Abstract
Serological assays are commonly used in wildlife health studies to screen for exposure of an individual or a population to infectious agents. Such assays can therefore provide useful information regarding the health status of an individual or for determining the prevalence of a pathogen within a population. In this study, serological assays of three viral agents have been conducted on the Adélie Penguin (Pygoscelis adeliae) on Ross Island, Ross Sea, Antarctica. We sampled adult Adélie Penguins during three consecutive summer breeding seasons (2010–2011, 2011–2012 and 2012–2013), and tested those samples for antibodies to avian influenza A virus, Newcastle disease virus, and infectious bursal disease virus. No antibodies were detected for avian influenza A virus in any season. Two samples in 2012–2013 were positive for Newcastle disease virus antibodies and a total of 10 samples were positive for infectious bursal disease virus antibodies during this study. This information establishes baseline data for these three viruses in Adélie Penguins at this location and can be used for future comparisons of disease prevalence in this population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The introduction of pathogens to the Antarctic is considered a threat to the animals inhabiting this region due to either natural or unintentional means (Woehler et al. 2014) aided by a warming climate (Turner et al. 2014). Recently, the presence of three different strains of avian influenza A viruses has been confirmed in the Western Antarctic (Hurt et al. 2014, 2016; Barriga et al. 2016; de Souza Petersen et al. 2017). Prior to the confirmation of these viruses, serological testing and virus isolation were the primary means of investigating for these and other viruses in Antarctic avifauna. Past investigations reported in Antarctic penguins have targeted viral agents that primarily affect commercially raised poultry (Fuller et al. 2012; Grimaldi et al. 2014). The earliest report on viral antibodies in Antarctic birds, and the first to include one of the three viruses assayed in this study, described finding Newcastle disease virus antibody in the sera from 60 Antarctic birds (Sladen 1962). Subsequent investigations for antibodies to avian influenza A virus, Newcastle disease virus and infectious bursal disease virus in penguins followed in the late 1970s with the studies of Morgan et al. (1978, 1981) and Morgan and Westbury (1981). Surveys for these three viruses represent the majority of penguin serological studies within the three main biogeographic regions of the Antarctic Treaty area (Continental, Maritime, Sub-Antarctic; Frenot et al. 2005; Convey 2010), and are summarized in Appendix A, Tables A1–A3. Infrequently, antibodies to less well-studied agents have been investigated such as Kemerovo and Sakhalin viruses (Doherty et al. 1975), or Reovirus and Adenovirus (St. George et al. 1985).
Overall, however, reports of the health and disease status of Antarctic penguin species remain scarce (Woods et al. 2009; Grimaldi et al. 2014) when compared to the much larger body of knowledge related to other aspects of their life history (see, e.g., Borboroglu and Boersma 2013). In an effort to address the gap in knowledge about virus prevalence in Antarctic penguins, we conducted serological surveys for antibodies to avian influenza A, Newcastle disease and infectious bursal disease viruses in adult Adélie Penguins (Pygoscelis adeliae) from two colonies on Ross Island, Antarctica. This sea-ice-obligate species is the most abundant penguin of the high-latitude Antarctic; it breeds on sparsely located snow- and glacier-free coastal areas scattered around the continent, present at colonies during October–February and otherwise dwells in the surrounding sea-ice-covered ocean (Ainley 2002). We present these benchmark viral data for use in future assessments of health/disease status trends in this species at southern Ross Sea locations in anticipation of future changes in climate and an increase in the human footprint in this region.
Materials and methods
Adélie Penguin serum samples
Up to 5.0 mL venous blood per individual was collected from adult Adélie Penguins during two field seasons (2010–2011 and 2011–2012) at Cape Crozier (77°27′S, 169°23′E) and three field seasons (2010–2011, 2011–2012, and 2012–2013) at Cape Bird, Ross Island (Fig. 1) during the austral summer, December-January. Cape Bird is comprised of three partitions: the northern-most, henceforth referred to as CB North (77°13′S, 166°28′E), where adult Adélie penguins were sampled during all three seasons; the middle and southern-most partitions [CB Middle (77°14′S, 166°25′E) and CB South (77°16′S, 166°20′E)] were only sampled during the 2012–2013 season. Adults were distinguished from juveniles (< 2 years old) by having completely black plumage below the throat and neck; the plumage of juveniles is instead white in these areas plus they lack the white eye ring (Trivelpiece et al. 1985).
Adult Adélie Penguins at Cape Bird were caught with a long-handled net along the beach when they had likely been relieved of nest-attending duties based on their appearance and movements, i.e. guano-stained ventral feathers, and leaving the colony in the direction of the water. Sampling took place between 09h30 and 18h00; sites varied by dates, though these were dictated strictly by weather conditions, and was usually carried out along the beach well away from nests. Birds were caught at approximately 20 min intervals approximating non-probability (haphazard) sampling (Stevenson 2008). Blood was collected from a digital foot vein by venipuncture using sterile 23-gauge needles attached to sterile 5.0 mL syringes; 3.0–5.0 mL of whole blood was collected in this manner and promptly dispensed into sterile blood collection tubes without anti-coagulant (Becton–Dickinson; BD). Birds were briefly checked for the presence of any ectoparasites on the head and around the vent and for any external signs of injury. After sampling, and prior to being released, each bird was marked with livestock spray on the ventrum and “permanent” marker on the tops of each foot with a unique identification number. Both the livestock spray and marker would eventually wear off or be lost at moult.
