Abstract
Fatty acid analysis for estimating dietary sources in marine predators is a powerful tool in food web research. However, questions have been raised about using fatty acids as dietary indicators from whole lipid samples, rather than from separate lipid classes. A drawback of scientific field-based studies is that samples are rarely collected over extended periods, precluding seasonal dietary comparisons. We used fisheries samples obtained over one year to investigate seasonal variations in the fatty acid composition of separated phospholipids and triacylglycerols of Antarctic krill (Euphausia superba). Seasonal variation was observed in fatty acid biomarkers within triacylglycerol and phospholipid fractions of krill. Fatty acids in krill triacylglycerols (thought to best represent recent diet), reflected omnivorous feeding with highest percentages of flagellate biomarkers (18:4n-3) in summer, and diatom biomarkers (16:1n-7c) in autumn, winter and spring. Carnivory biomarkers (∑ 20:1 + 22:1 and 18:1n-9c/18:1n-7c) in krill were higher in autumn. Phospholipid fatty acids were less variable and higher in 20:5n-3 and 22:6n-3, which are essential components of cell membranes. Sterol composition did not yield detailed dietary information, but percentages and quantities of cholesterol, the major krill sterol, were significantly higher in winter and spring compared with summer and autumn. Copepod markers ∑ 20:1 + 22:1 were not strongly associated with the triacylglycerol fraction during some seasons, and neither was 18:4n-3. Krill might mobilise 18:4n-3 from triacylglycerols to phospholipids for conversion to long-chain (≥ C20) polyunsaturated fatty acids, which would have implications for its role as a dietary biomarker. For the first time, we demonstrate the dynamic seasonal relationship between specific biomarkers and krill lipid classes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Euphausia superba (Antarctic krill, hereafter ‘krill’) play a vital role in the Southern Ocean food web, as a lipid-rich food source for higher predators (Murphy et al. 1988; Kattner et al. 2007). Recent estimates suggest that krill predators consume 55 million tonnes of krill per year in the Scotia Sea region alone (Trathan and Hill 2016). Krill are also harvested by a commercial fishery to produce aquaculture feeds and nutraceutical ‘krill oil’ products for human consumption, as their lipids (oils) have high levels of essential omega 3 fatty acids 20:5n-3 (eicosapentaenoic acid) and 22:6n-3 (docosahexaenoic acid) (Gigliotti et al. 2011; Nicol et al. 2011).
Lipids can exceed 40% of the dry mass of krill and the proportion of lipids in krill varies seasonally (Hagen et al. 2001). The major lipid classes in krill are triacylglycerols (neutral lipids) and phospholipids (polar lipids), but lower percentages of sterols, monoacylglycerols, diacylglycerols, free fatty acids, sterol esters and wax esters can also be present in the lipid profile (Clarke 1980; Hellessey et al. 2018). The overall lipid class composition is largely determined by the percentages of triacylglycerols (TAG) (Fricke et al. 1984; Hellessey et al. 2018), because TAG is the primary depot lipid in krill (Clarke 1984; Falk-Petersen et al. 2000; Schmidt and Atkinson 2016). TAG increases in krill when food is readily available (summer and late autumn; Hellessey et al. 2018) and krill also utilise TAG for energy (Mayzaud et al. 1998), therefore, TAG content decreases at times when food is scarce (winter and early spring; Hellessey et al. 2018) or when excess energy is needed during the reproductive period (Falk-Petersen et al. 2000). Phospholipid (PL) content fluctuates inversely to TAG (Hellessey et al. 2018), and PL (such as phosphatidyl-ethanolamine; see Mayzaud 1997) are tightly conserved by krill (Virtue et al. 1993a) as they have an important structural role in cell membranes and control membrane fluidity (Parrish et al. 2000). The phosphatidyl-choline fraction of the phospholipids may also be used as storage lipid (Mayzaud 1997; Hagen et al. 2001). Reasons for the extra storage capacity of phosphatidyl-choline are unknown, but may be linked to reproductive processes and development (Mayzaud 1997).
Krill lipids are composed of polyunsaturated (PUFA), monounsaturated (MUFA) and saturated (SFA) fatty acids, and their fatty acid content and composition has been well studied (Bottino 1974; Fricke et al. 1984; Falk-Petersen et al. 2000; Hagen et al. 2001; O’Brien et al. 2011; Schmidt and Atkinson 2016; Ericson et al. 2018). Selected fatty acids can be used as dietary ‘biomarkers’ as they are primarily derived from items in the diet of krill (Stübing and Hagen 2003; Schmidt and Atkinson 2016). The fatty acids 20:5n-3 and 16:1n-7c have been used as biomarkers for diatoms, while 22:6n-3 and 18:4n-3 have been used as flagellate biomarkers (Stübing and Hagen 2003; Ericson et al. 2018). The ∑ 20:1 + 22:1 isomers are found in high levels in selected Antarctic copepods [e.g. Metridia gerlachei (Ju and Harvey 2004) and Calanoides acutus (Hagen et al. 1993; Graeve et al. 1994; Ju and Harvey 2004)] and may be used as indicators of copepod consumption by krill (Kattner and Hagen 1995; Ju and Harvey 2004; Schmidt et al. 2014). Higher ratios of 18:1n-9c / 18:1n-7c also infer greater levels of carnivory (Stübing and Hagen 2003).
The fatty acid profile of the total lipid can be used to investigate the diet of krill and this is preferred in studies with large sample sizes, as the separation of lipid classes is laborious (Stübing and Hagen 2003). In studies with smaller sample sizes, the krill lipid classes can be separated and the fatty acid profile is obtained for each lipid class. Fatty acid biomarkers located in the TAG lipid fraction are thought to be the best indicators of krill diet (Bottino 1974; Virtue et al. 1993a). Studies most often use fatty acid data from the total lipid of whole krill or dissected body parts (Virtue et al. 1993b, 1996; Atkinson et al. 2002; Alonzo et al. 2003, 2005a; Schmidt et al. 2006, 2014; O’Brien et al. 2011; Auerswald et al. 2015; Virtue et al. 2016; Ericson et al. 2018) or fatty acid data from the TAG fraction only (Ju and Harvey 2004; Reiss et al. 2015) to make inferences about the diet of krill. A lesser number of early studies also reported the fatty acid composition of the more tightly conserved PL fraction (Clarke 1980; Fricke et al. 1984; Virtue et al. 1993a; Falk-Petersen et al. 2000; Mayzaud et al. 2000; Hagen et al. 2001; Stübing et al. 2003).
