Abstract
Glacial lakes and their sediments are highly sensitive temporal markers of environmental variability. The combination of particular geographical conditions with climate changes makes the Múlajökull lakes an appropriate model for revealing some site-specific and common relationships in psychrophilic microbial ecology. The aim of this study was to evaluate the taxonomic and functional diversity of microbial community structures in five glacial lake sediments situated at different distances from the glacier, i.e., 50 m to 1750 m from the ice margin. The Shannon diversity indices varied in the range from 1.99 to 2.94 (with the lowest in C sample) tested by EcoPlates™ and from 1.69 to 1.89 at the phylum level (with the lowest in A sample) tested by the shotgun metagenomic sequencing, respectively. An inter-sample comparison was also conducted. Overall, six bacteria phyla (Proteobacteria, Cyanobacteria, Bacteroidetes, Actinobacteria, Verrucomicrobia and Planctomycetes) and one microalgae phyla (Bacillariophyta) were detected in five lake sediments tested at abundancy that exceeded 1%. Obtained data brings new knowledge related to the cold biosphere on the local and global Earth systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Glaciers, ice caps and ice sheets occupy a significant area of our planet, covering about 10% of the total land area (National Snow and Ice Data Center). Biological processes in glaciers are dominated almost exclusively by microbial communities (Anesio et al. 2017), and cold environments, such as ice and permafrost, are the biggest reservoirs of preserved microbial biomass (Simon et al. 2009). Psychrophilic microorganisms use various environmental, physiological and molecular adaptations to thrive under adverse conditions (De Maayer et al. 2014; García-Laviña et al. 2019; Gura and Rogers 2020).
Great variations of bacterial communities that are dependent on the physicochemical conditions and dissolved organic matter quality have been revealed across the Greenland ice sheet (Cameron et al. 2016) and Antarctica (Smith et al. 2018). The Arctic, Antarctic, and Alpine ecosystems are recognised as sensors and sentinels of global change (Teufel et al. 2017). Microorganisms, dissolved gases and nutrients in the runoff inevitably enter the englacial drainage system (Dieser et al. 2014), and climate changes influence the permafrost microbial ecology due to this additional organic matter, which becomes available for microbial degradation (Mackelprang et al. 2016; Ibrar et al. 2020). Nevertheless, climate-associated increases in phytoplankton production and concomitant shifts in diversity can influence bacterial community structure by altering the availability and composition of autochthonous carbon for heterotrophic production (Teufel et al. 2017). Photoheterotrophic bacterioplankton community is able to utilise solar energy along with organic and inorganic substrates, being a key function in oligotrophic mountain lakes (Echeverría-Vega et al. 2018). Analysis of biogeochemical cycles occurring on glaciers provides new knowledge related to the local and global Earth system. The primary and secondary carbon production (Säwström et al. 2002; Anesio et al. 2009; Yallop et al. 2012), nitrogen fixation (Telling et al. 2012), viral infectivity (Bellas et al. 2013) and other processes detected on glacier surfaces, characterise the biogeochemical cycles of the terrestrial cold biosphere (Anesio et al. 2017).
However, the presence of liquid water in a glacial environment is considered to be the major limiting factor for the development of microbial processes (Anesio et al. 2017). In this respect, oligotrophic glacial lakes serve as models to study the effects of climatic conditions (e.g., the persistent high UV regime, deglaciation) on natural biological systems on the Earth and other planetary bodies (Parro et al. 2018). Studies on microbial communities that are derived from glacial and periglacial environments bring new knowledge for approving various biogeographic hypotheses (Papke et al. 2003; Nemergut et al. 2007; Rodrigues et al. 2009; Schmidt et al. 2011).
Glacial lakes and their sediments are highly sensitive temporal markers of environmental variability related to the multistage process of deglaciation; this, in turn, influences the sediment load, and therefore, the transparency and UV levels of the water column. Therefore, the environmental processes combined with climatic factors notably affect the biota community structure in glacial lakes (Callieri et al. 2007; Cameron et al. 2017). This aspect is little understood, hence, further studies may provide us with new knowledge regarding ecology, water resources and the evolution of glacier lakes at planetary scales (Parro et al. 2018).
Development and application of various molecular ‘omics’ methods during last two decades have greatly impacted in developing an improved understanding of the processes related to the microbial ecology in permafrost and in the soil layers activated as the permafrost thaws (Mackelprang et al. 2016; Belov et al. 2020; Zhou et al. 2020).
Parro et al. (2018) studied the microbial community structure of oligotrophic Encanado lakes (Region Metropolitana, Chile), using an on-site immunoassay-based life detector chip, as well as bacterial and archaeal 16S rRNA gene sequences (Parro et al. 2018). The presence of sulfate-reducing bacteria, Alpha, Beta-, Gamma-, and Deltaproteobacteria, as well as cyanobacteria and methanogenic archaea, were detected. Yang et al. (2016) reported on the abundance of nitrifying bacteria of Nitrospira, nitrogen-fixing bacteria of Bradyrhizobium and Rhizobium, methane-oxidising bacteria of Methylobacterium and Methylopila, and sulphur- and sulphate-reducing bacteria of Desulfococcus in the Chongce glacier (Yang et al. 2016).
To date, microbiological aspects related to the variability of glacial habitats dependent on natural and anthropological factors remain poorly understood. The combination of particular geographical conditions with climate changes makes the Múlajökull lakes in central Iceland an appropriate model for revealing some site-specific and common relationships in psychrophilic bacterial ecology.
In this study, we aimed to compare the bacterial and archaeal community structures of five glacial lake sediments situated at different distances from the glacier, i.e., 50–1,750 m from the ice margin, representing various environmental conditions. The lakes were sampled in August 2017, just following the warmest season in this region (July). Comparison of metabolic biodiversity of culturable microorganisms, as well as the shotgun metagenomic sequencing in the sediments of five tested lakes, was accompanied by physicochemical testing of water and sediment samples.
Materials and methods
Sampling
Lake sediments were sampled on 26 August, 2017, and were stored in new polyethylene zipper plastic bags. Samples were collected from the lake bottom sediments near the lakeshore at the depth not deeper than 0.5 m. The composite sample for each lake was obtained by mixing three sub-samples, which were taken at a distance of 10 m apart; sampling was conducted using a sterile sampling cup. During the transport to the laboratory, samples were put in a black bag and hidden away from the direct sunlight. The air temperature in the field during the expedition in Iceland was mostly between 5 and 10 °C. Average air temperatures in the nearest weather station (Hveravellir), which is located 50 km away but at the approximately same height as the study area (641.0 m a.s.l.) are shown in Fig. S1. Water temperature in sampled lakes is shown in Table 1. Laboratory samples were stored at the temperature of –80 °C.
Geological setting
Múlajökull is a surge-type outlet glacier in central Iceland. It drains the Hofsjökull ice cap and has a known history of surges since 1924. During each surge, the glacier margin advances several hundred metres on average every 10 years (Björnsson et al. 2003). Nowadays, the glacier is retreating and the ice-thinning at its marginal zone is reported to be > 5 m per year (Jóhannesson et al. 2013) during 1999–2008 and 2.5 m during 2008–2015 (Lamsters et al. 2016). The glacier margin is located approximately 2 km away from its maximum extent during the Little Ice Age (LIA) when the prominent Múlar terminal moraine formed (Benediktsson et al. 2015). The forefield of Múlajökull is characterised by numerous drumlins and proglacial lakes between them (Johnson et al. 2010; Jónsson et al. 2014; Benediktsson et al. 2015), and is a well-known site for the studies of drumlin formation (Benediktsson et al. 2016; Lamsters et al. 2016; McCracken et al. 2016; Iverson et al. 2017; Finlayson et al. 2019; Ives and Iverson 2019), providing a good opportunity for ecological and microbiological studies of glacial lake environments of different ages.
