Abstract
Zooplankton (meso- and macrozooplankton) distributions and biomass are poorly known in the Ross Sea despite their importance in energy transfer within food webs and biogeochemical cycles. Mesozooplankton abundance and biomass on the continental shelf are spatially variable and span two orders of magnitude during austral summer. Selected sub-regions (near the shelf break or ice shelf) show similar variability, suggesting that other processes, either oceanographic or biological, influence zooplankton on smaller scales. Biomass at one location (76.5° S, 172° E) was consistently elevated throughout January, although the causes of this “hotspot” were unclear. At a station near the ice shelf, abundance and biomass of the pteropod Limacina antarctica was very high. Zooplankton biomass at this location was sevenfold greater than any other station, and while the high biomass was driven by pteropod contributions, copepods were also abundant. Copepods dominated the mesozooplankton composition at all other stations, comprising 90 and 78% on average of the total abundance and biomass. Zooplankton biomass comprised on average 3.96% of the total particulate carbon (0–200 m) and was weakly correlated with chlorophyll and biogenic silica. We suggest that summer zooplankton growth and biomass, while linked to organic matter concentrations, are regulated by other factors (e.g., predation by crystal krill and Antarctic silverfish), as both grazers may be responsible for significant losses. Our data indicate that, contrary to other suggestions, summer zooplankton biomass and abundance in the Ross Sea are similar to those in other Antarctic coastal regions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Zooplankton are essential components of food webs and biogeochemical cycles in that they process particulate matter generated by phytoplankton into energy available to higher trophic levels. They also metabolize and transfer organic carbon within the water column, hence playing an important role in the biological pump (Turner 2015; Steinberg and Landry 2017). Historically, mesozooplankton have been collected using towed nets or towed plankton recorders, which provide abundance and composition data; recently acoustic and optical techniques have been used to increase the temporal and spatial scales of sampling (Davis et al. 1996; Foote and Stanton 2000). Net sampling, however, yields information that at present cannot be easily collected in any other manner and provides insights into the dynamics of secondary producers.
Southern Ocean macrozooplankton have received substantial attention, largely because of the important role of large forms such as Antarctic krill (Euphausia superba). Krill are considered a keystone species in the Antarctic because numerous higher trophic level species (baleen whales, seals, fish, penguins, and pelagic birds) depend on them for food. Krill also are commercially harvested in some locations, and their stocks are spatially and temporally variable (Atkinson et al. 2004). However, lesser known euphausiid species occur in coastal systems, such as crystal krill (Euphausia crystallorophias). There are fewer studies of other meso- and macrozooplankton groups that co-occur with Antarctic krill. Indeed, the role of other zooplankton in Antarctic food webs is often overlooked, despite the fact that a direct comparison of metabolic rates of copepods and krill suggests that energetically copepods process more food (on an areal basis) than do krill, at least at some times of the year (Ashjian et al. 2004). Because krill have an extremely heterogeneous distribution, the importance of copepods relative to krill can be expected to vary widely.
Antarctic krill (E. superba) require deep (>1000 m) water columns to complete their life cycle (Knox 2006), and hence are often absent from shallower continental shelf areas such as the Ross Sea. E. crystallorophias is found in shelf areas, but its biomass appears to be much lower than stocks of Antarctic krill in other regions (Sala et al. 2002; Deibel and Daly 2007). It may co-occur with E. superba, particularly at the shelf break. Other mesozooplankton such as copepods may play critical roles within continental shelf food webs (Pinkerton and Bradford-Grieve 2014). On an annual basis, the Ross Sea is the most productive and highest phytoplankton biomass location in the Southern Ocean (Smith et al. 2014). It also has substantial standing stocks of higher trophic level species, for example supporting ca. 38% of the world’s Adélie penguins, 25% of the world’s Emperor penguins, and 42% of global stocks of Antarctic petrels (Ballard et al. 2011). Given the importance of this area, there are surprisingly few studies of mesozooplankton distribution and abundance in the Ross Sea. It has been suggested that zooplankton are the most important factor in regulating food web dynamics and ecological interactions in the Ross Sea (Ainley et al. 2010, 2015), so understanding energy transfer within the food web, and the response of this continental shelf food web to climate change, is dependent on knowledge of meso- and macrozooplankton abundance and distribution.
