Abstract
Global warming, increase of the atmospheric temperature leading to changes in climate, is a growing environmental concern for all organisms including marine organisms, and many efforts have been made to reveal the potential adverse effects on the systematics of aquatic organisms in response to the temperature changes. To examine the effects of temperature shifts on copepods in temperate and polar regions, we compared the life parameters and gene expression profiles of the de novo lipogenesis (DNL) pathway and heat shock protein (hsp) genes in the temperate copepod Tigriopus japonicus (T. japonicus) and the Antarctic copepod Tigriopus kingsejongensis (T. kingsejongensis). The median lethal temperature (LT50) and no observed effect level (NOEL) in the temperate copepod T. japonicus were determined to be 35.3 and 32 °C, respectively, in response to a temperature increase of 2 °C a day. In the Antarctic copepod T. kingsejongensis, the LT50 and NOEL were determined to be 24.8 and 12 °C, respectively. In addition, delayed developmental time and impaired fecundity were observed (P < 0.05) in response to temperature changes in T. japonicus. T. japonicus DNL pathway genes were down-regulated in response to high temperature, whereas T. kingsejongensis DNL pathway genes showed up-regulation in response to high temperature, indicating that these two Tigriopus species have different modes of action in response to temperature shifts. In both copepods, transcription of heat shock proteins (hsps) was mostly up-regulated in response to temperature shifts, but it showed moderate expression at 15 °C for T. japonicus and 4 °C for T. kingsejongensis. These findings indicate temperature shift-mediated species-specific modulations of the DNL pathway and hsps gene expression, leading to alteration of lipid synthesis and chaperoning with deleterious effects on the life parameters of these two copepods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The warming effects associated with climate change are becoming a matter of great concern for marine organisms and ecosystems (Walther et al. 2002; Hoegh-Guldberg and Bruno 2010). The Fourth Assessment Report of the Intergovernmental Panel on Climate Change (IPCC) predicted that aquatic water temperatures would increase by approximately 2–3 and 3–5 °C by the years 2050 and 2100, respectively (IPCC 2007). Temperature is a key abiotic factor affecting the biological processes of aquatic species (van Dooremalen and Ellers 2010). Therefore, temperature changes will result in alteration of the life traits of affected aquatic organisms (e.g., growth rate and fecundity) (Lee et al. 2003; Rhyne et al. 2009; Pankhurst and King 2010).
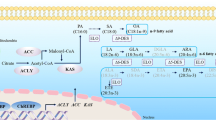
Fatty acids (FA) play a crucial role in the sustenance of life parameters (e.g., survival, growth, and reproduction) (Tessier et al. 1983; Sargent et al. 2002) of diverse living organisms (Mansour et al. 2005), such as the water flea Daphnia pulex (Schlechtriem et al. 2006) and the copepods Platychelipus littoralis (Werbrouck et al. 2016) and Paracyclopina nana (Lee et al. 2017a, b). The relationship between temperature shifts and the FA composition of aquatic organisms is a big concern (Downer and Kallapur 1981; Schlechtriem et al. 2006; Masclaux et al. 2009; Gladyshev et al. 2011), since FA play essential roles in thermal signaling (Ghosh et al. 1997). In general, aquatic organisms in polar regions have high lipid contents to maintain their body temperature, but this is not true in temperate region-inhabiting species (Nichols et al. 1994; Kattner and Hagen 2009; Murzina et al. 2013). However, despite the ease of applying temperature shifts to produce thermal stress, studies on physiological variation of FA and lipids are limited. In particular, it is important to understand the mechanisms of the de novo lipogenesis (DNL) pathway, which is associated with FA synthesis, in marine invertebrates in order to evaluate the effects of temperature changes in both temperate and Antarctic-dwelling copepods. Thus, the finding that DNL pathway genes (e.g., sterol regulatory element-binding protein [SREBP], carbohydrate response element-binding protein [ChREBP], ATP-citrate lyase [ACLY], acetyl-CoA carboxylase [ACC], and β-ketoacyl-acyl-carrier-protein synthase [KAS]) playing a central role in FA synthesis and their expression profile are important to understand the process of fat accumulation under temperature change in copepods. However, compared to the temperate species, the collection and maintenance of the Antarctic species in laboratory-based and molecular studies are challenging compared to field studies, since differences in the laboratory cultivation environment and the actual environment are clearly inevitable.