Sterol composition can also be used to make inferences about an organism’s diet, and can complement fatty acid biomarker data (Virtue et al. 1993a, b; Martin-Creuzburg and von Elert 2009). Cholesterol is the major sterol in crustaceans, comprising up to 95% of the sterol profile (Kanazawa 2001). Minor sterols of algal origin are often used as dietary biomarkers (Parrish et al. 2000).
A recent study (Ericson et al. 2018) provides data on the seasonal and interannual fluctuations in fatty acid composition of the total lipid of krill. The present study further investigates the fatty acid composition of the separate lipid classes (TAG and PL) in krill, to establish whether lipid class separation is necessary when using fatty acids as dietary biomarkers. There is currently limited data on how the distribution of krill fatty acid biomarkers in the TAG and PL of krill varies between seasons, and no collated data on seasonal fluctuations in krill sterols. Seasonal studies have been difficult because of a lack of samples from seasons other than summer, and because at-sea scientific sampling rarely covers the entire seasonal cycle in sufficient detail.
We utilised samples collected by a krill fishing vessel during 2016 to obtain more comprehensive seasonal data on the sterol composition of krill, and the distribution of fatty acids in the major lipid classes.
Materials and methods
Krill sample collection
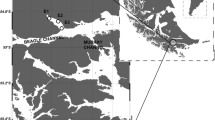
Krill sample collection is described in detail in Ericson et al. (2018). Briefly, krill were caught on board the FV Saga Sea (Aker Biomarine) during their 2016 fishing season (December 2015–September 2016), from three different locations; the West Antarctic Peninsula (WAP), South Orkney Islands (SOI) and South Georgia (SG) (Fig. 1). Twenty krill day−1 were randomly sampled from the catch by a fisheries observer (there was no selection by size or maturity stage). These samples were transported to Hobart, Tasmania on dry ice and stored at – 80 °C.

modified from Hellessey et al. (2018)
Map of locations where Euphausia superba samples were collected by the krill fishery in (open circle) summer (Jan–Feb), (open triangle) autumn (Mar–May), and (+) winter/spring (Jun–Sep) of 2016, at the West Antarctic Peninsula (WAP), South Orkney Islands (SOI) & South Georgia (SG). Map
Initial lipid extraction and fatty acid analyses
Krill were subsampled for lipid extraction from the fisheries samples at 2-week intervals. They were individually extracted in separation funnels using a modified method of Bligh and Dyer (1959), with a solvent mixture of methanol (MeOH)/dichloromethane (CH2Cl2)/water (H2O) at 20:10:7, by vol. To separate phases, 10 mL CH2Cl2 and 10 mL saline MilliQ H2O were added the following day. The lower lipid layer was drained into a round bottomed flask and solvent was removed using a rotary evaporator, to concentrate the total lipid extract (TLE). The TLE was stored at – 20 °C in a pre-weighed glass vial with added solvent (CH2Cl2), to ensure that oxidation of the sample did not occur.
These total lipid extracts (TLE) obtained from Ericson et al. (2018) were used for the present study. Krill with a large range of selected biomarker percentages in the TLE were included. Only males were included to eliminate gender as a potential confounding variable (Clarke 1980; Mayzaud et al. 2000). Males that had < 25% TAG (as % of total lipids) were excluded, as low TAG percentages during the reproductive season may make them less suitable for dietary analysis (Virtue et al. 1996; Stübing and Hagen 2003). Total lipid extracts from males in the 2016 catch were selected from each season (summer, autumn and combined winter/early spring; n = 12 for each season). Summer krill were sampled from the WAP and the SOI in January and February 2016, autumn krill were sampled from the WAP between March–May 2016, and winter/early spring (referred to as ‘winter/spring’ hereafter) krill were sampled from SG between June–September 2016. Samples selected for this study are shown in Supplementary Material, along with their total lipid (mg g −1 DM; dry mass), TAG and PL percentage data (% of total lipid) obtained from their TLE. Krill dry mass was obtained by multiplying the krill wet mass by 0.2278, to correct for the 77.2% water content in krill (Virtue et al. 1993a). Detailed analyses for the full suite of krill collected in 2016 can be found in Hellessey et al. (2018).
Separation of lipid classes via column chromatography
Aliquots were taken from the TLE and analysed via column chromatography, to separate the lipid classes of krill. The separated lipid classes were then used to investigate the fatty acid composition within the TAG and PL classes. For all chosen samples (n = 36), one gram of activated silica was added to a glass column and washed through using chloroform (CHCl3) to pack the column. Ten milligrams of total lipid extract were added to the packed column. Triacylglycerols were eluted with 10 mL CHCl3, followed by elution of glycolipids with 20 mL acetone (C3H6O), and elution of phospholipids with 20 mL methanol (MeOH), to produce extracts for triacylglycerol, phospholipid and glycolipid fractions (total lipid class fractions; TLCF). All TLCF were reduced via rotary evaporation and added to 1.5 mL glass vials with Teflon® caps. Accurate lipid class separation was confirmed by running 1 µL aliquots of all lipid class fractions through an Iatroscan TLC–FID analyser (see Hellessey et al. 2018 for detailed methods) following column chromatography. Once accurate separation was verified, TAG and PL lipid fractions were used to prepare fatty acid methyl esters (FAME) for fatty acid analysis.
