Abstract
Crustacean decapods are key components that structure the benthic ecosystems in the Subarctic/Arctic regions and support one of the largest fishery industries, but their larval dynamics are largely unknown. To investigate variability in decapod larvae community in this region, we analysed plankton samples collected during the summers of 2007 and 2008 along the southeastern Bering and Chukchi Seas. Distribution of adult population was studied using bottom trawling during 2008 cruise. Larvae of Pagurus spp., Hyas spp., and the commercially important Chionoecetes bairdi and Chionoecetes opilio were the most abundant species. The distribution of benthic adults linked to those of planktonic larvae and may favour recruitment near suitable habitats and the maintenance of the populations. Earlier larval stages of C. bairdi, C. opilio, and Hyas spp. were more abundant in 2008 than in 2007. The body size of C. opilio showed a significant latitudinal pattern, in which larger sizes occurred at higher latitudes in association with distinct temperature and food conditions. We argue that annual changes in abundance and developmental stage structure of planktonic larvae seemed to be related to the 1 month delay in the sampling period and are not determined by the contrasting environmental conditions observed in both years.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pacific Subarctic/Arctic seas are experiencing profound ecosystem changes due to human (direct and indirect) pressures, including global warming and the overexploitation of marine resources (Grebmeier et al. 2006). In this sense, warming and the loss of sea-ice cover have large implications for the ecosystem functioning, likely related to alterations in growth, abundance, distribution, and phenology of marine organisms (Grebmeier 2012). Many of these substantial changes are already evident. In the pelagic ecosystem, earlier sea–ice breakup affects the promotion of the phytoplankton bloom and may cause higher levels of primary production (Arrigo et al. 2008). Zooplankton also respond to interannual changes, showing higher abundance and biomass during recent warmer periods with low sea-ice coverage in both the Chukchi (Matsuno et al. 2011) and Bering Seas (Ohashi et al. 2013). These alterations were also detected in Arctic benthic ecosystems. For example, significant populations of a dominant clam declined in the Pacific Arctic (Grebmeier 2010), and the trophic structure of crustacean decapods changed towards generalistic feeders due to environmental disturbances in a North Atlantic fjord (Berge et al. 2009).
The Bering and Chukchi Seas are among the most productive ecosystems and the former sustains one of the largest fishery industries. The overfishing has led to the collapse of the crab fishery in the southeastern Bering Sea in 1980, closing most of the stocks for harvesting, and it also modified the distribution pattern of the crabs and changed the population structure (Loher and Armstrong 2005). One key component in conservation and fishery management is the understanding of the mechanisms involved with larval dynamics (Cowen et al. 2000). Most marine crustacean decapods release dispersive larvae that act as the primary connectivity agents among populations (Morgan et al. 2009). Larval assemblages result from complex interactions of biological traits (e.g., spawning time and location, larval behaviour, and planktonic larval duration) and hydrodynamic processes, and both vary at several spatial and temporal scales (Pineda et al. 2007). Normally, mortality is high during the planktonic period, because larvae are very sensitive to predation, pollutants, starvation, and suboptimal conditions of temperature and salinity (Cowen and Sponaugle 2009). The persistence of adult populations requires their replacement by larval recruits; therefore, the effect of the ongoing changes in the Arctic and subarctic areas on planktonic phases has significant consequences for fishery management. Although larval studies are a useful tool for understanding the dynamics of crustacean populations, there is a little published information of decapod larvae in the Pacific Subarctic/Arctic seas. In the SE Alaska, few studies have been conducted on the temporal distribution of the larvae in the plankton (e.g., Shirley and Shirley 1987; Park and Shirley 2005; Fisher 2006) and some valuable data on spatial variability of larvae can be derived from monitoring programs (Armstrong et al. 1981; Wolotira et al. 1984; Incze et al. 1987). Further to the north, in the N Bering Sea and Chukchi Sea, the gap of information is even wider, but recently, research groups are making efforts on selected taxa (Eisner et al. 2013; Weems et al. 2016).
