Abstract
The first non-coastal Sub-Antarctic Marine Protected Area (Namuncurá) in Argentina was created in 2013, at Burdwood Bank (MPAN-BB), an undersea plateau located about 200 km south from Malvinas/Falkland Islands, SW Atlantic Ocean. The main contribution of this work was to explore fish species composition and the structure of fish assemblages in three different zones of the MPAN-BB with different conservation strategies and different surrounding areas. Twenty-two fishing trawls were performed using a demersal bottom trawl pilot net at depths between 71 and 608 m. A total of 667 fish belonging to 30 species were collected in the surveyed area. The richest family in terms of species number was Nototheniidae (seven species), followed by Macrouridae, Myxinidae and Zoarcidae (four species each), then Moridae and Arhynchobatidae (three species each), and finally Muraenolepididae and Psychrolutidae (two species each). The remaining families were represented by a single species. Three significantly different fish assemblages were detected. These distinct assemblages were largely circumscribed at the plateau, the shelf-break slope, and the area west of the BB. The results showed that fish diversity in the MPAN-BB is relatively high constituting ~ 10% of the fish composition reported for the Atlantic sector of the Magellanic Province. The present data suggest that fishes are an important component of the benthic community planned to be protected by the implementation of the MPAN-BB. These findings have important implications for habitat preservation and threatened species conservation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Marine Protected Areas (MPAs) are powerful management tools used around the world to conserve marine species and habitats (Dehens and Fanning 2018). Some or all activities performed beyond MPAs of the oceans are limited or forbidden in MPAs to protect natural and cultural resources found within the boundaries of the MPA (Lubchenco et al. 2003). Moreover, the formation of reserves aids to restore the structure and complexity of the ecosystem in those MPAs that have been under overexploitation pressure previous to reserve formation (Sala and Giakoumi 2017). A bulk of the research has demonstrated that after intense fishing activities, ecosystems usually undergo drastic changes in fish assemblage structure and composition (Hinz et al. 2009). For example, fish biomass in marine reserves is, on average, 670% greater than in adjacent unprotected areas, and approximately 343% greater than in partially-protected MPAs (Sala and Giakoumi 2017). Research focused on fish assemblages, especially in MPAs, have been significantly recognized and are highly encouraged, since they are sensitive indicators of habitat deterioration, ecosystem productivity and general environmental change (Holbrook et al. 1994). Indeed, MPAs must be at the center of any scenario intending to insert fisheries on an ecologically sustainable basis (Pauly 2009). The number of MPAs in Latin America have increased since the 1980s (Guarderas et al. 2008), but they are still far short of the United Nations goal of having 10% of the world’s oceans protected by 2020 (Sala and Giakoumi 2017).
In 2013, Argentina declared the first MPA within its Exclusive Economic Zone (EEZ) named “Namuncurá” (NMPA), located at Burdwood Bank (BB) (Argentina National Congress Law 26,875) (hereafter MPAN-BB). After creation of the MPAN-BB, coverage of marine protected waters within the EEZ of Argentina increased from 0.9 to 2.8%. The MPAN-BB is located about 200 km south of the Malvinas/Falkland Islands and 150 km east of Isla de los Estados (Argentina). The BB is a large plateau of 34,000 km2 bounded by the 200-m isobath between 53°30′ S-55° S; 55° W-62° W in the SW Atlantic Ocean. The BB is part of the North Scotia Ridge, where its shallow location, together with the influence of sub-Antarctic waters (between 4 and 7 °C) and seabed features, generate fronts and upwelling zones supporting high concentrations of nutrients and oxygen saturation throughout the year (Martin and Veccia 2014). Given such features, the MPAN-BB plays a significant role as a barrier to circumpolar oceanic flow, promoting high productivity (Matano et al. 2019) and sustaining a rich and unique oceanic hot spot of benthic biodiversity (Schejter et al. 2016). The MPAN-BB is considered a sub-Antarctic area of ecological importance, where several biological processes are highlighted as ecologically relevant and the region identified as deserving of primary attention for its conservation (López Gappa 2000; Tatián et al. 2005; Zamponi 2008a, b; Schejter et al. 2012, 2016; Falabella et al. 2013).
The demersal fish assemblages of the MPAN-BB and its continental slope are poorly known. Previous studies have provided preliminary taxonomic checklists of the fish diversity (Zunino and Ichazo 1979; Gosztonyii 1981; Shejter et al. 2016) reporting a total of 12 fish species. The ichthyofauna in the MPAN-BB is dominated by sub-Antarctic species such as Patagonotothen elegans, Patagonotothen guntheri, Patagonotothen ramsayi, Dissostichus eleginoides, Cottoperca trigloides and Micromesistius australis (Laptikhovsky 2004; Knust et al. 2012).
