatom assemblages (1.701 SD). Changes in biological communities demonstrated a correlation with meteorologically recorded climatic parameters (air temperature and precipitation). The strongest taxonomic shifts in biological communities took place in 1880 and 1980. Both dates can be linked with prominent and recent climatic events: 1880 can be related to the end of the Little Ice Age in the region and 1980 is the beginning of the modern accelerating warming.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recent studies have shown that the Arctic has warmed at more than twice the rate of the rest of the planet during the past decades and the magnitude of ecological changes exerts major influences on biological dynamics in the Arctic (IPCC 2013). Greening trends of tundra vegetation derived from satellite images have identified the tundra as one of the clearest examples of the terrestrial impacts of climate change (IPCC 2014). Associated with greening vegetation, climate feedbacks at high latitudes alter global soil carbon storage, surface energy budgets and local hydrological regimes (Forkel et al. 2016; Myers-Smith et al. 2020). Some of the most rapid ecological changes associated with warming have occurred in freshwater environments (Arctic Biodiversity Trends 2010). Warmer air temperatures exert considerable control over numerous processes, including lake chemistry, ice and permafrost dynamics (Magnuson et al. 1997; Kumke et al. 2007; Fritz et al. 2016; Palagushkina et al. 2017a) and have important implications for biological communities (Smol et al. 2005; Heino et al. 2009; Hoff et al. 2015; Wetterich et al. 2018). There is substantial evidence that lake ecosystems in the Canadian Arctic and Fennoscandia are undergoing changes that have been linked to human-induced climate warming during the past 150–100 years (Overpeck et al. 1997; Wolfe 2003; Rühland and Smol 2005; DeSellas et al. 2008; Douglas and Smol 2010; Medeiros et al. 2012, 2014). However, only few data that link recent climate changes with the changes in biological communities are available from the Russian Arctic (Smol et al. 2005; Solovieva et al. 2005); the majority of palaeoecological studies in the Russian Arctic concentrate on longer time-periods (Melles et al. 2012; Biskaborn et al. 2012; Meyer et al. 2015; Solovieva et al. 2015; Subetto et al. 2017; Syrykh et al. 2017, 2021; Nazarova et al. 2020). Whether the recent changes in the biological communities can be solely explained by global warming is still to be understood (Engels et al. 2019).
The Bol`shezemelskaya Tundra (Fig. 1) is a swampy, hilly plain situated in the Russian Arctic west of the Ural Mountains, within the zone of continuous permafrost. This is the most eastern region of the European Arctic. Few data are available on recent changes in biological communities and vegetation from the area and most are published in Russian (Baranovskaya 1978; Martynenko and Getsen 1978; Sekretareva 2004; Teteryuk 2012a,b; Fefilova et al. 2012, 2014, etc.). The only sub-recent palaeoecological data available from the region are partially controversial (Solovieva et al. 2005). The investigation of lacustrine sediment records from two lakes of the region, Mitrofanovskoe and Vanuk-ty (Solovieva et al. 2005), has demonstrated that recent diatom and chironomid changes at both lakes have been driven, largely, by climate warming. At Mitrofanovskoe Lake the evidence is clearer: the major compositional changes in diatom and chironomid communities are synchronous, and they are supported by increases in total diatom accumulation rate and loss-of-ignition. At Vanuk-ty Lake, diatoms show a clearer response to temperature changes during the past decades, but chironomid fauna demonstrates only a weak reaction (Solovieva et al. 2005). Nonetheless, an investigation of the modern chironomid ecology in the Pechora river basin, which includes Bol`shezemelskaya Tundra and adjacent areas, have confirmed a high importance of air temperature as the main ecological driver of chironomid distribution in the region (Nazarova et al. 2017a). For a better understanding of the response of freshwater and terrestrial (vegetation) biological communities of the region to the recent global climatic changes, more investigations that apply more proxies and include further lakes should be conducted.
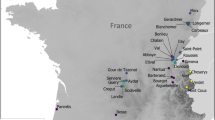
Location of the investigated Lake Bolshoy Kharbey. White star shows sampling location. Maps
Our study aimed to trace the effects of the recent climatic changes on biological communities of a big glacial lake and surrounding vegetation in this poorly studied region of Russian Arctic. We performed a multi-proxy investigation of a short sediment core from Lake Bolshoi Kharbei (BK), the biggest lake of Bol`shezemelskaya Tundra, using chironomid, Cladocera, diatom and pollen analyses. We compared our data with the available meteorological and modern hydrobiological records from the region.
Materials and methods
Regional settings
The Bol`shezemelskaya Tundra (67°31′15′' to 67°36′9′' N; 62°50′43′' to 62°55′20′' E) is a large lowland plain situated to the north-west of the Ural Mountains (Fig. 1). The area is underlain by Permian rocks and Quaternary deposits (Vlasova 1976). The relief is hilly, with maximum altitudes reaching 226 m a.s.l. The region is characterised by continuous permafrost that is up to 130 m thick, averaging 40–80 m; the active layer is 0.3–2.5 m thick (Getsen 2011). The climate is severe, with 8 months of winter when the mean monthly temperatures are below 0 °C. The coldest months are January and February with minimum temperatures of about − 55 °C. The warmest month is July with maximum temperatures (TJuly) reaching 31 °C, and mean TJuly 12.9 °C (Getsen 2011). Mean annual T (Tann) is − 5.5 °C and varies between − 2.8 (2007) and − 8.8 °C (1998). Mean annual precipitation (Precann) is 534 mm and varies between 349 mm (1997) and 762 mm (1962), with 60% of precipitation falling during the summer months, and maximum precipitation in August (Getsen 2011).
Lake Bolshoy Kharbey
Lake Bolshoy Kharbey (surface area 21.3 km2) is located in the eastern part of the Bol`shezemelskaya Tundra (67°33′22"N, 62°53′23"E). The lake has a glacial origin and is surrounded by a swampy watershed (Zvereva et al. 1970). The greatest depth (18.5 m) was recorded in the southern part of the lake, but most of the lake is 3–6 m deep. Due to relatively shallow depths, Lake B. Kharbey differs from the other large tundra lakes in the region by having less stable temperature stratification (Goldina 1972). In some years, a lack of summer stratification has been observed (Gladyshev et al. 2011). The water of the lake has low conductivity and mineralisation (17.7–76.9 mg l−1) and high oxygen saturation (98–100%), with pH varying in different years between 6.5 and 7.4 (Table 1). The Ntotal:Ptotal ratio in the lake varies between 2 and 240, averaging at 110, which characterises the lake as oligotrophic (Fefilova et al. 2012).
According to the geobotanical division of the European part of Russia (Vegetation 1980), the lake system is located in the European – West Siberian tundra province. It is within the circumpolar tundra belt and lies in the shrub tundra subzone, which is dominated by Betula nana, with some Empetrum nigrum and Vaccinium vitis-idaea.
The lake is far from any industrial sources. The nearest source of possible human-induced pollution is the industrial area of the city of Vorkuta, which is ca 80 km from the lake. The lake has no permanent settlements in the vicinity; therefore, it is classified as ‘undisturbed’ and oligotrophic according to comprehensive ecological monitoring surveys (Dauvalter and Khloptseva 2008; Stenina 2009; Gladyshev et al. 2011; Fefilova et al. 2012; Baturina et al. 2012, 2014). Although results of sediment analyses suggest that the lakes of Bol`shezemelskaya Tundra have been influenced by global lead pollution during the past ca 150 years (Dauvalter 2004; Solovieva et al. 2005, 2008), lead concentrations in the sediments of Lake B. Kharbey are low (mean 9 μg g−1) and do not exceed 20 μg g−1, which is within the threshold of the background lead concentration in soils (12.5–20 μg g−1) (Clarke and Washington 1924; Taylor 1964).
During our expedition at the end of July to early August of 2012, the water temperature in the lake was 9.6–13.0° C.
Sediment coring and radiometric dating
A short sediment core was collected in the southern part of Lake B. Kharbey from a depth of 6 m using a UWITEC sampler (Austria) during the summer expedition in 2012 (Nazarova et al. 2017a). The core was sectioned in the field at 1 cm intervals for further laboratory analyses. Selected sediment intervals were analysed for 210Pb at Geochronology Laboratory of St. Petersburg State University. The samples for 210Pb were selected upon the availability of the material in order to gather sufficient data for a highly reliable age–depth model. The age-depth model for the core is based on the results of 210Pb analysis and was made with the Bacon 2.2 package (Blaauw and Christen 2011) using R software (R Core Team 2013).
Climate data
The meteorological data used in this study were obtained from a Vorkuta meteorological station. The monthly temperature record is available since 1937 and the precipitation record is available since 1947 (Fig. 2; Getsen 2011). The instrumental record of Vorkuta meteorological station shows that during the last ca 80 years, there has been a steady growth of the mean annual and mean monthly temperatures of nearly all months. An especially strong increase occurred in 1980–1985 (Fig. 2). The coldest period was recorded for the 1960s and 1970s. Precipitation in the region shows a slight negative trend, with the driest period at the beginning of the record (end of the 1940s), the wettest period between 1955 and 1965, and a slightly decreasing precipitation rate towards the twenty-first century (Fig. 2) (Getsen 2011).
Data on a mean monthly and annual (Tann) air temperatures and b mean monthly and annual (Pann) precipitation from Vorkuta weather station (Getsen 2011). The trend lines are fitted by LOESS smoothing with span 0.5
Biological indicators
Chironomids
Treatment of sediment samples for chironomid analysis followed standard techniques described in Brooks et al. (2007). To capture the maximum diversity of the chironomid population, 74 to 138 chironomid larval head capsules were extracted from each sample. Several studies have demonstrated that this sample size is adequate for a reliable estimate of inferred temperature (Heiri and Lotter 2001; Quinlan and Smol 2001). The total number of chironomid head capsules was taken as 100%. Chironomids were identified to the highest taxonomic resolution possible with reference to Wiederholm (1983) and Brooks et al. (2007). Information on the ecology of chironomid taxa was taken from Brooks et al. (2007), Moller Pilot (2009, 2013) and Nazarova et al. (2011, 2015, 2017a, 2017b).