Investigations of mesozooplankton in the Ross Sea have largely focused on locations near research stations, such as McMurdo Sound and Terra Nova Bay. Hopkins (1987) found the mesozooplankton of McMurdo Sound consisted primarily of copepods (Calanoides acutus, Metridia gerlachei, and Paraeuchaeta antarctica) and pteropods (Limacina helicina antarctica). Elliot et al. (2009) found a similar mesozooplankton composition under the summer sea ice at McMurdo. The overall biomass of mesozooplankton in the Ross Sea may be exceptionally low when compared to other Antarctic systems; indeed, Deibel and Daly (2007) concluded that total zooplankton biomass in the Ross Sea was <1% that in the Croker Passage of the Antarctic Peninsula. Such low levels may be due to intense predation pressure by the crystal krill, E. crystallorophias, and the Antarctic silverfish, P. antarcticum (Ainley et al. 2007, 2015). However, Stevens et al. (2015) found that mesozooplankton abundances at two stations on the shelf were similar to those found in other Antarctic coastal systems. Tagliabue and Arrigo (2003) modeled zooplankton biomass in the Ross Sea, and suggested that the low mesozooplankton biomass was due to the temporal decoupling of primary and secondary production. However, data on mesozooplankton abundances and distributions are far too scarce to adequately test this hypothesis.
We report herein the results of an investigation of mesozooplankton biomass and abundance over broad areas of the Ross Sea continental shelf. The study was part of a larger oceanographic program to investigate the mesoscale variations in iron supply to the surface layer (PRISM—Processes Regulating the Iron Supply at the Mesoscale) (McGillicuddy et al. 2015). Mesozooplankton tows were not meant to provide mesoscale distributions, but to assess broader patterns of mesozooplankton abundance and to investigate their potential role in Ross Sea biogeochemical cycles.
Methods
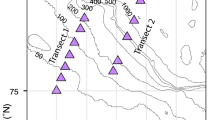
Zooplankton were collected during NBP12-01 from the R.V.I.B. N.B. Palmer as part of PRISM. Sampling occurred from January 9 through February 6, 2012 (Fig. 1). Mesozooplankton were sampled with oblique bongo tows from the surface to 200 m in ice-free waters of the Ross Sea. Tows were conducted at all times of the day without regard to solar angle (photoperiods were 24 h during the entire cruise). The nets were 60-cm-diameter bongos with mesh sizes of 200 and 500 µm; both had calibrated flow meters (General Oceanics) suspended 15 cm into the mouth of the net ring. Only data from the 500-µm net are reported here, as there was no substantial difference between the two mesh sizes, likely because both nets were substantially clogged by phytoplankton, reducing the effective filtration dimension. The vertical net speed was 15 m min−1 and ship speed was two knots. These collection methods were designed to collect copepod-sized organisms and not krill, although juvenile krill were occasionally captured in our tows. Upon recovery, nets were rinsed with cold seawater, the samples recovered from the cod ends, and the sample volume and volume filtered measured. All further manipulations were conducted in a cold room (0 °C).
The total volume of each tow was quantitatively subsampled using a Folsom plankton splitter. The volumes of the subsamples and the rinses were measured, and a subsample was preserved in formalin (10 mL 37% formaldehyde per 200 mL sample) and returned to the laboratory for microscopic evaluation. Water column particulate organic carbon (POC) samples were filtered through 25-mm combusted Whatman GF/F filters, folded in half, placed in combusted glass vials, covered with combusted aluminum foil, and dried at 60 °C. In the laboratory, the POC on the filters was assessed using a Fisons elemental analyzer. Samples for chlorophyll were filtered through 25-mm GF/F filters, extracted in 90% acetone in the dark at −20 °C for 24 h, and the fluorescence determined before and after acidification (JGOFS 1996). The fluorometer was calibrated using a commercial standard (Sigma).
All samples were analyzed microscopically in the laboratory. Large copepods, chaetognaths, amphipods, pteropods, euphausiids, polychaete larvae, ostracods, and siphonophores were separated from phytoplankton manually and placed in seawater with preservative. The entire sample was processed. The preserved mesozooplankton were then enumerated with identification software using a Zooscan optical imaging system at a resolution of 2400 dpi (Grosjean et al. 2004). In brief, pre-sorted samples were placed in filtered seawater with formalin to remove loosely attached particles. The sample was then sieved through a 150-µm mesh to further remove any remaining phytoplankton, poured into the counting chamber, and digitized. The Zooscan produces high-resolution, digitized images of a sample and distinguishes individual animals. If the sample was too dense for accurate digitizing due to overlapping animals, it was re-split with the plankton splitter and reanalyzed. Samples were enumerated only to broad taxonomic groups. Mesozooplankton diversity in the Ross Sea appears to be quite low, but the dominant copepods (Calanoides acutus, Oncaea sp., Oithona sp., Metridia gerlachei, and Paraeuchaeta antarctica) were similar to those found in previous studies (Hopkins 1987; Elliot et al. 2009; Stevens et al. 2015). All averages reported (e.g., Table 2) are based on non-zero values. Because euphausiids were not sampled quantitatively, they were not included in the various estimates.
Biomass of broad taxonomic groups was estimated using length–carbon relationships from the literature (Comeau et al. 2010; Atkinson et al. 2012; Thompson et al. 2013; Kiørboe 2013; Mayzaud and Pakhomov 2014). Lengths of animals were estimated from the digitized images by comparison to a line gauge included in each image. Copepod sizes also varied, but were designated as either large or small forms. Rare mesozooplankters (comprising less than 1% of animal numbers, such as larval fish or polychaete larvae) were not included. Similarly, siphonophores contributed only trivial amounts of carbon and also were not included.