Baseline information on biodiversity, species abundance, and distribution in protected areas is increasingly essential to contribute to decisions about MPAs and to assess future changes resulting from global and regional processes. The key roles that MPAs can play in mitigating and adapting to the effects of climate change are currently being evaluated (Roberts et al. 2017). Due to the rate of habitat degradation and effects of climate warming being observed in different regions of the world, and the associated shift in species distributions, improving our knowledge about biodiversity and biological traits of fish species in MPAs is mandatory for monitoring and assessment purposes. In this respect, the MPAN-BB is a very recent reserve, which deserves priority in biodiversity research if this reserve is intended to be an effective area of conservation for marine organisms. A comprehensive baseline study is needed to update and improve our knowledge about the fish fauna occurring in this area. As a first step towards this end, the main contribution of this work is to explore the composition and structure of the assemblage of fish species inhabiting three different zones of the MPAN-BB that differ from each other in conservation strategies and surrounding areas.
Materials and methods
Study area
The study area spans a longitudinal range from Tierra del Fuego (Argentina) to the MPAN-BB (67°–55° W) where waters of different properties and origin occur (Fig. 1). For instance, estuarine waters of 26–30 psu from the mixing of Pacific salt waters, ice melting and continental runoff characterize the Beagle Channel (Balestrini et al. 1998). Water masses on the northern region of the Drake Passage are diluted owing to a surplus of rainfall over the southeastern Pacific Ocean and the continental discharging throughout coastal South America. Flow towards the Atlantic Ocean, called the Cape Horn Current, enters onto the continental shelf through the Le Maire strait, which contributes to the waters surrounding the Isla de los Estados and to the low salinity waters south of the Magellan Strait (Acha et al. 2004). On the other hand, the MPAN-BB is part of the North Scotia Ridge, where the Malvinas/Falklands current with its cooler and more saline waters influences the area (Acha et al. 2004). Also, the Antarctic Circumpolar Current is then deflected towards the north-east, splits into two branches, which contours the bank. Likewise, the Burdwood Bank acts as an active center for the obduction of deep waters and the flow of deep waters is primarily driven by tides and local wind (Matano et al. 2019).
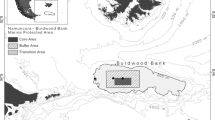
Location of the study area and sampling stations along the longitudinal gradient from Tierra del Fuego to the Namuncurá Sub-Antarctic Marine Protected Area—Burdwood Bank (NMPA-BB) during the March–April-2016 research cruise on board the RV “Puerto Deseado”. LMS: Le Maire Strait, CH: Cape Horn. Inset shows the three areas of the NMPA-BB according to the management zones based on the Law 26,875
Criteria of the ecological vulnerability of MPAs are mainly based on the existence of necessary high biomass of sessile taxa (Falabella et al. 2013; Schejter et al. 2016). According to these criteria, the MPAN-BB is divided into three-grade levels of protection: The core area (Fig. 1), which has strict protection due to the vulnerable characteristics presented by the ecosystem components and, therefore, no activities are allowed, except for control and monitoring. The buffer area, surrounding the core area where authorized activities (like scientific research, explorations dealing with natural resources and biodiversity, sustainable resources management, restoration and monitoring of global change) are allowed. And the third and last zone, the external transition area, which surrounds the buffer area out to the 200 m-isobath, where productive and extractive activities considered in the management plan are allowed (Falabella et al. 2013; Schejter et al. 2016).
Biological and environmental sampling
Fishes were collected in the austral autumn (March–April, 2016) onboard the Oceanographic Vessel “Puerto Deseado” (CONICET, Argentina). A demersal bottom trawl pilot net (6 m in total length, with 25 mm mesh on the wings and 10 mm in the cod-end, with 0.25 m2 of otter board surface, 12 kg of otter board weight, 0.6 m and 1.8 m for vertical and horizontal aperture, respectively) was used to collect fishes (Llompart et al. 2015). A total of 22 fishing trawls were performed (Table 1), with a 15-min-target for bottom time for each bottom trawl. The speed of trawling ranged between 2 and 3.7 knots. Three main zones of sampling were surveyed to compare fish assemblages restricted to- or outside of- the MPAN-BB. Trawls were performed in 13 sampling stations at the BB plateau (BBP), in 6 sampling stations at the Tierra del Fuego shelf and Isla de los Estados (TDS), and in 3 sampling stations at the shelf-break immediately south of the limit of the reserve (BBSB). At each sampling station, all fish were counted and identified to the lowest possible taxonomic level following Gon and Heemstra (1990), Cousseau and Perrotta (2004) and Cousseau et al. (2007). For species names and their higher classification, Eschmeyer (2017) was followed.
Fish abundance was expressed as the total number of each species collected per 15 min of trawling. Temperature and salinity data were collected using a logging CTD (SBE-19, Sea-Bird Electronics Inc., Bellevue, USA), while depth was measured with a single-beam Kongsberg echo-sounder.