Cladocera
Sediment samples at 1-cm intervals were prepared for cladoceran analysis using the methods described in Szeroczyńska and Sarmaja-Korjonen (2007). The most abundant body part was chosen for each species to represent the number of individuals. A minimum of 100 individuals were encountered, which is satisfactory to characterise cladoceran assemblages (Kurek et al. 2010). The percentages for all cladoceran species were calculated from this sum of individuals. Taxa constituting at least 10% of the total number of Cladocera individuals per sample were regarded as dominant.
Diatoms
Diatom slide preparation followed standard methods using the water-bath technique (Battarbee 1986). Diatom slides were mounted using Naphrax, identified and counted 300 to 500 valves per sample under an Axioplan Zeiss light microscope equipped with an oil-immersion objective. Diatoms were identified at the lowest possible taxonomic level following mainly Krammer and Lange-Bertalot (1986–1991), in accordance with modern taxonomy from the Algaebase database (Guiry and Guiry 2015) and classification of diatoms used in Russia (Glezer et al. 1988) with the latest revisions (Genkal et al. 2013; Guiry and Guiry 2019). The total number of valves was taken as 100%. We defined taxa with abundances of ≥ 10% and ≥ 5% as dominant and subdominant, respectively (Palagushkina et al. 2012, 2017b). Biogeographical and ecological characteristics of the taxa with respect to preferences of habitat, pH and water salinity, as well as changes in the ice-cover duration and the spring/autumn turbulence period were described following Davydova (1985), Van Dam et al. (1994), Fallu et al. (2000), Barinova et al. (2006) and other sources from case studies.
Pollen
A total of 25 samples with an interval of 1 cm and each consisting of ca 1–4 g of dry sediment, were processed for pollen analysis using a standard procedure that included treatment with HCl and KOH, sieving (250 µm), treatment with HF, acetolysis and mounting in glycerine (Faegri and Iversen 1989). One Lycopodium spore tablet was added to each sample to calculate total pollen and spore concentrations (Stockmarr 1971). Identification of the pollen and spores was performed using a reference pollen collection and pollen atlases (Kuprianova and Alyoshina 1972; Reille 1992, 1995, 1998). In this study, we did not separate the pollen attributed to Betula into two sections (Betula sect. nanae / fruticosae and Betula sect. albae). The morphology of birch pollen is variable (Blackmore et al. 2003), which makes it difficult to separate shrubby and tree taxa by means of a pollen analysis with a high degree of confidence. Non-pollen palynomorphs (NPPs) were identified using descriptions, pictures and photographs published by van Geel et al. (2001). The microscopic analysis revealed moderately high pollen concentration and generally good preservation of pollen grains, allowing an easy counting of up to 300 terrestrial pollen grains per sample. Percentages of all taxa were calculated based on setting the total of all pollen and spore taxa equal to 100%.
Numerical analysis
The metheorological data and the results of chironomids, cladocerans, diatom and pollen analyses are displayed in diagrams produced with C2 ver. 1.7.7 (Juggins 2007). Zonation of the stratigraphies was done using cluster analysis in PAST (Hammer et al. 2001).
A principal component analysis (PCA) was used to assess the overall changes in species composition throughout the sediment core for chironomids, cladocerans, diatoms and pollen (ter Braak and Prentice 1988) based on square-root transformed data. Detrended canonical correspondence analysis (DCCA), the direct form of DCA, with species assemblage changes constrained to sediment age as the sole environmental variable, was used to develop quantitative estimates of compositional turnover as beta-diversity (BD), scaled in standard deviation (SD) units for each taxonomic group. This technique has been previously used in palaeolimnological studies in arctic regions to quantitatively assess the response of biological indicators to environmental stressors, including climate warming and permafrost thaw (Smol et al. 2005; Thienpont et al. 2013). Species diversity was estimated using Hill’s N2 index, which is commonly used as a measure of ‘effective’ diversity (Hill 1973).
To find potential statistically significant relationships between species composition and diversity (chironomids, cladocerans, diatoms and pollen) and the climatic variables, we performed an ordinary least square regression using PAST (Hammer et al. 2001). This method was previously used in the only palaeoecological study conducted in the region (Solovieva et al. 2005); application of the same method enables a better comparison of the results. Due to the relatively short time span, we assumed a linear response of the species composition along the major underlying gradient. The species composition data were summarised as PCA axes before being used as response variables. All ordinations were performed using CANOCO 4.0 for Windows (ter Braak and Šmilauer 2002).
Results
Age-depth model
Data on the content of 210Pb in the core are presented in Table 2. Concentrations of 210Pb in the core decreased exponentially with the depth, which was determined the by half-life of 210Pb (T1/2 = 22.2 years). The mean sedimentation rate calculated for the first 20 cm of the core was 1.34 ± 0.12 mm yr−1 and, accordingly, the age of the 19–20 cm layer was 149 ± 13 years. Deeper layers (22–23 and 24–25 cm) contain only traces of 210Pb, showing almost complete decay of this radionuclide over ~ 150 years, which is the age limit for the 210Pb method (Fig. 3). All dates in the manuscript are expressed as years CE.
Chironomids
We found 38 chironomid taxa in the core. Micropsectra insignilobus-type is present in all investigated horizons. This taxon is characteristic of oligotrophic waters of cold regions and, probably, is acidophobic. We found acidification-tolerant taxa (Heterotrissocladius grimshawi-type, Heterotrissocladius marcidus-type and Heterotrissocladius maeaeri-type), taxa that are indicative of moderate temperatures littoral-sublittoral (Microtendipes pedellus-type) and taxa that are usually rare in the Russian Arctic (Constempellina – Thienemanniola) (Nazarova et al. 2008, 2015), which is attributed to lentic and lotic ecosystems (Brooks et al. 2007). The chironomid records were subdivided into three statistically significant chironomid assemblage zones (Ch I–III) (Fig. 4).
Ch I (25–18 cm, 1830–1880). The dominant taxa are Microtendipes pedellus-type, the abundance of which gradually increases toward the end of the zone from 19 to 31%, Micropsectra insignilobus-type, Heterotrissocladius grimshawi-type and Heterotrissocladius maeaeri-types 1 and 2, which slightly decrease at the upper part of the zone (Fig. 4).
Ch II (18–4 cm, 1880–1980). After 1880, the abundance of Microtendipes pedellus-type dropped sharply while Micropsectra insignilobus-type becomes dominant, although its abundance gradually declines towards the upper layers of the core together with the abundances of several acid-tolerant Heterotrissocladius taxa (Heterotrissocladius macridus-type, Heterotrissocladius maeaeri-type, Heterotrissocladius grimshawi-type). Constempellina – Thienemanniola becomes quite frequent within Ch II.
Ch III (4–1 cm, 1980–2002). Abundances of thermophilic taxa with high nutrient demands increase Heterotrissocladius macridus-type, Tanytarsus mendax-type, Psectrocladius sordidellus-type and Chironomus plumosus-type. Abundances of phytophilic taxa (Cricotopus intersectus-type and Cricotopus cylindraceus-type) also increase.
Cladocera
A total of 20 cladoceran taxa were identified, of which, 13 taxa belonged to the family Chydoridae (Chydorids). Cladocera stratigraphy was split into three assemblage zones (CLZ I–III; Fig. 5).
CLZ I (25–18 cm, 1830–1880) is characterised by the strong dominance of planktonic Bosmina spp. (55–72%) and littoral Chydorus cf. sphaericus (19–32%). Cold-tolerant, phytophylous Acroperus harpae, littoral Alona affinis and benthic Alona quadrangularis occurred in this zone mostly at low abundances (mean 2–3%). Alona quadrangularis increases towards a maximum of 5% at ca 1845 and nearly disappears from the record afterwards. Bosmina longirostris has maximum abundances in the zone around the same time. Chydorus cf. sphaericus and Alona affinis increases distinctly towards the top of the zone.
In CLZ II (18–4 cm, 1880–1980), littoral Alona affinis increases to 21% and became dominant. Another littoral species, Alonopsis elongatus, also increases towards the middle of the zone and reaches 10% ca 1925. Similar to the underlying zone, Bosmina (Eubosmina) cf. longispina prevails over Bosmina longirostris among Bosmina species. Eurycercus sp. and small species of Alona are permanently present. Abundances of Bosmina longirostris increase up to 26% towards the top of the zone. This is a small planktonic species that is common in the littoral zone of lakes (Luoto et al. 2008). Planktonic species Bosmina (E) cf. longispina increases to its maximum abundance in the core (45%) in the upper part of the zone. Only in this zone, we found a moderately thermophilic species, Camptocercus rectirostris, which is rare for the Bol`shezemelskaya Tundra.
In CLZ III (4–0 cm, 1980–2010), abundances of Bosmina species vary and increase from 56% in the previous zone to 65% in this zone. In CLZ III, the abundances of Chydorus cf. sphaericus and Alona affinis first decrease, then sharply increase toward the top of the zone. Bosmina longirostris increases to its maximum abundance.
Diatoms
In total, 122 diatom taxa were identified in the sediment core. Diatom assemblages were composed of predominantly benthic (74 taxa), oligohalobic indifferent (74 species), cosmopolitan (73) and alkaliphilic (61) taxa. In relation to water temperature, few prevalent diatom species preferred moderate conditions. With respect to the water-flow factor, the prevalent species preferred stagnant-flowing waters. We identified three significant diatom zones (D I–III; Fig. 6).
In D I (25–18 cm, 1830–1880), the diatom flora are represented by cosmopolitan benthic and planktonic-benthic alkaliphilic species, which are indifferent to water salinity and prefer moderate temperature conditions and stagnant waters (Fig. 6). At the top of the zone, Achnanthidium minutissimum (92% of the total number of valves) dominates the diatom assemblages.
In D II (18–4 cm; 1880–1980), the share of planktonic and planktonic-benthic species increase. The planktonic subdominant Aulacoseira islandica reaches the highest abundance in 1917 when planktonic Aulacoseira subarctica and planktonic–benthic Ellerbeckia arenaria dominate the diatom assemblages. The share of halophobic taxa increases from 0.2 to 23.7%. Cold-stenothermic species Gyrosigma acuminatum is still present in this zone, alongside with other taxa that are characteristic of cool conditions (Eunotia praerupta, Aulacoseira islandica and Pinnularia brevicostata).