At Cape Crozier, 60 adult penguins were caught during each of the two field seasons using long-handled nets when they returned from foraging at sea and before they reached nest sites. Blood was collected from the right jugular vein using sterile 21-gauge needles on 5.0 mL syringes. For each individual, up to 3.0 mL of blood was immediately placed into sterile blood collection tubes (BD). Birds were not marked before release.
The blood samples collected were allowed to clot and subsequently centrifuged at 4000 g for 30 min, usually no longer than 4 h after collection. The serum was removed aseptically and placed into labeled 1.5 mL microcentrifuge tubes; these tubes were frozen in a − 20 °C freezer at Cape Crozier and a 0 °C freezer at Cape Bird. These samples were later stored in − 20 °C freezers at McMurdo Station and Scott Base until shipped to New Zealand in a dry shipper. All samples were placed into a − 70 °C freezer on arrival in New Zealand. In total, 424 serum samples were sent to the Australian Animal Health Laboratory (AAHL) in Geelong, Victoria, Australia for testing for antibodies for the three viral agents. A total of 144 samples collected in 2010–2011 and 2011–2012 were submitted in 2012, and 280 samples collected in 2012–2013 were submitted in 2013. Samples < 0.5 mL and/or those that were haemolysed were not sent for testing; the minimum volume of serum required was 0.5 mL and haemolysis can interfere with the tests (AAHL protocol). Each sample submitted was tested for each of the three viral antibodies.
Test methods
The tests used for detecting antibodies to influenza A, infectious bursal disease and Newcastle disease virus are Australian Animal Health Laboratory proprietary methods based on established principles of commonly used serological tests. A blocking enzyme-linked immunosorbent assay (b-ELISA) was used to test for antibodies for influenza A antibodies and a competitive enzyme-linked immunosorbent assay (c-ELISA) was used to test for antibodies for infectious bursal disease virus. Any sera testing positive or inconclusive for infectious bursal disease were tested further with a virus neutralization test (VNT). The test utilized for Newcastle disease was a hemagglutination-inhibition (HI) assay. The main characteristics of each test are given in Table 1.
Statistical parameters
Calculations for apparent prevalence were performed using Epi Tools—Estimating prevalence at: http://epitools.ausvet.com.au.
Results
The usable samples (not haemolysed and/or ≥ 0.5 mL) collected at Cape Bird during the first field season, 2010–2011 (n = 14) and submitted for testing did not yield any positive results for any of the tests reported in this study, Table 2. In general, while birds caught during this study could be described as being healthy, observations of birds exhibiting feather loss at all of the colonies on Ross Island during the field seasons of 2011–2012 and 2012–2013 were unprecedented. Birds observed with a feather loss condition appeared otherwise healthy (Grimaldi et al. 2015). No ectoparasites (ticks or lice) were seen on any of the penguins at any colony during this study.
Avian influenza A virus
No samples were positive for avian influenza A virus antibody during the study period, Table 2.
Newcastle disease virus
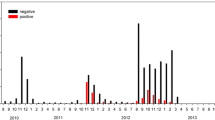
The test results for Newcastle disease virus (NDV) antibody yielded 2 positives, both with low titres. One sample (1/110 samples) was from CB North with a titre of 1:8, the second sample (1/100 samples) was collected at CB Middle and was reported as 1:16, Table 2. All other samples were negative.
Infectious bursal disease virus
Of the 424 samples collected over the course of three field seasons and tested for IBDV antibody, four were considered positive and seven produced inconclusive results (exhibiting 40-60% inhibition; AAHL procedure), Table 3. All positive samples and those with inconclusive results were additionally analyzed by the virus neutralization test (VNT). The majority of positive samples had titres of either 1:20 or 1:40; two had titres as high as 1:160, Table 3. Only one inconclusive sample was determined to be negative by the VNT.
Discussion
In spite of positive antibody levels to two of the three viruses for which serological assays were performed, clinical disease was not obvious in any of the birds sampled in this study. Almost without exception, all of the previous reports regarding infectious disease agents of Antarctic Treaty area penguins describe the birds as being in good health (Grimaldi et al. 2014). Recently, however, Barriga et al. (2016) described young Chinstrap Penguins (P. antarctica) from which influenza A virus was detected, as appearing ill even though the strain was determined to be a low pathogenicity type. It is also not uncommon for viruses to be isolated from a number of bird and mammal species (culture positive) and be described as “healthy”(e.g., Pfitzer et al. 2000; Bakker et al. 2006; Pantin-Jackwood et al. 2008; Damiani et al. 2012). During the timeframe of this study, serological tests would indicate that Adélie Penguins on Ross Island were not exposed to influenza A virus. On the other hand, there has apparently been exposure to Newcastle disease virus at Cape Bird, and Adélie Penguins at both Cape Bird and Cape Crozier had been exposed to infectious bursal disease virus.