The harpacticoid copepods Tigriopus spp. are widely distributed marine invertebrates and are an important link in the transfer of energy and toxicants from primary producers to higher trophic level consumers (Raisuddin et al. 2007). Of the harpacticoid copepods, Tigriopus japonicus have been widely used as a model species for ecotoxicological and environmental genomics research due to their small size (< 1 mm), short life cycle (< 2 weeks), sexual dimorphism, and easy maintenance (Raisuddin et al. 2007). The Antarctic harpacticoid copepod Tigriopus kingsejongensis has recently been used as a promising model species for Antarctic marine environmental research (Park et al. 2014; Kim et al. 2016; Lee et al. 2016).
Recently, comparisons of these two Tigriopus copepods have been reported to assess the effects of environmental stressors (ultraviolet [UV] radiation and water accommodated fraction [WAF] of crude oil) on life parameters (mortality and fecundity) with molecular responses (Han et al. 2016, 2017). In this study, we investigated how temperature shifts in T. japonicus and T. kingsejongensis affect the life parameters (e.g., survival, developmental time, and fecundity) with the changes in FA through transcriptional regulation of lipogenesis pathway genes (i.e., SREBP, ChREBP, ACLY, ACC, and KAS). In addition to these lipogenesis pathway genes, we measured the transcriptional regulation of heat shock proteins (hsps) that play the role of cell chaperoning due to environmental stressors in response to different temperatures. Overall, the results of this study contribute to our understanding of the effects of temperature changes on life parameters, lipogenesis, and stress defense mechanisms in temperate and Antarctic copepods.
Materials and methods
Culture and maintenance of T. japonicus and T. kingsejongensis
Tigriopus japonicus (collected from Haeundae beach in Busan, South Korea) and T. kingsejongensis (kindly provided by Dr. Sanghee Kim, Korea Polar Research Institute, Incheon, South Korea) were maintained in 10 L tanks containing filtered (0.2-μm) artificial seawater (ASW) (TetraMarine Salt Pro, Tetra™, Cincinnati, OH, USA) adjusted to a salinity of 32 practical salt units (psu) and pH 7.8–8.2. The tanks were kept in an automatically controlled incubator (MIR-553, Sanyo, Gunma, Japan) under a 12:12 h (light:dark) photoperiod. The oxygen concentration was regulated by aeration to 7–8 mg O2 L−1. The temperature was maintained at 20 and 8 °C for T. japonicus and T. kingsejongensis, respectively. Two groups of T. japonicus and T. kingsejongensis were maintained at 25 and 14 °C, respectively, before the experiment (Han et al. 2016). Copepods were fed with the green algae Tetraselmis suecica (T. suecica) (~ 6 × 104 cells mL−1) daily, and the ASW was renewed every 3 days. Species identity was confirmed by morphometric analysis, followed by molecular characterization of the mitochondrial cytochrome oxidase 1 (mtCO1) gene as a universal barcode marker (Jung et al. 2006; Park et al. 2014).
Acute toxicity tests in response to an increase in temperature in temperate and Antarctic copepods
A mortality test was conducted by exposing 10 adult individuals of T. japonicus and T. kingsejongensis to a temperature increase (2 °C a day). This test was replicated three times. Each 12-well tissue culture test plate (30012, SPL Life Science Co. Ltd., Seoul, South Korea) contained 10 adult copepods in 4-mL ASW. The copepods were not fed during the acute toxicity tests. Mortality was recorded once every 24 h. The no observed effective level (NOEL), 10% lethal temperature (LT10), and median lethal temperature (LT50) values were computed using Probit analysis (ToxRat® Ver.2.09, ToxRat Solutions GmbH, Alsdorf, Germany).
Expression of heat shock proteins and de novo lipogenesis pathway genes in response to different temperatures
To investigate the expression patterns of de novo lipogenesis (DNL) pathway genes (SREBP, ChREBP, ACLY, ACC, and KAS) and heat shock protein (hsp) genes, we measured mRNA expression in response to different temperatures (25 °C [control], 15 °C, and 35 °C for T. japonicus; 14 °C [control], 4 °C, and 24 °C for T. kingsejongensis) over 30, 60, and 120 min. The total RNA was extracted from each sample (200 and 20 individuals for T. japonicus and T. kingsejongensis, respectively) with TRIZOL® reagent (Invitrogen, Paisley, Scotland, UK) according to the manufacturer’s instructions. To synthesize cDNA for real-time quantitative reverse transcriptase-polymerase chain reaction (RT-PCR), two μg of total RNA and of oligo(dT)20 primer were used for reverse transcription (SuperScript™ II RT kit, Invitrogen, Carlsbad, CA, USA). Real-time qRT-PCR was conducted using the following conditions: 95 °C for 4 min; 35 cycles of 95 °C for 30 s, 58 °C for 30 s, and 72 °C for 30 s; and 72 °C for 10 min using SYBR Green as a probe (Molecular Probes, Invitrogen) on a CFX96™ real-time PCR system (Bio-Rad, Hercules, CA, USA). To confirm amplification of specific products, melting curve cycles were run as follows: 95 °C for 1 min; 55 °C for 1 min; and 80 cycles of 55 °C for 10 s with a 0.5 °C increase for each cycle using real-time qRT-PCR F or R primers (Online Resource 1). All experiments were performed in triplicate. Fold change in expression relative to the controls was evaluated using the \(2^{-\Delta\Delta C_{t}}\) method (Livak and Schmittgen 2001).