Near the ice margin, glacial lakes are mainly located in the linear depressions between drumlins (Fig. 1), but further away they also occupy erosional basins between end moraine ridges. Distant from the LIA terminal moraine, there are numerous small lakes, which can be mainly referred to as thermokarst lakes. These are located in an extensive area of Þjórsárver bog, characterised by discontinuous permafrost and small palsas (Thorhallsdóttir 1996; Saemundsson et al. 2012; Benediktsson et al. 2015): the age of these small thermokarst lakes is unknown. Knowing that the temperature in Iceland during LIA was 1–2 ° lower than today, we can assume that the oldest lakes are at least younger than LIA maximum, which is correlated with the formation of the terminal moraine in the first half of the eighteenth century (Benediktsson et al. 2015). However, it must be taken into the account that the climate has also been changing since LIA, and some palsas, as well as thermokarst lakes, were known to disappear and emerge again between 1930 and 1950 (Thorhallsdóttir 1996).
The examples of glacial lakes near the Múlajökull glacier. A, B The regional setting of the study areas in the Arctic. C Distal lake near the LIA terminal moraine (proximal side). D Small thermokarst lakes in the Þjórsárver bog area (in the distal direction from LAI terminal moraine). E Proglacial and distal lakes near the ice margin. Note that the water in distal lakes that are not connected to the glacier are more transparent and blue due to the lesser amount of suspended particles
The sampling site A is a small lake at the beginning of the Þjórsárver bog. From one side, it is bordered by the terminal moraine, so it most likely formed as a moraine-dammed lake at the LIA maximum, although we cannot exclude that it could evaporate or drain away, and fill by water later due to changes of climate and distribution of permafrost. The sedimentary sequence in the Þjórsárver bog consists of loess, peat and tephra. It could be one of the reasons why the chemical composition of water in lake A differs from the rest of the lakes, which are located in depressions incised in glacial or outwash sediments.
The glacial lakes in the forefield of the Múlajökull (Fig. 1) can be classified as; (1) ice-marginal lakes dammed by the glacier margin and receiving water by direct input from melting ice or from subglacial channels, (2) distant lakes fed from meltwater streams, (3) precipitation and possibly groundwater-fed remnant lakes with no contemporary surficial input of glacial meltwater, and (4) thermokarst lakes developed after the degradation of palsas.
The only lake fed by glacial meltwaters today is lake E (Fig. 1). It was still connected to the glacier in 2008, when the ice-margin advanced some 20 m creating end moraine. (Jónsson et al. 2014) Lakes B, C, D are remnant lakes, which are fed only by precipitation and possibly groundwater. The maximum ages of the sampled lakes could be attributed to the known ages of surge moraines. The maximum age of the lake A could be almost 300 years according to the possible formation time for the terminal moraine between A.D. 1717 and 1760 as reported by (Benediktsson et al. 2015). Lakes B, C, D are definitely younger than the formation age of the terminal moraine; the ages of the lakes D and C could be quite similar because there are no end moraine ridges separating them, but lake B is older because there are end moraine ridges separating it from the lakes D and C. The age of this end moraine is unknown (Benediktsson et al. 2015). Lake D is visible on an aerial photograph from 1945, (Sigurkarlsson 2015) so it has existed at least 73 years. As the variations of the glacier snout have been measured by the Icelandic Glaciological Society since 1932 (Benediktsson et al. 2015), we can calculate that the glacier has retreated a little bit more than 400 m from its position in 1932 up to its position in the 1954 surge; the 1954 surge moraine almost coincides with the 1992 end moraine. Most likely, there was ice in the place of lake D in 1932, so we can assume that this lake originated in the years after 1932. Lake C could be a little bit older than 1932, and Lake E is younger than 1992, because it is located 400 m away in the glacier direction from the 1992 end moraine.
Chemical characterisation of benthic sediments
Air-dried samples of benthic sediments were sieved through a 2-mm sieve. To determine the particle sizes, the samples were treated with 0.1 M NaOH to break down the aggregates. Sands were sieved and fractions finer than 0.05 mm were determined by pipette analysis. On the basis of the soil texture classes, the fractions from 0.063 to 2.0 mm were classified as sand, those from 0.002 to 0.063 mm as silt, and those finer than 0.002 mm as clay. The percentage of sand, silt and clay was calculated from fine earth as less than 2 mm fraction (ISO 11277:1998).
Chemical analyses (pHBaCl2 value, concentrations of exchangeable elements (Ca2+, Mg2+, K+, Na+) (cmol kg−1), sum of exchangeable bases (cmol kg−1), and P2O5 concentration (mg kg−1, total nitrogen and total carbon content (%), total nitrogen and total carbon ratio)) of the sediment samples were done in three replicates according to internationally used methods of the International Organization for Standardization (ISO) ((ISO 11260:2018; ISO 14254:2018; ISO 10694:1995; ISO 13878:1998; ISO 11263:1994). Exchangeable elements (Ca2+, Mg2+, K+, Na+) (cmol kg−1) were determined in BaCl2 solution using an atomic absorption spectrometer Perkin Elmer Analyst 200. The soil pHBaCl2 value was measured with a glass electrode in 1 M BaCl2 (1:2.5 mass-to-volume ratio), using a pH-meter WTW inoLab. Total nitrogen and total carbon content (%) were determined by an element analyser “EuroVector”. The reliability of the obtained results was assessed after chemical analysis. The laboratory results were considered acceptable when the difference between the values obtained was less than ± 10%.
Estimation of microbial functional diversity by Biolog EcoPlate™
Catabolic diversity of the microbial community in the activated sludge was determined using Biolog EcoPlate™ (Biolog, Inc., USA). The measurement of substrate metabolism with EcoPlate™ is based on the colour formation from tetrazolium dye, a redox indicator. A 10 mL aliquot of lake sediment was suspended in 40 mL of sterile 0.85% NaCl solution, then inoculated (180 μL) into each well and afterwards incubated for 96 h at 28 °C. The microbial activity in each well was expressed as average well-colour development measured at 620 nm after 48, 72 and 96 h incubation, using microplate reader TECAN Infinite ® F50 (Switzerland). The results of Biolog profiles were presented by the Shannon diversity index, which was calculated by the following Eq. 1:
where pj = relative colour intensity of individual well (Gabor et al. 2003).