A subsample of each TLCF was transferred to a glass test tube with a Teflon® screw-cap and 3 mL of methylating solution (MeOH /CH2Cl2/hydrochloric acid (HCl), 10:1:1, by vol) was added. Each test tube was then heated at 90–100 °C for 75 min, then cooled for 5 min before addition of 1 mL H2O and 1.8 mL hexane/CH2Cl2 solution (4:1, by vol) to extract the FAME. Samples were centrifuged for 5 min, and the upper layer (FAME) was transferred to a vial. An additional 1.8 mL of hexane/CH2Cl2 solution was added to the test tube, and the sample was centrifuged again, before adding the top layer of FAME to the vial. This process was carried out three times in total, to ensure that all of the FAME had been extracted and added to the vial (samples in the vial were blown down with nitrogen (N2) gas in between transfers).
To prepare samples for gas chromatography (GC-FID), 1.5 mL of internal injection standard (23:0 FAME) was added to each vial. Samples were analysed via GC-FID using an Agilent Technologies 7890A System equipped with a non-polar Equity®-1 fused silica capillary column (15 m length × 0.1 mm internal diameter × 0.1 μm film thickness). Samples (0.2 μL) were injected in splitless mode at an oven temperature of 120 ºC with helium as the carrier gas. The oven temperature was raised at a rate of 10 ºC min−1 up to 270 °C, then a rate of 5 °C min−1 up to 310 °C. Quantification of fatty acid peaks (expressed as a % of the total fatty acid area) was conducted using Agilent Technologies ChemStation software. Initial identification was based on comparison of retention times with known (Nu Check Prep) and fully characterized laboratory (tuna oil) standards.
Gas chromatography-mass spectrometry (GC–MS) was carried out using a Thermo Scientific 1310 GC–MS coupled with a TSQ triple quadruple, to further confirm component identification. Selected samples were injected using a Tripleplus RSH auto sampler using a non-polar HP-5 Ultra 2 bonded-phase column (50 m length × 0.32 mm internal diameter × 0.17 μm film thickness). The HP-5 column was a similar polarity to the column used for GC-FID analyses. The initial oven temperature (45 ºC) was held for 1 min, then rose at a rate of 30 °C min−1 to 140 °C, then at a rate of 3 °C min−1 to 310 °C, and held for 12 min. Helium was the carrier gas. Operating conditions of the GC–MS were as follows: electron impact energy 70 eV; emission current 250 μamp; transfer line 310 °C; source temperature 240 °C; scan rate 0.8 scans s−1; mass range 40–650 Da. Mass spectra were acquired and processed with the software Thermo Scientific Xcalibur™. Nu Check Prep and tuna oil standards were also used for assistance in identification of peaks.
Sterol analysis
An additional 300 µL aliquot was taken from each of the TAG fractions for saponification. Each aliquot was transferred into a glass test tube fitted with a Teflon® lined screw cap, blown down under N2 gas and treated with 2 mL of saponifying solution (5% potassium hydroxide (KOH) in MeOH/MilliQ H2O, 80:20, by vol), then heated at 60 °C for 3 h. Samples were cooled and 1 mL of MilliQ H2O and 1.8 mL of hexane:CH2Cl2 solution was added to extract the total non-saponifiable neutral lipids (TSN). Samples were then centrifuged for 5 min and the upper layer containing TSN was transferred to a vial, and another 1.8 mL of hexane/CH2Cl2 was added to the test tube. This process was carried out three times and samples were blown down each time using N2 gas.
Samples of TSN lipids obtained above were silylated by treatment with N2 gas and addition of 50 µL N,O-bis (Trimethylsilyl) trifluoroacetamide, then heated overnight at 60 °C. Prior to analysis, samples were blown down using N2 gas and 1000 µL of internal injection standard (23:0 FAME) was added to each vial. Samples were blown down again under N2 gas and transferred to glass inserts with 200 µL CH2Cl2. Samples were then run through a GC-FID and GC–MS as described above, to obtain sterol composition and content, and to confirm component identifications.
Statistical analyses
Principal components analyses (PCA) of fatty acid data were carried out in PRIMER 6 using Pearson correlation, due to large differences in fatty acid variances. Data were transformed (log x + 1) prior to PCA analysis. Fatty acid biomarker data were analysed in RStudio (version 1.1.453) using one-way ANOVA with either season or lipid class as a factor, or two-way ANOVA with season and lipid class as factors, and a season*lipid class interaction. Tukey comparisons were used to investigate significant differences between levels of season. Sterol data were also analysed in RStudio, using one-way ANOVA with season as a factor. Data for all analyses were log or square root transformed when they did not meet assumptions of normality or homogeneity of variances. A Welch’s test was used for sterol data that had heterogeneous variances and data transformation did not normalise the data.
Results
The fatty acid composition (as a percentage of total fatty acids) of the TAG and PL fractions of krill in summer, autumn and winter/spring of 2016 are shown in Table 1. Total percentages of omega-3 fatty acids, polyunsaturated (PUFA), monounsaturated (MUFA) and saturated (SFA) fatty acids are also shown in Table 1. Principal components analysis (PCA) of krill from all seasons combined is shown in Fig. 2. The fatty acid composition (%) of TAG and PL in krill are clearly separated along PC1, which explains 85.3% of the variation. Phospholipids were higher in the long-chain omega-3 PUFA, particularly 20:5n-3 and 22:6n-3. Triacylglycerols were higher in 14:0, and 16:1n-7c and 18:1n-9c. PC2 explains a minor (5.9%) amount of the fatty acid variation, separating krill with higher percentages of 18:4n-3 from krill with higher percentages of 20:1n-9c and 22:1n-9c. The fatty acid 16:0 was evenly distributed between TAG and PL with substantial percentages (~ 20%) in both lipid classes.