Of the three previous studies on Ross Island to investigate influenza A antibodies in Adélie Penguins, the two earlier reports found antibodies to specific hemagglutinins and neuraminadases of this virus (Morgan and Westbury 1981; Austin and Webster 1993). In the third study (Morgan and Westbury 1988), no antibodies were detected as in this study. Miller and Shellam (2010) likewise did not find antibodies to Influenza A in Adélie Penguins in the Australian sector. This is similar to reports for antibody investigations to this virus in the sub-Antarctic. Antibodies were detected in Gentoo Penguins (P. papua) on Bird Island, South Georgia (Wallensten et al. 2006); Southern Rockhopper (Eudyptes chrysomcome) and Macaroni Penguins (E. chrysolophus) on Marion Island; and in Northern Rockhopper Penguins (E. moseleyi) on Gough Island (Abad et al. 2013), Table A1.
In contrast to the sporadic occurrence of influenza A antibodies in penguins on Ross Island, there are not only more reports of antibodies being detected in penguin colonies on the Antarctic Peninsula, but as of 2013, sequences from two different strains of influenza A have been detected in samples from several locations in three different penguin species (Adélie, Gentoo, and Chinstrap Penguins; Hurt et al. 2014, 2016; Barriga et al. 2016). Nearly identical sequences to the Adélie Penguin influenza A virus were recovered from a Snowy Sheathbill (Chionis albus) in 2014 (Hurt et al. 2016). A third avian influenza A strain (H4N7) has been confirmed recently from a Southern Giant Petrel (Macronectes giganteus) sampled also in this region (the South Shetland Islands; de Souza Petersen et al. 2017). These authors support the idea that migratory birds may have introduced and spread infectious agents to the Antarctic (de Souza Petersen et al. 2017).
Studies on Newcastle disease virus have also only been infrequently conducted on Ross Island. A report from the United States Department of Agriculture (Pierson and Pfow 1975) describes a research collection of Adélie Penguins caught in McMurdo Sound (Ross Island) becoming ill with the neurotropic form of NDV allegedly during quarantine in the United States. Pierson and Pfow (1975) concluded that these birds had been exposed to or infected with this virus at the time of capture in Antarctica, and therefore, the virus was present in these populations. Subsequent follow-up viral culture and serology tests on Adélie Penguins in McMurdo Sound were negative, however, for this virus (Morgan and Westbury 1988).
In 1978, Austin and Webster (1993) recovered a paramyxovirus from an Adélie Penguin cloacal sample, designated APMV/179/78, and detected antibodies to paramyxoviruses in blood samples from 17 of 100 Adélie Penguins (17%) and South Polar Skuas (Stercorarius maccormicki) in 1978 and 1986, respectively. It was later determined that APMV/179/78 was more closely related phylogenetically to APMV-1 serotypes (Alexander et al. 1989) to which NDV belongs. In East Antarctica, antibody to NDV was detected in Adélie Penguins near Wilkes Base (Morgan and Westbury 1981) and in 3 of 17 (17.6%) Adélie Penguins on Béchervaise Island (Morgan and Westbury 1988). Morgan et al. (1985) experimentally infected Little Penguins (Eudyptula minor) with a strain of paramyxovirus (designated APMV-IM), isolated from Royal Penguins (Eudyptes schlegeli) on Macquarie Island (Morgan et al. 1981). HI antibody titres of 1:8 and 1:16 were detected 2–3 weeks after infection (Morgan and Westbury 1981). As noted, similar titres were detected in two Adélie Penguins at Cape Bird sub-colonies in this study. The Macquarie Island strain was considered non-pathogenic (lentogenic) for Little Penguins (E. minor) at that location (Morgan and Westbury 1981; Morgan et al. 1985). This might likely be the case for the NDV strain apparently circulating sporadically in Adélie Penguins at Cape Bird.
This is the first study to report results of IBDV antibodies in Adélie Penguins on Ross Island. Across all samples collected in the three field seasons, there were 11 samples with either a positive or inconclusive ELISA result, Table 3. These samples were then tested using the virus neutralization test (VNT), Table 3. An advantage of the VNT is that the serum of any avian species can be used and this test is both very sensitive and very specific (Phalen 2002). It is considered to be the “gold standard” for IBDV antibody testing (De Wit et al. 2001). The only other effort to detect antibodies to the IBDV virus in Adélie Penguins on Ross Island was conducted in 1999. However, because these results were negative, these findings were never published (Ritchie pers. comm.).