DNA extraction and shotgun metagenomic sequencing
The total DNA from sediment samples was extracted using PowerSoil ® DNA Isolation kit (Mo Bio Laboratories, Carlsbad, CA, USA) obtaining an average of 1.17 ± 0.75 (n = 5) ngμL−1 of DNA per sample. In case of an insufficient total amount of DNA, the extraction procedure was repeated and followed by vacuum concentration with Eppendorf Concentrator Plus (Eppendorf, Hamburg, Germany) to obtain the total yield of 150 ng. Quantification of the DNA was performed with a Qubit 2.0 fluorometer and Qubit® dsDNA HS assay kit (Thermo Fisher Scientific, Waltham, MA, USA). For each sediment sample, 150 ng of the extracted DNA was shared to 300 bp long fragments and further processed using an Ion Plus Fragment Library Kit (Thermo Fisher Scientific, Waltham, MA, USA), Ion Xpress™ Barcode Adapters 49–64 Kit (Thermo Fisher Scientific, Waltham, MA, USA) and NGS clean-up and size selection (MACHEREY–NAGEL GmbH & Co, Düren, Germany). Size-selection of 400 bp long fragments was performed on the Pippin Prep system using BluePippin reagent kit (Sage Science, Beverly, MA, USA). The quality and quantity of shotgun metagenomic libraries were determined with Agilent 2100 Bioanalyzer and Agilent High Sensitivity DNA Kit (Agilent Technologies Inc., Santa Clara, CA, USA). Next-generation sequencing was performed on Ion Proton™ System and Ion PI™ Chip (Thermo Fisher Scientific, Waltham, MA, USA), following the manufacturer’s instructions.
Bioinformatic and statistical analysis
Adapters were removed with cutadapt v1.16 (Martin 2011). Read trimming was performed with trimmomatic 0.38 using 5 bp sliding window with quality threshold 15, average quality threshold 15 and minimum read length after trimming 100 bp (Bolger et al. 2014). Reads mapping to human reference genome GRCh38 (Ensembl release 90) were removed with bowtie2-2.3.5.1 (Langmead and Salzberg 2012). Functional characterisation of metagenomes was performed with humann2 v0.11.2 (Franzosa et al. 2018) using the Uniref50 database (Suzek et al. 2015) with copies-per-million (CPM) as the abundance value.
Taxonomical assignments were performed with kraken2 2.0.8-beta with a confidence threshold of 0.2 (Wood and Salzberg 2014). Improvement of taxonomical assignments was performed with bracken 2.5 at the species level. Reads classified as Homo sapiens were removed from the analysis of taxonomies. Taxonomies with median and maximum of ≥ 10 reads were kept for further analyses.
To facilitate sample comparison of taxonomic data, a rarefaction procedure was performed by constructing multinomial distribution from the taxonomic frequencies, followed by generating n samples from the distribution, where n equalled the read count of sample with the fewest reads. To enable compositional data analysis methods on the taxonomical data, imputation of zero values was performed with the Bayesian-multiplicative replacement method, as implemented in R 3.6.2 package zCompositions 1.3.4 with default parameters (Martín-Fernández et al. 2015). Isometric log-ratio (ilr) transformation was then applied to the resulting taxonomical compositions and the compositional environmental variables (Egozcue et al. 2003). A principal component analysis biplot was constructed from the transformed compositions with scikit-learn 0.22. Loading vectors were translated from ilr representation to centred log-ratio (clr) (Aitchison, 1982) represented by multiplying obtained PCA loadings with the orthonormal basis of ilr space as described by (Egozcue et al. 2003). Taxonomic data and the compositional environmental variables were transformed separately. Noncompositional variables (distance and temperature) were normalised by dividing these features with their maximum values.
Alpha and beta diversities were expressed as Shannon index and Aitchison’s distance, respectively. Calculations were performed with scikit-bio (0.5.6).
The creation of beta diversity matrix and Euclidean distance-based hierarchical clustering analysis was carried out using MeV software (Saeed et al. 2003).
Results
Chemical analysis of benthic sediments
Relatively older wetland lake (A) was characterised by low pHBaCl2 value, and higher concentrations of exchangeable elements (Na+, Mg2+, K+, Ca2+), sum of exchangeable bases, total nitrogen and total carbon content as well as higher P2O5 concentration than other younger remnant lakes and glacial lake (Table 2).
Sum of exchangeable bases in the remnant lakes and glacial lake varied from 64.93 to 84.00 cmol kg−1, except small remnant lake (C), which was 108.21 cmol kg−1, but in the old wetland lake (A) it reached 235.49 cmol kg−1.
The total carbon/total nitrogen ratio was relatively low (7.66) in the older wetland lake (A), however, it increased in the relatively younger remnant lakes and in the glacial lake (8.26–10.95), indicating higher decomposition and organic matter turnover processes that probably could also be explored by pH value (Table 2). pHBaCl2 value in the analysed lake sediment samples varied from 4.18 in the relatively older wetland lake (A) to 4.81 in the relatively younger small remnant lake (C) and glacial lake (E).
Higher P2O5 concentrations were detected in the youngest glacial lake (E) (15.85 mg kg−1) than in older remnant lakes B, C, and D (2.55–3.13 mg kg−1), however, the highest concentration was in old wetland lake (A).
Metabolic biodiversity of culturable microorganisms tested by EcoPlates™
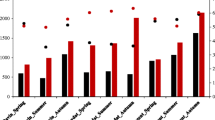
Microbial community structure in five tested lakes sediments was compared by the evaluation of a metabolic profile diversity. Preliminary testing of sediments samples for the number of CFU has revealed that the amount of culturable aerobic heterotrophic microorganisms varied in the range of 105–107 g−1 dw−1. The data on the ability of a microbial community to utilise 31 distinct carbon substrates in EcoPlate™ revealed considerable changes in microbial community composition amongst the tested sediment samples. After 48 h incubation, the highest biodiversity was detected in lake A (H’ = 2.34), whilst in the sediments of lake C, metabolic activity of microorganisms was not revealed at all. Further incubation resulted in an increasing number of substrates utilised by the microbial community. Particularly, after 96 h, the biodiversity index H’ of the microbial community in five tested lakes varied in range from 1.99 (in lake C) to 2.94 (in lake B), with the substrate richness of 8 and 22, respectively (Fig. 2).
Metabolic biodiversity of the Múlajökull lakes sediment samples tested by EcoPlates™. A Shannon index, B substrate richness. Data are average of three replicates. Sampling sites are shown in Fig. 1
Additionally, the substrate utilisation patterns were compared based on the substrate categories according to (Urakawa et al. 2013). These data have been summarised in our earlier paper (Gaidelene et al. 2018). According to this analysis, the lakes B and C were characterised by the greatest utilisation of polymers and amines after 96 h incubation. Amongst the 10 carbohydrates provided by EcoPlate™, only 6, 8, 2, 3 and 5 substrates were positive for the samples from A, B, C, D and E lakes, respectively. The most frequently used carbohydrates were D-mannitol and n-acetyl-d-glucosamine (Gaidelene et al. 2018).
Analysis of bacterial and archaeal communities based on shotgun metagenomic sequencing
Shotgun metagenomic sequencing produced an average of 18.2 ± 8.3 (n = 5) million reads per sample, providing access to the composition of microbial communities at various levels of taxonomy. A Composition of microbial DNA isolated from five lakes sediments was compared using different approaches. The Shannon diversity indices at the phylum level varied in range from 1.69 to 1.89, whilst at the species level from 4.02 to 5.40, with the lowest value at both levels observed in sample A (Fig. 3).
Alpha diversity metrics of microbial communities in the sediments of Múlajökull lakes, based on observed Shannon’s diversity indices at (A) Phylum and (B) Species level. Sampling sites are shown in Fig. 1
An inter-sample comparison was also conducted. Clustering analysis of beta diversities revealed that at the phylum level, microbial populations of sediments from lake A and lake E displayed the highest level of difference from each other and the rest of the samples, whilst at the species level, such distinguishing degree of difference was presented only by lake A sediments (Fig. 4).