Composition of fatty acid biomarkers in the triacylglycerols and phospholipids of krill
Percentages (mean ± SE) of the major fatty acid biomarkers in the TAG and PL of krill are shown in Fig. 3. Percentages of essential omega 3 PUFA 20:5n-3 and 22:6n-3 and carnivory markers ∑ 20:1 + 22:1 in krill were higher in PL, while percentages of phytoplankton biomarkers 16:1n-7c, 18:4n3 and the carnivory ratio 18:1n9-c / 18:1n-7c were higher in TAG (Fig. 3). Percentages of 18:4n-3 in both lipid classes were more variable than the other fatty acid biomarkers (Fig. 3).
Percentages (mean ± SE) of fatty acid biomarkers 20:5n-3, 22:6n-3, 18:4n-3, 16:1n-7c, ∑ 20:1 + 22:1, & ratios of 18:1n-9c/18:1n-7c in male Euphausia superba, in triacylglycerols (TAG) & phospholipids (PL). Data from all seasons are combined. Significant differences between the TAG and PL are denoted by asterisks where * p < 0.05, **p < 0.001. For each lipid class n = 36
The composition of fatty acid biomarkers in TAG and PL also varied seasonally (Fig. 4). Percentages of 20:5n-3 in krill were higher in TAG in summer than in winter/spring (One-Way ANOVA, df = 2, Tukey p = 0.007) and higher in PL in the autumn than in winter/spring (One-Way ANOVA, df = 2, Tukey p = 0.011). Percentages of 22:6n-3 were lower in TAG in autumn compared with the other seasons (One-Way ANOVA, df = 2, Tukey p < 0.04), while there were no seasonal differences in the PL (One-Way ANOVA, df = 2, p = 0.134).
Percentages (mean ± SE) of fatty acid biomarkers 20:5n-3, 22:6n-3, 18:4n-3, 16:1n-7c, ∑ 20:1 + 22:1, & ratios of 18:1n-9c/18:1n-7c in triacylglycerols (TAG) & phospholipids (PL) of male Euphausia superba, in summer (Jan–Feb), autumn (Mar–May) & winter/spring (Jun–Sep) of 2016. For each lipid class & season n = 12
Percentages of 18:4n-3 within both TAG and PL were higher in summer than in winter/spring (One-Way ANOVA, df = 2, Tukey p < 0.001), with intermediate percentages in autumn (Fig. 4). The TAG and PL of krill had similar percentages of 18:4n-3 in autumn and winter/spring (Two-Way ANOVA Lipid Class*Season, df = 2, Tukey p > 0.925). Percentages of 16:1n-7c in TAG were lowest in summer (One-Way ANOVA, df = 2, Tukey p < 0.001), while no seasonal differences were observed in PL (One-Way ANOVA, df = 2, Tukey p = 0.368).
Seasonal differences were also observed for the carnivory biomarkers ∑ 20:1 + 20:1 and 18:1n-9c/18:1n-7c. The ∑ 20:1 + 22:1 in TAG were lower in summer than the other seasons (One-Way ANOVA, df = 2, Tukey p < 0.04) and highest in PL in autumn (One-Way ANOVA, df = 2, Tukey p < 0.02). Ratios of 18:1n-9c/18:1n-7c in TAG were higher in autumn than in winter/spring (One-Way ANOVA, df = 2, Tukey p = 0.042), but no seasonal differences were seen in PL (One-Way ANOVA, df = 2, Tukey p = 0.311).
The relationship between TAG and PL fatty acid biomarkers in krill is summarised by the TAG/PL ratios in Table 2. The high ratios of 16:1n-7c and 18:1n-9c/18:1n-7c in TAG, and strong association of 20:5n-3 and 22:6n-3 with PL, have also been consistently observed in other studies (see references in Table 2). The ratios of 18:4n-3 and ∑ 20:1 + 20:1 isomers in TAG and PL are variable and these biomarkers do not show a consistent association with either TAG or PL. Percentages of 18:4n-3 are consistently higher in TAG of krill in summer, but this relationship does not hold in autumn or in studies when the season is not stated (Table 2). The ratio of ∑ 20:1 + 20:1 isomers in TAG/PL is highly variable between studies, ranging from 0.7–7.7 (Table 2).
Seasonal variations in krill sterols
Four sterols were identified in the neutral lipids of krill (Table 3). Cholesterol (cholest-5-en-3β-ol) was the major sterol, making up 50–89% of the sterol composition, while desmosterol (cholesta-5,24-dien-3β-ol) made up 11–33% of the composition. Brassicasterol (24-methylcholesta-5,22E-dien-3β-ol) percentages were variable, and present at 0–12% of sterols. Thirty-three of the sampled krill had brassicasterol percentages < 5%, but three krill sampled in autumn had percentages exceeding 9%. Trans-22-dehydrocholesterol (24-cholesta-5,22E-dien-3β-ol) was a minor component making up 0–3% of the total sterol composition. Other unidentified sterols made up 0–13% of the sterol composition.
Average cholesterol levels were up to 14% and 22 µg/g higher in winter/spring compared with the other seasons (Table 3). Desmosterol percentages were up to 9% lower in winter/spring, with quantities 2–3 µg/g higher in winter/spring (Table 3). Average percentages and quantities of brassicasterol were highest in autumn, but seasonal differences were not statistically significant due to large variances in autumn data. Trans-22-dehydrocholesterol percentages were lowest in autumn, and quantities were slightly higher in winter/spring (Table 3).
Discussion
The composition of fatty acids and fatty acid biomarkers in krill differed between lipid classes (TAG and PL) and seasons (summer, autumn and winter/spring). The strong association of 20:5n-3 and 22:6n-3 with PL is consistent with previous studies (Clarke 1980; Fricke et al. 1984; Virtue et al. 1996; Mayzaud et al. 2000; Hagen et al. 2001; Stübing et al. 2003; Alonzo et al. 2005b), as these long chain PUFA have an important structural role in the cell membranes and are tightly conserved (Dalsgaard et al. 2003). Higher percentages of MUFA and SFA in the TAG fraction, namely 14:0, 16:1n-7c and 18:1n-9c, are also consistent with previous studies (Falk-Petersen et al. 2000; Hagen et al. 2001; Alonzo et al. 2005b). More variable and less predictable percentages of 18:4n-3 in krill (compared with other biomarkers) have also been previously reported (Schmidt et al. 2006; Ericson et al. 2018).