Data analysis
In order to determine the degree of dominance of fish species, the Olmstead-Tukey test (Sokal and Rohlf 1979) was calculated, taking into account the abundance and relative frequency of each species. To assess all of the fish species related to the fishing effort invested, an asymptotic Clench model was fitted (Soberón and Llorente 1993). It is recommended to apply this model mainly in large areas or for those taxa with high numbers of species, and therefore allows the possibility of adding new species over time, until the upper limit is reached. Non-linear regression was used to fit the model for the smoothed curve of the observed data obtained by rearranging the sampling stations 100 times using EstimateS 9.1.0 software (Colwell 2013). The predicted asymptote was calculated as a/b. The total of our fish inventory was assessed by calculating the proportion of the maximum number of species (asymptote) registered at the end of the fishing sampling (Moreno and Halffter 2000).
To assess spatial variations in the fish assemblage, a cluster analysis (CA) (Package clustsig; Whitaker and Christman 2014) using Bray/Curtis dissimilarity index, group average sorting (Krebs 1989) and the associated Similarity Profiles (SIMPROF) permutation test (Clarke et al. 2008) were applied using R 3.3.1 software (R Core Team 2017). This methodology provides a statistical basis for recognizing those points in the clustering performance at which further subdivision of samples is unwarranted (Clarke et al. 2008). The null hypothesis of no significant differences among groups of sampling stations and of species was rejected if the significance level (p) was < 0.01. Species abundances were log-transformed (log(x + 1)), and those species with less than 5% average occurrence frequency were omitted to reduce the effects of rare species in the analyses. The species composition between groups of sampling stations was compared using one-way analysis of similarities. If differences between the groups defined by the CA-SIMPROF were found, the similarity percentage analysis (SIMPER) (Package vegan, Oksanen et al. 2016) was used to determine which species were most responsible for the dissimilarity of Bray–Curtis.
In addition, the distance-based gradient redundancy analysis (dbRDA) based on Bray–Curtis resemblance matrix was used to model the relationship between species composition and temperature, salinity, depth and sampling groups as predictor variables. Fish data was log-transformed (log(x + 1)) and the environmental variables were square-root transformed to reduce their magnitude and normalize due to differences in measurements units. Sampling groups were treated as a dummy variable. The competitive models were compared using the AIC criterion, and the model with the lowest value of BIC was selected (Package vegan; Oksanen et al. 2016).
Results
Environmental sampling
The overall lowest bottom temperature values were recorded at the BBSB zone and ranged from 4.13 °C to 4.23 °C (n = 3, mean and SD: 4.19 °C ± 0.04). However, at the TDS and BBP zones the bottom temperature was more variable ranging from 3.79 °C to 9.01 °C (n = 6, mean and SD: 6.95 °C ± 2.02) and from 5.82 °C to 7.17 °C (n = 13, mean and SD: 6.45 °C ± 0.32), respectively (Table 1).
Bottom salinity at the TDS zone was variable, ranging from 32.34 to 34.24 psu (n = 6, mean and SD: 33.56 ± 0.63), whereas at the two remaining areas bottom salinity values were less variable. At the BBP zone, bottom salinity ranged from 33.98 to 34.12 psu (n = 13, mean and SD: 34.05 ± 0.04), and at the BBSB zone bottom water was saltier with a range of values from 34.18 to 34.24 psu (n = 3, mean and SD: 34.21 ± 0.03) (Table 1).
In terms of depth, shallower depths characterized the sampling stations at the BBP (n = 13, mean and SD: 130.61 m ± 35.38) compared to those at the TDS zone (n = 6, mean and SD: 238.5 m ± 192.15) and at the BBSB zone (n = 3, mean and SD: 602.33 m ± 151.09) (Table 1).
Fish assemblages of the NMPA-BB and surrounding areas
A total of 667 fish specimens, belonging to 13 families, 20 genera and 30 species were collected in the surveyed area. The richest family in terms of species number was Nototheniidae (seven species), followed by Macrouridae, Myxinidae and Zoarcidae (four species each), then Moridae and Arhynchobatidae (three species each), and finally Muraenolepididae and Psychrolutidae (two species each). The remaining families were represented by a single species each. The Olmstead-Tukey test revealed that only eight species were considered dominant (24%), while most species were considered rare (67%), and relatively few were considered occasional (9%) (Table 2). A comparison (Table 2) between species collected and bottom types reported in the surveyed area ( Schejter et al. 2016, 2017, 2018, 2019) showed that hard bottoms (rocky, coral garden) supported most species (n = 18) as would be expected from an environment offering a wide range of ecological niches. The next highest diversities of fishes were followed by those found on soft bottoms (sand, mud, silt substrates), which were relatively poor in species composition (n = 10). Only 6 species were collected on the two bottom types.
The longtail southern cod, P. ramsayi, had the widest distribution and this species was collected at all sampling stations, with its highest abundance at the Transition area. Of the seven species occurring only in the MPAN-BB, two (Bathyraja magellanica and Careproctus sp.) were restricted to the core area. Conversely, 11 species were only registered outside the MPAN-BB. Among them, Coelorinchus marinii, Macrourus carinatus, Muraenolepis orangiensis, Myxine debueni and Salilota australis were only collected at the BBSB. The species that were collected at the MPAN-BB as well as outside the BB were: Agonopsis chiloensis, C. trigloides, P. elegans, P. ramsayi and P. guntheri.