Effects of temperature on developmental time and fecundity of T. japonicus
To examine the effects of different temperatures on developmental time, we collected the nauplii (< 12 h post-hatching) of T. japonicus. Subsequently, 10 newly hatched nauplii were transferred to each well, containing 4-mL ASW, in a 12-well tissue culture test plate. There were three temperature treatment groups (25 °C [control], 15 °C, and 35 °C) in three replicates for each treatment (a total of 90 nauplii). The animals were fed once per day by adding 100 of µg L−1 T. suecica (approximately 200,000 cells mL−1) to each well throughout the experiment. Developmental stages were observed once every 24 h under a stereomicroscope (SZX-ILLK200, Olympus, Tokyo, Japan) for 25 days.
To examine the effects of temperature on fecundity, adult ovigerous females were individually exposed to different temperatures (25 °C [control], 15 °C, and 35 °C) for 10 days with 4 mL ASW in a 12-well tissue culture test plate. The number of nauplii was counted daily for 10 days. Throughout the temperature exposure period, 50% of the ASW was renewed once every 24 h. The copepods were fed once per day by adding 100 µg L−1 of live T. suecica (approximately 200,000 cells mL−1).
Statistical analysis
All data are expressed as mean value with standard error (mean ± SE). Normal distributions and homogeneity of variance were assessed using Levene’s test. Data were analyzed using one-way ANOVA, followed by Tukey’s honest significant difference test at a significance level of P < 0.05. All statistical analyses were performed using SPSS® version 21 software (SPSS Inc., Chicago, IL, USA).
Results
Effects of temperature increases on mortality in T. japonicus and T. kingsejongensis
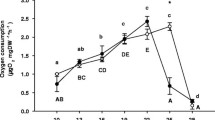
The survival rate of both copepod species gradually decreased as temperature increased. The LT50 and NOEL in T. japonicus were determined to be approximately 35.3 and 32 °C, respectively. In T. kingsejongensis, the LT50 and NOEL in response to temperature increases were 24.8 and 12 °C, respectively (Fig. 1; Online Resource 2).

Reproduced with permission from Han et al. (2018)
Effects of increasing temperature (2 °C a day) on the survival rate in a Tigriopus japonicus and b Tigriopus kingsejongensis. Error bars indicate the standard errors.
Effects of temperature on the developmental timing and fecundity of T. japonicus
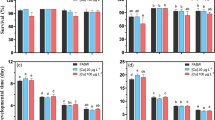
In T. japonicus, the developmental times from the nauplius stage to the copepodite stage and the nauplius to adult stage increased relative to the control (25 °C), suggesting an overall retardation in their development rate. At 35 °C, nauplii did not survive (Fig. 2a). Fecundity was significantly decreased (P < 0.05) at both experimental temperatures (15 and 35 °C) compared to the control (25 °C) in T. japonicus, although the nauplii produced all subsequently died at 35 °C (Fig. 2b). These results indicate that increased temperature led to adverse effects on developmental time and fecundity of this copepod species.
Effects of different temperatures (25 °C [control], 15 °C, and 35 °C) on the lifecycle parameters of Tigriopus japonicus. a Developmental times from nauplius to copepodite (N–C) and nauplius to adult (N–A) and b Average number of hatched nauplii from ovigerous females over 10 days. Significant differences were analyzed by ANOVA supported by a Tukey’s post hoc test at a significance level of P < 0.05
Expression of De Novo Lipogenesis pathway genes in response to different temperatures
To examine the effects of increasing temperature on T. japonicus and T. kingsejongensis, we measured the mRNA expression of DNL pathway genes in response to different temperatures (25 °C [control], 15 °C, and 35 °C for T. japonicus; 14 °C [control], 4 °C, and 24 °C for T. kingsejongensis) after 30, 60, 90, and 120 min. In T. japonicus, the mRNA of DNL pathway genes was not significantly expressed in the low-temperature group (15 °C), whereas the mRNA expression of DNL pathway genes significantly decreased (P < 0.05) in the high-temperature treatment (35 °C). In T. kingsejongensis, the mRNA expression of DNL pathway genes significantly decreased (P < 0.05) in the low-temperature treatment (4 °C), while the mRNA expression of DNL pathway genes significantly increased (P < 0.05) in the high-temperature group (24 °C) (Fig. 3; Online Resource 3).