A visual depiction of Múlajökull lakes sediment microbial population structure beta diversity matrix table with Euclidean distance-based hierarchical clustering tree, (A) At the phylum level and (B) at the species level. The coloured squares represent Beta diversity number which ranges from 0 (green), through median value (black) to maximal value (red). The scale with specific values is depicted at the top of each figure
The rarefied taxonomic resolution of microbial communities at phylum and species levels is presented in Fig. 5. As shown in Fig. 5A, overall, six bacteria phyla (Proteobacteria, Cyanobacteria, Bacteroidetes, Actinobacteria, Verrucomicrobia and Planctomycetes) and one microalgae phyla (Bacillariophyta) were detected in five lake sediments tested at an abundancy that exceeded 1%. Nevertheless, each lake was characterised by a unique abundance profile of these microorganisms (Fig. 5A). In general terms, the abundance for some of these organisms decreased (for Cyanobacteria, from 29.8% in lake E to 9% in lake A, and for Actinobacteria, from 9% in lake E to 1.3% in lake A) or on the contrary increased (for Proteobacteria, from 49.5% in lake E to 64.2% in lake A, and for Bacillariophyta from 0.16% in lake E to 15.6% in lake A) with the distance from the glacier (Fig. 5B).
Microbial community analysis plots based on shotgun metagenomic sequencing. Histogram representing taxonomic composition and relative abundance (over 1%) at phylum (A) and species (C) levels for each sediment sample in five Múlajökull lakes, normalised relative abundancy changes for phylum Cyanobacteria, Actinobacteria, Proteobacteria and Bacillariophyta (B), selected species from these phyla (D) and numbers of taxa that are shared between samples on species level (E). Sampling sites are shown in Fig. 1
At the species level, a relative abundance of Cyanobacteria was represented by Anabaena sp. and Dolichospermum sp. with the highest concentration in the lake E (23.2 and 15.7%, respectively), and Rippkaea orientalis and Calothrix parietina with the highest concentration in the lake C (11.8 and 1.9%, respectively, the first being absent from lakes A and E) (Fig. 5C). Amongst microalgae, Thalassiosira pseudonana and Phaeodactylum tricornutum were identified with the highest concentration in lake A (14.88 and 2.7%, respectively), whilst Proteobacteria were represented by Escherichia coli (highest in lake C, 27.48%), Sphingomonas sp. (highest in lake E, 11.31%, also high in lake D, low in lake B, but absent from lake A and C), Acidovorax sp. (highest in lake C, 3.55%), Undibacterium parvum (highest in lake D, 7.29%), Polaromonas sp. (highest in lake A, 1.86%), Janthinobacterium sp. (highest in lake D, 2.52%) and methanotrophic Methylomicrobium alcaliphilum (highest in lake A, 21.01%, and also found in lakes B and C, but not in D and E) (Fig. 5C). Regarding the abundance of other bacterial species, the highest abundance of Flavobacterium sp. and Opitutus sp. was identified in lake E (9.91 and 2.06%, respectively) (Fig. 5C). Interestingly, the same tendency of abundancy relation to distance from glacier can also be observed within the number of species from previously mentioned phyla, thus abundancy of Escherichia coli, Methylomicrobium alcaliphilum, Phaeodactylum tricornutum and Thalassiosira pseudonana increased whilst abundancy of Sphingomonas sp. MM-1, Anabaena sp. WA102, Dolichospermum compactum, Dolichospermum sp. UHCC 0315A and Flavobacterium sp. GS03 decreased (Fig. 5D). Pairwise cross-comparison of shared microbial species revealed that in all cases majority of taxa were present in both samples; however, the identity level varied between 63.01% and 98.2% (Fig. 5D).
To further assess the factors that contribute most to observed variations within collected sediment samples, a PCA biplot analysis was carried out. In general terms, the acquired results fitted well with data from beta diversity analysis. In the phylum level, spots that represented lakes B, C and D clustered together, whilst spots representing lakes A and E were located a considerable distance away from other data points. Factors that contributed most to lake E placement within the plot were pOH (calculated from pH value) and pHBaCl2 values, the concentration of Ca2+ as well as the abundance of Gemmatimonadetes, Actinobacteria, Cyanobacteria and Verrucomicrobia, whilst factors contributing most to the placement of lake A were concentration of K+, total nitrogen content, as well as the abundance of Basidiomycota, Proteobacteria, Firmicutes and Bacteroidetes. Due to their location near the boundary of two adjacent sectors, the placement of the lakes B, C and D cluster was affected by contributing factors of both lower sectors (Fig. 6A). On the species level, the spots that represented lake A were located a considerable distance away from spots of other lakes, whilst the distance between lake B and C spots and lake D and E spots were located in pairs that were closer to each other than to other three lake spots, thus indicating greater resemblance. The factors that displayed the greatest contribution to placement were various minor representation taxa (Fig. 6B).
PCA biplot of the chemical and environmental measurements as well as bacterial communities at phylum (A) and species (B) level. Coloured circles represent analysed lakes, whilst radial arrows represent factors that contribute to sample location within the plot. The length of arrows indicates the significance of contribution. Top 10 factors contributing to placement within a specific sector are depicted with black arrows and listed within the corresponding sector
Taken together, these results indicate that there is a specific core environmental microbiome which is characteristic to specific geographical regions and its members from existing niches are rapidly seeding the newly created ones. Results were acquired by employing taxonomic data.
Discussion
The first observation that we made during data analysis was related to surprisingly high proportion of E. coli within our microbial population estimations. Although such situation could be considered as possible within predominant majority of densely populated areas, we believed that due to scarce population and relatively low tourism activity in Iceland the proportion of E.coli should be lower. Therefore, to test this hypothesis we performed an additional comparative investigation of intermediate read assignment data that was acquired employing kraken2 and final taxonomical population structure data that was acquired employing bracken 2.5. As expected acquired results revealed that there were only few E.coli reads detected by kraken2 whilst bracken 2.5 abundancy count was significantly higher. Similar situation was also observed in the case of other microorganisms (Online Resource 1). The most probable explanation to this phenomenon lies in bracken 2.5 taxonomic abundancy calculation approach, where reads that display specific allocation at higher taxonomic level (for example genus of phylum) are distributed (or “pushed”) to lower taxonomic level through statistical calculations based on data that was reliably assigned to the higher taxonomic level. Reads assigned to lower taxonomic levels are reassigned to the target level. Therefore bracken 2.5 calculations are highly reliant on quality and completeness of employed databases and in our case observed high proportion of E. coli could be explained by presence of yet unidentified taxonomically related bacteria or taxonomically related bacteria without known genomic sequence. Although not perfect, we believe that employment of bracken 2.5 for abundance of species calculations is essential, because kraken2 was not designed to estimate relative abundances and, although some groups employ directly kraken2 derived data for such purposes, these are not taking into consideration the genome size of microorganisms and fact that number of genomic regions are highly conservative between various species of the same taxa. Even more due drawbacks of our current microbial knowledge (only 2.1% of the global prokaryotic taxa are represented by sequenced genomes (Zhang et al. 2020) our data once more highlights the necessity to consider metagenomics derived knowledge at species level only in the context with data of lower (genus, phylum etc.) taxonomic level.