The species accumulation model fit well to the observed data (R = 0.99), and the fish inventory reached 70% of the theoretical species richness predicted by the Clench model at the level of fishing effort invested (22 fishing stations). The slope value of the asymptotic curve was greater than 0.1. Within our study area and with our methodology, the minimum extra effort required to reach the predicted lower limit of species richness was 63 sample stations (data not shown).
Three significantly (global R = 0.6, p < 0.01 for all pairwise comparisons) different fish assemblage associations were detected (I to III, Fig. 2). Group I constituted almost all sampling stations outside of MPAN-BB (TDS sampling stations: 4, 5, 11, 12 and 39). Group II consisted of sampling stations from the three areas (core, buffer and transition) of the BBP (sampling stations, 17, 23, 26, 27, 28, 30, 31, 32, 33, 34, 35, 36, and 38) and only one of the sampling stations of BBSB (sampling station: 40). Group III constituted nearly all sampling stations from the BBSB (18 and 21) and one close to Isla de los Estados (sampling station: 13).
Relationship between clusters of sampling stations and species dissimilarities and fish abundance (log (x + 1) transformed) along the longitudinal gradient from Tierra del Fuego (Argentina) to the NMPA-BB. Clustering of species and sampling stations were done using the Bray/Curtis dissimilarity measure and group average sorting (Krebs 1989). Colored squares reflect the abundance of each taxon in each sampling station. Open square 0 individuals, filled square 1 to 10 individuals, filled square 11 to 20 individuals, filled square 21 to 30 individuals and filled square > 31 individuals. See Table 2 for species abbreviations
Four fish species associations (a to d) were defined by the CA-SIMPROF procedure (Fig. 2). Fish species grouped in assemblage A (Zoarcidae, Myxinidae and Patagonotothen. cornucola) were more abundant at the BBP (Group II). Eleven fish species constituted fish assemblage B, most of which were more abundant at BBSB. Myxine debueni, S. australis and M. holotrachys were only present in this zone. Fish species constituting assemblage C (P. wiltoni and M. australis) were more abundant at the sampling stations west of the MPAN-BB. Finally, fish species in assemblage D (P. ramsayii, P. guntheri, P. elegans, C. trigloides and A. chiloensis) were mostly responsible for the observed differences between groups of sampling stations (SIMPER contribution > 74%; Table 3). Patagonotothen ramsayii, P. guntherii and P. elegans were more abundant at the BBP, while C. trigloides and A. chiloensis were more abundant at sampling stations from the TDS zone.
The biplot ordination diagram (Fig. 3) from the dbRDA axes analysis explained 38.5% of the total variation for the fish assemblages in the surveyed area. The marginal test was significant for each predictor variable (p < 0.02) and the best solution model only included the zone and depth. The dbRDA1 mainly discriminated sampling stations from BBP and BBSB and was 0.99 correlated with depth factor. The dbRDA2 axis separated those sampling stations outside the MPAN-BB with respect to the sampling stations within the BBP and was strongly correlated (- 0.99) with the zone variable.
Discussion
This work provides for the first time a fish checklist covering the entire surface of the unique MPA located within Argentinean Exclusive Economic Zone. General and historical information on oceanography, biodiversity and fisheries exploitation in the BB was compiled by Falabella et al. (2013), but only partial information of several taxonomic groups has been analyzed (Falabella et al. 2013). It is known that the MPAN-BB should be considered a hot spot of benthic richness and biodiversity, because of its high productivity (Schejter et al. 2016). This high productivity is affected by an upwelling, which occurs at the southeast of the Malvinas/Falkland Islands around Beauchêne Island and the BB, bringing nutrient-rich waters and plankton to the surface, resulting in abundant marine life (Acha et al. 2004). A similar situation occurs to the northwest near the Jason Islands. Waters from the west of the BB are influenced by a close shelf-break front; a recognized area of high productivity (Guerrero et al. 1999). As a result, the coastal waters of the shelf of Tierra del Fuego and Islas de los Estados have large biomasses of zooplankton and ichthyoplankton (Sánchez and Ciechomski 1995; Sabatini et al. 1999). Marine productivity in this area is particularly high and supports some of the largest concentrations of penguins, albatrosses, and sea mammals in the archipelago (Acha et al. 2004). Our results revealed that fish diversity in the MPAN-BB is relatively high constituting ~ 10% of the fish composition reported for the Atlantic sector of the Magellanic Province (Menni et al. 1984; Gon and Heemstra 1990; Wisner and Mc Millan 1995; Cousseau et al. 2007, 2012; Menni and Lucifora 2007). Moreover, a fish community characterized by a low number of dominant species and a majority of either uncommon or rare species is a widespread and general pattern described for several groups of organisms (Magurran et al. 2011). Recently, this pattern was also observed in an environment close to the MPAN-BB, the West Antarctic Peninsula (Llompart et al. 2015), where similar percentages of dominant and rare fish species were observed.