Expression patterns of de novo lipogenesis pathway genes in response to different temperatures over 30, 60, 90, and 120 min in a Tigriopus japonicus and b Tigriopus kingsejongensis. ChREBP carbohydrate regulatory element-binding protein, SREBP sterol regulatory element-binding protein, ACLY ATP-citrate lyase, ACC acetyl-CoA carboxylase, KAS β-ketoacyl-(acyl-carrier-protein) synthase. Expression profiles are represented as a heat map. Blue and red indicate down- and up-regulation, respectively, of mRNA expression compared to the control (white). (Color figure online)
Expression of heat shock proteins in response to different temperatures
From the mRNA expression of hsps in different test temperatures after specific treatment intervals, the hsps mRNA transcripts of T. japonicus were significantly increased (P < 0.05) at 15 and 35 °C compared to the control. Similarly, in T. kingsejongensis, expression of most of the measured hsps was significantly increased (P < 0.05) at 4 and 24 °C compared to the control. The exceptions were hsp40, hsp70, and hsp90 (Fig. 4; Online Resource 3).
Expression patterns of heat shock protein genes in response to different temperatures over 30, 60, 90, and 120 min in a Tigriopus japonicus and b Tigriopus kingsejongensis. Expression profiles are represented as a heat map. Blue and red indicate down- and up-regulation, respectively, of mRNA expression compared to the control (white). (Color figure online)
Discussion
Survival rate was negatively affected by increasing culture temperature in both the temperate and Antarctic copepods. T. japonicus and T. kingsejongensis copepods experienced 100% mortality at 38 and 30 °C, respectively (Fig. 1) (Han et al. 2018). During the experiment, a decreased survival rate of the live prey T. suecica was also observed as the culture temperature rose (data not shown). Previous studies have found similar results. Rhyne et al. (2009) observed that the microalga Rhodomonas lens, a common copepod food source, was susceptible to temperatures above 26 °C, which led to a declining population or even complete culture eradication. In the calanoid copepod Pseudodiaptomus pelagicus, significant reductions in survival were observed at 32 and 34 °C compared to low temperatures (Rhyne et al. 2009). The benthopelagic calanoid copepod Pseudocyclops xiphophorus was completely eradicated at 34 °C (Brugnano et al. 2008). This collective body of evidence indicates that rising temperatures have fatal effects on the survival of marine copepod species, which may also suggest a species-specific temperature threshold, resulting in the indicated increase in mortality.
Delayed development of T. japonicus was observed in the low temperature treatment (15 °C) compared to the control (Fig. 2a). Temperature shifts can affect the metabolic rate and development of aquatic invertebrates (Sweeney 1984). For example, in the calanoid copepods Acartia clausi and Pseudocalanus newmani, developmental retardation was observed with decreasing temperature (Klein Breteler and Schogt 1994; Lee et al. 2003). Similar developmental retardation has been observed in the copepod Paracyclopina nana at low temperature (Lee et al. 2017a). However, within tolerable temperature ranges, high temperatures can accelerate the development of copepods. For example, Pseudodiaptomus dubia had shorter developmental times at high temperatures (30–35 °C) than those at low temperatures (15–25 °C) (Li et al. 2009). Overall, it is apparent that temperature shifts can affect the basal metabolism rate, leading to delayed and/or accelerated copepod development. In addition to these impacts on development, we observed that the fecundity of T. japonicus was significantly decreased (P < 0.05) in the low temperature treatment (Fig. 2b). In the copepod P. nana, a reduced reproductive rate was observed in response to rapid temperature change. At a low temperature, fecundity was reduced by 20% compared to that at a higher temperature (Lee et al. 2017a). Similarly, in the copepod P. dubia, fecundity and reproductive frequency decreased at low temperatures (Li et al. 2009). These findings indicate that low temperature can cause substantial reproductive impairment in copepods, in comparison to high temperature, as long as these high temperatures fall within their optimal temperature conditions. Energy allocation (EA), a well-known survival strategy of diverse organisms, may explain this phenomenon. In this paradigm, trade-offs in the energy investment in development and reproduction are a response to environmental stressors (Kooijman and Troost 2007). For example, dysfunction in the development and fecundity of the copepod T. japonicus was observed in response to the water accommodated fraction (WAF) of crude oil, although no mortality was observed even in 100% WAF (Han et al. 2014). Thus, developmental retardation and reduction in fecundity in response to rapid temperature changes could be linked to a survival strategy for homeostatic maintenance of metabolism in suboptimal temperatures. Although we did not examine the effects of rapid temperature change on the development and fecundity of T. kingsejongensis due to its slow developmental time (approximately 30 days at 6 °C), this species may have a similar pattern of reduced fecundity and developmental delays at low temperature. Further investigations are needed to examine this possibility.