In D III (4–0 cm, 1980–2010), there is a consecutive increase in the proportion of planktonic and planktonic–benthic species and an increase of the standing-flowing water taxa, such as Aulacoseira subarctica, Tabellaria fenestrata, Pseudostaurosira brevistriata.
Pollen
The pollen diagram (Fig. 7) is subdivided into four pollen zones (PZ I–IV).
Stratigraphic diagram showing distribution of the main pollen taxa in sediment core from the Lake B. Kharbey, principal component analysis axis 1 scores (PCA 1) and N2 diversity. “C” after taxon name means concentration (grain g−1). Pollen % data are presented in black; pollen concentration data are presented on grey. AP – arboreal pollen; NAP – non-arboreal pollen
PZ I (25–18 cm, 1830–1880) is characterised by the dominance of Pinus (up to 40%) and Picea (up to 35%). Among other conifers, Larix pollen are constantly present at very low abundances (0.4–1.4%). Betula is also abundant in this zone (up to 21%). Cyperaceae dominates the herbaceous taxa (up to 16%).
In PZ II (18–12 cm, 1880–1920), the composition of the dominant taxa is the same as in the PZ I; however, the abundances of Betula decrease and Cyperaceae slightly increases among the herbaceous plants. Amarantaceae (Chenopodiaceae) has high abundances ca 1880–1890.
PZ III (12–4 cm, 1920–1980) is characterised by a significant increase of Pinus (up to 60%) and a decrease of Picea. Larix almost disappeared (0 to 0.3%). Betula and Cyperaceae also continue decreasing in this zone. In the upper part of the zone (from ca 1950), the abundance of planktonic infusorium Staurophrya elegans significantly increases, reflecting a rise of the water level in the lake.
PZ IV (4–0 cm, 1980–2010) is distinguished by a sharp increase of Betula (up to 25%) and decrease of Pinus and Picea percentages. The concentration of pollen sharp increases especially for Pinus, Picea, Betula, Alnaster fruticosa, Cyperaceae and Poaceae. Spores of Sphagnum and Polypodiophyta and remnants of Staurophrya elegans also increase (both percentages and concentrations). The pollen analysis revealed that, despite decreasing percentages of Pinus and Abies, the concentration of these taxa increase significant (Fig. 7, in grey).
Changes in biological communities
N2 diversity of chironomid communities increased from the bottom of the core (N2 = 11–12) towards the surface of the sediment (N2 = 17–20). However, between 1990 and 2005, it declined to 11–14. Cladoceran N2 diversity increased from its minimum values in the lowest part of the core (mean N2 = 5.9 between 1822 and 1844) towards the surface of the core with the highest values at ca 1980 (N2 = 7.6) and decreased thereafter to 6. Diversity of diatom communities had a clear trend to increase from N2 = 9–10 at the lower horizons of the core to N2 = 18–22 around 1950 and a decreasing trend thereafter. N2 diversity of pollen assemblages was 9.7 (median) before 1880. Between 1880 and 1980, it decreased to 7.0 (median) and increased to 10 (median) after 1980.
The overall changes in species composition during the ca 180 years was the smallest for terrestrial vegetation (pollen 0.485 SD). All investigated components of hydrobiological communities (chironomids, cladocerans, diatoms) demonstrated a high rate of species turnover. The smallest changes took place in cladoceran communities (0.966 SD). Chironomid taxonomic turnover was 1.331 SD, and the greatest rate of change was observed in diatom assemblages (1.701 SD).
Regression analysis shows that mean July air temperatures from instrumental records (1937–2009) had a significant relationship (p < 0.01) with variations in chironomid communities (R2 = 0.62, span = 0.2 and 0.3, chironomid PCA axis 1; Table 3). For Cladocera, the strongest relationship was found for TJuly (R2 = 0.39, span = 0.5 and 0.3, PCA axis 1) and precipitation (R2 = 0.36, span = 0.5, PCA axis 1). Diatoms demonstrated the strongest correlation with TJanuary (R2 = 0.53, span = 0.2), annual precipitation (R2 = 0.41, span = 0.3, PCA axis 1) and no significant correlation with summer temperature. Pollen data demonstrated a significant relationship with TJuly (R2 = 0.51, span = 0.3) and a positive correlation with Tann (R2 = 0.46, span = 0.2).
Discussion
Investigation of the short sediment core from Lake B. Kharbey, performed by a complex of palaeobiological methods, including chironomids, cladocerans, diatom and pollen analyses, showed that notable changes took place in both in the in-lake biological communities and in the surrounding vegetation. The sediment core covered a period from the nineteenth century to the present day. This time interval is known as a time of considerable ecological changes, which includes climate change due to the end of the Little Ice Age (LIA) to modern climate warming (Miller et al. 2012) and industrialisation in many areas, including acidification of many lakes throughout the Northern Hemisphere (Battarbee 1994).
However, the overall level of pollution in the region of this investigation is very low (Solovieva et al. 2008; Fefilova 2014), and during modern monitoring studies no evidence of any acidification or eutrophication of other lakes in the Bol`shezemelskaya Tundra (Solovieva et al. 2002, 2005, 2008) and specifically in Lake B. Kharbey have been found (Baturina et al. 2012; Fefilova et al. 2012). Therefore, it is unlikely that global and regional atmospheric contamination had any effect on the lake ecosystem and its catchment, and we can suppose that the changes in the ecosystem of the lake and the catchment must be caused by predominantly climate-related reasons. The late nineteenth and twentieth centuries are characterized by generally increasing temperatures, especially in recent decades (MacDonald et al. 2008). The shift in hydrobiological communities at ca 1880 can be related to the beginning of the gradual climatic warming in the region associated with the end of the LIA. In Bol`shezemelskaya Tundra there is an overall rise in chironomid-inferred mean July air temperatures starting from the mid-nineteenth century (Solovieva et al. 2005) with the especially prominent increase in temperature in 1980–1985 (Fig. 2; Getsen 2011) when the second strong shift was observed in the biological records. The influence of the climate change on biological communities in our investigation is further supported by strong correlations between the air temperature and the taxonomic shifts in chironomids, cladocerans, diatom and pollen (vegetation) data (Table 3).
The chironomid communities of the lake demonstrate that substantial changes occurred over the past 180 years, which is reflected in the high BD (1.33 SD). Our results complement earlier studies in the region. Chironomid communities of the glacial lakes Mitrofanovskoe and Vanuk-ty from the Bol`shezemelskaya Tundra (Solovieva et al. 2005; Smol et al. 2005) demonstrated slightly lower species turnover in both lakes from 1850 to 2000: 1.08 and 1.15 SD, respectively. A higher estimate of the total species turnover (BD = 1.47 SD) for the same period is known only for Col Pond (Ellesmere Island, high Canadian Arctic) (Smol et al. 2005).
Up until 1880, the chironomid communities were dominated by taxa indicative of cold to moderate temperatures and mainly littoral-sublittoral taxa; the highest representation was from Microtendipes pedellus-type. This taxon prefers waters with higher oxygen content, low phosphorus and is frequently, but not exclusively, found in flowing waters with sandy and stony sediments where it feeds on algae or fine particulate organic material (Moller Pillot 2009; Płóciennik et al. 2015). Between 1880 and 1930, abundances of Microtendipes pedellus-type varied significantly, and after 1930, it declined up to the end of the record, where it remains present only at lower abundances, being replaced by taxa that are more characteristic for profundal conditions.
The general trend in compositional changes of chironomid communities have a significant relationship (p < 0.01) with the mean July air temperatures from instrumental records (1937–2009). The strongest taxonomic change occurred in 1980, when the cold-stenotherm taxa (Heterotrissocladius maeaeri-type, Heterotrissocladius grimshawi-type and Micropsectra insignilobus-type) decline. At the same time, around 1980, Microtendipes pedellus-type was replaced by Heterotrissocladius macridus-type, the most thermophilic species of Heterotrissocladius (Brooks et al. 2007). Larvae of Heterotrissocladius macridus-type feed on detritus and prefer organic muddy bottoms and substrate with vegetation; they are seldom found in sands. Although Heterotrissocladius macridus-type is reported to be oligotrophic, it has been often collected in mesotrophic lakes, is common in humic waters and tolerates lower oxygen content (Moller Pillot 2013). Heterotrissocladius macridus-type is acidophilic, probably because as detritus feeder, it is dependent on particular decomposition products, which are affected by pH (Moller Pillot 2013, and references therein). After 1980, several other temperate-thermophilic, phytophilic or tolerant-to-eutrophication taxa appeared or increased in the lake (Chironomus plumosus-type, Psectrocladius sordidellus-type, Stempelinella-Zavrelia, Cricotopus taxa and Tanytarsus mendax-type).
These clear taxonomic changes strongly indicate a response of chironomid communities to climate warming. The prevalence of the profundal fauna reflects lake infilling. A similar transition has been observed at ca 1985 in chironomid and diatom assemblages of small lakes near the Alaskan tundra–taiga boundary (Medeiros et al. 2014) and is seen in many Arctic and subarctic lakes (Medeiros et al. 2014; Hamerlik, et al. 2017; Engels et al. 2020).
The diversity of chironomids had an increasing trend, with a short-term decline between 1990 and 2000. Similar dynamics were reconstructed from the chironomid record of the two earlier investigated lakes from the region: Mitrofanovskoe and Vanuk-ty (Solovieva et al. 2005). In both lakes, N2 diversity of chironomid communities remained relatively stable until the 1980s–1990s, then considerably declined and increased again after ca 2000. In Lake Mitrofanovskoe (Solovieva et al. 2005) the most apparent taxonomic changes were observed after the 1980s when several taxa that are characteristic for warm productive lakes appeared or increased in chironomid fauna. However, in Lake Vanuk-ty, changes in chironomid communities were not so well pronounced (Solovieva et al. 2005). Here a simultaneous increase in thermophilic (Paratanytarsus penicillatus-type, Psectrocladius sordidellus-type), temperate (Microtendipes pedellus-type) and taxa preferring cool conditions (Tanytarsus lugens-type and Psectrocladius septentrionalis-type) were observed at the end of the twentieth century. However, the Vanuk-ty Lake has been used for small-scale commercial fishing since the late 1940s (Solovkina and Sidorov 1966). During the 1960s the fish catches were more than two times higher than in the 1980s, decreasing further by nearly ten times by 2002 (Solovieva et al. 2005). Decreasing fish catches and increasing fishing pressure since the 1960s suggests that fish populations in Vanuk-ty Lake may have declined substantially in recent decades. Therefore, it was supposed that chironomid communities of Lake Vanuk-ty were responding mainly to strong changes in fish populations, connected to industrial fishing and not to climatic factors (Solovieva et al. 2005).