Nevertheless we believe that our data represents well the microbial population structure and remarkable shifts in the structure of bacterial communities across five Múlajökull glacial lakes, which are different by a distance from a glacier, and thus relative age and hydrological conditions, were detected in this study. Apparently, the differences in physicochemical and hydrological characteristics of the tested lakes should be the major drivers of these changes. Leon et al. (2012) attributed the differences in bacterial activity and community structure in the sediments of three Patagonian Chilean oligotrophic lakes of Quaternary glacial origin to the unique geomorphological pattern of each lake due to both local (e.g., volcanic activity) and global (climate change) disturbances (Leon et al. 2012). In a study of bacterial community diversity in two oligotrophic Andean lakes fed by meltwater from the Echaurren glacier in central Chile, the watershed characteristics (area and length, slope, lithology, resistance to erosion), as well as nutrient concentration and turbidity were found to strongly influence the limnology of the lakes (Echeverría-Vega et al. 2018).
Anesio et al. (2017) recently reviewed the data on the microbiome of cold regions; a comparison of glacial habitats across glaciers and ice sheets worldwide showed their similarity. Meanwhile, bacterial production on glacial surfaces in the Arctic was found to be higher than that in Antarctica, probably due to a longer growing season, higher temperatures and other factors (Telling et al. 2012; Anesio et al. 2017).
As shown in Tables 1 and 2, the tested Múlajökull lakes differed by temperature, pH, EC, the concentration of biogenic elements and exchangeable cations. The highest concentrations of the total organic carbon and nitrogen were found in the lake A, i.e., 1.15 and 8.83%, respectively. The other four lakes contained these elements in significantly lower concentrations, i.e., 10–20 times less than in lake A (Table 2).
In this respect, the dominance of Cyanobacteria in the lake sediments of B, C, D, and E could be explained by oligotrophic conditions. Cyanobacteria dominate in aquatic/sediment systems as a carbon producer (Anesio et al. 2017). In our study, Cyanobacteria was represented almost in all lake sediments by Anabaena sp., Dolichospermum sp. and Calothrix sp., although their relative abundance was different (Fig. 5C).
The changes in Cyanobacteria abundance in the sediments of glacial lakes could be attributed to the differences in hydrological connectivity during glacier melting with respect to the turbidity gradient. Thus, changes in water turbidity lead to changes in temperature and light availability (Gallegos et al. 2008; Egholm et al. 2012; Rose et al. 2014; Peter and Sommaruga 2016). These changes affect the abundance of primary producers.
An unexpectedly low abundance of Polaromonas was found in the sediments of the tested lakes (max. 2.06% in lake E) (Fig. 5C). According to (Darcy et al. 2011; Nash et al. 2018; Kohler et al. 2020), the genus Polaromonas are amongst the dominant bacteria of glacial ice and sediments worldwide. Strains of Polaromonas from seasonally cold soils are able to oxidise a wide array of unusual energy sources including H2 (Sizova and Panikov 2007) and a broad range of recalcitrant organic compounds (Jeon et al. 2003; Mattes et al. 2008; Yagi et al. 2009). A high metabolic versatility allows Polaromonas to adapt to shifting availabilities of energy sources in periglacial environments (Jeon et al. 2003; Sizova and Panikov 2007; Mattes et al. 2008; Yagi et al. 2009; Osborne et al. 2010; Yang et al. 2015).
Lake A, which is situated at the longest distance away from the glacier, was characterised by contrasting physicochemical parameters and bacterial community structure, as compared to the other tested lakes. As was mentioned above, the highest concentration of C, N, P, and other elements in lake A should provide a bacterial community with completely different conditions. It is important to note that this lake differed from other tested lakes by swamping (Table 1, Online Resource 2). According to (Lin et al. 2012), who compared the composition and activity of microbial communities at bog and fen sites in the Glacial Lake Agassiz Peatland of northwestern Minnesota, USA, where both bacterial and archaeal richness and rRNA gene abundance were > 2 times higher on average in the fen than in the bog (Lin et al. 2012). Our data indicated that the bacterial community diversity and richness in the lake A were lower than in other tested lakes (Figs. 3 and 5).
At the same time, lake A exhibited a completely different bacterial community structure in comparison with the other lakes. Indeed, only this lake was characterised by the highest relative abundance of Proteobacteria (64.23%) and Bacillariophyta (15.62%) (Fig. 5A, B). Proteobacteria have been reported to dominate in microbial communities of glacial lakes environments (Kohler et al. 2020; Sułowicz et al. 2020). Despite the contrasting physicochemical characteristics of the tested lakes, the abundance of Proteobacteria in the lake E was lower (15%) as compared to lake A. Another dominant phylum Bacillariophyta, found in all tested lakes, had a similar trend in its abundance, i.e., decreasing towards the glacier. The factors affecting diatom diversity in glacial environments has recently been studied in the context of ongoing warming in cold environments (Huang et al. 2020). Phytoplankton community succession in a temperate aquatic ecosystem was recently studied by (Kim et al. 2020). The decrease in biomass of Bacillariophyta over 24 years was proposed to be due precipitation, water level and total nitrogen for taxonomic groups, whilst for functional groups, it was total nitrogen, total phosphorus and euphotic zone depth (Kim et al. 2020).
Methylomicrobium alcaliphilum was detected in lake A with a rather high concentration, i.e., 21.01%. (Fig. 5C). These bacteria belong to the family Methylococcaceae. Other authors reported that Methylococcales was the most abundant and active component of the subglacial microbial community in the meltwater of the Russell Glacier situated at the western margin of the Greenland Ice Sheet (Dieser et al. 2014). Osudar et al. (2016) studied the processes of methane oxidation in five Arctic lake systems on Samoylov Island (Lena Delta, Siberia). A high variation in the methane concentrations was shown, with the highest values detected in the lake outlets and in a lake complex in a flood plain area (Osudar et al. 2016). In our study, the Methylococcaceae family was found almost exclusively in the lake A (data not shown). Most probably, this fact can be attributed to the formation of anaerobic conditions and, therefore, the presence of methane.
It is important to note, that lake A is likely not connected with glacial meltwater. It is situated in a swampy territory with completely different hydrological characteristics, as compared to the other tested lakes. Particularly the area in which lake A is located, is known as an area of discontinuous permafrost (Thorhallsdóttir 1996; Saemundsson et al. 2012; Benediktsson et al. 2015). Besides, a river fed by glacial meltwater at a distance of 300 m apart from lake A, is expected to influence the physicochemical and microbiological conditions in lake A, due to the possible hydrological connectivity in this bog area.
Another important factor for analysis of bacterial ecology in glacial lakes is seasonal changes. As shown in Online Resource 3, the lake sediments for this study were collected in August 2017, at the average air temperature 11.4 ± 2.6 °C and lake water temperatures 7–11 °C (Table 1). The lowest average air temperature in 2017 was detected in January (–0.5 °C) and the highest was in July (13.2 °C), respectively (Online Resource 3). Besides, diurnal changes could notably increase the influence of temperature changes on microbial community structure and activity, especially at short distances from the glacier. Bertilsson et al. (2013) reviewed the current knowledge on microorganisms in seasonally frozen lakes and found that seasonal changes considerably influenced the concentration of dissolved oxygen, density-driven vertical and horizontal mixing processes and other parameters, that directly impact the bacterial community structure (Bertilsson et al. 2013).
Conclusions
Summarising the results on microbial diversity in five glacial lakes near the Múlajökull glacier, the following conclusions were made:
-
1.