In the present study, we analysed, for the first time, the large-scale geographical distribution of the decapod larvae community from the south Bering Sea to the Chukchi Seas during two consecutive summers of 2007 and 2008. Specifically, this study examined the variability in abundance, diversity, larval stage composition and body size throughout this wide, ecological transition area in the Pacific Subarctic/Arctic sector, as well its relationship with environmental conditions. Moreover, using data from bottom trawls during a 2008 cruise, we evaluated the coupling/decoupling between the benthic adult distribution and the larval patterns. The present study established a baseline for assessing future changes in the Arctic communities of decapods that may occur in response to variations in hydrography, circulation, or ice retreat associated with climatic change, as well as impacts from fisheries activities.
Materials and method
Field sampling
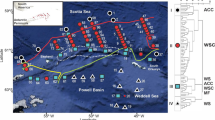
Zooplankton samplings were conducted from the shelves and upper slopes of the southeastern Bering Sea (55N) to Chukchi Sea (71N) during the summers of 2007 (20 July−13 August, n = 68) and 2008 (24 June–13 July, n = 70) (Fig. 1a, b). The temperature and salinity were measured by a Seabird SBE-911plus CTD at each station. To estimate the chlorophyll a (Chl. a) concentration, water samples were collected from 0, 5, 10, 20, 30, 40, 50, 75, 100, 125, and 150 m (varied depending on the bottom depth). The samples were filtered through GF/F filters and measured using a Turner Designs fluorometer, after extraction with N,N-dimethyl-formamide. Zooplankton samples were collected by the vertical tow of a NORPAC net (45 cm mouth diameter, 0.335 mm mesh; Motoda 1957) from 150 m depth or near the bottom (where the depth was shallower than 150 m) to the surface. The net was equipped with a flowmeter in the mouth, estimating the volume of water filtered. Plankton samples were immediately preserved with 5% v/v borax buffered formaldehyde-seawater.
During the 2008 cruise, benthic adult population of decapods were collected using an Otter trawl (with 10 mm mesh size inner net installed in the codend, a 24.5 m foot-rope and 4 m opening) at three stations in the southeastern Bering Sea, five stations around St. Lawrence Island, and seven stations in the Chukchi Sea (Fig. 1c). Bottom depth varied between 42 and 140 m and was monitored acoustically using a Net Sounder (Furuno Electric) mounted on the upper head-rope of the trawl. The average speed towing was 3.7 knots (7 Km h−1) and the tow duration was 10–30 min depending on the bottom topography. Distance of tow was calculated by multiplying the duration by the speed of tow, and was confirmed from GPS positions recorded at the beginning and end of each tow.
Sample processing
Zooplankton samples were divided once using a Motoda box splitter (Motoda 1959). One-half of the sample is weighed for wet weight (WW), with a precision of 0.01 g, using an electronic balance (Mettler PM4000). The remaining subsample was used to estimate the zooplankton abundance under a stereomicroscope. From this subsample (1/2 of total), all of the decapod larvae were sorted, staged, and identified to the lowest taxonomic level possible, following several specific descriptions and identification keys (see Table 1 for details). Some larvae were categorized as taxonomic groups, due to limitations associated with identifying larval stages to species level (e.g., Pagurus spp. Hippolytidae sp.1). To make a quantitative comparison possible, the decapod larvae abundance was expressed per square meter (ind. m−2). For the abundant and commercially important species, snow crab (Chionoecetes opilio), all larvae present in the samples were measured to a precision of 0.05 mm using a dissection stereomicroscope equipped with a calibrated eyepiece micrometer. Zoea I (ZI) and zoea II (ZII) were measured for rostral–dorsal length (RDL distance between the apexes of rostral and dorsal spines) and cephalothorax length (CL from frontal margin, between the eyes, to the posterolateral carapace margin). Megalopae (M) were only measured for CL, due to its different morphology. Benthic adults collected by bottom trawling were also identified on board to the lowest taxonomic level possible and weighed. The abundances were standardized with the capture per unit of effort (CPUE: kg h−1). It was calculated for each taxonomic group and trawl haul by dividing catch in weight by tow duration. The general taxonomic nomenclature in the present study followed De Grave et al. (2009).