Thus, in chironomid communities of the lakes in the Bol`shezemelskaya Tundra that do not experience any anthropogenic load (Mitrofanovskoe and B. Kharbey), recent climatic changes caused similar taxonomic shifts and a similar pattern of diversity variations.
Cladoceran communities demonstrated lower species turnover (0.966 SD) and a positive correlation with the TJuly and precipitation. The moderate compositional turnover of cladoceran communities in the lake was determined by the prevalence of species acclimated to variable ecological conditions. In general, over the entire investigated time interval, the cladoceran communities were dominated by the taxa that are characteristic for large water bodies, mainly from the Bosminidae family. Typical inhabitants of open pelagic biotopes dominated: Bosmina (Eubosmina) cf. longispina, Bosmina (B) longirostris, Bosmina sp., Chydorus cf. sphaericus, and Alona affinis.
Before 1880, cladoceran assemblages were typical for cold high latitudes (Bosmina (Eubosmina) cf. longispina, Acroperus harpae, Alona affinis and Chydorus cf. sphaericus) (Hofmann 2000; Nevalainen and Luoto 2010). After 1880, the abundances of moderately thermophilic species such as Bosmina longirostris and Eurycercus lamellatus (Kamenik et al. 2007; Nevalainen and Luoto 2010; www.artsdatabanken.no) increased. Bosmina longirostris and Chydorus cf. sphaericus (Smirnov 2010) are also attributed to the higher trophic state of the lake. All these species are widespread in the Arctic (e.g. Rautio 2001; Kotov et al. 2010; Frolova et al. 2013, 2014, 2017), occur at high abundance in large lakes of the Eastern Europe (Fefilova et al. 2014) and are dominant in the modern zooplankton of Lake B. Kharbey (Kononova et al. 2014).
No detailed palaeoecological studies of sub-recent changes in cladoceran communities have been conducted on the lakes of the region until now. An earlier investigation of Holocene sediments from two lakes from the Bol`shezemelskaya Tundra (Vankavad and Mezhgornoe) (Sarmaja-Korjonen et al. 2003; Kultti et al. 2003) had only low resolution of the upper part of the cores and could not be used as reference material for our current study. However, at the end of the twentieth century, several comprehensive studies of zooplankton were carried out on Lake B. Kharbey (Baranovskaya 1978; Fefilova et al. 2012, 2014; Kononova et al. 2014; etc.). Our results complement these modern hydrobiological investigations, which also revealed an increase in abundances of the same taxa (Bosmina longirostris, Daphnia) from the end of the twentieth century to the present. Higher percentages of Daphnia, Bosmina longirostris and Chydorus cf. sphaericus in zooplankton suggest chemical or thermal eutrophication, while an increase of Bosmina cf. longispina is indicative of the oligotrophication (O’Brien et al. 2005; Tsugeki et al. 2003; Smirnov 2010; Guilizzoni et al. 2012). The dynamics of Bosmina longirostris was uneven, with the last and highest peak in abundance observed in the late 1990s and some decrease in the upper layers of the core, which corresponded to the 2000s (Fig. 5). This decrease in abundance of Bosmina longirostris was recorded in an analysis of modern zooplankton samples in the 2000s (Fefilova et al. 2012, 2014).
The present study showed that changes in the quantitative parameters of Cladocera populations in the lake were at least partly determined by temperature and precipitation. Temperature has been identified as an important factor in the structuring of cladoceran assemblages in Finnish Lapland (Korhola 1999; Sarmaja-Korjonen et al. 2006), Norway (Hessen et al. 2006) and the Yukon and Northwest Territories, Canada (Swadling et al. 2000; Sweetman et al. 2010). This is in agreement with data from East Siberia (Frolova et al. 2013, 2014), which found that TJuly was significantly correlated with the distribution of subfossil Cladocera in the lakes of north-western Yakutia; 17.4% of the variance in the taxa data was explained by this TJuly. Precipitation induces water drainage from the catchment area, supplying a lake with additional allochthonous mineral and organic substances. It has been reported that an increase in atmospheric precipitation at the beginning of the growing season caused changes in the littoral zooplankton, similar to that what is usually observed when the amounts of organic matter and nutrients increase (Krylov et al. 2014). In the tundra, the precipitation-induced surface run-off from the catchment is facilitated by the thawing of permafrost (Adrian et al. 2009).
The largest rate of change was observed in diatom communities (1.701 SD) and, similarly to chironomids, was higher than in two earlier studied lakes from the Bol`shezemelskaya Tundra: Lake Mitrofanovskoe (1.23 SD) and Lake Vanuk-ty (1.49 SD) (Solovieva et al. 2005; Smol et al. 2005).
During the past ca 180 years, the main trend in diatom turnover is an increase in the proportion of planktonic species, which starts after ca 1880 (Aulacoseira subarctica, Tabellaria fenestrata, Cocconeis placentula, etc.), and further increases after 1980. A strong dominance of Achnanthidium minutissimum at ca 1870 reflects an increase of water flow and flooding of shore zones that can be associated with the end of LIA in this region at that time, as was hypothesised in an earlier study (Solovieva et al. 2005). Changes in the species composition and complex of dominants reflect the ongoing processes of lake-level rise associated with the influx of meltwater or permafrost thawing and related active shoreline thermokarst processes and increasing supply of dissolved inorganic carbon and nitrogen to the lake (Bouchard et al. 2017). This has been confirmed by field observations that demonstrated a considerable increase in the depth of the active layer in the region after 1996 (Mazhitova and Kaverin 2007).
For diatoms, the strongest correlation was found with TJanuary and with annual precipitation. Although winter temperature does not influence diatoms directly, the air temperature was found to be a driver of temporal variability of ice cover (Marszelewski and Skowron 2006). Even a slight decrease in winter air temperature can cause a significant shift of a lake’s freeze-up (Palecki and Barry 1986; Gronskaya 2000; Vuglinsky et al. 2002; Menard et al. 2002; Hampton et al. 2017). Duration of ice cover and loss affects the available light, mixing depth and input of nutrients from rainfall and runoff (Reynolds 1980, 1984, 2003). All of these factors affect the abundance and dynamics of algae, thus linking winter temperatures with variations in diatom assemblages. Our findings are also supported by an earlier observation, where the abundance of Aulacoseira subarctica, which increasingly dominate diatom assemblages in Lake B. Kharbey, was positively related to short ice cover, early ice-out and a long-lasting spring circulation (Horn et al. 2011). Aulacoseira subarctica prefer mild, short winters and can develop a high initial biomass under low-light conditions while the abundances of other diatoms decreased over winter. However, further warming leading to an early summer stratification can cause a decline of Aulacoseira subarctica (Horn et al. 2011).
The dominance of Tabellaria fenestrata, which was especially abundant in Lake B. Kharbey after 1980, may be associated with a further increase in the water level in the lake (Trifonova and Afanasyeva 2008). A sufficient increase in the proportion of planktonic diatoms since 1970 has been found in other lakes of the Bol`shezemelskaya Tundra (Solovieva et al. 2005, 2008). This trend reflects climate warming and the associated increase of the open water period, strengthening the development of planktonic centric diatoms from Aulacoseira that require a sufficient mixing of the water column for their development (Ruhland and Smol 2005).
Our study reveals that the pollen spectra of the investigated core showed low species turnover over the study period (0.485 SD). Little variations took place between 1830 and 1980 and the strongest change started after 1980. The concentration of pollen increased sharply, especially for Pinus, Picea, Betula, Alnaster fruticosa, Cyperaceae, Poaceae, ferns and Sphagnum. The abundance of planktonic infusorium Staurophrya elegans increased significantly as well. According to Rebristaya (1977), lakes in the Bol`shezemelskaya Tundra are surrounded by sedge bogs, which explains the high percentages of Cyperaceae in every pollen zone. Increasing abundances of planktonic Staurophrya elegans, ferns and mosses that are indicative for wet conditions suggest an increase of the soil moisture content or further spreading of marshy environments, which could be related to climate-induced thawing permafrost in the region.
Variations in pollen data in our study demonstrate a significant relationship with air temperature (both TJuly and Tann). Although no study on the succession of vegetation in the Bol`shezemelskaya Tundra or Northern Ural during the past two centuries has been done before, several low-resolution Holocene sediment sequences from different parts of the region demonstrated that cooling during the LIA influenced the vegetation in the north of the region and led to a dominance of the tundra vegetation. In the south of the region, north-taiga forests dominate during the entire period (Klimanov and Sirin 1997; Volkova et al. 1989; Elina et al. 2000, 2005; Golubeva 2008).
In our study, the presence of Pinus, Picea and partially Betula pollen in sediments is a result of long-distance transportation. Pollen of Pinus can be transported for 3000 km (Campbell et al. 1999); Picea and Betula can be transported for 300–400 km and 250–300 km, respectively (Sladkov 1967). Picea and Betula form the tree line in north-east Europe (MacDonald et al. 2008) and occurred 30 km south of Vorkuta (Rebristaya 1977). Increases of Picea and Betula pollen concentrations may reflect a shift of the tree line northwards after 1980. A detailed survey of recent dispersion of Pinus sibirica, Picea obovata, Larix sibirica and Larix cajanderi at nine sites in north-eastern European Russia and Central Siberia revealed that their population growth started during the 1970s and was linked to rise of annual and summer temperatures (Esper and Schweingruber 2004). Increase of pollen concentrations of Betula, Alnaster fruticosa and Poaceae after 1980 indicates the development of erect dwarf-shrubs and low shrub tundra (the southern type of Arctic tundra, according to Walker 2000).