It is imperative to be able to achieve a complete inventory of the fish species present in the study area. The Olmstead Tukey’s test showed that nearly 70% of the species occurring in MPAN-BB should be considered as rare. According to the a/b value of the asymptotic curve estimated by the Clench model, more sampling effort using the pilot net and larger fishing nets, is needed to accomplish a more complete inventory of the fish species. We are aware that using the pilot net is not enough to fully characterize the fish diversity of the MPAN-BB, but still, in the present scenario, these data represent a valuable source of information and a very helpful starting point for upcoming research, conservation efforts and management. The use of the pilot bottom trawl net probably oversampled small and benthic fishes. Moreover, the goal of obtaining a more complete inventory of fishes is also hindered by the remoteness and large extent of the study area together with the unusual bathymetric pattern of the Scotia Arc region. In this sense, given the conservation priority that has been granted to MPAN-BB and surrounding areas, for the next five years expeditions researching fishes are planned that will include much more intensive sampling, including assessments of seasonal variability, different fishing gears (pelagic nets, baited traps, etc.), and stratification by zone and depth, to achieve a more comprehensive description of fish assemblage composition and dynamics. As a whole, the main goal of future expeditions is to determine the role of structuring organisms or ecosystem engineers (environment-forming community) to provide a distribution map of the assemblages that will allow a new re-zoning of the MPAN-BB and to produce indicators to evaluate the health status of the ecosystems found in the reserve and nearby areas.
The distributional ranges of several marine fish species have been affected by changes in seawater temperature (Attrill and Power 2002; Perry et al. 2005; Dulvy et al. 2008). Water temperature is a key issue in structuring species assemblages in sub-Antarctic environments (Pakhomov et al. 2006; Llompart et al. 2015). In the present work, water temperature was not significant in driving the composition of the fish assemblages of the MPAN-BB and adjacent area. When comparing fish assemblages at a larger biogeographic scale, it would be expected that a major role in determining spatial differences in fish assemblages would be exerted by thermophilic fishes, such as P. ramsayi; however, P. ramsayi was present at stations in all temperature ranges. This demonstrates that fishes occurring within a distinct habitat (e.g., oil platform, open seafloor) are part of a subset of individuals from a larger population distributed over a wider area of space and time (Schroeder and Love 2004).
Differences in fish assemblages between the MPAN-BB and adjacent areas indicated significant patterns of broad-scale variation in the assemblage structure. These patterns considerably changed with latitudinal and longitudinal gradients. This information is of primary importance for developing effective conservation strategies (Dayton 2003). Our results showed that topography plays a key role in driving the observed patterns in community structure, as indicated by the presence of three well-defined, separate demersal fish assemblages. These distinct assemblages were largely circumscribed at the plateau, the shelf-break and upper slope, and the region west of the BB. Indeed, fish distribution is known to be influenced by depth as well as bottom complexity in relation to predator avoidance or food access (e.g., Friedlander and Parrish 1998; Claudet et al. 2011).
Marine platforms influence topography of benthic habitats, patterns of water flow around them, and form substrata utilized by benthic organisms and their predators. Understanding the role that these marine platforms perform, especially in partnership with large-scale environmental drivers, on the ecology of fish populations and on the seasonal movement patterns of fishes is necessary to understand the impacts of such structures on the ecology of the fish community. Information about the size of populations and their genetics as well as more detailed studies on their ecology are necessary to explain the population and assemblage dynamics. This, in turn, is necessary to establish the conservation status and management requirements of the MPAN-BB species. Schejter et al. (2016, 2017) stated that the bottom of the plateau of the BB is highly heterogeneous presenting patches of rocky areas, (suitable substrates for most of the fish species studied here) dominated by bryozoans, hydrozoans, brachiopods, serpulid tubeworms, stylasterid corals and patches of coarse biogenic sand over which king crabs and some sponges occur. On the other hand, the rocky slope of the shelf-break (which reaches more than 3000 m in depth in some regions) of the BB is characterized by cold-water coral reefs, which perform a three-dimensional structure that may offer refuge from predators and increase food availability for many marine species, including fishes. These cold-water coral reefs are endemic organisms with high fragility and slow recovery following disturbances (Schejter et al. 2016). Therefore, extension of the area of the MPAN-BB to include the east and west slopes of the BB, where a rich benthic community was found (Arntz and Brey 2003), is highly recommended (Schejter et al. 2016).
In summary, our results suggest that fishes are an important component of the benthic community planned to be protected by the implementation of the MPAN-BB, and that some members of this group are restricted to the BB, just like other benthic organisms with less mobility (Schejter et al. 2016). These results have important implications for habitat preservation and threatened species conservation and suggest differences among mainland and undersea plateau locations. The implementation of the MPAN-BB within the margins of the BB (bounded by the 200-m isobath) is appropriate to protect the biodiversity inhabiting this offshore environment, including fishes. However, as fishes perform regular movements, the border effect must be taken into account.