The expression patterns of DNL pathway genes were examined in response to temperature changes in two related copepods. As shown in Fig. 3, no significant modulation of DNL pathway genes was observed in T. japonicus at 15 °C compared to the control, whereas the down-regulation of DNL pathway genes was observed at 35 °C (Fig. 3a). These results are consistent with a report that temperature changes led to modulation of DNL pathway genes and altered FA composition in P. nana (Lee et al. 2017a, b). Generally, copepods in colder regions exhibit higher lipid contents than those in warmer environments (Kattner and Hagen 2009). Indeed, another temperate harpacticoid copepod, Platychelipus littoralis, demonstrated elevation of the product of the DNL pathway, C16:0 (Werbrouck et al. 2016), which is commonly observed in bacteria (Hazel and Williams 1990). In temperate species, increased expression of DNL pathway-related genes also leads to an increase in the overall FA content under cold temperature (Brodte et al. 2008). These observations suggest that up- and down-regulation of DNL pathway genes in response to temperature changes are closely associated with FA synthesis in copepods. However, in this study, the Antarctic copepod T. kingsejongensis demonstrated significant reduction in the mRNA expression of DNL-pathway-related genes under cold temperatures, whereas the DNL pathway genes were up-regulated at 24 °C (Fig. 3b). In a previous study, different expression patterns of fatty acid metabolism genes were shown in response to different temperatures in three rice planthoppers (Nilaparvata lugens, Sogatella furcifera, and Laodelphax striatellus). For instance, fatty acid synthesis-related genes (e.g., fatty acyl-CoA reductase and fatty acid synthase) were up-regulated at high temperatures only in N. lugens, whereas down-regulation was observed in both S. furcifera and L. striatellus (Huang et al. 2017). These findings suggest that the susceptibility and adaptability of FA synthesis to temperature changes are likely species-specific. We are the first to study the identification and transcriptional regulation of DNL pathway genes in response to temperature changes in two congeneric copepods. Temperature changes may induce transcriptional regulation of DNL pathway genes, leading to FA synthesis and/or accumulation in these two copepods. The slower decline in mortality of T. kingsejongensis could possibly be linked to the up-regulation of DNL pathway regulating genes, since DNL is associated with effects on the systemic metabolism, and tissue-specific regulation of this pathway is critical for metabolic homeostasis (Yilmaz et al. 2016), implicating that T. kingsejongensis is relatively more adaptable to extreme conditions than the temperate species T. japonicus. Further in-depth analysis on the various types of fatty acids in addition to the final product of the DNL pathway (palmitic acid, 16:0) is required to better understand the relationship between the species-specific acclimating modulations of the DNL pathway.
The transcriptional regulation of hsps was examined in response to rapid temperature changes in two congeneric copepods. The expression of most hsps significantly increased (P < 0.05) in the high-temperature treatment. The low-temperature treatment did not result in significantly increased hsps expression, except for hsp20 (Fig. 4). Heat shock proteins are well-studied molecular chaperones that play important roles in the cellular defense response to environmental stressors (e.g., UV radiation and salinity), helping to preserve a normal physiological state (Hartl 1996; Sarkar 2006). For example, a significant increase in hsps was observed in the rotifer B. koreanus when it was exposed to UV light (Kim et al. 2011). Similarly, in the copepod P. nana, the mRNA expression of hsps was increased under salinity stress (Lee et al. 2017b). It has been demonstrated that the mRNA expression of hsps in T. japonicus and T. kingsejongensis was significantly modulated (P < 0.05) in response to UV radiation (Han et al. 2016), indicating that hsps are involved in preventing and repairing cellular damage induced by temperature changes in these related species. In addition, we found that the hsp90 genes in these copepods were significantly increased (P < 0.05) under high-temperature conditions. This protein has an important role in cell protection, where it repairs misfolded proteins that have been induced by environmental stressors (Kalmar and Greensmith 2009). For example, in the marine crab Charybdis japonica, the hsp90 gene was significantly induced in response to bisphenol A and 4-nonylphenol (Park et al. 2014). In the sea cucumber Apostichopus japonicus, the mRNA expression of the hsp90 gene was increased under heat stress (Zhao et al. 2011). Thus, we suggest that hsp90 is likely a key protective protein and a useful molecular biomarker in the high-temperature response of T. japonicus and T. kingsejongensis.