In this study, the positive IBDV results were unexpected because of the previous negative results obtained in the investigation by Ritchie (pers. comm.) and the fact that this disease is only recognized in chickens (Saif 1998). However, a review of Table A3 shows the finding of antibodies to both of the two known serotypes of this virus in all but two instances. In one study, seroprevalence was as high as 100% (Watts et al. 2009). Seropositivity to this virus would therefore seem to be the rule rather than the exception. Watts et al. (2009) suggest that Emperor Penguins (Aptenodytes forsteri) are natural carriers of IBDV due to the inability of the virus to persist in the sea-ice of their breeding colonies as these undergo seasonal melt (Watts et al. 2009). Thus, other sources or means of introduction to their colonies, including introduction by human activity, have been ruled out. Emperor Penguins do occasionally appear at Adélie Penguin colonies on Ross Island, with colonies at Cape Crozier and nearby Beaufort Island during their breeding season (Kooyman et al. 2007). It would only be speculation, but drawing on the conclusion by Watts et al. (2009) that Emperor Penguins are natural carriers of IBDV, they may be the source of this virus that is periodically infecting Adélie Penguins on Ross Island.
Alternative means of introduction of pathogenic organisms to birds in Antarctica have been offered, however. Host switching is one proposed method and results when either a food source or other animal introduces its pathogens into new susceptible hosts (Morgan et al. 1981; Wallensten et al. 2006; Hurt et al. 2014). Prior to the implementation of the Madrid Protocol in 1998 (the Protocol on Environmental Protection to the Antarctic Treaty, 1991), it was not unusual for live domestic poultry to be brought to mainly sub-Antarctic islands to supplement food supplies (e.g., Morgan et al. 1978; Gauthier-Clerc et al. 2002). Though no longer allowed, this practice has been offered as one of the means of the possible direct introduction of poultry viruses to which antibodies have been detected in certain penguin populations (Morgan et al. 1978; Gauthier-Clerc et al. 2002).
It has also been proposed that migratory birds such as skuas or giant petrels might introduce viruses or bacteria directly or by transporting virus-bearing ticks with them, namely the bi-hemispheric seabird tick, Ixodes uriae, into colonies (e.g. Morgan and Westbury 1981; Austin and Webster 1993; Gauthier-Clerc et al. 2002; Baumeister et al. 2004; Wallensten et al. 2006; Chang et al. 2009; Abad et al. 2013; Hurt et al. 2014, 2016). De Souza Petersen et al. (2017) provide direct support for the migratory bird hypothesis with the recovery of an avian influenza A strain in a southern giant petrel. In this case, this bird had been tracked and had credible movements from the South Shetland Islands and potential opportunity for contacts during its seasonal migration into more northerly temperate waters (de Souza Petersen et al. 2017).
At Cape Bird, the only other resident avian species at these colonies was the South Polar Skua though a few other bird species were observed occasionally but always in flight, e.g. Snow Petrels (Pagodroma nivea), Antarctic Petrels (Thalassoica antarctica) and Wilson’s Storm Petrels (Oceanites oceanicus). Other species such as Jaegers (Stercorarius sp.) and Gulls (Larus sp.) have also been documented as occasional visitors around Ross Island in the past (Ainley et al. 1978). There is no support for involvement of migratory species and positive IBDV titres in Emperor Penguins in East Antarctica, however, as other avian species are not present during their winter breeding season when antibodies have been detected (Watts et al. 2009; Miller and Shellam 2010).
An additional, but as yet unproven, source of potentially pathogenic microorganisms is from the personnel and materials of both research and tourist groups (Grimaldi et al. 2011; Woehler et al. 2014). Although some have implicated past human actions (Gardner et al. 1997; Gauthier-Clerc et al. 2002; Abad et al. 2013), human involvement has also been deemed unlikely (Watts et al. 2009; Abad et al. 2013). Gardner et al. (1997) expressed concerns that non-penguin avian species’ access to contaminated poultry products may have led to antibodies to infectious bursal disease in penguin populations in East Antarctica. Improper food disposal practices at research stations could have allowed skuas to pilfer poultry and other food waste and contaminate nearby penguin colonies (Gardner et al. 1997). However, Watts et al. (2009) found neutralizing antibodies to IBDV (serotype 1) in the serum of Adélie Penguins in remote colonies away from human activity thereby absolving careless humans of culpability in this case.
From a serological perspective, infectious bursal disease virus is circulating among not only the Adélie Penguins on Ross Island, but most of the penguins sampled elsewhere, Table A3. However, the confirmed recovery of this virus has yet to be accomplished in spite of numerous attempts (Watts et al. 2009; Miller and Shellam 2010). Positive serological results may well follow exposure to a particular agent but they might also be the result of cross-reactivity to very similar antigens of closely related viruses (Gauthier-Clerc et al. 2002; Gilbert et al. 2013). This has been well documented with the hemagglutination-inhibition test and different serotypes of paramyxoviruses (Miller et al. 2010). The possibility of cross-reacting antigen–antibody reactions resulting in seropositivity to IBDV in (Emperor) penguins from consuming prey infected with a closely related birnavirus had been proposed (Critchlie 1998). There was speculation that the first outbreak of IBD in the United States was attributed to infected but unspecified crustaceans mixed in feed given to poultry (Swarbrick 1998). There is some merit to that line of thinking as the lesser mealworm beetle (Alphitobius diaperinus) is recognized as a reservoir of the IBDV (Saif 1998) and is also known as a pest of poultry production facilities (McAllister et al. 1995).