References
Acha EM, Mianzan HW, Guerrero RA, Favero M, Bava J (2004) Marine fronts at the continental shelves of austral South America: physical and ecological processes. J Marine Syst 44:83–105. https://doi.org/10.1016/j.jmarsys.2003.09.005
Arntz W, Brey T (2003) Expedition Antarktis XIX/5 (LAMPOS) of RV ‘‘Polarstern’’ in 2002. Ber Polarforsch Meeresforsch 462:124
Attrill MJ, Power M (2002) Climatic influence on a marine fish assemblage. Nature 417:275. https://doi.org/10.1038/417275a
Balestrini C, Manzella G, Lovrich GA (1998) Simulacion de corrientes en el Canal Beagle y Bahia Ushuaia, mediante un modelo bidimensional. Serv Hidrog Naval Dto Oceanog Inf Tec 98:1–58
Clarke KR, Somerfield PJ, Gorley RN (2008) Testing of null hypotheses in exploratory community analyses: similarity profiles and biota-environment linkage. J Exp Mar Biol Ecol 366:56–69. https://doi.org/10.1016/j.jembe.2008.07.009
Claudet J (2011) Marine protected areas: a multidisciplinary approach. Cambridge University Press, London
Colwell RK (2013) EstimateS: Statistical estimation of species richness and shared species from samples. Version 9. User's Guide and application published at: https://purl.oclc.org/estimates
Cousseau MB, Perrotta RG (2004) Peces marinos de Argentina. Biología, distribución, pesca. Publishers INIDEP, Mar del Plata
Cousseau MB, Barbini SA, Figueroa DE (2012) The presence of southern fishes in the Argentinian continental shelf and adjacent areas. Mar Biodivers 42:73–78. https://doi.org/10.1007/s12526-011-0088-x
Cousseau MB, Figueroa DE, Díaz De Astarloa JM, Mabragaña E, Lucifora LO (2007) Rayas, chuchos y otros batoideos del Atlántico Sudoccidental (34°S-55°S). Publishers INIDEP, Mar del Plata
Dayton PK (2003) The importance of the natural sciences to conservation. Am Nat 162:1–13. https://doi.org/10.1086/376572
Dehens LA, Fanning LM (2018) What counts in making marine protected areas (MPAs) count? The role of legitimacy in MPA success in Canada. Ecol Indic 86:45–57. https://doi.org/10.1016/j.ecolind.2017.12.026
Dulvy NK, Rogers SI, Jennings S, Stelzenmüller V, Dye SR, Skjoldal HR (2008) Climate change and deepening of the North Sea fish assemblage: a biotic indicator of warming seas. J App Ecol 45:1029–1039. https://doi.org/10.1111/j.1365-2664.2008.01488.x
Eschmeyer WN, Fricke R, Van der Laan R (2017) Catalog of Fishes. California Academy of Sciences, San Francisco. https://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp
Falabella V, Campagna C, Caille G, Krapovickas S, Moreno D, Michelson A, Piola A, Schejter L, Zelaya D (2013) Banco Burdwood: Contribuciones para el establecimiento de una línea de base y plan de manejo de la futura Área Marina Protegida. Preliminar Report, p 51
Friedlander AM, Parrish JD (1998) Habitat characteristics affecting fish assemblages on a Hawaiian coral reef. J Exp Mar Biol Ecol 224:1–30. https://doi.org/10.1016/S0022-0981(97)00164-0
Gon O, Heemstra PC (1990) Fishes of the Southern Ocean. JLB Smith Inst Ichthyol, Grahamstown
Gosztonyi AE (1981) Results of the research cruises of FRV "Walther Herwig" to South America. LIX. Lycodonus malvinensis n. sp. (Pisces, Blennioidei), another new zoarcid fish from the western South Atlantic Ocean. Arch Fischereiwiss 31:151–159
Guarderas AP, Hacker SD, Lubchenco J (2008) Current Status of Marine Protected Areas in Latin America and the Caribbean. Conserv Biol 22:1630–1640. https://doi.org/10.1111/j.1523-1739.2008.01023.x
Guerrero RA, Baldoni A, Benavides HR (1999) Oceanographic conditions at the southern end of the Argentine continental slope. In: Sánchez RP (ed) Reproductive habitat, biology and acoustic biomass estimates of the southern blue whiting (Micromesistius australis) in the sea off southern Patagonia. Publishers INIDEP, Mar del Plata, pp 7–22
Hinz H, Prieto V, Kaiser MJ (2009) Trawl disturbance on benthic communities: chronic effects and experimental predictions. Ecol Appl 19:761–773. https://doi.org/10.1890/08-0351.1
Holbrook SJ, Kingsford MJ, Schmitt RJ, Stephens JS (1994) Spatial and temporal patterns in assemblages of temperate reef fish. Am Zool 34:463–475. https://doi.org/10.1093/icb/34.3.463
Knust R, Gerdes D, Mintenbeck K (2012) The expedition of the research vessel Polarstern to the Antarctic in 2011 (ANTXXVII/3) (CAMBIO). Rep Pol Mar Res 644:1–202