The Shannon diversity indices for microbial communities of five lake sediments were determined using both shotgun metagenomic sequencing and metabolic activity of culturable microorganisms. The Shannon diversity indices at the phylum level varied in range from 1.69 to 1.89, whilst at the species level from 4.02 to 5.40, with the lowest value at both levels being in sample A. The lowest Shannon index of metabolic diversity (i.e., 1.99) was found in sample C, compared with other lakes where the Shannon index varied from 2.6 to 2.9.
-
2.
Each lake was characterised by a unique assemblage. PCA biplot analysis showed that on the phylum level, the spots representing lakes B, C and D clustered together, whilst spots representing lakes A and E were located a considerable distance away from other data points.
-
3.
Cyanobacteria were represented in all lake sediments tested, however, their diversity and relative abundance were different. Thus, a relative abundance of Cyanobacteria was represented by Anabaena sp. with the highest concentration in the lake E (23.23%), Dolichospermum sp. with the highest concentration in the lake E (15.7%) and Calothrix parietina with the highest concentration in the lake C (1.9%).
-
4.
A relatively low abundance of Polaromonas was found in the sediments of the tested lakes (max. 1.86% in lake A). These data differed from those shown for glacial lakes situated at other geographical locations worldwide, where Polaromonas were found to be amongst the dominant bacteria of glacial ice and sediments. This fact indicates to the site-specific relationships in the psychrophilic microbial ecology of Múlajökull lakes. The revealing of such differences brings new knowledge related to the cold biosphere on the local and global Earth systems.
Data availability
On request.
Code availability
Not applicable.
References
Aitchison J (1982) The statistical analysis of compositional data. J R Statist Soc B 44(2):139–177
Anesio AM, Hodson AJ, Fritz A et al (2009) High microbial activity on glaciers: importance to the global carbon cycle. Glob Chang Biol 15(4):955–960. https://doi.org/10.1111/j.1365-2486.2008.01758.x
Anesio AM, Lutz S, Chrismas NAM, Benning LG (2017) The microbiome of glaciers and ice sheets. NPJ Biofilms Microbiomes 3:10. https://doi.org/10.1038/s41522-017-0019-0
Bellas CM, Anesio AM, Telling J et al (2013) Viral impacts on bacterial communities in Arctic cryoconite. Environ Res Lett 8:9pp. https://doi.org/10.1088/1748-9326/8/4/045021
Belov AA, Cheptsov VS, Manucharova NA, Ezhelev ZS (2020) Bacterial communities of novaya zemlya archipelago ice and permafrost. Geosci 10(2):67. https://doi.org/10.3390/geosciences10020067
Benediktsson ÍÖ, Schomacker A, Johnson MD et al (2015) Architecture and structural evolution of an early Little Ice Age terminal moraine at the surge-type glacier Múlajökull, Iceland. J Geophys Res F Earth Surf 120(9):1895–1910. https://doi.org/10.1002/2015JF003514
Benediktsson ÍÖ, Jónsson SA, Schomacker A et al (2016) Progressive formation of modern drumlins at Múlajökull, Iceland: stratigraphical and morphological evidence. Boreas 45(4):567–583. https://doi.org/10.1111/bor.12195
Bertilsson S, Burgin A, Carey CC et al (2013) The under-ice microbiome of seasonally frozen lakes. Limnol Oceanogr 58(6):1998–2012. https://doi.org/10.4319/lo.2013.58.6.1998
Björnsson H, Pálsson F, Sigurdsson O, Flowers GE (2003) Surges of glaciers in Iceland. Ann Glaciol 36:82–90. https://doi.org/10.3189/172756403781816365
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30(15):2114–2120. https://doi.org/10.1093/bioinformatics/btu170
Callieri C, Modenutti B, Queimaliños C et al (2007) Production and biomass of picophytoplankton and larger autotrophs in Andean ultraoligotrophic lakes: differences in light harvesting efficiency in deep layers. Aquat Ecol 41:511–523. https://doi.org/10.1007/s10452-007-9125-z
Cameron KA, Stibal M, Zarsky JD et al (2016) Supraglacial bacterial community structures vary across the Greenland ice sheet. FEMS Microbiol Ecol 92(2). https://doi.org/10.1093/femsec/fiv164
Cameron KA, Stibal M, Hawkings JR et al (2017) Meltwater export of prokaryotic cells from the Greenland ice sheet. Environ Microbiol 19(2):524–534. https://doi.org/10.1111/1462-2920.13483
Darcy JL, Lynch RC, King AJ et al (2011) Global distribution of Polaromonas phylotypes - evidence for a highly successful dispersal capacity. PLoS ONE 6(8):e23742. https://doi.org/10.1371/journal.pone.0023742
De Maayer P, Anderson D, Cary C, Cowan DA (2014) Some like it cold: Understanding the survival strategies of psychrophiles. EMBO Rep 15:508–517. doi:https://doi.org/10.1002/embr.201338170
Dieser M, Broemsen ELJE, Cameron KA et al (2014) Molecular and biogeochemical evidence for methane cycling beneath the western margin of the Greenland Ice Sheet. ISME J 8:2305–2316. https://doi.org/10.1038/ismej.2014.59
Echeverría-Vega A, Chong G, Serrano AE et al (2018) Watershed-induced limnological and microbial status in two oligotrophic andean lakes exposed to the same climatic scenario. Front Microbiol 9:357. https://doi.org/10.3389/fmicb.2018.00357
Egholm DL, Pedersen VK, Knudsen MF, Larsen NK (2012) Coupling the flow of ice, water, and sediment in a glacial landscape evolution model. Geomorphology 141–142:47–66. https://doi.org/10.1016/j.geomorph.2011.12.019
Egozcue JJ, Pawlowsky-Glahn V, Mateu-Figueras G, Barceló-Vidal C (2003) Isometric logratio transformations for compositional data analysis. Math Geol 35:279–300. https://doi.org/10.1023/A:1023818214614
Finlayson A, Phillips E, Benediktsson ÍÖ et al (2019) Subglacial drumlins and englacial fractures at the surge-type glacier, Múlajökull, Iceland. Earth Surf Proc Land 44(1):367–380. https://doi.org/10.1002/esp.4485
Franzosa EA, McIver LJ, Rahnavard G et al (2018) Species-level functional profiling of metagenomes and metatranscriptomes. Nat Methods 15:962–968. https://doi.org/10.1038/s41592-018-0176-y
Gabor EM, De Vries EJ, Janssen DB (2003) Efficient recovery of environmental DNA for expression cloning by indirect extraction methods. FEMS Microbiol Ecol 44(2):153–163. https://doi.org/10.1016/S0168-6496(02)00462-2
Gaidelene J, Lamsters K, Karušs J, Krievāns M, Rečs A. Muter O (2018) A metabolic profile of microbial communities in benthic sediments originated from glacial meltwater and ice-dammed lakes. Abstract of the 75th Scientific Conference of the University of Latvia. Environ Exp Biol 16:93–94. doi:https://doi.org/10.22364/eeb.16.09
Gallegos CL, Davies-Colley RJ, Gall M (2008) Optical closure in lakes with contrasting extremes of reflectance. Limnol Oceanogr 53(5):2021–2034. https://doi.org/10.4319/lo.2008.53.5.2021