In reference to the possibility of a birnavirus found in fish, Watts et al. (2009) discredit the infected-food theory referring to a study by Dobos et al. (1979) who state, “There are no serological cross-reactions between IBDV and other birnaviruses.” However, the possibility of cross-reacting antibodies has since been given support by documentation of recombination events occurring in strains of IBDV serotype 1 (Jackwood 2012). Such a scenario was postulated as the origin for the emergence of hypervirulent strains of IBDV (van den Berg 2000). Genetic re-assortment of birnaviruses from other birds or fish could perhaps be a source of an as yet uncharacterized strain of avibirnavirus that is circulating in Antarctic penguins. Re-assortment of cross-species strains of the influenza A virus leading to pandemics (Neumann and Kawaoka 2011) is testimony to the possibility of such an event.
Additionally, the IPNV (Infectious Pancreatic Necrosis Virus; affecting fish) and IBDV strains used in the Dobos et al. (1979) study were not wild strains and the serum used to produce the antiserum in these tests originated in rabbits (anti-IPNV) and chickens (anti-IBDV). One of the concerns of using a particular test validated for one species is the uncertainty of how the serum of another species may react in that test due to unknown properties of the serum (Tizard 2002). The tests utilized in this study have been validated for use mainly in poultry (Table 1). While the detection of antibodies may truly indicate exposure to particular microorganisms, the possibility exists that other confounding factors may result in a “positive” test. These and other issues related to serology testing are more thoroughly discussed in Gardner et al. (1996), Fierz (1999), Phalen (2001) and Tizard (2002). This, too, is addressed in the report accompanying the positive results for the IBDV test. The following comment was provided,
“The outliers should not be represented as evidence for exposure to IBDV, but as indications to the need for additional work in both diagnostics and surveillance.” (AAHL report).
This sentence succinctly addresses the use of serology and its interpretation in this and other wildlife viral serology studies. This sentiment regarding the performance of serological testing outside of its original validated target species is likewise echoed in Vandalen et al. (2009). Despite some of the inherent problems with serological tests, they still remain a powerful tool for investigating infectious diseases in wildlife (Gilbert et al. 2013).
Conclusions and recommendations
The data presented herein establish a benchmark for future viral studies on Adélie Penguins on Ross Island and can be used to compare to penguin populations in other regions of the Antarctic Treaty area. While longitudinal health studies of penguins are infrequent in the three main biogeographic regions of the Antarctic (Grimaldi et al. 2014), they are even less common on Ross Island as at least 30 years has passed since the last study. However, there may well have been other studies, not only on Ross Island, but other regions that have not been published. All findings, both positive and negative alike, should be published.
Paramyxoviruses and infectious bursal disease viruses are believed to be endemic in Antarctic avian species (Morgan and Westbury 1988; Watts et al. 2009; Miller et al. 2010; Soñora et al. 2015). The results of the antibody tests in this study reaffirm the seemingly transient nature of viruses associated with Antarctic penguins as previous studies have revealed. While serological evidence for avian influenza A was negative for the Adélie Penguin populations on Ross Island during this study, confirmation of the presence of different strains of avian influenza A virus has recently been established within the Antarctic Treaty area (Hurt et al. 2014, 2016; Barriga et al. 2016; de Souza Petersen et al. 2017).
With the recent confirmation of three different strains of avian influenza A viruses in multiple species of seabirds in West Antarctica, ongoing monitoring should be a priority within the Antarctic Treaty area. This is especially relevant in the Western Antarctic Peninsula region given the warming trend there, the relatively large mix of avian species, the high concentration of research bases and a growing tourism presence (Chown et al. 2012; Turner et al. 2014). The increased potential for the distribution of non-indigenous microorganisms through natural means and an increasing human presence, aided by ameliorating climatic conditions (Turner et al. 2014; Woehler et al. 2014), likely enhance exposure to pathogens, both endemic and novel, to the animal inhabitants of the Antarctic regions. Additionally, the time gaps in investigations should encourage national programme managers to establish regular, long-term surveillance for these important diseases in all regions. Establishing baseline datasets and monitoring schemes would therefore make it possible to be aware of future incursions in keeping with the aims of both SCAR and CCAMLR. These would also aid in guiding decision-making policies for managing the ice-free areas frequently used for tourism and research purposes alike within the Antarctic Treaty area.
Data availability
Information regarding all the samples used in this study (bird identification number, colony location, date collected, test results) can be found in Online Resource 1, ESM_1.xlsx.