Krebs CJ (1989) Ecological methodology. Addison and Wesley Longman Pp, California
Laptikhovsky V (2004) A comparative study of diet in three sympatric populations of Patagonotothen species (Pisces: Nototheniidae). Polar Biol 27:202–205. https://doi.org/10.1007/s00300-003-0573-1
Llompart F, Delpiani M, Lattuca E, Delpiani G, Cruz-Jiménez A, Orlando P, Ceballos S, Díaz de Astarloa JM, Vanella F, Fernández D (2015) Spatial patterns of summer demersal fish assemblages around the Antarctic Peninsula and South Shetland Islands. Antarc Sci 27:109–117. https://doi.org/10.1017/S0954102014000352
López Gappa J (2000) Species richness of marine Bryozoa in the continental shelf and slope off Argentina (south-west Atlantic). Divers Distrib 6:15–27. https://doi.org/10.1046/j.1472-4642.2000.00067.x
Lubchenco J, Palumbi SR, Gaines S, Andelman S (2003) Plug- ging a hole in the ocean: the emerging science of marine reserves. Ecol Appl 13:S3–S7. https://doi.org/10.1890/1051-0761(2003)013[0003:PAHITO]2.0.CO;2
Magurran AE, Khachonpisitsak S, Ahmad AB (2011) Biological diversity of fish communities: pattern and process. J Fish Biol 79:1393–1412. https://doi.org/10.1111/j.1095-8649.2011.03091.x
Martin J, Veccia M (2014) Estudio oceanográfico del Canal Beagle y Atlántico Sudoccidental. Informe campaña Área Protegida Namuncurá Banco Burdwwod, CONICET, Argentina
Matano RP, Palma ED, Combes V (2019) The Burdwood Bank circulation. J Geophys Res Oceans 124:6904–6926. https://doi.org/10.1029/2019JC015001
Menni RC, Lucifora LO (2007) Condrictios de la Argentina y Uruguay. FCNyM, Universidad Nacional de La Plata, Serie Técnica-Didáctica, La Plata, Argentina, ProBiota
Menni RC, Ringuelet RA, Aramburu RH (1984) Peces marinos de la Argentina y Uruguay. Editorial Hemisferio Sur, Buenos Aires
Moreno CE, Halffter G (2000) Assessing the completeness of bat biodiversity inventories using species accumulation curves. J Appl Ecol 37:149–158. https://doi.org/10.1046/j.1365-2664.2000.00483.x
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, Mcglinn D, Minchin PR, O'hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H (2016) Vegan: Community Ecology Package. R package version 2.4–1. https://CRAN.R-project.org/package=vegan
Pakhomov EA, Bushula T, Kaehler S, Watkins BP, Leslie RW (2006) Structure and distribution of the slope fish community in the vicinity of the sub-Antarctic Prince Edward Archipelago. J Fish Biol 68:1834–1866. https://doi.org/10.1111/j.1095-8649.2006.01076.x
Pauly D (2009) Beyond duplicity and ignorance in global fisheries. Sci Mar 73(2):215–224. https://doi.org/10.3989/scimar.2009.73n2215
Perry AL, Low PJ, Ellis JR, Reynolds JD (2005) Climate change and distribution shifts in marine fishes. Science 308:1912–1915. https://doi.org/10.1126/science.1111322
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/
Roberts C, O’leary B, Mccauley D, Cury P, Duarte C, Lubchenco J, Pauly D, Sáenz-Arroyo A, Sumaila U, Wilson R, Worm B, Castilla JM (2017) Marine reserves can mitigate and promote adaptation to climate change. PNAS Early Edition: 1–9
Sabatini M, Álvarez Colombo G, Ramirez FC (1999) Zooplankton biomass in the reproductive area of the southern blue whiting (Micromesistius australis). In: Sánchez RP (ed) Reproductive habitat, biology and acoustic biomass estimates of the southern blue whiting (Micromesistius australis) in the sea off southern Patagonia. Publishers INIDEP, Mar del Plata, pp 23–35
Sala E, Giakoumi S (2017) No-take marine reserves are the most effective protected areas in the ocean. ICES J Mar Sci 75:1166–1168. https://doi.org/10.1093/icesjms/fsx059
Sánchez RP, Ciechomski JD (1995) Spawning and nursery grounds of pelagic fish species in the sea-shelf off Argentina and adjacent areas. Sci Mar 59:455–478
Schejter L, Acuña FH, Garese A, Cordeiro R, Pérez CD (2018) Sea pens (Cnidaria: Pennatulacea) from Argentine waters: New distributional records and first report of associated sea anemones. Pan-Am J Aquat Sci 13:292–301
Schejter L, Bertolino M, Calcinai B, Cerrano C, Pansini M (2012) Banco Burdwood: resultados preliminares sobre composición y riqueza específica de esponjas (Phylum Porifera), a partir de muestras colectadas en la campaña del buque rompehielos estadounidense ‘‘Nathaniel B. Palmer’’, abril-mayo 2008. INIDEP Research Report, Argentina