In addition to mesozooplankton tows, CTD casts were conducted at every station, and water samples collected throughout the upper 200 m. Continuous measurements of fluorescence and optical transmission were made on each cast, and the optical transmission data were converted to POC concentrations by regressing the discrete sample values with the transmissometer data (Cp, the beam attenuation due to particles; Gardner et al. 2000) at that depth. The resulting regression was
and from this relationship POC concentrations were determined at 1-m intervals at all stations where mesozooplankton were sampled. POC concentrations were then integrated over the same depths where mesozooplankton were collected. Samples for nutrients, chlorophyll, and particulate organic matter were collected and processed as described in Smith et al. (2013). All cruise data are available from the Biological and Chemical Oceanography Data Management Office (http://www.bco-dmo.org/project/2155).
Results
Oceanographic conditions
The oceanographic conditions encountered in the Ross Sea during this study were similar to the long-term means for ice concentrations, iron and nutrient concentrations, phytoplankton biomass, and vertical stratification (Smith et al. 2003; Schine et al. 2016; Table 1). Ice was limited to coastal Victoria Land and near the shelf break (Fig. 1), and nearly all of our mesozooplankton tows were in open water. Mixed layers were modest, averaging 26.4 m, but ranged from 6 to 92 m, which was indicative of the large spatial variations encountered during austral summer. Greatest mixed layer depths were found near the Ross Ice Shelf. Surface temperatures ranged from −1.25 to +2.48 °C. Nitrate at 10 m averaged 19.3 µM and dissolved iron 0.11 nM; chlorophyll averaged 5.44 µg L−1, but ranged from 0.22 to 22.4 µg L−1 (Table 1). The largest chlorophyll concentrations observed at 10 m depth were near the ice shelf and closer to the Victoria Land coast (Fig. 2a, e); conversely, the lowest concentrations were found in the northern Ross Sea at ca. 74ºS (Fig. 2c, d).
Spatial distribution of zooplankton abundance (individuals m−3), zooplankton biomass (µg C L−1), chlorophyll (Chl; mean concentration in upper 30 m; µg L−1), and biogenic silica (BSi; mean concentration in upper 30 m; µmol L−1). Stations are grouped by location in the following boxes: a between 170 and 174ºE and south of 76ºS (n = 14); b at the continental slope (n = 1); c west of 170ºE and south of 76ºS (n = 7); d within 25 km of the shelf break (n = 9); e near the Ross Ice Shelf (n = 3); and f on the Ross Bank (n = 6). Biogenic silica not measured at all stations. See Fig. 1 for exact stations locations
Mesozooplankton abundance and distribution
The abundance of mesozooplankton varied greatly over the continental shelf, and averaged 24.4 individuals m−3 (Table 2). Copepods were the dominant type, averaging 21.9 individuals m−3 or nearly 90% of all mesozooplankton (Fig. 3). Chaetognaths were the next most abundant group. They represented on average 9.4% of the total mesozooplankton abundance and 6.5% of mesozooplankton biomass (when present) (Table 2, Fig. 4). Pteropods were collected in 68% of the tows, and represented 9.8% of mesozooplankton abundance and 19.1% of biomass (when present); ostracods (found at eight stations) represented 7.8% of total abundance and 3.8% of total biomass at those stations. All other groups were less than 5% of the total number mesozooplankton abundance and biomass.
One station was extremely anomalous—Station 65, located near the Ross Ice Shelf. Pteropod (Limacina) abundances were extremely high (4480 ind m−3), which were higher than any other mesozooplankton group encountered anywhere on the shelf. Limacina can reproduce rapidly under optimal conditions in the Ross Sea, and substantial abundances in the Ross Sea have been noted by others (Maas et al. 2011). Abundances of Limacina at St. 65 were nearly 28 times greater than pteropod abundances at any other station. In addition, biomass of copepods and chaetognaths were also elevated there (5.72 and 0.54 µg L−1, respectively), and both were higher than at any other station. Because of the extreme abundance of pteropods at this one location, it was not included in computing zooplankton averages for the shelf. The location of this pteropod “bloom” was an area of substantial mixing (mixed layers of ~90 m), low temperatures (average −1.76 °C), and high biomass and abundance of colonial P. antarctica (Smith and Jones 2015; Smith et al. 2017).