Seasonal changes in TAG fatty acids allowed inferences about the diet of krill. The 1.2–1.8-fold increase in 20:5n-3 and 22:6n-3 in TAG of krill in summer indicated that higher percentages of phytoplankton were consumed during summer. The biomarkers 18:4n-3 and 16:1n-7c were more seasonally variable in TAG, with highest percentages of 18:4n-3 in summer and highest percentages of 16:1n-7c in autumn and winter/spring. This suggests that krill were feeding on higher percentages of flagellates in the summer and switched to a more diatom-enriched diet in the autumn and winter. Elevated summer and autumn ratios of 18:1n-9c/18:1n-7c in TAG of krill indicated that some carnivorous feeding was occurring during these seasons, while copepods (∑ 20:1 + 22:1 isomers in TAG) comprised a higher proportion of the diet in autumn and winter/spring. It should be noted that high percentages of 18:1n-9c have also been found in krill fed cryptophytes (Alonzo et al. 2005a) and krill are able to biosynthesize it (Reiss et al. 2015), therefore, this may partially confound its use as a carnivory marker.
Because the sampling regime for this study was dictated by fishing operations, samples could not be collected for all season and location combinations. This implies that although these dietary signatures show seasonal trends, they may also reflect the region in which the krill were caught (Reiss et al. 2015). The ocean surrounding South Georgia is known to have high concentrations of diatoms (Schmidt and Atkinson 2016), which may also explain the high percentages of 16:1n-7c in the winter/spring krill caught there. In the WAP region, flagellates are also often found in krill stomachs (Schmidt and Atkinson 2016), and krill caught in this region had elevated percentages of the flagellate marker 18:4n-3. Changes in the fine-scale fatty acid composition of krill are likely to reflect a range of spatial and temporal factors including season and location. However, the overarching trend of increasing omnivory from summer through to winter is likely to be seasonally driven, as abundances of krill prey are closely linked to seasonal changes in their environment (Murphy et al. 2007). Our observed pattern of increasing omnivory from summer through to autumn and winter also agrees with previous seasonal studies conducted at a range of locations (see review in Schmidt and Atkinson 2016). On a larger scale, the specific fatty acid composition of krill is also known to differ interannually (Ericson et al. 2018), therefore this dietary information from a single year cannot be applied universally.
Our TAG fatty acid data corresponds well with the findings of Ericson et al. (2018) which reported the fatty acid composition of the total lipid for these 2016 samples. This confirms that the fatty acid composition from the total lipid (TAG + PL) and TAG lipid class are similar and can both be used to make inferences about krill diet.
The fatty acid composition of the PL fraction of krill also displayed some seasonal variation, particularly for the biomarkers 18:4n-3 and ∑ 20:1 + 22:1. Percentages of 18:4n-3 in PL followed the same trend as TAG, with percentages decreasing as the seasons progressed from summer to winter/spring. Percentages of ∑ 20:1 + 22:1 isomers in PL also showed a similar trend to TAG, but the increased proportion in autumn was more pronounced in PL.
Our study is the first to identify a variable relationship in the TAG/PL ratio of 18:4n-3 in krill. We were able to detect this variability because our study, unlike others, had sufficient breadth of coverage to allow comparisons between all seasons. Most studies with more limited or no seasonal comparisons have reported that 18:4n-3 is higher in the TAG fraction of krill (Clarke 1980; Falk-Petersen et al. 2000; Stübing et al. 2003), and other euphausiid species (Kattner and Hagen 1998; Mayzaud et al. 1999; Virtue et al. 2000) and this has justified its suitability as a trophic marker (Hagen et al. 2001). We found that this TAG/PL relationship is not consistent between studies, and that 18:4n-3 may be found in equal or higher proportions in PL during some seasons. This suggests that 18:4n-3 may also be conserved or mobilised to the PL fraction of krill under certain conditions.
Some marine invertebrates are able to elongate and desaturate the C18 PUFA 18:3n-3 to synthesize long-chain omega 3 PUFA such as 20:5n-3 and 22:6n-3 (Monroig et al. 2013). This has been demonstrated in marine crustaceans (Bell et al. 2007) and implies that 20:5n-3 and 22:6n-3 may not solely have a dietary origin. Virtue et al. (1993a) suggested that krill may use this elongation-desaturation pathway, as krill fed diets low in 20:5n-3 in the laboratory still had high percentages of this fatty acid. Levels of 20:5n-3 and 22:6n-3 in krill are also often higher than what could be obtained from the diet alone (Virtue et al. 1993b). Bell et al. (2007) found that larval krill fed an 18:3n-3 tracer had a very limited ability to synthesize long-chain PUFA and concluded that this may not be a pathway that is readily utilised by krill. Larvae in their study already had sufficient levels of 20:5n-3 and 22:6n-3, however, which may have suppressed synthesis of these fatty acids from the tracer (Bell et al. 2007). We hypothesize that 18:4n-3 could be mobilised to PL of krill for conversion to 20:5n-3, and possibly 22:6n-3, during seasons when these important membrane lipids are less available from the diet. Results from a study on an Antarctic copepod species Calanus propinquus indicated that such lipid biosynthesis could explain decreased levels of 16:1n-7 and 18:4n-3 in these copepods (Kattner and Hagen 1995). Such mobilisation of 18:4n-3 to PL for conversion to long-chain PUFA could explain why percentages of 18:4n-3 did not differ between the TAG and PL of krill in autumn and winter/spring, and why inconsistent TAG/PL ratios of 18:4n-3 are found in different studies. While 18:4n-3 may still be a useful biomarker in krill, it may be modified in TAG and not just replaced by other dietary fatty acids. Further studies are needed to confirm the extent to which adult krill are able to carry out conversion of 18:4n-3 to long-chain PUFA.