References
Abad FX, Busquets N, Sanchez A, Ryan PG, Majó N, Gonzalez-Solís J (2013) Serological and virological surveys of the influenza A viruses in Antarctic and sub-Antarctic penguins. Antarct Sci 25:339–344
Ainley D (2002) The Adélie penguin: bellwether of climate change. Columbia University Press, New York
Ainley DG, Wood RC, Sladen WJ (1978) Bird life at Cape Crozier, Ross Island. Wilson Bull 90:492–510
Alexander D, Manvell R, Collins M, Brockman S, Westbury H, Morgan I, Austin F (1989) Characterization of paramyxoviruses isolated from penguins in Antarctica and sub-Antarctica during 1976–1979. Arch Virol 109:135–143
Austin F, Webster R (1993) Evidence of ortho-and paramyxoviruses in fauna from Antarctica. J Wildl Dis 29:568–571
Bakker VJ, Van Vuren DH, Crooks KR, Scott CA, Wilcox JT, Garcelon DK (2006) Serologic survey of the island spotted skunk on Santa Cruz Island. West N Am Naturalist 66:456–461
Barriga GP, Boric-Bargetto D, San Martin MC, Neira V, Van Bakel H, Thompsom M, Tapia R, Toro-Ascuy D, Moreno L, Vasquez Y (2016) Avian influenza virus H5 strain with North American and Eurasian lineage genes in an Antarctic Penguin. Emerg Infect Dis 22:2221–2223
Baumeister E, Leotta G, Pontoriero A, Campos A, Montalti D, Vigo G, Pecoraro M, Savy V (2004) Serological evidences of influenza A virus infection in Antarctica migratory birds. Int Congr Ser 1263:737–740
Borboroglu PG, Boersma PD (eds) (2013) Penguins: natural history and conservation. Washington University Press, Seattle & London
Chang C-M, Lebarbenchon C, Gauthier-Clerc M, Le Bohec C, Beaune D, Le Maho Y, Van Der Werf S (2009) Molecular surveillance for avian influenza A virus in king penguins (Aptenodytes patagonicus). Polar Bio 32:663–665
Chown SL, Huiskes AH, Gremmen NJ et al (2012) Continent-wide risk assessment for the establishment of nonindigenous species in Antarctica. Proc Natl Acad Sci USA 109:4938–4943
Convey P (2010) Terrestrial biodiversity in Antarctica-Recent advances and future challenges. Polar Sci 4:135–147
Critchlie K (1998) Infectious bursal disease, penguins—Antarctica (04). ProMED 19970607:1191
Damiani A, Kalthoff D, Beer M, Müller E, Osterrieder N (2012) Serological survey in dogs and cats for influenza A (H1N1) pdm09 in Germany. Zoonoses Pub Health 59:549–552
de Souza Petersen E, de Arauj J, Krüger L, Seixas MM, Ometto T, Thomazelli LM, Walker D, Durigon EL, Petry MV (2017) First detection of avian influenza virus (H4N7) in Giant Petrel monitored by geolocators in the Antarctic region. Mar Biol 164:62
De Wit J, Heijmans J, Mekkes D, Van Loon A (2001) Validation of five commercially available ELISAs for the detection of antibodies against infectious bursal disease virus (serotype 1). Avian Path 30:543–549
Dobos P, Hill B, Hallett R, Kells D, Becht H, Teninges D (1979) Biophysical and biochemical characterization of five animal viruses with bisegmented double-stranded RNA genomes. J Virol 32:593–605
Doherty R, Carley J, Murray M, Main A Jr, Kay B, Domrow R (1975) Isolation of arboviruses (Kemerovo group, Sakhalin group) from Ixodes uriae collected at Macquarie Island, Southern Ocean. Am J Trop Med Hyg 24:521–526
Fierz W (1999) Basic problems with serological laboratory diagnosis. Mol Biotechnol 13:89–111
Frenot Y, Chown SL, Whinam J, Selkirk PM, Convey P, Skotnicki M, Bergstrom DM (2005) Biological invasions in the Antarctic: extent, impacts and implications. Biol Rev 80:45–72
Fuller T, Bensch S, Müller I, Novembre J, Pérez-Tris J, Ricklefs RE, Smith TB, Waldenström J (2012) The ecology of emerging infectious diseases in migratory birds: an assessment of the role of climate change and priorities for future research. EcoHealth 9:80–88
Gardner IA, Hietala S, Boyce WM (1996) Validity of using serological tests for diagnosis of diseases in wild animals. Rev Sci Tech 15:323–335
Gardner H, Kerry K, Riddle M, Brouwer S, Gleeson L (1997) Poultry virus infection in Antarctic penguins. Nature 387(6630):245–245
Gauthier-Clerc M, Eterradossi N, Toquin D, Guittet M, Kuntz G, Le Maho Y (2002) Serological survey of the king penguin, Aptenodytes patagonicus, in Crozet Archipelago for antibodies to infectious bursal disease, influenza A and Newcastle disease viruses. Polar Bio 25:316–319