Data analysis
The Shannon’s diversity index,\(H' = \sum\limits_{i = 1}^S {{p_i}} \ln {p_i}\) (where S is the number of taxa and p i is the proportion of individuals of taxa i), was computed based on all of the identified taxa of decapod larvae (Shannon and Weaver 1949). The mean larval stages of the selected species were calculated following the equation: \(\overline {LS} = \sum {\frac{{i \cdot Ni}}{N}}\), where i is the larval stage (e.g. i = 1 for ZI; i = 2 for ZII…), Ni is the abundance of the larval stage, and N is the total decapod larvae abundance of each species. The smaller and larger \(\stackrel{-}{LS}\)indicates the dominance of early and late larval stages, respectively. Differences in \(\stackrel{-}{LS}\), zooplankton abundance, temperature, and decapod larvae diversity and abundance between 2007 and 2008 were assessed using a Mann–Whitney U test, because the data did not conform to parametric test assumptions of normality and homogeneity of variance. The spatial relationship between larval abundance and potential food resources (integrated mean chlorophyll a and zooplankton biomass) was explored using the Spearman’s rank correlation.
A regression analysis and ANCOVA were used to evaluate potential variations in the body size of C. opilio larvae with latitude. The ANCOVA was performed with a separate-slope model for each body size measurement (CL and RDL) to test whether the relationships between the size (covariate) and latitude (dependent variable) varied among larval stages (ZI, ZII, M). Spearman’s rank correlation was used to analyse the factors forcing the observed size plasticity of C. opilio larvae, relationships between the body size (CL and RDL) of these larvae (ZI, ZII, and M), and environmental variables (latitude, surface temperature: ST, bottom temperature: BT, integrated mean temperature: IMT, integrated mean salinity: IMS, integrated mean chlorophyll a: IMCh, zooplankton abundance: ZooAb and zooplankton biomass: ZooBio).
Results
Environmental conditions
Over the study area, temperature decreased offshore, due to the heating of the shallow waters (Fig. 2). Furthermore, thermal stratification in the water column was evident, since sea surface temperature (SST) ranged from 3.7 to 13.9 °C, and the bottom temperature (BT) showed lower values between 1.7 and 8.0 °C. The salinity increased offshore influenced by the ice-melting and river runoff, with values between 26.2 and 32.9 PSU in the surface, and denser values ranged from 30.4 to 33.2 PSU in the bottom. Total zooplankton abundance and biomass showed an irregular horizontal distribution. The zooplankton abundance ranged from 1,103 to 316,409 ind. m−2 and was dominated by copepods and barnacle larvae, whereas the biomass showed values from 0.12 to 226 g m−2 (Fig. 3). The integrated mean chlorophyll (IMC) ranged from 0.03 to 21.4 µg L−1, showing maximum concentrations around the Bering Strait at both the surface (19.4 µg L−1) and bottom (23.7 µg L−1).
Comparison of environmental conditions among cruises revealed differences in temperature and zooplankton dynamics (Figs. 2, 3). Thus, the integrated mean temperature (IMT) was significantly higher (Mann–Whitney U test, p < 0.01) in July−August 2007 (5.11 ± 2.15 °C) than in June−July 2008 (2.07 ± 1.67 °C). This differences were especially evident in the horizontal maps of SST, but the maximum SST values (>8.5 °C) in both years were observed in the northeast of St. Lawrence Islands. Regarding zooplankton biomass, annual differences were also found (Mann–Whitney U test, p < 0.01) with higher average values during 2008 (55.85 ± 45.52 g m−2) than 2007 (35.05 ± 37.08 g m−2). The biomass and abundance of zooplankton showed similar horizontal distribution patterns in 2007, with higher values northward in the Chukchi Sea (Fig. 3). However, during 2008, the biomass and abundance displayed opposite patterns. Thus, higher abundances were located in the Chukchi Sea due to the dominance of small organisms, such as barnacle larvae, whereas the biomass peaked at the southern Bering Sea, where copepods were the most abundant group.
Pelagic larvae
Abundance and distribution
In total, 1254 decapod larvae were sorted, and 18 different species belonging to eight families were identified (Table 1). The larval composition was characterized by the dominance of benthic species and the absence of pelagic taxa. Families with the most species were Pandalidae (five species), Crangonidae (three species), and Oregoniidae (three species) (Table 1). The Shannon–Wiener diversity index ranged between 0 and 1.76 and displayed a homogenous spatial distribution throughout the study area (Fig. 4). Although 2008 showed higher diversity values than 2007, there was no significant difference between the years (Mann–Whitney U test, p > 0.05).