Distributions of mesozooplankton did not show a clear pattern in space, although our sampling locations were not designed to assess their mesoscale spatial patterns (Fig. 2). However, high abundances of mesozooplankton were found at Stations 12 and 31 (St. 65 had the greatest abundance due to large numbers of pteropods and copepods), which are located within 20 km of each other. Large numbers of copepods and chaetognaths were found nearly at the same location (76.73S, 169.82E). Primary or secondary maxima of amphipods and pteropods were also located there, suggesting that this location was a local “hotspot” for mesozooplankton. Chlorophyll concentrations were also elevated in this region and averaged 12.3 µg L−1 for Stations 10, 29, 31, 32, and 96. Biogenic silica concentrations at these stations averaged 25.2 µmol L−1, indicating that much of the chlorophyll was associated with diatoms (Nelson and Smith 1986). Mesozooplankton abundance throughout the entire region was weakly correlated with both chlorophyll (R 2 = 0.18, p < 0.001; n = 82) and biogenic silica concentrations (Fig. 3; R 2 = 0.06; p < 0.001; n = 84). Copepod abundance showed similar weak correlations, with a slightly stronger relationship to biogenic silica. The weak correlations of both indices of phytoplankton carbon indicate that other factors play a significant role in regulating summer mesozooplankton biomass. Chaetognaths and copepods were also linearly related (R 2 = 0.31), indicating that chaetognaths feed on copepods.
Mesozooplankton abundance also varied substantially within specific oceanographic and smaller geographic regions (Fig. 2). In a box south of 76° S and between 170 and 174º E, mesozooplankton abundance ranged from 44 to 4163 individuals m−3, and mesozooplankton biomass from 0.25 to 2.8 µg L−1. Similarly, abundance varied by two orders of magnitude within the shelf break domain, and one order of magnitude near the ice shelf and closer to the Victoria Land coast (Fig. 2). There was no relationship between mesozooplankton abundance and sampling date, suggesting that the extreme spatial differences were the result of smaller scale processes rather than temporal development.
Mesozooplankton carbon
Copepods contributed by far the largest percentage of carbon to the total mesozooplankton carbon pool (Table 3). Total mesozooplankton carbon averaged 207 ± 176 mg C m−2 (integrated over 200 m), and copepod carbon averaged 193 ± 214 mg C m−2. On average copepods represented 78.0% of total mesozooplankton carbon. Pteropods and ostracods, despite occasionally being numerous, contributed little to total organic carbon due to their small sizes. Amphipods and chaetognaths contributed a similarly low amount of carbon (~6% of the total). It is clear that copepods comprise the majority of mesozooplankton biomass in the Ross Sea.
Integrated particulate organic carbon concentrations were substantially greater than mesozooplankton carbon, averaging 33.3 ± 7.46 g C in the upper 200 m of the water column (Table 3), similar to integrated concentrations found in previous summers (e.g., Smith et al., 2000). Mesozooplankton biomass represented, on average, 3.96% of the integrated particulate carbon (Table 3). Station 12 had the maximum mesozooplankton contribution to POC as well as the maximum abundance. There was no relationship between sampling date and either POC or mesozooplankton biomass.
Discussion
The abundance and biomass of mesozooplankton in the Ross Sea are extremely variable, ranging over two orders of magnitude. The cause(s) of such variability are uncertain, although food availability (phytoplankton concentrations) may be important, as it also varies to a similar extent in both time and space (e.g., Table 1; Smith et al. 2011a, 2013). Different zooplankton also have different growth rates, and consequently integrate oceanographic variability over a longer period of time than do phytoplankton. Abundance and biomass can also be influenced by physical features such as ice concentrations and mesoscale features (eddies, jets) as well as by biological interactions (e.g., predation by higher trophic levels). We sampled a great range of hydrographic and biological conditions, and since zooplankton have variable growth rates (smaller copepods grow faster than the larger forms such as euphausiids; Pinkerton and Bradford-Grieve 2014), this might result in substantial variability in space and time, in a manner similar to phytoplankton (Smith et al. 2006, 2011a, b). Our study cannot resolve the dominant mechanisms that generate this spatial variability, but such variations undoubtedly play significant roles in biogeochemical cycles (for example, the spatial variations of vertical flux driven by fecal material) of the region.
It is difficult to compare our study with previous efforts because the spatial extent of our sampling was considerably greater than others, and different studies use slightly different methodologies. Also, our study encompassed only summer conditions, while others included spring. However, it is worth noting the similarities and differences among the results of various field efforts. Elliot et al. (2009) sampled a shallow (<25 m), under-ice region in McMurdo Sound. Given its location, the waters sampled under ice were likely advected from the open water Sound area, and hence represent summer conditions in that broader area. Their study found an average mesozooplankton abundance of 1183 individuals m−3, with a maximum of 4246 individuals m−3 in early February. Copepods were by far the most abundant taxon and at times comprised 100% of the total mesozooplankton abundance, although pteropods were occasionally observed in substantial numbers as well. Pane et al. (2004) sampled in Terra Nova Bay and found mesozooplankton numbers ranging from 45 to 3965 individuals m−3, with an average of 1450 individuals m−3. Again, copepods were overwhelmingly dominant in all samples. Foster (1987) sampled under ice in McMurdo Sound in spring, and Hopkins (1987) sampled in summer in the open waters of McMurdo Sound. Foster (1987) found an average abundance of 76 individuals m−3, and Hopkins (1987) found dry weights to range from 1.5 to 3.4 g over the entire 800 m water column. Both reported that the mesozooplankton assemblage was dominated by copepods.