Conclusions
This is the first multi-proxy investigation in the region of sub-recent (ca 180 years) variations in chironomids, cladocerans, diatoms and pollen in sediments of Lake B. Kharbey. We revealed that the main changes in the biological communities took place synchronously around ca 1880 and 1980. Both dates can be linked with prominent and recent climatic events: 1880 can be related to the end of the LIA in the region and 1980 is the beginning of the modern accelerating warming. This, together with the results of the comparative analysis involving modern meteorological records and the results of the scarcely available regional palaeoecological and hydrobiological studies, provides support that recent taxonomic changes in chironomid, cladoceran, diatom and vegetation assemblages are largely driven by changing climate. For all investigated biological proxies, we observed general growth of diversity from the beginning of the record towards modern time; however, from 1980 to 2010, the diversity of chironomids, cladocerans and diatoms demonstrate a slight decline. This can be associated with modern eutrophication of Lake B. Kharbey caused by warming, increasing run-off from the adjacent area and release of nutrients from the melting permafrost. The first-ever detailed pollen record of the past ca two centuries has shown a decline in pollen diversity between 1880 and 1980. The increase in the pollen concentration, especially for Picea and Betula, and increase of the pollen diversity after 1980 can be caused by the northwards shift of the treeline. Changes of the diversity appear together with strong taxonomic shifts in all biological proxies. The observed taxonomic trends in chironomid, cladoceran and diatom communities since the beginning of the nineteenth century constitutes a shift from predominantly cold to moderate stenotherm, oligotrophic- to mesotrophic taxa and an increase in the contribution of littoral and thermophilic taxa tolerant to eutrophication and a wide range of pH. The smallest rate of change was observed in vegetation and the highest rate of change occurred in the diatom assemblages.
References
Adrian R, O’Reilly CM, Zagarese H, Baines SB, Hessen DO, Keller W, Livingstone DM, Sommaruga R, Straile D, van Donk E, Weyhenmeyer GA, Winder M (2009) Lakes as sentinels of climate change. Limnol Oceanogr 54:2283–2297
Arctic Biodiversity Trends 2010 – Selected indicators of change. CAFF International Secretariat. Iceland, Akureyri
Baranovskaya VK (1978) Crustaceans (Crustacea). In: Getsen MV (ed) Flora and fauna of reservoirs of the European North on the example of the lakes of the Bolshezemelskaya tundra. Nauka, Leningrad, pp 174–177 (in Russian)
Barinova SS, Medvedeva LA, Anisimova OV (2006) Biological Diversity of Algae—Environmental Indicators. Pilies Studio, Tel Aviv (in Russian)
IPCC. Climate Change 2013: The Physical Science Basis (eds Stocker TE et al.) (Cambridge Univ. Press, 2013). Cambridge, United Kingdom and New York, NY
Battarbee RW (1986) Diatom analysis. In: Berglund BE (ed) Handbook of Holocene Palaeoecology and Palaeohydrology. J Wiley and Sons, NY, pp 527–570
Battarbee RW (1994) Diatoms, lake acidification and the Surface Water Acidification Programme (SWAP): a review. Hydrobiologia 274:1–7. https://doi.org/10.1007/978-94-017-2095-3_1
Baturina MA, Loskutova OA, Fefilova EB, Khokhlova LG (2012) Zoobenthos of the lake Bolshoi Kharbei (Bolshezemelskaya tundra): modern state and analysis of retrospective data. Bulletin of the Komi Science Center, Ural Branch of the Russian Academy of Sciences 4(12):21–29 (in Russian)
Baturina MA, Loskutova OA, Shchanov VM (2014) Structure and distribution of Zoobenthos of the Kharbey lake system. Journal of Siberian Federal University Biology 4(7):332–356 (in Russian)
Biskaborn BK, Herzschuh U, Bolshiyanov D, Savelieva L, Diekmann B (2012) Environmental variability in northeastern Siberia during the last similar to 13,300 yr inferred from lake diatoms and sediment-geochemical parameters. Palaeogeogr Palaeocl 329:22–36. https://doi.org/10.1016/j.palaeo.2012.02.003
Blaauw M, Christen JA (2011) Flexible paleoclimate age-depth models using an autoregressive gamma process. Bayesian Anal 6(3):457–474. https://doi.org/10.1214/ba/1339616472
Blackmore S, Steinmann JAJ, Hoen PP, Punt W (2003) The northwest european pollen Flora 65: Betulaceae and Corylaceae. Rev Palaeobot Palynol 123:71–98
Bouchard F, MacDonald LA, Turner KW, Thienpont JR, Medeiros AS, Biskaborn BK, Korosi J, Hall RI, Pienitz R, Wolfe BB (2017) Paleolimnology of thermokarst lakes: a window into permafrost landscape evolution. Arctic Science 3:91–117. https://doi.org/10.1139/as-2016-0022
Brooks SJ, Langdon PG, Heiri O (2007) Using and identifying chironomid larvae in palaeoecology. QRA Technical Guide № 10, Quaternary Research Association, London
Campbell ID, McDonald K, Flannigan M, Kringayark J (1999) Longdistance transport of pollen into the Arctic. Nature 399:29–30. https://doi.org/10.1038/19891
Clarke FW, Washington HS (1924) The Composition of the Earth’s Crust. U.S. Dep. Interior Geol Surv. https://doi.org/10.3133/pp127
Dauvalter VA (2004) Effect of atmospheric emission of the Vorkuta Industrial Region on the chemical composition of lake sediments. Water Res 31:668–672
Dauvalter VA, Khloptseva EV (2008) On hydrological and hydrochemical features of lakes of Bolshezemelskaya tundra. Nat Engineering Sci 1(3):407–414 (in Russian)
Davydova NN (1985) Diatoms as indicators of natural conditions of water bodies in Holocene. Nauka, Leningrad (in Russian)
DeSellas AM, Paterson AM, Sweetman JN, Smol JP (2008) Cladocera assemblages from the surface sediments of south-central Ontario (Canada) lakes and their relationships to measured environmental variables. Hydrobiologia 600:105–119. https://doi.org/10.1007/s10750-007-9180-4
Douglas MSV, Smol JP (2010) Freshwater Diatoms as Indicators of Environmental Change in the High Arctic. In: Smol JP, Stoermer EF (eds) The Diatoms: Application for the Environmental and Earth Sciences. Cambridge University Press, Cambridge, pp 249–266
Elina GA, Lukashov AD, Yurkovskaya TK (2000) Late Glacial and Holocene of East Fennoscandia (paleo-vegetation and paleogeography). KarNC RAN, Petrozavodsk (in Russian)
Elina GA, Lukashov AD, Tokarev PN (2005) Kartography of vegetation and landscapes on temporal sequences of Holocene in taiga zone of East Fennoscandia. Nauka, St. Petersburg (in Russian)
Engels S, Medeiros AS, Axford Y, Brooks SJ, Heiri O, Nazarova L, Luoto TP, Porinchu DP, Quinlan R, Self AE (2019) Climate change as a driver of biodiversity: subfossil chironomids as an indicator of long-term trends in insect diversity. Global Change Biol. https://doi.org/10.1111/GCB.14862
Esper J, Schweingruber FH (2004) Large-scale treeline changes recorded in Siberia. Geophys Res Lett 31:L06202. https://doi.org/10.1029/2003GL019178
Faegri K, Iversen J (1989) Textbook of pollen analysis. John Wiley and Sons, Chichester. https://doi.org/10.1002/jqs.3390050310
Fallu MA, Allaire N, Pienitz R (2000) Freshwater diatoms from northern Québec and Labrador (Canada). Bibliotheca Diatomologica. Gebr, Borntraeger, Berlin
Fefilova EB, Kononova ON, Dubovskaya OP, Khokhlova LG (2012) The current state of Zooplankton in the lake system of Bol’shezemel’skaya Tundra. Inland Water Biol 5:333–341. https://doi.org/10.1134/S1995082912040074
Fefilova EB, Baturina MA, Kononova ON, Loskutova OA, Khokhlova LG, Dubovskaya OP (2014) Long-term changes of aquatic communities in the Kharbeyskie lakes. Biology 3(7):240–266
Forkel M, Carvalhais N, Rödenbeck C, Keeling R, Heimann M, Thonicke K, Zaehle S, Reichstein M (2016) Enhanced seasonal CO2 exchange caused by amplified plant productivity in northern ecosystems. Science 351:696–699. https://doi.org/10.1126/science.aac4971
Fritz M, Wolter J, Rudaya N, Palagushkina O, Nazarova L, Obu J, Rethemeyer J, Lantuit H, Wetterich S (2016) Holocene ice-wedge polygon development in the northern Yukon permafrost peatlands Canada. Quat Sci Rev 147:279–297. https://doi.org/10.1016/j.quascirev.2016.02.008
Frolova LA, Nazarova LB, Pestryakova LA, Herzschuh U (2013) Analysis of the effects of climate-dependent factors on the formation of zooplankton communities that inhabit Arctic lakes in the Anabar River basin. Contemp Probl Ecol 6(1):1–11. https://doi.org/10.1134/S199542551301006X
Frolova L, Nazarova L, Pestryakova L, Herzschuh U (2014) Subfossil cladoceran remains from sediment in thermokarst lakes in northeastern Siberia, Russia. J Paleolimnol 52:107–119. https://doi.org/10.1007/s10933-014-9781-7
Frolova LA, Ibragimova AG, Ulrich M, Wetterich S (2017) Reconstruction of the history of a thermokarst lake in the Mid-Holocene based on an analysis of subfossil Cladocera (Siberia, Central Yakutia). Contemp Probl Ecol 10(4):423–430. https://doi.org/10.1134/S1995425517040023
Genkal SI, Kulikovsky MS, Mikheeva TM, Kuznetsova IV, Lukyanova EV (2013) Diatoms of plankton of Svisloch River and its reservoirs. Scientific World, Moscow (in Russian)
Getsen MV (2011) Vorkuta – city on coal, city in the Arctic. Syktyvkar (in Russian)
Gladyshev MI, Semenchenko VP, Dubovskaya OP, Fefilova EB, Makhutova ON, Buseva ZhF, Sushchik NN, Razlutskij VI, Lepskaya EV, Baturina MA, Kalachova GS, Kononova ON (2011) Effect of temperature on contents of essential highly unsaturated fatty acids in freshwater zooplankton. Limnologica 41:339–347. https://doi.org/10.1016/j.limno.2011.03.001
Glezer ZI, Karaeva NI, Makarova IV et al. (1988) Classification of diatoms. In: Diatoms of the USSR (fossil and modern). Leningrad 2(1):31–35 (in Russian)
Goldina LP (1972) Geography of the lakes of the Bolshezemelskaya tundra. Nauka, Leningrad (in Russian)
Golubeva YuV (2008) Holocene climate and vegetation of Komi Republic. Litosphera 2:124–132 ((in Russian))
Gronskaya TP (2000) Ice thickness in relation to climate forcing in Russia. Verh Int Verein Limnol 27:2800–2802. https://doi.org/10.1080/03680770.1998.11898176
Guilizzoni P, Levine SN, Manga M, Marchetto A, Lami A, Ambrosetti W, Brauer A, Gerli S, Carrara EA, Rolla A, Guilizzoni L, Vignati DAL (2012) Ecological effects of multiple stressors on a deep lake (Lago Maggiore, Italy) integrating neo and palaeolimnological approaches. J Limnol 71:1–22. https://doi.org/10.4081/jlimnol.2012.e1
Guiry MD, Guiry GM (2015) AlgaeBase. World-wide electronic publication, National University of Ireland, Galway http://www.algaebase.org.