The abundance of decapod larvae had a maximum value of 1139 larvae m−2 and was characterized by a large quantity of negative samples (those with 0 larvae), especially in 2007. The decapod larvae assemblage was dominated, in terms of abundance, by species of the families Paguridae (Pagurus spp.) and Oregoniidae (C. opilio, C. bairdi, and Hyas spp.), which accounted for more than half of the total larval catches (Table 1). During 2008, the larval abundance was significantly higher than in 2007 (Mann–Whitney U test, p < 0.01) (Table 1). The distribution of the decapod larvae in 2007 and 2008 showed clearly different patterns. Thus, during 2007, the decapod larvae were more abundant in the eastern Bering Sea. In 2008, the Chukchi Sea was the region that accounted for the highest densities, mainly forced by the distribution of the dominant species (Fig. 4). In this area, the maximum values were 571 larvae m−2 of C. opilio, 275 larvae m−2 of Hyas spp. and 186 larvae m−2 of Pagurus spp. (Fig. 4).
It should be noted that the very low abundance observed around the Bering Strait gave the appearance of a disjunct horizontal distribution in many taxa. However, the horizontal distribution of decapod larvae differed among taxa. The larvae of C. opilio, Hyas spp., and Pandalus spp., showed this disjunct pattern in both years, whereas Telmesus cheiragonus and Pagurus spp. only displayed such distribution during 2007 (Fig. 4). During 2008, T. cheiragonus larvae were restricted to the northern area, whereas Pagurus spp. larvae showed a wider distribution throughout the study area, with higher abundances in the Chukchi Sea (Fig. 4). On the contrary, C. bairdi larvae were only collected in the south Bering Sea and showed significant abundance values in both years (Table 1; Fig. 4). Rare species (those found in less than 3 samples) were also present. For example, larvae of the commercially important crabs Paralithodes camtschaticus and P. brevis were also found in south Bering Sea at very low densities, as well as the larvae of Hippolytidae spp. or Oedignathus inermis which were recorded in the Bering Strait and Chukchi Sea respectively (Table 1).
Larval stage composition
The analysis of the larval stages did not show spatial differences, but indicated that in 2007, late-stage larvae were more abundant, whereas during 2008, the population structure was dominated by early stage larvae (Fig. 4). During 2007, the population structure was characterized by a greater proportion of ZII and M in C. opilio (\(\overline {LS}\) = 2.33 ± 0.49), C. bairdi (\(\overline {LS}\) = 2.09 ± 0.2), and Hyas spp. (\(\overline {LS}\) = 2.75 ± 0.41), whereas during 2008, ZI accounted for almost 100% (\(\overline {LS}\) =1) of these three species (Mann–Whitney U–test, p < 0.01). In contrast, the population structure of T. cheiragonus and Pandalus goniurus did not show significant differences between the years (Mann–Whitney U test, p > 0.05). The low larval abundance of these species was insufficient (as was also suggested by the absence of all larval stages in the samples) to detect changes in the population structure (Fig. 5).
Composition of the larval stages (ZI zoea I, ZII zoea II, ZIII zoea III, ZIV zoea IV, ZV zoea V, and M megalopa) of selected species of crustacean decapods during 2007 (white bars) and 2008 (black bars). Numbers in parentheses indicate the number of larvae for each year. **p < 0.01, NS not significant
Body size
The body size (CL and RDL) of C. opilio showed a significant positive correlation with the latitude for each larval stage: ZI, ZII, and M (Fig. 6a, b; Table 2). However, the ANCOVA analysis showed that the parallelism of the regression lines was rejected, because the slopes significantly varied for CL (F 2, 156 = 13.07, p < 0.0001) and RDL (F 1, 148 = 38.83, p < 0.0001) with larval stages. The correlation analysis with environmental parameters showed that the size of ZI had a significant negative correlation with temperatures: ST (CL: p < 0.0001; RDL: p = 0.004), BT (CL: p < 0.0001; RDL: p < 0.0001) and IMT (CL: p < 0.0001; RDL: p < 0.0001). For ZII stage, the size had no correlation with temperature, but it had a positive correlation with chlorophyll a (CL: p = 0.016; RDL: p = 0.043). The body size had no significant correlation with any of these environmental parameters for the M larval stage.