In summary, we demonstrated that temperature changes can affect life parameters (e.g., survival, growth, and fecundity) through modulation of DNL pathway genes and heat shock response genes in response to temperature changes in two Tigriopus species. To date, comparative studies on the effects of temperature changes have not been applied in either in vivo or in vitro study, nor has there been a previous comparison of related temperate and Antarctic copepods. Therefore, the results of this study provide a better understanding of how rapid temperature changes affect life parameters and the underlying biochemical and molecular responses of T. japonicus and T. kingsejongensis.
References
Brodte E, Graeve M, Jacob U, Knust R, Pörtner HO (2008) Temperature-dependent lipid levels and components in polar and temperate eelpout (Zoarcidae). Fish Physiol Biochem 34:261–274. https://doi.org/10.1007/s10695-007-9185-y
Brugnano C, Guglielmo L, Ianora A, Zagami G (2008) Temperature effects on fecundity, development and survival of the benthopelagic calanoid copepod, Pseudocyclops xiphophorus. Mar Biol 156:331–340. https://doi.org/10.1007/s00227-008-1086-9
Downer RGH, Kallapur VL (1981) Temperature-induced changes in lipid composition and transition temperature of flight muscle mitochondria of Schistocerca gregaria. J Therm Biol 6:189–194. https://doi.org/10.1080/17451000903042461
Ghosh S, Strum JC, Bell RM (1997) Lipid biochemistry: functions of glycerolipids and sphingolipids in cellular signaling. FASEB J 11:45–50
Gladyshev MI, Semenchenko VP, Dubovskaya OP, Fefilova EB, Makhutova ON, Buseva ZF, Sushchik NN, Razlutskij VI, Lepskaya EV, Baturina MA, Kalachova GS, Kononova ON (2011) Effect of temperature on contents of essential highly unsaturated fatty acids in freshwater zooplankton. Limnologica 41:339–347. https://doi.org/10.1016/j.limno.2011.03.001
Han J, Won E-J, Hwang D-S, Shin K-H, Lee YS, Leung KMY, Lee J-S (2014) Crude oil exposure results in oxidative stress-mediated dysfunctional development and reproduction in the copepod Tigriopus japonicus and modulates expression of cytochrome P450 (CYP) genes. Aquat Toxicol 152:308–317. https://doi.org/10.1016/j.aquatox.2014.04.027
Han J, Puthumana J, Lee M-C, Kim S, Lee J-S (2016) Different susceptibilities of the Antarctic and temperate copepods Tigriopus kingsejongensis and Tigriopus japonicus to ultraviolet (UV) radiation. Mar Ecol Prog Ser 561:99–107. https://doi.org/10.3354/meps11946
Han J, Kim H-S, Kim I-C, Kim S, Hwang U-K, Lee J-S (2017) Effects of water accommodated fractions (WAFs) of crude oil in two congeneric copepods Tigriopus sp. Ecotoxicol Environ Saf 145:511–517. https://doi.org/10.1016/j.ecoenv.2017.07.065
Han J, Jeong C-B, Byeon E, Lee J-S (2018) Effects of temperature changes on the generation of reactive oxygen species and the expression and activity of glutathione-S transferases in two congeneric copepods Tigriopus japonicus and Tigriopus kingsejongensis. Fish Sci. https://doi.org/10.1007/s12562-018-1224-3
Hartl FU (1996) Molecular chaperones in cellular protein folding. Nature 381:571–579. https://doi.org/10.1038/381571a0
Hazel JR, Williams EE (1990) The role of alterations in membrane lipid composition in enabling physiological adaptation of organisms to their physical environment. Prog Lipid Res 29:167–227. https://doi.org/10.1016/0163-7827(90)90002-3
Hoegh-Guldberg O, Bruno JF (2010) The impact of climate change on the world’s marine ecosystems. Science 328:1523–1528. https://doi.org/10.1126/science.1189930
Huang HJ, Xue J, Zhuo JC, Cheng RL, Xu HJ, Zhang CX (2017) Comparative analysis of the transcriptional responses to low and high temperatures in three rice planthopper species. Mol Ecol 26:2726–2737. https://doi.org/10.1111/mec.14067
IPCC (Intergovernmental Panel on Climate Change) (2007) Summary for policymakers. In: Parry ML, Canziani OF, Palutilof JP, van der Linden PJ, Hanson GE (eds) Climate change 2007: impacts, adaptation and vulnerability. Contribution of Working Group II to the fourth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge, pp 7–12