Guiry MD, Guiry GM (2019) AlgaeBase. World-wide electronic publication, National University of Ireland, Galway https://www.algaebase.org.
Hamerlik L, Svitok M, Novikmec M, Veslská M, Bitušik P (2017) Weak altitudinal pattern of overall chironomid richness is a result of contrasting trends of subfamilies in high-altitude ponds. Hydrobiologia 793:67–81
Hammer Ø, Harper DAT, Raan PD (2001) PAST: Palaeontological statistics software package for education and data analysis. Palaeontol Electronica 41:1–9
Hampton SE, Galloway AWE, Powers SM, Ozersky T, Woo KH, Batt RD, Labou SG, O’Reilly CM, Sharma S, Lottig NR, Stanley EH, North RL, Stockwell JD, Adrian R, Weyhenmeyer GA, Arvola L, Baulch HM, Bertani I, JrLL B, Carey CC, Catalan J, Colom-Montero W, Domine LM, Felip M, Granados I, Gries C, Grossart H-P, Haberman J, Haldna M, Hayden B, Higgins SN, Jolley JC, Kahilainen KK, Kaup E, Kehoe MJ, MacIntyre S, Mackay AW, Mariash HL, McKay RM, Nixdorf B, Nõges P, Nõges T, Palmer M, Pierson DC, Post DM, Pruett MJ, Rautio M, Read JS, Roberts SL, Rücker J, Sadro S, Silow EA, Smith DE, Sterner RW, Swann GEA, Timofeyev MA, Toro M, Twiss MR, Vogt RJ, Watson SB, Whiteford EJ, Xenopoulos MA (2017) Ecology under lake ice. Ecol Lett 20:98–111
Heino J, Virkkala R, Toivonen H (2009) Climate change and freshwater biodiversity: detected patterns, future trends and adaptations in northern regions. Biol Rev 84:39–54
Heiri O, Lotter AF (2001) Effect of low count sums on quantitative environmental reconstructions: an example using subfossil chironomids. J Paleolimnol 26:343–350. https://doi.org/10.1023/A:1017568913302
Hessen DO, Faafeng BA, Smith VA, Bakkestuen V, Walseng B (2006) Extrinsic and intrinsic controls of zooplankton diversity in lakes. Ecology 87:433–443. https://doi.org/10.1890/05-0352
Hill MO (1973) Diversity and evenness: A unifying notation and its consequences. Ecology 54:427–432. https://doi.org/10.2307/1934352
Hoff U, Biskaborn BK, Dirksen V, Dirksen O, Kuhn G, Meyer H, Roth NL, A, Diekmann B, (2015) Holocene environment of Central Kamchatka, Russia: Implications from a multi-proxy record of Two-Yurts Lake. Global Planet Change 134:101–117. https://doi.org/10.1016/j.gloplacha.2015.07.011
Hofmann W (2000) Response of the chydorid faunas to rapid climatic change in four alpine lakes at different altitudes. Palaegeogr Palaeoclim Palaeoecol 159:281–292. https://doi.org/10.1016/S0031-0182(00)00090-0
Horn MG, Beucher CP, Robinson RS, Brzezinski MA (2011) Southern Ocean nitrogen and silicon dynamics during the last deglaciation. Earth Planet Sci Lett 310(3–4):334–339. https://doi.org/10.1016/j.epsl.2011.08.016
IPCC. Climate Change 2014: Impacts, Adaptation, and Vulnerability (eds Field CB et al.) (Cambridge Univ. Press, 2014). Cambridge, United Kingdom and New York, NY
Juggins S (2007) C2 Version 1.5 User guide. Software for ecological and palaeoecological data analysis and visualisation. Newcastle University, Newcastle upon Tyne, UK
Kamenik C, Szeroczynska K, Schmidt R (2007) Relationships among recent Alpine Cladocera remains and their environment: implications for climate-change studies. Hydrobiologia 594:33–46. https://doi.org/10.1007/s10750-007-9083-4
Klimanov VA, Sirin AA (1997) Peat accumulation dynamics in the bogs of Northern Eurasia for the last 3,000 years. Dokl Akad Nauk 354(5):683–686
Kononova ON, Dubovskaya OP, Fefilova EB (2014) Zooplankton and dead zooplankton in Kharbeiskie lakes of Bolshezemelskaya tundra (period from 2009 to 2012). Journal Siberian Federal University 4:24–30 (in Russian)
Korhola A (1999) Distribution patterns of Cladocera in subarctic Fennoscandian lakes and their potential in environmental reconstruction. Ecography 22:357–373
Kotov AA, Sinev AYu, Glagolev SM, Smirnov NN (2010) Cladocerans (Cladocera). In: Alekseev VR and SYa Tsalolihin (eds) Opredelitel zooplanktona I zoobentosa presnyh vod Evropeyskoy Rossii. T.1. Zooplankton. Tovarischestvo nauchnyh izdaniy KMK, Moscow, pp 151–283 (in Russian)
Krammer K, Lange-Bertalot H (1986) Bacillariophyceae. 1. Teil: Naviculaceae: Suesswasserflora von Mitteleuropa. Gustav Fischer Verlag, Stuttgart, Jena
Krammer K, Lange-Bertalot H (1988) Bacillariophyceae. 2. Teil: Bacillariaceae, Epitemiaceae, Surirellaceae: Suesswasserflora von Mitteleuropa. Gustav Fischer Verlag, Stuttgart, Jena
Krammer K, Lange-Bertalot H (1991) Bacillariophyceae. 3. Teil: Centrales, Fragilariaceae, Eunotiaceae: Suesswasserflora von Mitteleuropa. Gustav Fischer Verlag, Stuttgart, Jena
Krylov AV, Kulakov DV, Tsvetkov A, Papchenkov VG (2014) Effect of atmospheric precipitation and the abundance of semiaquatic bird colonies on zooplankton in the littoral of a small high-trophic lake. Biology Bulletin 41(10):862–868. https://doi.org/10.1134/S1062359014100069(InRussian)
Kultti S, Väliranta M, Sarmaja-Korjonen K, Solovieva N, Virtanen T, Kauppila T, Eronen M (2003) Palaeoecological evidence of changes in vegetation and climate during the Holocene in the pre-Polar Urals, northeast European Russia. J Quat Sci 18(6):503–520. https://doi.org/10.1002/jqs.765
Kumke T, Ksenofontova M, Pestryakova L, Nazarova L, Hubberten H-W (2007) Limnological characteristics of lakes in the lowlands of Central Yakutia, Russia. J Limnology 66:40–53. https://doi.org/10.4081/jlimnol.2007.40
Kuprianova LA, Alyoshina LA (1972) Pollen and spores of plants of the flora of the European part of the USSR. Nauka, Leningrad (in Russian)
Kurek J, Korosi JB, Jeziorski A, Smol JP (2010) Establishing reliable minimum count sizes for cladoceran subfossils sampled from lake sediments. J Paleolimnol 44:603–612. https://doi.org/10.1007/s10933-010-9440-6
Luoto TP, Nevalainen L, Sarmaja-Korjonen K (2008) Multi-proxy evidence for the ‘Little Ice Age’ from Lake Hampträsk, Southern Finland. J Paleolimnol 40:1097–1113. https://doi.org/10.1007/s10933-008-9216-4
MacDonald GM, Kremenetski KV, Beilman DW (2008) Climate change and the northern Russian treeline zone. Philos T Roy Soc B 363:2285–2299. https://doi.org/10.1098/rstb.2007.2200
Magnuson JJ, Assel RA, Bouser CJ, Dillon PJ, Eaton JG, Evans HE, Fee EJ, Hall RI, Mortsch LR, Schindler DW, Quinn FH, Webster KH (1997) Potential effects of climate changes on aquatic systems: Laurentian Great Lakes and Precambrian Shield region. Hydrol Process 11:825–872
Marszelewski W, Skowron R (2006) Ice cover as an indicator of winter air temperature changes: case study of the Polish Lowland lakes. Hydrol Sc J 51(2):336–349. https://doi.org/10.1623/hysj.51.2.336
Martynenko VA, Getsen MV (1978) Equisetophyta, Anthophyta. In: Flory and fauna of the water bodies of the European North (Bol`shezemelskaya Tundra). Nauka, Leningrad, pp 161–165 (in Russian)
Mazhitova GG, Kaverin DA (2007) Thaw depth dynamics and soil surface subface subsidance at a circumpolar active layer monitoring (CALM) site, the European North of Russia. Kriosfera Zemli 11:20–30 (in Russian)
Medeiros AS, Friel CE, Finkelstein SA, Quinlan R (2012) A high resolution multi-proxy record of pronounced recent environmental change at Baker Lake, Nunavut. J Paleolimnol 47:661–676. https://doi.org/10.1007/s10933-012-9589-2
Medeiros AS, Taylor DJ, Couse M, Quinlan R, Hall RI, Wolfe BB (2014) Biologic and nutrient responses to catchment disturbance and warming in small lakes near the Alaskan tundra-taiga boundary. The Holocene 24:1308–1319. https://doi.org/10.1177/0959683614540955
Melles M, Brigham-Grette J, Minyuk PS, Nowaczyk NR, Wennrich V, DeConto RM, Anderson PM, Andreev AA, Coletti A, Cook TL, Haltia-Hovi E, Kukkonen M, Lozhkin AV, Rosen P, Tarasov P, Vogel H, Wagner B (2012) 2.8 Million Years of Arctic Climate Change from Lake El’gygytgyn. NE Russia Science 337:315–320. https://doi.org/10.1126/science.1222135
Menard P, Duguay C, Flato G, Rouse W (2002) Simulation of ice phenology on a large lake in the Mackenzie River basin (1960–2000). In: Proceedings of the 59th Eastern Snow Conference 3–12. Vermont, USA, Stowe.