In a more recent study in the Ross Sea, Stevens et al. (2015) observed abundances between 120 and 360 individuals m−3 at two stations on the continental shelf and two on the slope (St. 122 and 158, with depths less than 930 m) during summer (February), and biomass between 0.31 and 1.32 g C in the upper 200 m, with the two continental shelf stations having 0.32 and 1.09 g C (0–200 m). These samples were again dominated by copepods. Our study found lower average biomass, but our maximum biomass value exceeded these two measurements. Given the much greater spatial domain that we sampled and the range of hydrographic and biological conditions on the continental shelf, substantial spatial variability would be expected. Our total POC concentrations were similar to those found in other years (Smith et al. 2000, 2011a), suggesting that 2012 was a relatively “normal” year with relatively “normal” biological growth in response to oceanographic conditions. The range of POC concentrations was not as great as mesozooplankton biomass, largely because the depth-integrals to 200 m tended to obscure surface layer variability.
It has been suggested that the Ross Sea supports low zooplankton biomass when compared to other regions (Tagliabue and Arrigo 2003; Deibel and Daly 2007). Indeed, Deibel and Daly (2007) reported that mesozooplankton biomass in the Ross Sea was an order of magnitude lower than in the Antarctic Peninsula region, and the data tabulated by Tagliabue and Arrigo (2003) suggested a similar disparity. Tagliabue and Arrigo (2003) argued this was the result of a decoupling of phytoplankton production with mesozooplankton ingestion and production. A comparison between the phytoplankton composition of Terra Nova Bay and the Ross Sea polynya suggested that the coupling was greater at locations where diatoms were more prevalent than Phaeocystis. However, P. antarctica regularly occurs in Terra Nova Bay as well (Goeyens et al. 2000; DiTullio unpubl.), so that the differences in phytoplankton composition (and hence coupling to mesozooplankton) between the Ross Sea polynya and Terra Nova Bay with regard to phytoplankton composition (and hence coupling to mesozooplankton) may be less than suggested. Absolute phytoplankton growth rates in Terra Nova Bay may be lower than in other regions of the Ross Sea due to greater vertical mixing induced by strong winds off the continent, which would allow phytoplankton–mesozooplankton coupling to be greater in the model used by Tagliabue and Arrigo (2003). Conversely, Ainley et al. (2006) suggested that “palatable” phytoplankton production (that is, non-P. antarctica biomass) in the Ross Sea limits mesozooplankton ingestion and growth, and that intense predation from higher trophic levels, especially Antarctic silverfish and omnivorous species of krill, keeps mesozooplankton biomass low (Ainley 2007).
Smith et al. (2011b) estimated that on average 60% of the total annual phytoplankton production in the Ross Sea polynya was due to haptophytes. If Phaeocystis indeed is a poor food source for zooplankton (Nejstgaard et al. 2007), then the total amount of carbon available for mesozooplankton growth is far less than productivity estimates might imply. Our data suggest that mesozooplankton abundance is weakly correlated with both chlorophyll and biogenic silica (Fig. 3). This does not imply that mesozooplankton (and primarily copepods) use haptophyte carbon, but the very weak correlation with biogenic silica suggests that diatomaceous food is not a dominant factor controlling mesozooplankton biomass. The large contribution of Limacina to mesozooplankton abundance near the ice shelf, a region that was conspicuous due to the high biomass and abundance of P. antarctica colonies (Smith and Jones 2015; Smith et al. 2017), suggests that at least some mesozooplankton are able to utilize carbon from Phaeocystis (either in colonial or solitary form). Experimental data on grazer use of P. antarctica is inconsistent. For example, Antarctic krill used P. antarctica but at greatly reduced efficiency relative to diatoms (Haberman et al. 2002), and Hansen and van Boekel (1991) found that copepods had greatly reduced (but non-zero) ingestion rates when phytoplankton were dominated by P. antarctica. Weisse et al. (1994) found different responses to the haptophyte that were dependent on the zooplankton species tested and the size and physiological state of the alga. These and other reports suggest that zooplankton do not efficiently incorporate the carbon of P. antarctica for either palatability or size reasons (colonies can reach diameters of 2 mm or more; Nejstgaard et al. 2007). Alternatively, it is possible that mesozooplankton cannot respond rapidly to the increased food availability at the low temperatures at which P. antarctica is found (<0 °C) (Liu and Smith 2012; Kaufman et al. 2014). However, copepods and chaetognaths also reached their maximum biomass near the ice shelf, a region with an average temperature of −1.76 °C. Taken together, it appears that at least some haptophyte carbon is used by various mesozooplankton, although further information is needed to fully assess the role of P. antarctica in the Ross Sea food web.