García-Laviña CX, Castro-Sowinski S, Ramón A (2019) Reference genes for real-time RT-PCR expression studies in an Antarctic Pseudomonas exposed to different temperature conditions. Extremophiles 23:625–633. https://doi.org/10.1007/s00792-019-01109-4
Gura C, Rogers SO (2020) Metatranscriptomic and metagenomic analysis of biological diversity in subglacial lake vostok (Antarctica). Biology (Basel) 9(3):55. https://doi.org/10.3390/biology9030055
Huang S, Herzschuh U, Pestryakova LA et al (2020) Genetic and morphologic determination of diatom community composition in surface sediments from glacial and thermokarst lakes in the Siberian Arctic. J Paleolimnol 64:225–242. https://doi.org/10.1007/s10933-020-00133-1
Ibrar I, Yadav S, Altaee A et al (2020) Treatment of biologically treated landfill leachate with forward osmosis: investigating membrane performance and cleaning protocols. Sci Total Environ 744:140901. https://doi.org/10.1016/j.scitotenv.2020.140901
ISO 11277:1998 Soil quality — determination of particle size distribution in mineral soil material — method by sieving and sedimentation. In: 13.080.20 Physical properties of soils. p 30
Iverson NR, McCracken RG, Zoet LK et al (2017) A theoretical model of drumlin formation Based on Observations at Múlajökull Iceland. J Geophys Res Earth Surf 122(12):2302–2323. https://doi.org/10.1002/2017JF004354
Ives LRW, Iverson NR (2019) Genesis of glacial flutes inferred from observations at Múlajökull. Iceland Geology 47(5):387–390. https://doi.org/10.1130/G45714.1
Jeon CO, Park W, Padmanabhan P et al (2003) Discovery of a bacterium, with distinctive dioxygenase, that is responsible for in situ biodegradation in contaminated sediment. Proc Natl Acad Sci 100(23):13591–13596. https://doi.org/10.1073/pnas.1735529100
Jóhannesson T, Björnsson H, Magnússon E et al (2013) Ice-volume changes, bias estimation of mass-balance measurements and changes in subglacial lakes derived by lidar mapping of the surface of Icelandic glaciers. Ann Glaciol 54(63):63–74. https://doi.org/10.3189/2013AoG63A422
Johnson MD, Schomacker A, Benediktsson ÍÖ et al (2010) Active drumlin field revealed at the margin of Múlajökull, Iceland: a surge-type glacier. Geology 38(1):943–946. https://doi.org/10.1130/G31371.1
Jónsson SA, Schomacker A, Benediktsson ÍÖ et al (2014) The drumlin field and the geomorphology of the Múlajökull surge-type glacier, central Iceland. Geomorphology 207:213–220. https://doi.org/10.1016/j.geomorph.2013.11.007
Kim HG, Hong S, Kim DK, Joo GJ (2020) Drivers shaping episodic and gradual changes in phytoplankton community succession: taxonomic versus functional groups. Sci Total Environ 734:138940. https://doi.org/10.1016/j.scitotenv.2020.138940
Kohler TJ, Vinšová P, Falteisek L et al (2020) Patterns in microbial assemblages exported from the meltwater of Arctic and Sub-Arctic glaciers. Front Microbiol 11:669. https://doi.org/10.3389/fmicb.2020.00669
Lamsters K, Karušs J, Rečs A, Bērziņš D (2016) Detailed subglacial topography and drumlins at the marginal zone of Múlajökull outlet glacier, central Iceland: Evidence from low frequency GPR data. Polar Sci 10(4):470–475. https://doi.org/10.1016/j.polar.2016.05.003
Langmead B, Salzberg SL (2012) Fast gapped-read alignment with Bowtie 2. Nat Methods 9:357–359. https://doi.org/10.1038/nmeth.1923
Leon C, Campos V, Urrutia R, Mondaca MA (2012) Metabolic and molecular characterization of bacterial community associated to Patagonian Chilean oligotrophic-lakes of quaternary glacial origin. World J Microbiol Biotechnol 28:1511–1521. https://doi.org/10.1007/s11274-011-0953-6
Lin X, Green S, Tfaily MM et al (2012) Microbial community structure and activity linked to contrasting biogeochemical gradients in bog and fen environments of the glacial lake agassiz peatland. Appl Environ Microbiol 78(19):7023–7031. https://doi.org/10.1128/AEM.01750-12
Mackelprang R, Saleska SR, Jacobsen CS et al (2016) Permafrost meta-omics and climate change. Annu Rev Earth Planet Sci 44:439–462. https://doi.org/10.1146/annurev-earth-060614-105126
Martin M (2011) Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J 17(1):10–12. doi:https://doi.org/10.14806/ej.17.1.200
Martín-Fernández JA, Hron K, Templ M et al (2015) Bayesian-multiplicative treatment of count zeros in compositional data sets. Stat Modell 15(2):134–158. https://doi.org/10.1177/1471082X14535524
Mattes TE, Alexander AK, Richardson PM et al (2008) The genome of Polaromonas sp. strain JS666: insights into the evolution of a hydrocarbon- and xenobiotic-degrading bacterium, and features of relevance to biotechnology. Appl Environ Microbiol 74(20): 6405–6416. doi:https://doi.org/10.1128/AEM.00197-08
McCracken RG, Iverson NR, Benediktsson Í et al (2016) Origin of the active drumlin field at Múlajökull, Iceland: new insights from till shear and consolidation patterns 148: 243–260. Quat Sci Rev. https://doi.org/10.1016/j.quascirev.2016.07.008
Nash MV, Anesio AM, Barker G et al (2018) Metagenomic insights into diazotrophic communities across Arctic glacier forefields. FEMS Microbiol Ecol 94(9). doi:https://doi.org/10.1093/femsec/fiy114
Nemergut DR, Anderson SP, Cleveland CC et al (2007) Microbial community succession in an unvegetated, recently deglaciated soil. Microb Ecol 53:110–122. https://doi.org/10.1007/s00248-006-9144-7
Osborne TH, Jamieson HE, Hudson-Edwards KA et al (2010) Microbial oxidation of arsenite in a subarctic environment: diversity of arsenite oxidase genes and identification of a psychrotolerant arsenite oxidiser. BMC Microbiol 10:205. https://doi.org/10.1186/1471-2180-10-205
Osudar R, Liebner S, Alawi M et al (2016) Methane turnover and methanotrophic communities in arctic aquatic ecosystems of the Lena Delta, Northeast Siberia. FEMS Microbiol Ecol 92(8). doi:https://doi.org/10.1093/femsec/fiw116
Papke RT, Ramsing NB, Bateson MM, Ward DM (2003) Geographical isolation in hot spring cyanobacteria. Environ Microbiol 5(8):650–659. https://doi.org/10.1046/j.1462-2920.2003.00460.x
Parro V, Blanco Y, Puente-Sanchez F, Rivas LA, Moreno-Paz M, Echeverrıa A, Chong-Dıaz G, Demergasso C, CNA, (2018) Biomarkers and metabolic patterns in the sediments of evolving glacial lakes as a proxy for planetary lake exploration. Astrobiology 18(5):586–606. https://doi.org/10.1089/ast.2015.1342
Peter H, Sommaruga R (2016) Shifts in diversity and function of lake bacterial communities upon glacier retreat. ISME J 10:1545–1554. https://doi.org/10.1038/ismej.2015.245
Rodrigues DF, Da C Jesus E, Ayala-Del-Río HL et al (2009) Biogeography of two cold-adapted genera: psychrobacter and Exiguobacterium. ISME J 3:658–665. Doi:https://doi.org/10.1038/ismej.2009.25
Rose KC, Hamilton DP, Williamson CE et al (2014) Light attenuation characteristics of glacially-fed lakes. J Geophys Res Biogeosciences 119(2):1446–1457. https://doi.org/10.1002/2014JG002674