Meyer H, Chapligin B, Hoff U, Nazarova L, Diekmann B (2015) Oxygen isotope composition of diatoms as Late Holocene climate proxy at Two-Yurts-Lake, Central Kamchatka, Russia. Global and Planet Change 134:118–128. https://doi.org/10.1016/j.gloplacha.2014.04.008
Miller GH, Geirsdóttir Á, Zhong Y, Larsen DJ, Otto-Bliesner BL, Holland MM, Bailey DA, Refsnider KA, Lehman SJ, Southon JR, Anderson C, Björnsson H, Thordarson T (2012) Abrupt onset of the Little Ice Age triggered by volcanism and sustained by sea-ice/ocean feedbacks. Geophys Res Lett 39(2):L02708. https://doi.org/10.1029/2011GL050168
Moller Pillot HKM (2009) Chironomidae Larvae. KNNV Publishing, Zeist, The Netherlands, Biology and ecology of the Chironomini. https://doi.org/10.1163/9789004278042
Moller Pillot HKM (2013) Chironomidae Larvae. KNNV Publishing, Zeist, The Netherlands, Biology and ecology of the aquatic Orthocladiinae. https://doi.org/10.1163/9789004278059
Myers-Smith IH, Kerby JT, Phoenix GK, Bjerke JW, Epstein HE, Assmann JJ, John C, Andreu-Hayles L, Angers-Blondin S, Beck PSA, Berner LT, Bhatt US, Bjorkman AD, Blok D, Bryn A, Christiansen CT, Cornelissen JHC, Cunliffe AM, Elmendorf SC, Forbes BC, Goetz SJ, Hollister RD, de Jong R, Loranty MM, Macias-Fauria M, Maseyk K, Normand S, Olofsson J, Parker TC, Parmentier F-JW, Post E, Schaepman-Strub G, Stordal F, Sullivan PF, Thomas HJD, Tømmervik H, Treharne R, Tweedie CE, Walker DA, Wilmking M, Wipf S (2020) Complexity revealed in the greening of the Arctic. Nat Clim Change 10:106–117. https://doi.org/10.1038/s41558-019-0688-1
Vegetation of the European part of the USSR (1980) Nauka, Leningrad (in Russian)
Nazarova LB, Pestryakova LA, Ushnitskaya LA, Hubberten H-W (2008) Chironomids (Diptera: Chironomidae) in lakes of Central Yakutia and their indicative potential for paleoclimatic research. Contemp Probl Ecol 1:335–345. https://doi.org/10.1134/S1995425508030089
Nazarova L, Herzschuh U, Wetterich S, Kumke Th, Pestrjakova L (2011) Chironomid-based inference models for estimating mean July air temperature and water depth from lakes in Yakutia, northeastern Russia. J Paleolimnol 45:57–71. https://doi.org/10.1007/s10933-010-9479-4
Nazarova L, Self AE, Brooks SJ, van Hardenbroek M, Herzschuh U, Diekmann B (2015) Northern Russian chironomid-based modern summer temperature data set and inference models. Glob Planet Chang 134:10–25. https://doi.org/10.1016/j.gloplacha.2014.11.015
Nazarova LB, Self AE, Brooks SJ, Solovieva N, Syrykh LS, Dauvalter VA (2017a) Chironomid fauna of the lakes from the Pechora River basin (East of European part of Russian Arctic): ecology and reconstruction of recent ecological changes in the region. Contemp Probl Ecol 4:350–362. https://doi.org/10.1134/S1995425517040059
Nazarova L, Bleibtreu A, Hoff U, Dirksen V, Diekmann B (2017b) Changes in temperature and water depth of a small mountain lake during the past 3000 years in Central Kamchatka reflected by chironomid record. Quat Internat 447:46–58. https://doi.org/10.1016/j.quaint.2016.10.008
Nazarova L, Syrykh LS, Mayfield RJ, Frolova LA, Ibragimova A, Grekov IM, Subetto DA (2020) Palaeoecological and palaeoclimatic conditions in Karelian Isthmus (north-western Russia) during the Holocene: multi-proxy analysis of sediments from the Lake Medvedevskoe. Quaternary Res 95:65–83. https://doi.org/10.1017/qua.2019.88
Nevalainen L, Luoto TP (2010) Temperature sensitivity of gamogenesis in littoral cladocerans and its ecological implications. J Limnol 69:120–125. https://doi.org/10.4081/jlimnol.2010.120
O’Brien WJ, Barfield M, Bettez N, Hershey AE, Hobbie JE, Kipphut G, Kling G, Miller MC (2005) Long-term response and recovery to nutrient addition of a partitioned arctic lake. Freshwater Biol 50:731–741. https://doi.org/10.1111/j.1365-2427.2005.01354.x
Overpeck J, Hughen K, Hardy D, Bradley R, Case R, Douglas M, Finney B, Gaewski K, Jacoby G, Jennings F, Lamoureux S, Lasca A, MacDonald G, Moore J, Retelle M, Smith S, Wolfe A, Zielinski G (1997) Arctic environmental change of the last four centuries. Science 278:1251–1256. https://doi.org/10.1126/science.278.5341.1251
Palagushkina OV, Nazarova LB, Wetterich S, Schirrmeister L (2012) Diatoms of modern bottom sediments in Siberian Arctic. Contemp Probl Ecol 5:413–422. https://doi.org/10.1134/S1995425512040105
Palagushkina O, Wetterich S, Biskaborn B, Nazarova L, Lenz J, Schwamborn G, Schirrmeister L, Grosse G (2017a) Diatom records and tephra mineralogy in pingo deposits of Seward Peninsula, Alaska. Palaeogeogr Palaeocl 479:1–15. https://doi.org/10.1016/j.palaeo.2017.04.006
Palagushkina O, Wetterich S, Schirrmeister L, Nazarova L (2017b) Modern and fossil diatom assemblages from Bol’shoy Lyakhovsky Island (New Siberian Archipelago, Arctic Siberia). Contemp Probl Ecol 4:380–394. https://doi.org/10.1134/S1995425517040060
Palecki DE, Barry RG (1986) Freeze-up and break-up of lakes as an index of temperature changes during the transition seasons: a case study for Finland. J Clim Appl Met 25:893–902
Płóciennik M, Kruk A, Michczynska DJ, Birks HJB (2015) Kohonen artificial neural networks and the indval index as supplementary tools for the quantitative analysis of palaeoecological data. Geochronometria 42:189–201. https://doi.org/10.1515/geochr-2015-0036
Quinlan R, Smol JP (2001) Setting minimum head capsule abundance and taxa deletion criteria in chironomid-based inference models. J Paleolimnol 26:327–342. https://doi.org/10.1023/A:1017546821591
R Core Team (2013) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/.