The increased PL percentages of ∑ 20:1 + 22:1 in krill our study is also an unusual finding, as previous studies on krill (Clarke 1980; Fricke et al. 1984) and other euphausiids (Virtue et al. 2000) have found higher percentages of these fatty acids in TAG. When the individual fatty acids with 20:1 and 22:1 isomers are separated, the fatty acids 20:1n-9c and 20:1n-7c are higher in the TAG fraction, but 22:1n-9c is higher in the PL fraction. The fatty acid 22:1n-9c was present in proportions of up to 2.7% in krill, which skewed the ratio of TAG/PL towards higher PL composition for this biomarker. It is unknown why 22:1n-9c is higher in the PL, but it may also be the product of elongation-desaturation pathways as it is in copepods (Kattner and Hagen 1995). Differences in percentages of 18:4n-3 and ratios of ∑ 20:1 + 22:1 in the TAG and PL of krill may also relate to whether the experimental protocol separates the phospholipids into separate phosphatidyl-choline and phosphatidyl-ethanolamine fractions. If the majority of 18:4n-3 and ∑ 20:1 + 22:1 are being stored in the phosphatidyl-choline (storage) fraction of the PL, krill may also be storing these fatty acids for energy, rather than for structural use in cell membranes.
The four sterols identified in krill were cholesterol, desmosterol, brassicasterol, and trans-22-dehydrocholesterol. Crustaceans must obtain sterols from their diet as they lack the ability to synthesize them (Kanazawa 2001; Martin-Creuzburg and von Elert 2009). Sterols have a range of roles in organisms as essential constituents of cell membranes, precursors for moulting hormones and as important components for egg production and development (Martin-Creuzburg and von Elert 2009). Cholesterol was the major sterol in krill and appeared to drive the overall sterol composition, which is consistent with the findings of previous studies (Fricke et al. 1984; Virtue et al. 1993a, b; Phleger et al. 2002; Ju and Harvey 2004).
Our study is the first to document seasonal variations in the sterol composition of krill. We cannot establish from this study why krill had significantly higher percentages and quantities of cholesterol in winter, but it may relate to seasonally driven physiological processes. Organisms possess the ability to store cholesterol and may use these stores during periods when cholesterol levels are low (Martin-Creuzburg and von Elert 2009). The increased quantities of both cholesterol and its precursor desmosterol in krill in winter/spring support the hypothesis that krill may have been storing cholesterol. The decrease in desmosterol percentages in winter may also relate to increased conversion of desmosterol to cholesterol during this time.
Krill might store cholesterol during the winter months when moulting is less frequent and they are not reproductively active. Stored cholesterol may then be depleted during late spring, summer and autumn when krill growth rates increase and they are reproducing, then they are replenished and stored the following winter. Further research is needed to investigate potential storage mechanisms for sterols in krill.
One previous study has also identified only four sterols in krill (Virtue et al. 1993a). Others have identified additional algal sterols in krill such as the flagellate marker dinosterol (Virtue et al. 1996), and campesterol, stigmasterol, sitosterol, isofucosterol (Virtue et al. 1993b, 1996), cholestanol and brassicastanol (Phleger et al. 2002). Although sterols must be obtained from the diet, crustaceans may be able to convert a range of these sterols to cholesterol (Virtue et al. 1993a; Kanazawa 2001; Phleger et al. 2002; Martin-Creuzburg and von Elert 2009). This may explain why minor sterols were largely absent from the sterol profile of krill in this study, if they were being dealkylated to desmosterol or cholesterol.
The identified sterols, however, do provide some inferences about the krill diet. Brassicasterol has been found in high quantities in Phaeocystis pouchetii (now Phaeocystis antarctica; Nichols et al. 1991), therefore, this prymnesiophyte may have been present in the diet of krill particularly during autumn. However, it should also be noted that P. antarctica is rich in the fatty acids 14:0, 16:0, 18:0, and 18:1n-9c (Nichols et al. 1991; Virtue et al. 1993a) and these fatty acids were not significantly higher in krill in autumn compared with the other seasons.
In addition to its role as a precursor to cholesterol (Fricke et al. 1984; Virtue et al. 1993b), desmosterol is a major component of sterols in diatom ice algae (Barrett et al. 1995; Ju and Harvey 2004). Desmosterol percentages were highest in krill which were caught at the WAP and SOI in summer and autumn (regions with sea ice), and lowest in krill caught in winter/spring at SG (permanently ice-free). Higher percentages of desmosterol in summer and autumn krill may be explained by addition of ice-algae in the diet, and the physiological relationship of desmosterol-cholesterol described above.
Our study provides a detailed seasonal description on the composition of fatty acids in the TAG and PL fractions of krill. For the first time, we report variation in TAG and PL fatty acids and sterols across all seasons and demonstrate that the relationship between specific biomarkers and the lipid classes is more complex than previously thought. Further studies are needed to investigate the ability of krill to synthesize key sterols and fatty acids from other derivatives, and how this synthesis may relate to seasonal fatty acid and sterol composition.