Saeed AI, Sharov V, White J et al (2003) TM4: a free, open-source system for microarray data management and analysis. Biotechniques 34:374–378. https://doi.org/10.2144/03342mt01
Saemundsson T, Arnalds O, Kneisel C et al (2012) The Orravatnsrustir palsa site in Central Iceland-Palsas in an aeolian sedimentation environment. Geomorphology 167–168:13–20. https://doi.org/10.1016/j.geomorph.2012.03.014
Säwström C, Mumford P, Marshall W et al (2002) The microbial communities and primary productivity of cryoconite holes in an Arctic glacier (Svalbard 79 N). Polar Biol 25:591–596. https://doi.org/10.1007/s00300-002-0388-5
Schmidt SK, Lynch RC, King AJ et al (2011) Phylogeography of microbial phototrophs in the dry valleys of the high Himalayas and Antarctica. Proc R Soc B Biol Sci 278:702–708. https://doi.org/10.1098/rspb.2010.1254
Sigurkarlsson MF (2015) Surge history of Múlajökull, Iceland, since 1945 detected with remote sensing data. 10 ECTS Thesis, Faculty of Earth Sciences, University of Iceland, Reykjavik. 33p. https://skemman.is/bitstream/1946/20434/1/Magn%c3%bas%20Freyr%20Sigurkarlsson.pdf
Simon C, Wiezer A, Strittmatter AW, Daniel R (2009) Phylogenetic diversity and metabolic potential revealed in a glacier ice metagenome. Appl Environ Microbiol 75(23):7519–7526. https://doi.org/10.1128/AEM.00946-09
Sizova M, Panikov N (2007) Polaromonas hydrogenivorans sp. nov., a psychrotolerant hydrogen-oxidizing bacterium from Alaskan soil. Int J Syst Evol Microbiol 57:616–619. https://doi.org/10.1099/ijs.0.64350-0
Smith HJ, Dieser M, McKnight DM et al (2018) Relationship between dissolved organic matter quality and microbial community composition across polar glacial environments. FEMS Microbiol Ecol 94(7). doi:https://doi.org/10.1093/femsec/fiy090
Sułowicz S, Bondarczuk K, Ignatiuk D et al (2020) Microbial communities from subglacial water of naled ice bodies in the forefield of Werenskioldbreen. Svalbard Sci Total Environ 723:138025. https://doi.org/10.1016/j.scitotenv.2020.138025
Suzek BE, Wang Y, Huang H et al (2015) UniRef clusters: a comprehensive and scalable alternative for improving sequence similarity searches. Bioinformatics 31(6):926–932. https://doi.org/10.1093/bioinformatics/btu739
Telling J, Stibal M, Anesio AM et al (2012) Microbial nitrogen cycling on the Greenland Ice Sheet. Biogeosciences 9:2431–2442. https://doi.org/10.5194/bg-9-2431-2012
Teufel AG, Li W, Kiss AJ, Morgan-Kiss RM (2017) Impact of nitrogen and phosphorus on phytoplankton production and bacterial community structure in two stratified Antarctic lakes: a bioassay approach. Polar Biol 40:1007–1022. https://doi.org/10.1007/s00300-016-2025-8
Thorhallsdóttir TE (1996) Seasonal and annual dynamics of frozen ground in the central highland of Iceland. Arct Alp Res 28(2):237–243. https://doi.org/10.2307/1551765
Thorhallsdóttir TE (1998) Seasonal and annual dynamics of frozen ground, central highland of Iceland, Version 1. Boulder: National Snow and Ice Data Center, NSIDC. doi:https://doi.org/10.7265/m78z-mn18. Accessed 02 Oct 2020
Urakawa H, Ali J, Ketover RDJ, Talmage SD, Garcia JC, Campbell IS, Loh ANPML (2013) Shifts of bacterioplankton metabolic profiles along the salinity gradient in a subtropical estuary. Oceanography. https://doi.org/10.5402/2013/410814
Wood DE, Salzberg SL (2014) Kraken: ultrafast metagenomic sequence classification using exact alignments. Genome Biol 15:R46. https://doi.org/10.1186/gb-2014-15-3-r46
Yagi JM, Sims D, Brettin T et al (2009) The genome of Polaromonas naphthalenivorans strain CJ2, isolated from coal tar-contaminated sediment, reveals physiological and metabolic versatility and evolution through extensive horizontal gene transfer. Environ Microbiol 11(9):2253–2270. https://doi.org/10.1111/j.1462-2920.2009.01947.x
Yallop ML, Anesio AM, Perkins RG et al (2012) Photophysiology and albedo-changing potential of the ice algal community on the surface of the Greenland ice sheet. ISME J 6:2302–2313. https://doi.org/10.1038/ismej.2012.107
Yang J, Tezel U, Li K, Pavlostathis SG (2015) Prolonged exposure of mixed aerobic cultures to low temperature and benzalkonium chloride affect the rate and extent of nitrification. Bioresour Technol 179:193–201. https://doi.org/10.1016/j.biortech.2014.12.030
Yang GL, Hou SG, Le Baoge R et al (2016) Differences in bacterial diversity and communities between glacial snow and glacial soil on the Chongce Ice Cap. West Kunlun Mountains Sci Rep 6:36548. https://doi.org/10.1038/srep36548
Zhang Z, Wang J, Wang J, Wang J, Li Y (2020) Estimate of the sequenced proportion of the global prokaryotic genome. Microbiome 8:134. https://doi.org/10.1186/s40168-020-00903-z
Zhou L, Zhou Y, Yao X et al (2020) Decreasing diversity of rare bacterial subcommunities relates to dissolved organic matter along permafrost thawing gradients. Environ Int 134:105330. https://doi.org/10.1016/j.envint.2019.105330
Acknowledgements
The expedition to Iceland was realized in cooperation with SIA Armgate. We thank Agnis Rečs for assistance during fieldwork and Kristaps Auziņš and Ieva Kalka for laboratory analyses. We thank the anonymous reviewers for their valuable comments on our manuscript.
Funding
Financial support was received from the University of Latvia within the “Climate change and sustainable use of natural resources” programme and by the specific support objective activity 1.1.1.2. “Post-doctoral Research Aid” (Project id. No.1.1.1.2/16/I/001) of the Republic of Latvia, funded by the European Regional Development Fund, PostDoc Kristaps Lamsters research project No.1.1.1.2/VIAA/1/16/118.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. KL: conceived and designed research, collected the samples, described the geological setting, wrote the manuscript; JK, MK: designed research, collected the samples; MU, LB, IS, DF: performed the shotgun metagenome sequencing, bioinformatics and statistical analysis, wrote the manuscript; JG: tested the functional diversity of culturable microorganisms; RK – provided the chemical characterisation of samples; OM: tested the activity of culturable microorganisms, wrote the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Conflicts of interest
The Authors declare that there is no conflict of interest.
Consent to participate
All authors read and approved the manuscript.
Consent for publication
All authors agreed to publish these data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lamsters, K., Ustinova, M., Birzniece, L. et al. Bacterial and archaeal community structure in benthic sediments from glacial lakes at the Múlajökull Glacier, central Iceland. Polar Biol 43, 2085–2099 (2020). https://doi.org/10.1007/s00300-020-02770-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-020-02770-8