Gilbert AT, Fooks A, Hayman D, Horton D, Müller T, Plowright R, Peel A, Bowen R, Wood J, Mills J (2013) Deciphering serology to understand the ecology of infectious diseases in wildlife. EcoHealth 10:298–313
Grimaldi W, Jabour J, Woehler EJ (2011) Considerations for minimising the spread of infectious disease in Antarctic seabirds and seals. Polar Rec 47:56–66
Grimaldi WW, Seddon PJ, Lyver PO, Nakagawa S, Tompkins DM (2014) Infectious diseases of Antarctic penguins: current status and future threats. Polar Bio 38:591–606
Grimaldi WW, Hall RJ, White DD, Wang J, Massaro M, Tompkins DM (2015) First report of a feather loss condition in Adelie penguins (Pygoscelis adeliae) on Ross Island, Antarctica, and a preliminary investigation of its cause. Emu 115:185–189
Hurt AC, Vijaykrishna D, Butler J, Baas C, Maurer-Stroh S, Silva-de-la-Fuente MC, Medina-Vogel G, Olsen B, Kelso A, Barr IG, González-Acuña D (2014) Detection of evolutionarily distinct avian influenza A viruses in Antarctica. mBio 5:e01098-01014
Hurt AC, Su YC, Aban M, Peck H, Lau H, Baas C, Deng Y-M, Spirason N, Ellström P, Hernandez J (2016) Evidence for the introduction, reassortment, and persistence of diverse influenza A viruses in Antarctica. J Virol 90:9674–9682
Jackwood DJ (2012) Molecular epidemiologic evidence of homologous recombination in infectious bursal disease viruses. Avian Dis 56:574–577
Kooyman GL, Ainley DG, Ballard G, Ponganis PJ (2007) Effects of giant icebergs on two emperor penguin colonies in the Ross Sea, Antarctica. Antarct Sci 19:31–38
McAllister J, Steelman C, Newberry L, Skeeles J (1995) Isolation of infectious bursal disease virus from the lesser mealworm, Alphitobius diaperinus (Panzer). Poult Sci 74:45–49
Miller GD, Shellam GR (2007) Disease status of penguins on Macquarie Is., poster presented at the 6th International Penguin Conference, 2007, Hobart, Tasmania
Miller GD, Shellam GR (2010) Seasonal prevalence of viral antibodies in emperor penguins at Auster Colony, Antarctica. In: Poster presented at the 7th International Penguin Conference, 2010, Boston, Massachusetts
Miller PJ, Afonso CL, Spackman E, Scott MA, Pedersen JC, Senne DA, Brown JD, Fuller CM, Uhart MM, Karesh WB (2010) Evidence for a new avian paramyxovirus serotype 10 detected in rockhopper penguins from the Falkland Islands. J Virol 84:11496–11504
Morgan I, Westbury H (1981) Virological studies of Adelie penguins (Pygoscelis adeliae) in Antarctica. Avian Dis 25:1019–1026
Morgan I, Westbury H (1988) Studies of viruses in penguins in the Vestfold Hills. Hydrobiologia 165:263–269
Morgan I, Caple I, Westbury H, Campbell J (1978) Disease investigations of penguins and elephant seals on Macquarie Island [Australia]. Department of Agriculture. Victoria Res Proj Ser 47:1–51
Morgan I, Westbury H, Caple I, Campbell J (1981) A survey of virus infection in sub-Antarctic penguins on Macquarie Island, Southern Ocean. Aust Vet J 57:333–335
Morgan I, Westbury H, Campbell J (1985) Viral infections of little blue penguins (Eudyptula minor) along the southern coast of Australia. J Wildl Dis 21:193–198
Neumann G, Kawaoka Y (2011) The first influenza pandemic of the new millennium. Influenza Other Resp 5:157–166
Pantin-Jackwood MJ, Day JM, Jackwood MW, Spackman E (2008) Enteric viruses detected by molecular methods in commercial chicken and turkey flocks in the United States between 2005 and 2006. Avian Dis 52:235–244
Pfitzer S, Verwoerd D, Gerdes G, Labuschagne A, Erasmus A, Manvell R, Grund C (2000) Newcastle disease and avian influenza A virus in wild waterfowl in South Africa. Avian Dis 44:655–660
Phalen DN (2001) The use of serologic assays in avian medicine. Semin Avian Exot Pet Med 10:77–89
Phalen DN (2002) Virus neutralization assays used in exotic bird medicine. Semin Avian Exot Pet Med 11:19–24
Pierson G, Pfow C (1975) Newcastle disease surveillance in the United States. J Am Vet Med Assoc 167:801–803
Saif Y (1998) Infectious bursal disease and hemorrhagic enteritis. Poult Sci 77:1186–1189
Schlatter RP, Sladen WJL (1971) Nonbreeding south polar skuas: studies at Cape Crozier, 1969-1971. Antarc J US 6:103–104
Shellam G, Jones H, Iveson J (1983) A study of viral, bacterial and protozoal infections in Macquarie Island penguins. Austr Natl Antarctic Res Exped News 1:79
Sladen W (1962) Studies of respiratory pathogens in Antarctica. Polar Rec 11:318