Jung S-O, Lee Y-M, Park T-J, Park HG, Hagiwara A, Leung KMY, Dahms H-U, Lee W, Lee J-S (2006) The complete mitochondrial genome of the intertidal copepod Tigriopus sp. (Copepoda, Harpactidae) from Korea and phylogenetic considerations. J Exp Mar Biol Ecol 333:251–262. https://doi.org/10.1016/j.jembe.2005.12.047
Kalmar B, Greensmith L (2009) Induction of heat shock proteins for protection against oxidative stress. Adv Drug Deliv Rev 61:310–318. https://doi.org/10.1016/j.addr.2009.02.003
Kattner G, Hagen W (2009) Lipids in marine copepods: latitudinal characteristics and perspective to global warming. Lipids in aquatic ecosystems. Springer, New York, pp 257–280
Kim R-O, Rhee J-S, Won E-J, Lee K-W, Kang C-M, Lee Y-M, Lee J-S (2011) Ultraviolet B retards growth, induces oxidative stress, and modulates DNA repair-related gene and heat shock protein gene expression in the monogonont rotifer, Brachionus sp. Aquat Toxicol 101:529–539. https://doi.org/10.1016/j.aquatox.2010.12.005
Kim H-S, Lee B-Y, Han J, Lee YH, Min G-S, Kim S, Lee J-S (2016) De novo assembly and annotation of the Antarctic copepod (Tigriopus kingsejongensis) transcriptome. Mar Genomics 28:37–39. https://doi.org/10.1016/j.margen.2016.04.009
Klein Breteler WCM, Schogt N (1994) Development of Acartia clausi (Copepoda, Calanoida) cultured at different conditions of temperature and food. Hydrobiologia 292(293):469–479. https://doi.org/10.1007/BF00229974
Kooijman SALM, Troost TA (2007) Quantitative steps in the evolution of metabolic organisation as specified by the dynamic energy budget theory. Biol Rev Camb Philos Soc 82:113–142. https://doi.org/10.1111/j.1469-185X.2006.00006.x
Lee H-W, Ban S, Ikeda T, Matsuishi T (2003) Effect of temperature on development, growth and reproduction in the marine copepod Pseudocalanus newmani at satiating food condition. J Plankton Res 25:261–271. https://doi.org/10.1093/plankt/25.3.261
Lee SR, Lee JH, Kim AR, Kim S, Park H, Baek HJ, Kim H-W (2016) Three cDNAs encoding vitellogenin homologs from Antarctic copepod, Tigriopus kingsejongensis: cloning and transcriptional analysis in different maturation stages, temperatures, and putative reproductive hormones. Comput Biochem Physiol B 192:38–48. https://doi.org/10.1016/j.cbpb.2015.11.008
Lee S-H, Lee M-C, Puthumana J, Park JC, Kang S, Han J, Shin K-H, Park HG, Om A-S, Lee J-S, Han J (2017a) Effects of temperature on growth and fatty acid synthesis in the cyclopoid copepod Paracyclopina nana. Fish Sci 83:725–734. https://doi.org/10.1007/s12562-017-1104-2
Lee S-H, Lee M-C, Puthumana J, Park JC, Kang S, Hwang D-S, Shin K-H, Park HG, Soussi S, Om A-S, Lee J-S, Han J (2017b) Effects of salinity on growth, fatty acid synthesis, and expression of stress response genes in the cyclopoid copepod Paracyclopina nana. Aquaculture 470:182–189. https://doi.org/10.1016/j.aquaculture.2016.12.037
Li C, Luo X, Huang X, Gu B (2009) Influences of temperature on development and survival, reproduction and growth of a calanoid copepod (Pseudodiaptomus dubia). Sci World J 9:866–879. https://doi.org/10.1100/tsw.2009.96
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real time quantitative PCR and the \(2^{-\Delta\Delta C_{T}}\) method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Mansour MP, Frampton DMF, Nichols PD, Volkman JK, Blackburn SI (2005) Lipid and fatty acid yield of nine stationary-phase microalgae: applications and unusual C24–C28 polyunsaturated fatty acids. J Appl Phycol 17:287–300. https://doi.org/10.1007/s10811-005-6625-x
Masclaux H, Bec A, Kainz MJ, Desvilettes C, Jouve L, Bourdier G (2009) Combined effects of food quality and temperature on somatic growth and reproduction of two freshwater cladocerans. Limnol Oceanogr 54:1323–1332. https://doi.org/10.4319/lo.2009.54.4.1323