Schejter L, Bremec CS (2019) Stony corals (Anthozoa: Scleractinia) of Burdwood bank and neighboring areas, SW Atlantic Ocean. Sci Mar 63:247–260. https://doi.org/10.3989/scimar.04863.10A
Schejter L, Martin J, Lovrich G (2017) Unveiling the submarine landscape of the Namuncurá Marine Protected Area, Burdwood Bank, SW Atlantic Ocean. Pan Am J Aqua Sci 12:248–253
Schejter L, Rimondino C, Chiesa, Diaz De Astarloa J, Doti B, Elias R, Escolar M, Genzano G, Lopez Gappa J, Tatian M, Zelaya D, Cristobo J, Perez C, Cordeiro R, Bremec C (2016) Namuncurá Marine Protected Area: an oceanic hot spot of benthic biodiversity at Burdwood Bank, Argentina. Polar Biol 39:1–12. https ://doi.org/10.1007/s0030 0–016–1913–2
Schroeder DM, Love MS (2004) Ecological and political issues surrounding decommissioning of offshore oil facilities in the Southern California Bight. Ocean Coast Manage 47:21–48. https://doi.org/10.1016/j.ocecoaman.2004.03.002
Soberon J, Llorente J (1993) The use of species accumulation functions for the prediction of species richness. Conserv Biol 7:480–488. https://doi.org/10.1046/j.1523-1739.1993.07030480.x
Sokal RR, Rohlf JA (1979) Biometria. H. Blume, Barcelona, Principios y Métodos Estadisticos en la Investigación Biológica, p 832
Tatián M, Antacli JC, Sahade R (2005) Ascidians (Tunicata Ascidiacea) species distribution along the Scotia Arc. Sci Mar 62: 205–214.
Whitaker D, Christman M (2014) Clustsig: Significant Cluster Analysis. R package version 1.1. https://CRAN.R-project.org/package=clustsig.
Wisner RL, Mc Millan C (1995) Review of the new world hagfishes of the genus Myxine (Agnatha, Myxinidae) with descriptions of nine new species. Fish B-NOAA 93:530–550
Zamponi M (2008a) Informe Banco Burdwood. Cnidaria. Informe Tratado y Aprobado en el Acta CFP N° 18/2008, Mar del Plata
Zamponi M (2008) La corriente de Malvinas: ¿una vía de dispersión para cnidarios bentónicos de aguas frías? Rev Real Aca Gall Cs 7:183–203
Zunino G, Ichazo MM (1979) Los peces demersales del Banco Burdwood: distribución, abundancia de las especies y frecuencia de tallas (según datos de los B/I Walther Herwig y Shinkai Maru, campañas 1978–1979). Final report, Oceanografía Biológica, Universidad de Buenos Aires
Acknowledgements
We would like to thank the captain and crew of the OV ‘‘Puerto Deseado’’, and all the scientific staff for helping during sampling, as well as to Drs. Daniel Roccatagliata and Laura Schejter for their commitment to coordinating the work on board. Gustavo Lovrich, as scientific and academic coordinator, supported and encouraged all research projects dealing with the Namuncurá MPA, Burdwood Bank and the surrounding areas. We are especially indebted to D. Piepenburg, M. B Cousseau and an anonymous reviewer for their suggestions and corrections, which we believe greatly improved this manuscript. JMDA greatly appreciates the assistance of Thomas Munroe (National Systematics Laboratory, Smithsonian Institution, Washington, D.C.) for reading and correcting the English text.
Funding
This study was supported by grants from Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET-Argentina), PIP 0440 to DF Project-FONDO IBOL to SMD, EM, JMDA. D. M. Vazquez was supported by a CONICET fellowship. Operating costs were afforded by funds of the creation law of MPA Namuncurá—Banco Burdwood (Law 26.875). This is the scientific contribution 42 of the Marine Protected Area Namuncurá.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All permissions for collecting samples from the’Marine Protected Area Namuncurá (MPAN-BB) were issued by its Administrative Council by authorizing and funding the scientific missions. All sampling procedures and experimental manipulations follow the guidelines approved by the Universidad de Buenos Aires (Facultad de Ciencias Exactas y Naturales, Bioterio Central,https://exactas.uba.ar/cicual/).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Delpiani, S.M., Bruno, D.O., Vazquez, D.M. et al. Structure and distribution of fish assemblages at Burdwood Bank, the first Sub-Antarctic Marine Protected Area “Namuncurá” in Argentina (Southwestern Atlantic Ocean). Polar Biol 43, 1783–1793 (2020). https://doi.org/10.1007/s00300-020-02744-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-020-02744-w