Our data do not suggest that the Ross Sea has significantly lower mesozooplankton biomass than other regions in the Southern Ocean, a conclusion also reached by Stevens et al. (2015). Spatial variability of mesozooplankton is high, but similar to other surveys across relatively broad areas (e.g., the West Antarctic Peninsula; Steinberg, pers. comm.). The presence of elevated mesozooplankton biomass in selected areas in the Ross Sea (and the fact that it is maintained over time) suggests that there are indeed specific oceanographic and biological features that support zooplankton growth. Our study indicates the presence of these “hotspots” but not the mechanism(s) that generate them. Understanding the abundance, growth, and complex interactions among phytoplankton functional groups, mesozooplankton, and macrozooplankton remains a major uncertainty in modeling of Ross Sea food webs and predicting potential changes to these ecosystems in the future.
While the abundance of mesozooplankton does not appear different from other regions in the Southern Ocean, the uncertainty regarding P. antarctica as a food source and the lack of measured ingestion rates makes it difficult to assess their role in particulate carbon turnover and export to depth. Various studies in the Ross Sea have shown that zooplankton fecal pellets can play a large role in the flux of carbon to depth at selected times (Dunbar et al. 1998; Smith et al. 2011b; Manno et al. 2010), and their role does not appear to be markedly different from other Antarctic systems (Manno et al. 2015; Ducklow et al. 2015). The coupling between phytoplankton and zooplankton in the Ross Sea and the seasonal role of fecal pellets remains to be fully resolved.
Conclusions
Summer mesozooplankton abundance and biomass on the Ross Sea continental shelf is spatially variable and spans two orders of magnitude. While it has been suggested that zooplankton biomass in the Ross Sea is substantially lower than in other areas of the Southern Ocean, our data suggest that it is not markedly different from other regions. One location near the ice shelf was observed to support elevated mesozooplankton numbers (pteropods, copepods, and chaetognaths); the region was also characterized by massive concentrations of phytoplankton dominated by the haptophyte P. antarctica. Copepods overwhelmingly dominated the mesozooplankton composition, contributing on average 90 and 78% of the total mesozooplankton abundance and biomass, respectively. However, mesozooplankton contributed only 4% of the total particulate carbon in the water column. Biomass was positively correlated with chlorophyll and biogenic silica, suggesting that food was one factor in regulating mesozooplankton biomass and distribution. Further observations and experiments are needed to more fully assess the role of mesozooplankton in Ross Sea food webs and biogeochemical cycles.
References
Ainley DG (2007) Insights from study of the last intact neritic marine ecosystem. Trends Ecol Evol 22:444–445
Ainley DG, Ballard G, Dugger KM (2006) Competition among penguins and cetaceans reveals trophic cascades in the Ross Sea, Antarctica. Ecology 87:2080–2093
Ainley DG, Ballard G, Blight LK, Ackley S, Emslie SD et al (2010) Impacts of cetaceans on the structure of southern ocean food webs. Mar Mamm Sci 26:482–489
Ainley DG, Ballard G, Jones RM, Jongsomjit D, Pierce SD, Smith WO Jr, Veloz S (2015) Trophic cascades in the western Ross Sea, Antarctica: revisited. Mar Ecol Prog Ser 534:1–16
Ashjian C, Rosenwaks GA, Wiebe PH, Davis CS, Gallager SM, Copley NJ, Lawson GL, Alatalo P (2004) Distribution of zooplankton on the continental shelf off Marguerite Bay, Antarctic Peninsula, during austral fall and winter, 2001. Deep Sea Res II 51:2073–2098
Atkinson A, Siegel V, Pakhomov EA, Rothery P (2004) Long-term decline in krill stock and increase in salps within the Southern Ocean. Nature 432:100–103
Atkinson A, Ward P, Hunt EPV, Pakhomov EA (2012) An overview of Southern Ocean zooplankton data: abundance, biomass, feeding and functional relationships. CCAMLR Sci 19:171–218
Ballard G, Jongsomjit D, Veloz SD, Ainley DG (2011) Coexistence of mesopredators in an intact polar ocean ecosystem: the basis for defining a Ross Sea marine protected area. Biol Cons. doi:10.1016/j.biocon.2011.11.017