Benthic adults
Seven different taxa were identified in the 15 bottom trawl samples of decapods (Fig. 7). The mean CPUE of the total decapod assemblage ranged from 11.7 to 262.1 kg h−1 (Fig. 7), with an average value of 76.3 ± 101.2 kg h−1. The Chukchi Sea was the region with higher CPUEs, accounting for 98.5 kg h−1, followed by St. Lawrence Island (62.2 kg h−1) and the southeastern Bering Sea (18.9 kg h−1) (Fig. 7). In the study area, the most dominant decapods were the snow crab (C. opilio) and hermit crabs (mainly Paguridae species), with mean CPUE values of 46.9 kg h−1 and 17.0 kg h−1, respectively. The numerical importance of each taxa in relation with the overall composition varied among regions. The southeastern Bering Sea was characterized by the presence of C. bairdi (52.1%), which was absent northward. Catches from St. Lawrence Island and Chukchi Sea were dominated by C. opilio (78.4% and 43.7% respectively). In the Chukchi Sea, the contribution of Hyas spp. (20.1%) and Pandalus spp. (4.1%) increased in comparison with other southern areas (Fig. 7).
Discussion
Abundance and distribution of pelagic larvae
In comparison with other planktonic groups (such as copepods, chaetognaths, and apendicularians), decapod larvae showed lower abundance values in the samples collected during the summers of 2007 and 2008 (Matsuno et al. 2016). However, the abundance of decapod larvae was comparable to other studies conducted in the waters off the southeastern Bering Sea (Armstrong et al. 1981), the northern Bering Sea, and the central and southern Chukchi Sea (Eisner et al. 2013), but lower than those obtained in the nearshore waters of Kodiak Island (Wolotira et al. 1984), and the Glacier Bay, a high-latitude fjord in southeast Alaska (Fisher 2006). Regarding species composition, the larval dominance of C. opilio, C. bairdi, Hyas spp., Pagurus spp., and Pandalus spp. in our assemblages was also in agreement with the composition reported by Armstrong et al. (1981) and Wolotira et al. (1984) in the Bering Sea and by Eisner et al. (2013) and Weems et al. (2016) in the Chukchi Sea, but with a higher contribution of crangonid larvae (Argis lar). However, differences in sampling methods, time, and oceanographic variability in the area, make it difficult the quantitative comparison between studies. Larvae of the commercially important king crabs, P. brevipes and P. camtschaticus, were reported in this study but were only present in four stations at very low densities (Table 1). In the Bering Sea and near regions, larvae of king crabs occur in the plankton from March to June, peak in May, (Shirley and Shirley 1989; Otto et al. 1990) with a preference for nearshore waters (Armstrong et al. 1981; Wolotira et al. 1984), where the adults migrated for spawning and reproduction (Stevens and Swiney 2007; Dew 2008). It can explain the absence of significant abundance of king crab larvae, since our sampling mainly occupied offshore waters, and took place too late to find the larvae in the plankton.
Significant interannual differences were observed in the decapod larvae community. Thus, during summer 2007, we recorded higher larval abundance than during 2008. Furthermore, the larval distribution showed an opposite pattern, in which the larvae were more abundant in the Bering Sea during 2007 and in the Chukchi Sea during 2008. Inter-year differences in environmental conditions were associated with the development of the sea-ice associated spring phytoplankton bloom at high latitudes (Stabeno et al. 2012). During May–June, the melting and breaking up of sea-ice promote the phytoplankton bloom by enhancing light availability and maintaining nutrients in the surface waters (Arrigo et al. 2008; Grebmeier 2012). Changes in phytoplankton bloom phenology may influence the timing and duration of larval release, because decapods couple larval hatching rhythms with favourable periods of abundant food resources (Starr et al. 1990). This reproduction strategy ensures viable offspring, but it is especially sensitive, because the temporal window of favourable temperatures and food conditions can be narrow at high latitudes (Fisher 2006). The distribution of benthic invertebrates’ larvae often coincides with higher densities of phytoplankton and zooplankton, favouring optimal foraging (Metaxas and Young 1998; Brandão et al. 2015). In our case, we cannot test if larval mortality due to starvation can explain interannual differences in larval abundance. Spatial and temporal match/mismatch between larvae and their food is a key issue that need to be addressed in the future for better understand of larval dynamics in Pacific Subarctic/Arctic seas.