Soñora M, Moreno P, Echeverría N, Fischer S, Comas V, Fajardo A, Cristina J (2015) An evolutionary insight into Newcastle disease viruses isolated in Antarctica. Arch Virol 160:1893–1900
St. George T, Doherty R, Carley J, Filippich C, Brescia A, Casals J, Kemp D, Brothers N (1985) The isolation of arboviruses including a new flavivirus and a new Bunyavirus from Ixodes (Ceratixodes) uriae (Ixodoidea: Ixodidae) collected at Macquarie Island, Australia, 1975–1979. Am J Trop Med Hyg 34:406–412
Stevenson M (2008) An Introduction to Veterinary Epidemiology. EpiCentre, Massey University, Palmerston North
Swarbrick O (1998) Penguin mystery. Aust Vet J 76:67
Thomazelli LM, Araujo J, Oliveira DB, Sanfilippo L, Ferreira CS, Brentano L, Pelizari VH, Nakayama C, Duarte R, Hurtado R (2010) Newcastle disease virus in penguins from King George Island on the Antarctic region. Vet Microbiol 146:155–160
Tizard I (2002) The avian antibody response. Semin Avian Exot Pet Med 11:2–14
Trivelpiece SG, Trivelpiece WZ, Volkman NJ (1985) Plumage characteristics of juvenile pygoscelid penguins. Ibis 127:378–380
Turner J, Barrand NE, Bracegirdle TJ, Convey P, Hodgson DA, Jarvis M, Jenkins A, Marshall G, Meredith MP, Roscoe H (2014) Antarctic climate change and the environment: an update. Polar Rec 50:237–259
van den Berg TP (2000) Acute infectious bursal disease in poultry: a review. Avian Path 29:175–194
Vandalen KK, Shriner SA, Sullivan HJ, Root JJ, Franklin AB (2009) Monitoring exposure to avian influenza viruses in wild mammals. Mammal Rev 39:167–177
Wallensten A, Munster VJ, Osterhaus A, Waldenström J, Bonnedahl J, Broman T, Fouchier RA, Olsen B (2006) Mounting evidence for the presence of influenza A virus in the avifauna of the Antarctic region. Antarct Sci 18:353–356
Watts JM, Miller GD, Shellam GR (2009) Infectious bursal disease virus and Antarctic birds. In: Kerry KR, Riddle MJ (eds) Health of Antarctic wildlife: a challenge for science and policy. Springer-Verlag, Berlin, pp 95–105
Woehler EJ, Ainley D, Jabour J (2014) Human impacts to Antarctic wildlife: predictions and speculations for 2060. In: Tin T, Liggett D, Maher PT, Larners M (eds) Antarctic futures: human engagement with the Antarctic environment. Springer, New York, pp 27–60
Woods R, Jones H, Watts J, Miller G, Shellam G (2009) Diseases of Antarctic Seabirds. In: Kerry KR, Riddle MJ (eds) Health of Antarctic wildlife, a challenge for science and policy. Springer-Verlag, Berlin, pp 35–55
Acknowledgements
This work was funded by New Zealand’s Ministry for Business, Innovation and Employment (MBIE) under project C09X0510, “Protecting the Ross Sea Ecosystem”; a New Zealand Ministry of Science and Innovation grant, C01X1001; and a University of Otago Postgraduate Scholarship to WG. Funding for the serology tests was provided by MBIE Core Funding to Landcare Research. Logistics for personnel at Cape Bird were provided by Antarctica New Zealand. Sampling at Cape Crozier was funded by the US National Science Foundation Grant ANT-0944411, with logistics supplied by the U.S. Antarctic Program, permit ACA 2011-002. Helpful comments to improve this manuscript were provided by Dan Tompkins Landcare Research and two anonymous reviewers. We thank especially Elizabeth Porzig, Scott Jennings and Annie Pollard for their help with sampling at Cape Crozier and Catriona MacLeod and Amy Whitehead, both from Landcare Research, for collecting blood samples at Cape Bird North in 2010–2011. A very special thanks to Keven Drew, Landcare Research, whose help was invaluable with the fieldwork at Cape Bird for seasons 2011–2012 and 2012–2013.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Permits to capture and handle Adélie Penguins on Ross Island were conducted under Landcare Research Animal Ethics Permit 10/09/01, Animal Care Use Permit 4130 from Oregon State University. and the US Antarctic Conservation Act, permit 2010-002. The authors declare no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Grimaldi, W., Ainley, D.G. & Massaro, M. Multi-year serological evaluation of three viral agents in the Adélie Penguin (Pygoscelis adeliae) on Ross Island, Antarctica. Polar Biol 41, 2023–2031 (2018). https://doi.org/10.1007/s00300-018-2342-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-018-2342-1