Rautio M (2001) Zooplankton assemblages related to environmental characteristics in treeline ponds in Finnish Lapland. Arct Antarct Alp Res 33:289–298. https://doi.org/10.1080/15230430.2001.12003433
Rebristaya OV (1977) Flora of the Eastern part of Bol`shezemelskaya tundra. Nauka, Leningrad (in Russian)
Reille M (1992) Pollen and spores from Europe and North Africa. Marseille, France, Laboratory of Historical Botany and Palynology, URA CNRS ((in French))
Reille M (1995) Pollen and spores from Europe and North Africa Supplement 1. Marseille, France, Laboratory of historical botany and palynology, URA CNRS ((in French))
Reille M (1998) Pollen and spores from Europe and North Africa Supplement 2. Marseille, France, Laboratory of historical botany and palynology, URA CNRS ((in French))
Reynolds CS (1980) Phytoplankton assemblages and their periodicity in stratifying lake systems. Holarctic Ecol 3:141–159. https://doi.org/10.1111/j.1600-0587.1980.tb00721.x
Reynolds CS (1984) Phytoplankton periodicity: The interactions of form, function and environmental variability. Freshw Biol 14(2):111–142. https://doi.org/10.1111/j.1365-2427.1984.tb00027.x
Reynolds CS (2003) The development of perceptions of aquatic eutrophication and its control. International Journal of Ecohydrology and Hydrobiology 3(2):149–163
Rühland K, Smol JP (2005) Diatom shifts as evidence for recent Subarctic warming in remote tundra lake, NWT. Canada Palaeogeogr Palaeocl 226(1–2):1–16. https://doi.org/10.1016/j.palaeo.2005.05.001
Sarmaja-Korjonen K, Kultti S, Solovieva N, Väliranta M (2003) Mid-Holocene palaeoclimatic and palaeohydrological conditions in northeastern European Russia: a multi-proxy study of Lake Vankavad. J Paleolimnol 30:415–426. https://doi.org/10.1023/B:JOPL.0000007232.78172.97
Sarmaja-Korjonen K, Nyman M, Kultti S, Valiranta M (2006) Palaeolimnological development of Lake Njargajavri, northern Finnish Lapland, in a changing Holocene climate and environment. J Paleolimnol 35:65–81. https://doi.org/10.1007/s10933-005-7337-6
Sekretareva HA (2004) Vascular plants of the Russian Arctic. KMK, Moscow (in Russian)
Sladkov AN (1967) Introduction to Spore Pollen Analysis. Nauka, Moscow (in Russian)
Smirnov NN (2010) Historical Ecology of Freshwater Zoocenoses. KMK Sci. Press, Moscow (in Russian)
Smol JP, Wolfe AP, Birks HJB, Douglas MSV, Jones VJ, Korhola A, Pienitz R, Rühland K, Sorvari S, Antoniades D, Brooks SJ, Fallu M-A, Hughes M, Keatley B, Laing T, Michelutti N, Nazarova L, Nyman M, Paterson AM, Perren B, Quinlan R, Rautio M, Saulnier-Talbot É, Siitonen S, Solovieva N, Weckström J (2005) Climate-Driven Regime Shifts in the biological communities of Arctic lakes. P Natl Acad Sci USA 102(12):4397–4402. https://doi.org/10.1073/pnas.0500245102
Solovieva N, Jones VJ, Appleby PG, Kondratenok BM (2002) Extent, environmental impact and long-term trends in atmospheric contamination in the Usa basin of east-European Russian arctic. Water Air Soil Poll 139:237–260. https://doi.org/10.1023/A:1015812109207
Solovieva N, Jones VJ, Nazarova L, Brooks SJ, Birks HJB, Grytnes J-A, Appleby PG, Kauppila T, Kondratenok B, Renerg I, Ponomarev V (2005) Paleolimnological evidence for recent climatic change in lakes from the northern Urals, arctic Russia. J Paleolimnol 33:463–482
Solovieva N, Jones V, Birks JHB, Appleby P, Nazarova L (2008) Diatom responses to 20th century climate warming in lakes from the northern Urals, Russia. Palaeogeogr Palaeocl 259:96–106. https://doi.org/10.1016/j.palaeo.2007.10.001
Solovieva N, Klimaschewski A, Self AE, Jones VJ, Andrén E, Andreev AA, Hammarlund D, Lepskaya EV, Nazarova L (2015) Holocene environmental history of a small coastal lake from north-eastern Kamchatka Peninsula. Global Planet Change 134:55–66. https://doi.org/10.1016/j.gloplacha.2015.06.010
Solovkina LN, Sidorov GP (1966) Fish resources of lakes and rivers in Bol’shezemel’skaya Tundra. In: Belyaev GM, Vinberg GG, Gaevskaya NS, Zhadin VI, Zenkevich LA, Reznichenko OG, Scherbakov AP (eds) Hydrobiological Studies and Fish Resources of Lakes from Extreme North of the USSR. Nauka, Moscow, pp 164–169 (in Russian)
Stenina AS (2009) Diatom algae (Bacillariophyta) in the lakes of eastern Bolshezemelskaya tundra. Syktyvkar (in Russian).
Stockmarr J (1971) Tablets with spores used in absolute pollen analysis. Pollen Spores 13:615–621
Subetto DA, Nazarova LB, Pestryakova LA, Syrykh LS, Andronikov AV, Biskaborn B, Diekmann B, Kuznetsov DD, Sapelko TV, Grekov IM (2017) Palaeolimnological studies in Russian Northern Eurasia: A review. Contemp Probl Ecol 4:327–335. https://doi.org/10.1134/S1995425517040102
Swadling KM, Pienitz R, Nogrady T (2000) Zooplankton community composition of lakes in the Yukon and Northwest Territories (Canada): relationship to physical and chemical limnology. Hydrobiologia 431:211–224. https://doi.org/10.1023/A:1004056715976
Sweetman JN, Ruhland KM, Smol JP (2010) Environmental and spatial factors influencing the distribution of cladocerans in lakes across the central Canadian Arctic treeline region. J Limnol 69:76–87. https://doi.org/10.4081/jlimnol.2010.76
Syrykh LS, Nazarova LB, Herzschuh U, Subetto DA, Grekov IM (2017) Reconstruction of palaeoecological and palaeoclimatic conditions of the Holocene in the south of Taimyr according to the analysis of lake sediments. Contemp Probl Ecol 4:363–369. https://doi.org/10.1134/S1995425517040114
Syrykh L, Subetto D, Nazarova L (2021) Paleolimnological studies on the East European Plain and nearby regions: the PaleoLake Database. J of Paleolimnol 65:369–375. https://doi.org/10.1007/s10933-020-00172-8
Szeroczyńska K, Sarmaja-Korjonen K (2007) Atlas of Subfossil Cladocera from Central and Northern Europe. Friends of the Lower Vistula Society, Swiecie
Taylor SR (1964) Abundance of chemical elements in the continental crust; a new table. Geochim Cosmochim Acta 28(8):1273–1285. https://doi.org/10.1016/0016-7037(64)90129-2
ter Braak CJF, Prentice IC (1988) A theory of gradient analysis. Advan in Ecolog Research 18:271–317
ter Braak CJF, Šmilauer P (2002) CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination Version 4.5. Microcomputer Power, Ithaca, New York: www.canoco.com.
Teteryuk BJ (2012a) Flora of ancient lakes of the European North-East of Russia. Izvestia Samara Scientific Center 14(1):82–90 (in Russian)
Teteryuk BJ (2012b) Flora and vegetation of ancient lakes in the European North-East Russia. Nauka, St.Petersburg (in Russian)
Thienpont JR, Rühland KM, Pisaric MFJ, Kokelj SV, Kimpe LE, Blais JM, Smol JP (2013) Biological responses to permafrost thaw slumping in Canadian Arctic lakes. Freshwater Biol 58:337–353. https://doi.org/10.1111/fwb.12061
Trifonova IS, Afanasyeva AL (2008) Long-term changes in the phytoplankton of Lake Krasnoye. In: Long-term changes in the biological communities of the mesotrophic lake under climatic fluctuations and eutrophication. LEMA Publishing House, St. Petersburg (in Russian)
Tsugeki N, Oda H, Urabe J (2003) Fluctuation of the zooplankton community in Lake Biwa 618 during the 20th century: a paleolimnological analysis. Limnology 4:101–107. https://doi.org/10.1007/s10201-003-0097-y
Van Dam H, Mertens A, Sinkeldam J (1994) A coded checklist and ecological indicator values of freshwater diatoms from the Netherlands. Aquat Ecol 28(1):117–133. https://doi.org/10.1007/BF02334251
van Geel B (2001) Non-pollen palynomorphs. In: Smol JP, Birks HJB, Last WM (eds) Tracking environmental changes using lake sediments, vol 3. Terrestrial algal and siliceous indicators. Kluwer Academic Press, Dordrecht, pp 99–119
Vlasova TA (1976) Hydrological and Hydrochemical Conditions of Biological Productivity in Lakes of the Harbey System. In: Productivity of Lakes of the Eastern Part of the Bol’shaya Zemlya Tundra. Nauka, Leningrad, pp 6–26 (in Russian)
Volkova VS, Bahareva VA, Levina TP (1989) Vegetation and climate of Holocene in West Siberia. In: Paleoclimates of the Late Glacial and Holocene. Nauka, Moscow, pp 90–95 (in Russian)
Vuglinsky VS, Gronskaya TP, Lemeshko NA (2002) Long-term characteristics of ice events and ice thickness on the largest lakes and reservoirs of Russia. In: Squire V, Langhorne (eds) Ice in the Environment. Dunedin, New Zealand, pp 80–86
Walker DA (2000) Hierarchical subdivision of Arctic tundra based on vegetation response to climate, parent material and topography. Global Change Biol 6(S1):19–34. https://doi.org/10.1046/j.1365-2486.2000.06010.x
Wetterich S, Schirrmeister L, Nazarova L, Palagushkina O, Bobrov A, Pogosyan L, Savelieva L, Syrykh L, Matthes H, Fritz M, Gunther F, Opel T (2018) Holocene thermokarst and pingo development in the Kolyma Lowland 1 (NE Siberia). Permafrost Periglacial Processes 29(3):182–198. https://doi.org/10.1002/ppp.1979
Wiederholm T (1983) Chironomidae of the Holarctic region. Keys and diagnoses. Part 1: Larvae, Entomological Society of Lund, Sweden.
Wolfe AP (2003) Diatom community responses to late-Holocene climatic variability, Baffin Island, Canada: a comparison of numerical approaches. The Holocene 13:29–37. https://doi.org/10.1191/0959683603hl592rp
Zvereva OS, Vlasova TA, Goldina LP, Iz"yurova VK (1970) The results of limnological studies in the Bolshezemelskaya tundra. In: Biological basis of the use of nature of the North. Komi kn. izd-vo, Syktyvkar, pp 248–253 (in Russian)
Acknowledgements
Research was carried out within the frame and under financial support of the grant 20-17-00135 RSF (Russian Science Foundation). NR worked in the frame of project NIR № 0264-2019-0010. OL worked in the frame of the State Task No AAAA-A17-117112850235-2. LS is supported by the Ministry of Science and Higher Education of the Russian Federation (project No. FSZN-2020-0016). We warmly thank all Russian colleagues who helped us during the fieldwork in the region of investigation and the Geochronology Laboratory of St. Petersburg State University for performing the 210-Pb dating. Our sincere thanks to the anonymous reviewers and Dr. Mateusz Płóciennik for their valuable comments that helped us to improve the quality of our manuscript. All data will be lodged with PANGEA upon publication.
Author information
Authors and Affiliations
Contributions
LN performed data analysis, conceived and designed research, LF carried out Cladocera analysis, OP carried out diatom analysis, NR carried out pollen analsis, LS carried out chironomid analysis, IG performed graphic work and contributed to chironomid analysis, NS contributed to the statistical analysis and overall discussion of the data, OL lead the field work, provided regional data and contributed to analysical work. All authors wrote, read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nazarova, L.B., Frolova, L.A., Palagushkina, O.V. et al. Recent shift in biological communities: A case study from the Eastern European Russian Arctic (Bol`shezemelskaya Tundra). Polar Biol 44, 1107–1125 (2021). https://doi.org/10.1007/s00300-021-02876-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-021-02876-7