References
Alonzo F, Nicol S, Virtue P, Nichols PD (2003) Lipids as trophic markers in Antarctic krill, I. Validation under controlled laboratory conditions. In: Huiskes AHL, Gieskes WWC, Rozema J, Schorno RML, Vies SM, Wolff WJ (eds) Antarctic biology in a global context. Backhuys Publishers, Leiden, pp 121–128
Alonzo F, Virtue P, Nicol S, Nichols PD (2005a) Lipids as trophic markers in Antarctic krill. II. Lipid composition of the body and digestive gland of Euphausia superba in controlled conditions. Mar Ecol Prog Ser 296:65–79
Alonzo F, Virtue P, Nicol S, Nichols PD (2005b) Lipids as trophic markers in Antarctic krill. III. Temporal changes in digestive gland lipid composition of Euphausia superba in controlled conditions. Mar Ecol Prog Ser 296:81–91
Atkinson A, Meyer B, Stübing D, Hagen W, Schmidt K, Bathmann UV (2002) Feeding and energy budgets of Antarctic krill Euphausia superba at the onset of winter – II. Juveniles and adults. Limnol Oceanogr 47:953–966
Auerswald L, Meyer B, Teschke M, Hagen W, Kawaguchi S (2015) Physiological response of adult Antarctic krill, Euphausia superba, to long-term starvation. Polar Biol 38:763–780
Barrett S, Volkman J, Dunstan G (1995) Sterols in 14 species of marine diatoms (Bacillariophyta). J Phycol 31:360–369
Bell M, Dick J, Anderson T, Pond D (2007) Application of liposome and stable isotope tracer techniques to study polyunsaturated fatty acid biosynthesis in marine zooplankton. J Plankton Res 29:417–422
Bligh EG, Dyer W (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Bottino NR (1974) The fatty acids of Antarctic phytoplankton and euphausiids. Fatty acid exchange among trophic levels of the Ross Sea. Mar Biol 27:197–204
Clarke A (1980) The biochemical composition of krill, Euphausia superba Dana, from South Georgia. J Exp Mar Bio Ecol 43:221–236
Clarke A (1984) Lipid content and composition of Antarctic krill, Euphausia superba Dana. J Crustac Biol 4:285–294
Dalsgaard J, St John M, Kattner G, Muller-Navarra D, Hagen W (2003) Fatty acid trophic markers in the pelagic marine environment. Adv Mar Biol 46:225–340
Ericson JA, Hellessey N, Nichols PD, Kawaguchi S, Nicol S, Hoem N, Virtue P (2018) Seasonal and interannual variations in the fatty acid composition of adult Euphausia superba Dana, 1850 (Euphausiacea) samples derived from the Scotia Sea krill fishery. J Crustac Biol. https://doi.org/10.1093/jcbiol/ruy032
Falk-Petersen S, Hagen W, Kattner G, Clarke A, Sargent J (2000) Lipids, trophic relationships, and biodiversity in Arctic and Antarctic krill. Can J Fish Aquat Sci 57:178–191
Fricke H, Gercken G, Schreiber W, Oehlenschlager J (1984) Lipid, sterol and fatty acid composition of Antarctic krill (Euphausia superba Dana). Lipids 19:821–827
Gigliotti JC, Davenport MP, Beamer SK, Tou JC, Jaczynski J (2011) Extraction and characterisation of lipids from Antarctic krill (Euphausia superba). Food Chem 125:1028–1036
Graeve M, Hagen W, Kattner G (1994) Herbivorous or omnivorous? On the significance of lipid compositions as trophic markers in Antarctic copepods. Deep Sea Res Part I Oceanogr Res Pap 41:915–924
Hagen W, Kattner G, Graeve M (1993) Calanoides acutus and Calanus propinquus, Antarctic copepods with different lipid storage modes via wax esters or triacylglycerols. Mar Ecol Prog Ser 97:135–142
Hagen W, Kattner G, Terbruggen A, Van Vleet ES (2001) Lipid metabolism of the Antarctic krill Euphausia superba and its ecological implications. Mar Biol 139:95–104
Hellessey N, Ericson JA, Nichols PD, Kawaguchi S, Nicol S, Hoem N, Virtue P (2018) Seasonal and interannual variation in the lipid content and composition of Euphausia superba Dana 1850 (Euphausiacea) samples derived from the Scotia Sea fishery. J Crustac Biol. https://doi.org/10.1093/jcbiol/ruy053
Ju S-J, Harvey HR (2004) Lipids as markers of nutritional condition and diet in the Antarctic krill Euphausia superba and Euphausia crystallorophias during austral winter. Deep Sea Res Part II Top Stud Oceanogr 51:2199–2214
Kanazawa A (2001) Sterols in marine invertebrates. Fish Sci 67:997–1007
Kattner G, Hagen W (1995) Polar herbivorous copepods – different pathways in lipid biosynthesis. ICES J Mar Sci 52:329–335
Kattner G, Hagen W (1998) Lipid metabolism of the Antarctic euphausiid Euphausia crystallorophias and its ecological implications. Mar Ecol Prog Ser 170:203–213
Kattner G, Hagen W, Lee RF, Campbell R, Deibel D, Falk-Petersen S, Graeve M, Hansen BW, Hirche HJ, Jónasdóttir SH, Madsen ML, Mayzaud P, Müller-Navarra D, Nichols PD, Paffenhöfer GA, Pond D, Saito H, Stübing D, Virtue P (2007) Perspectives on marine zooplankton lipids. Can J Fish Aquat Sci 64:1628–1639
Martin-Creuzburg D, von Elert E (2009) Ecological significance of sterols in aquatic food webs. In: Arts MT, Brett MT, Kainz M (eds) Lipids in Aquatic Ecosystems. Springer, New york, pp 43–64
Mayzaud P (1997) Spatial and life-cycle changes in lipid and fatty acid structure of the Antarctic euphausiid Euphausia superba. In: Valencia J, Walton DWH (eds) Antarctic communities: species, structure and survival. Cambridge University Press, Cambridge, pp 284–294
Mayzaud P, Albessard E, Cuzin-Roudy J (1998) Changes in lipid compostion of the Antarctic krill Euphausia superba in the Indian sector of the Antarctic ocean: influence of geographical location, sexual maturity stage and distribution among organs. Mar Ecol Prog Ser 173:149–162
Mayzaud P, Virtue P, Albessard E (1999) Seasonal variations in the lipid and fatty acid composition of the euphausiid Meganyctiphanes norvegica from the Ligurian Sea. Mar Ecol Prog Ser 186:199–210
Mayzaud P, Albessard E, Virtue P, Boutoute M (2000) Environmental constraints on the lipid composition and metabolism of euphausiids: the case of Euphausia superba and Meganyctiphanes norvegica. Can J Fish Aquat Sci 57:91–103
Monroig O, Tocher D, Navarro J (2013) Biosynthesis of polyunsaturated fatty acids in marine invertebrates: recent advances in molecular mechanisms. Mar Drugs 11:3998–4018
Murphy EJ, Morris DJ, Watkins JL, Priddle J (1988) Scales of interaction between Antarctic krill and the environment. In: Sahrhage D (ed) Antarctic Ocean and Resources Variability. Springer, Berlin.