Comeau S, Ross J, Teyssié J-L, Gattuso J-P (2010) Response of the Arctic pteropod Limacina helicina to projected future environmental conditions. PLoS One. doi:10.1371/journal.pone.0011362
Davis CS, Gallager SM, Marra M, Stewart WK (1996) Rapid visualization of plankton abundance and taxonomic composition using the Video Plankton Recorder. Deep Sea Res I 43:1947–1970
Deibel D, Daly KL (2007) Zooplankton processes in Arctic and Antarctic polynyas. In: Smith WO Jr, Barber DG (eds) Polynyas: Windows to the World. Elsevier Press, Amsterdam, pp 271–332
Ducklow HW, Wilson SE, Post AF, Stammerjohn SE, Erickson M, Lee S, Lowry KE, Sherrell RM, Yager PL (2015) Particle flux on the continental shelf in the Amundsen Sea Polynya and Western Antarctic Peninsula. Elementa. doi:10.12952/journal.elementa.000046
Dunbar RB, Leventer AR, Mucciarone DA (1998) Water column sediment fluxes in the Ross Sea, Antarctica: atmospheric and sea ice forcing. J Geophys Res 103:10741–10760
Elliot DT, Tang KW, Shields AR (2009) Mesozooplankton beneath the summer sea ice in McMurdo Sound, Antarctica: abundance, species composition, and DMSP content. Polar Biol 32:113–123
Foote KG, Stanton TK (2000) Acoustical methods. In: Harris R, Wiebe PH, Lenz J, Skjoldal HR, Huntley M (eds) ICES zooplankton methodology manual. Academic Press, London, pp 223–258
Foster BA (1987) Composition and abundance of zooplankton under the spring sea-ice of McMurdo Sound, Antarctica. Polar Biol 8:41–48
Gardner WD, Richardson MJ, Smith WO Jr (2000) Seasonal build-up and loss of POC in the Ross Sea. Deep Sea Res II 47:3423–3450
Goeyens L, Elskens M, Catalano G, Lipizer M, Hecq JH, Goffart A (2000) Nutrient depletions in the Ross Sea and their relation with pigment stocks. J Mar Syst 27:195–208
Grosjean P, Picheral M, Warembourg C, Gorsky G (2004) Enumeration, measurement, and identification of net zooplankton samples using the ZOOSCAN digital imaging system. ICES J Mar Sci 61:518–525
Haberman KL, Ross RM, Quetin LB (2002) Diet of the Antarctic krill (Euphausia superba Dana): II. Selective grazing in mixed phytoplankton assemblages. J Exp Mar Biol Ecol 283:97–113
Hansen FC, van Boekel WHM (1991) Grazing pressure of the calanoid copepod Temora longicornis on a Phaeocystis dominated spring bloom in a Dutch tidal inlet. Mar Ecol Prog Ser 78:123–129
Hopkins TL (1987) Midwater food web in McMurdo Sound, Ross Sea, Antarctica. Mar Biol 96:93–106
JGOFS (1996) Protocols for the Joint Global Ocean Flux Study (JGOFS) core measurements. Report No. 19 of the Joint Global Ocean Flux Study, Scientific Committee on Oceanic Research, International Council on Scientific Unions, Intergovernmental Oceanographic Commission, Bergen, Norway
Kaufman DE, Friedrichs MAM, Smith WO Jr, Queste BY, Heywood KJ (2014) Biogeochemical variability in the southern Ross Sea as observed by a glider deployment. Deep Sea Res I 92:93–106
Kiørboe T (2013) Zooplankton body composition. Limnol Oceanogr 58:1843–1850
Knox GA (2006) Biology of the Southern Ocean. CRC Press, Boca Raton, FL
Liu X, Smith WO Jr (2012) A statistical analysis of the controls on phytoplankton distribution in the Ross Sea, Antarctica. J Mar Syst 94:135–144
Maas AE, Elder LE, Dierssen HM, Seibel BA (2011) Metabolic response of Antarctic pteropods (Mollusca: Gastropoda) to food deprivation and regional productivity. Mar Ecol Prog Ser 441:129–139
Manno C, Tirelli V, Accornero A, Fonda Umani S (2010) Importance of the contribution of Limacina helicina faecal pellets to the carbon pump in Terra Nova Bay (Antarctica). J Plankton Res 32:142–152
Manno C, Stowasser G, Enderlein P, Fielding S, Tarling GA (2015) The contribution of zooplankton faecal pellets to deep-carbon transport in the Scotia Sea (Southern Ocean). Biogeosci 12:1955–1965
Mayzaud P, Pakhomov EA (2014) The role of zooplankton communities in carbon recycling in the ocean: the case of the Southern Ocean. J Plankton Res 36:1543–1556
McGillicuddy DM Jr, Sedwick PN, Dinniman MS, Arrigo KR, Bibby TS, Greenan BJW, Hofmann EE, Klinck JM, Smith WO Jr, Mack SL, Marsay CM, Sohst BH, van Dijken G (2015) Iron supply and demand in an Antarctic shelf system. Geophys Res Lett 42:8088–8097
Nejstgaard JC, Tang KW, Steinke M, Dutz J, Koski M, Antajan E, Long JD (2007) Zooplankton grazing on Phaeocystis: a quantitative review and future challenges. Biogeochemistry 83:147–172