Murzina SA, Nefedova ZA, Falk-Petersen S, Ripatti PO, Ruokolainen TR, Pekkoeva SN, Nemova NN (2013) Lipid status of the two high latitude fish species, Leptoclinus maculatus and Lumpenus fabricii. Int J Mol Sci 14:7048–7060. https://doi.org/10.3390/ijms14047048
Nichols DS, Williams D, Dunstan GA, Nichols PD, Volkman JK (1994) Fatty acid composition of Antarctic and temperate fish of commercial interest. Comp Biochem Physiol B 107:357–363. https://doi.org/10.1016/0305-0491(94)90059-0
Pankhurst NW, King HR (2010) Temperature and salmonid reproduction: implications for aquaculture. J Fish Biol 76:69–85. https://doi.org/10.1111/j.1095-8649.2009.02484.x
Park E-O, Lee S, Cho M, Yoon SH, Lee Y, Lee W (2014) A new species of the genus Tigriopus (Copepoda: Harpacticoida: Harpacticidae) from Antarctica. Proc Biol Soc Washington 127:138–154. https://doi.org/10.2988/0006-324X-127.1.138
Raisuddin S, Kwok KWH, Leung KMY, Schlenk D, Lee J-S (2007) The copepod Tigriopus: a promising marine model organism for ecotoxicology and environmental genomics. Aquat Toxicol 83:161–173. https://doi.org/10.1016/j.aquatox.2007.04.005
Rhyne AL, Ohs CL, Stenn E (2009) Effects of temperature on reproduction and survival of the calanoid copepod Pseudodiaptomus pelagicus. Aquaculture 292:53–59. https://doi.org/10.1016/j.aquaculture.2009.03.041
Sargent JR, Tocher DR, Bell JG (2002) The lipids. In: Halver JE, Hardy RW (eds) Fish nutrition, 3rd edn. Academic Press, San Diego, pp 181–257
Sarkar A (2006) Biomarkers of marine pollution and bioremediation. Ecotoxicology 15:331–332. https://doi.org/10.1007/s10646-006-0073-5
Schlechtriem C, Arts MT, Zellmer ID (2006) Effect of temperature on the fatty acid composition and temporal trajectories of fatty acids in fasting Daphnia pulex (Crustacea, Cladocera). Lipids 41:397–400. https://doi.org/10.1007/s11745-006-5111-9
Sweeney BW (1984) Factors influencing life-history patterns of aquatic insects. In: Resh VH, Rosenberg D (eds) Ecology of aquatic insects. Praeger Scientific, New York, pp 56–100
Tessier AJ, Henry LL, Goulden CE, Durand MW (1983) Starvation in Daphnia: energy reserves and reproductive allocation. Limnol Oceanogr 28:667–676. https://doi.org/10.4319/lo.1983.28.4.0667
van Dooremalen C, Ellers J (2010) A moderate change in temperature induces changes in fatty acid composition of storage and membrane lipids in a soil arthropod. J Insect Physiol 56:178–184. https://doi.org/10.1016/j.jinsphys.2009.10.002
Walther G-R, Post E, Convey P, Menzel A, Parmesan C, Beebee TJC, Fromentin J-M, Hoegh-Guldberg O, Bairlein F (2002) Ecological responses to recent climate change. Nature 416:389–395. https://doi.org/10.1038/416389a
Werbrouck E, Van Gansbeke D, Vanreusel A, Mensens C, de Troch M (2016) Temperature-induced changes in fatty acid dynamics of the intertidal grazer Platychelipus littoralis (Crustacea, Copepoda, Harpacticoida): Insights from a short-term feeding experiment. J Therm Biol 57:44–53. https://doi.org/10.1016/j.jtherbio.2016.02.002
Yilmaz M, Claiborn KC, Hotamisligil GS (2016) De novo lipogenesis products and endogenous lipokines. Diabetes 65:1800–1807. https://doi.org/10.2337/db16-0251
Zhao H, Yang H, Zhao H, Chen M, Wang T (2011) The molecular characterization and expression of heat shock protein 90 (Hsp90) and 26 (Hsp26) cDNAs in sea cucumber (Apostichopus japonicus). Cell Stress Chaperon 16:481–493. https://doi.org/10.1007/s12192-011-0260-z
Acknowledgements
This work was supported by a Grant from the National Research Foundation (2017R1A2B4010155) funded to Jeonghoon Han and by a Grant from the Korea Polar Research Institute (PE18100) funded to Jae-Seong Lee.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Han, J., Lee, MC., Park, J.C. et al. Effects of temperature shifts on life parameters and expression of fatty acid synthesis and heat shock protein genes in temperate and Antarctic copepods Tigriopus japonicus and Tigriopus kingsejongensis. Polar Biol 41, 2459–2466 (2018). https://doi.org/10.1007/s00300-018-2382-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-018-2382-6