Murphy EJ, Watkins JL, Trathan PN, Reid K, Meredith MP, Thorpe SE, Johnston NM, Clarke A, Tarling GA, Collins MA, Forcada J, Shreeve RS, Atkinson A, Korb R, Whitehouse MJ, Ward P, Rodhouse PG, Enderlein P, Hirst AG, Martin AR, Hill SL, Staniland IJ, Pond DW, Briggs DR, Cunningham NJ, Fleming AH (2007) Spatial and temporal operation of the Scotia Sea ecosystem: a review of large-scale links in a krill centred food web. Philos Trans R Soc B 362:113–148
Nichols PD, Skerratt JH, Davidson A, Burton H, McMeekin TA (1991) Lipids of cultured Phaeocystis pouchetii: signatures for food-web, biogeochemical and environmental studies in Antarctica and the Southern ocean. Phytochemistry 30:3209–3214
Nicol S, Foster J, Kawaguchi S (2011) The fishery for Antarctic krill – recent developments. Fish Fish 13:30–40
O’Brien C, Virtue P, Kawaguchi S, Nichols PD (2011) Aspects of krill growth and condition during late winter-early spring off East Antarctica (110–130°E). Deep Sea Res Part II Top Stud Oceanogr 58:1211–1221
Parrish C, Abrajano T, Budge S, Helleur R, Hudson E, Pulchan K, Ramos C (2000) Lipid and phenolic biomarkers in marine ecosystems: analysis and applications. The Handbook of Environmental Chemistry, vol 5. Part D Marine Chemistry. Springer, Berlin, pp 193–223
Phleger CF, Nelson MM, Mooney BD, Nichols PD (2002) Interannual and between species comparison of the lipids, fatty acids and sterols of Antarctic krill from the US AMLR Elephant Island survey area. Comp Biochem Physiol Part B 131:733–747
Reiss CS, Walsh J, Goebel ME (2015) Winter preconditioning determines feeding ecology of Euphausia superba in the Antarctic Peninsula. Mar Ecol Prog Ser 519:89–101
Schmidt K, Atkinson A (2016) Feeding and food processing in Antarctic krill (Euphausia superba Dana). In: Siegel V (ed) Biology and Ecology of Antarctic Krill. Springer, Cham, pp 175–224
Schmidt K, Atkinson A, Petzke K, Voss M, Pond D (2006) Protozoans as a food source for Antarctic krill, Euphausia superba: complementary insights from stomach content, fatty acids, and stable isotopes. Limnol Oceanogr 51:2409–2427
Schmidt K, Atkinson A, Pond DW, Ireland LC (2014) Feeding and overwintering of Antarctic krill across its major habitats: the role of sea ice cover, water depth, and phytoplankton abundance. Limnol Oceanogr 59:17–36
Stübing D, Hagen W (2003) Fatty acid biomarker ratios – suitable trophic indicators in Antarctic euphausiids? Polar Biol 26:774–782
Stübing D, Hagen W, Schmidt K (2003) On the use of lipid biomarkers in marine food web analyses: an experimental case study on the Antarctic krill, Euphausia superba. Limnol Oceanogr 48:1685–1700
Trathan PN, Hill SL (2016) The importance of krill predation in the Southern Ocean. In: Siegel V (ed) Biology and Ecology of Antarctic Krill. Springer, Switzerland, pp 321–350
Virtue P, Nichols PD, Nicol S, McMinn A, Sikes EL (1993a) The lipid composition of Euphausia superba Dana in relation to the nutritional value of Phaeocystis pouchetii (Hariot) Lagerheim. Antarct Sci 5:169–177
Virtue P, Nicol S, Nichols PD (1993b) Changes in the digestive gland of Euphausia superba during short-term starvation – lipid class, fatty acid and sterol content and composition. Mar Biol 117:441–448
Virtue P, Nichols PD, Nicol S, Hosie G (1996) Reproductive trade-off in male Antarctic krill, Euphausia superba. Mar Biol 126:521–527
Virtue P, Mayzaud P, Albessard E, Nichols PD (2000) Use of fatty acids as dietary indicators in northern krill, Meganyctiphanes norvegica, from northeastern Atlantic, Kattegat, and Mediterranean waters. Can J Fish Aquat Sci 57:104–114
Virtue P, Meyer B, Freier U, Nichols PD, Jia Z, King R, Virtue J, Swadling KM, Meiners KM, Kawaguchi S (2016) Condition of larval (furcilia VI) and one year old juvenile Euphausia superba during the winter–spring transition in East Antarctica. Deep Sea Res Part II Top Stud Oceanogr 131:182–188
Acknowledgements
We would like to extend our warmest gratitude to the Captain and crew of Aker Biomarine’s FV Saga Sea for collecting, carefully packaging and storing the krill used for this study, so as to maintain premium sample integrity. We also thank Dr Andy Revill for facilitating this research, and Mina Brock for technical assistance in the laboratory. This research was funded by Australian Research Council Linkage Grant LP140100412 between the Australian Antarctic Division, Commonwealth Scientific and Industrial Research Organization, Institute for Marine and Antarctic Studies (University of Tasmania), Griffith University and Aker Biomarine.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ericson, J.A., Hellessey, N., Nichols, P.D. et al. New insights into the seasonal diet of Antarctic krill using triacylglycerol and phospholipid fatty acids, and sterol composition. Polar Biol 42, 1985–1996 (2019). https://doi.org/10.1007/s00300-019-02573-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-019-02573-6