Nelson DM, Smith WO Jr (1986) Phytoplankton bloom dynamics of the western Ross Sea II. Mesoscale cycling of nitrogen and silicon. Deep Sea Res 33:1389–1412
Pane L, Feletti M, Francomacaro B, Mariottini GL (2004) Summer coastal zooplankton biomass and copepod community structure near the Italian Terra Nova Base (Terra Nova Bay, Ross Sea, Antarctica). J Plankton Res 26:1479–1488
Pinkerton MH, Bradford-Grieve JM (2014) Characterizing foodweb structure to identify potential ecosystem effects of fishing in the Ross Sea, Antarctica. ICES J Mar Sci 71:1542–1553
Sala A, Azzali M, Russo A (2002) Krill of the Ross Sea: distribution, abundance and demography of Euphausia superba and Euphausia crystallorophias during the Italian Antarctic expeditions (January-February 2000). Sci Mar 66:123–133
Schine CMS, van Dijken G, Arrigo KR (2016) Spatial analysis of trends in primary production and relationship with large-scale climate variability in the Ross Sea, Antarctica (1997–2013). J Geophys Res. doi:10.1002/2015JC011014
Smith WO Jr, Jones RM (2015) Vertical mixing, critical depths, and phytoplankton growth in the Ross Sea. ICES J Mar Sci 72:1952–1960
Smith WO Jr, Marra J, Hiscock MR, Barber RT (2000) The seasonal cycle of phytoplankton biomass and primary productivity in the Ross Sea, Antarctica. Deep Sea Res II 47:3119–3140
Smith WO Jr, Dinniman MS, Klinck JM, Hofmann EE (2003) Biogeochemical climatologies in the Ross Sea, Antarctica: seasonal patterns of nutrients and biomass. Deep Sea Res II 50:3083–3101
Smith WO Jr, Shields AR, Peloquin JA, Catalano G, Tozzi S, Dinniman MS, Asper VL (2006) Biogeochemical budgets in the Ross Sea: variations among years. Deep Sea Res II 53:815–833
Smith WO Jr, Asper V, Tozzi S, Liu X, Stammerjohn SE (2011a) Surface layer variability in the Ross Sea, Antarctica as assessed by in situ fluorescence measurements. Prog Oceanogr 88:28–45
Smith WO Jr, Shields AR, Dreyer JC, Peloquin JA, Asper VL (2011b) Interannual variability in vertical export in the Ross Sea: magnitude, composition, and environmental correlates. Deep-Sea Res I 58:147–159
Smith WO Jr, Tozzi S, Long MC, Sedwick PN, Peloquin JA, Dunbar RB, Hutchins DA, Kolber Z, DiTullio GR (2013) Spatial and temporal variations in variable fluorescence in the Ross Sea (Antarctica): oceanographic correlates and bloom dynamics. Deep-Sea Res I 79:141–155
Smith WO Jr, Ainley DG, Arrigo KR, Dinniman MS (2014) The oceanography and ecology of the Ross Sea. Annu Rev Mar Sci 6:469–487
Smith WO Jr, McGillicuddy DM Jr, Olson EB, Kosnyrev O, Peacock EE, Sosik HM (2017) Mesoscale variability in intact and ghost colonies of P. antarctica in the Ross Sea: Distribution and abundance. J Mar Syst 166:97–107
Steinberg DK, Landry MR (2017) Zooplankton and the ocean carbon cycle. Annu Rev Mar Sci 9:413–444
Stevens CJ, Pakhamov EA, Robinson KV, Hall JA (2015) Mesozooplankton biomass, abundance and community composition in the Ross Sea and the Pacific sector of the Southern Ocean. Polar Biol 38:275–286
Tagliabue A, Arrigo KR (2003) Anomalously low zooplankton abundance in the Ross Sea: an alternative explanation. Limnol Oceanogr 48:686–699
Thompson GA, Dinfrio EO, Alder VA (2013) Structure, abundance and biomass size spectra of copepods and other zooplankton communities in upper waters of the Southwestern Atlantic Ocean during summer. J Plankton Res 35:610–629
Turner JT (2015) Zooplankton fecal pellets, marine snow, phytodetritus and the ocean’s biological pump. Prog Oceanogr 130:205–248
Weisse T, Tande T, Verity P, Hansen F, Gieskes W (1994) The trophic significance of Phaeocystis blooms. J Mar Syst 5:67–79
Acknowledgements
This research was supported by grants from the National Science Foundation (ANT-0944254 and ANT-1443258). We thank all of our PRISM colleagues who assisted in sampling: H. Doan, S. Charles, P. St. Laurent, and A. Mosby. Dr. J. Stone provided assistance with the Zooscan, and J. Cope confirmed many of our zooplankton identifications. This is VIMS contribution number 3642.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Smith, W.O., Delizo, L.M., Herbolsheimer, C. et al. Distribution and abundance of mesozooplankton in the Ross Sea, Antarctica. Polar Biol 40, 2351–2361 (2017). https://doi.org/10.1007/s00300-017-2149-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-017-2149-5