Larval stage composition
In the summer of 2007, larvae of C. opilio, C. bairdi, and Hyas spp. occurred in more advanced stages of development than during 2008. Alterations in the larval release timing not only affect the planktonic larval period but also settling on the bottom. This can be particularly important for species with extended planktonic larval durations, such as the majoid crabs C. bairdi, C. opilio, and Hyas spp., which spend up to 3–5 months in the water column before settling (Yamamoto et al. 2014). Late larval release implies a delay in megalopa settlement under suboptimal bottom temperatures that can lead to lower survivorship and recruitment (Parada et al. 2010). Moreover, temperature is also a key environmental variable determining the larval release time and the duration of larval stages (Yamamoto et al. 2014; González-Ortegón and Giménez 2014). The temperature was higher during 2007 cruise than 2008 cruise throughout the study area; therefore, it would be reasonable to assume that changes in the larval community could be influenced by the temperature regime. However, both cruises took place 1 month apart (July–August 2007 and June–July 2008), and at high latitudes, the increasing solar radiation during early summer heats up the upper water layers, so the differences in SST were only evident in one month.
Oceanographically, 2007 and 2008 were cold years, with strong similarities in terms of temperature and sea-ice extent (Stabeno et al. 2012). Considering the 1-month time lag between samplings, we can explain the differences in the larval stage composition observed in 2007 and 2008. In the case of C. opilio, one of the most abundant species in our samples, it seemed very clear. The hatching season of C. opilio starts in March and ends in June, but most hatching in the EBS occurs in April–May (Incze et al. 1987). Thus, ZI collected during 2008 could have corresponded to one of the last larval batches of the year. C. opilio takes approximately 20 days to pass from ZI to ZII, which was the exact time lag between the samplings. Consequently, the larvae in ZII stage collected during 2007 could also belong to one of the last batches of the year. This may also be applicable to other majoids, such as C. bairdi and Hyas spp., which have the same larval development mode (ZI, ZII, and M) and similar larval duration (Armstrong et al. 1981). Using the same samples as those that we used for the present study on decapod larvae, Matsuno et al. (2016) studied geographical changes in the mesozooplankton community in the Bering as Chukchi Seas and found similar trend for barnacle larvae. In this study, the authors reported the abundance peak in June-July (2008 cruise) and it decreases from July–August (2007 cruise) to September (2007 cruise by Eisner et al. 2013). In agreement with our observations, Matsuno et al. (2016) considered the differences in barnacle larvae abundance between 2007 and 2008 as a result of the 1-month sampling lag, rather than environmental variability.
Body size of larvae
Phenotypic plasticity in response to varying environmental conditions is a well-documented life history trait in many marine invertebrates (Hadfield and Strathmann 1996; Anger 2001). In crustacean decapods, larval size may be influenced by the maternal phenotypes (Sato and Suzuki 2010) and by the environmental conditions experienced during embryogenesis and during the planktonic larval period, such as temperature, salinity, and food availability (Giménez 2010; González-Ortegón and Giménez 2014). Latitudinal interpopulation variations were documented for wild larvae in many decapods. Poole (1966) and Shirley et al. (1987) found that dorsal carapace spine, rostral spine, and lateral spine of Metacarcinus magister ZI were 14, 14, and 29% longer, respectively, at higher latitudes off Alaska than those collected in Californian waters. Similarly, Marco-Herrero et al. (2012) reported the same plasticity trait when comparing the ZI larvae of Macropodia rostrata from UK, which had 32.6%, longer carapace length and 9.65% longer the rostral-dorsal spine length than those ZI from Spain populations. In our case, the body size variation between the Bering Sea and Chukchi Sea populations in C. opilio larvae may have had maternal influence since Webb et al. (2006) evaluated the effect of embryonic incubation temperature on the morphology and found that the body size and spine lengths were smaller at the warmest incubation temperature. This pre-hatching effect can be propagated throughout the larval development by a carry-over effect (Giménez 2010). However, the slopes of the relationship between latitude and body size were different among ZI, ZII, and M, suggesting that environmental components during the planktonic phase also had a strong effect on the observed pattern. In addition to the temperature effect on body size, we found that larger ZII larvae occurred at stations with significant higher phyto-zooplankton biomass, which may suggest that food conditions also influenced the observed phenotypic plasticity. Regarding the M stage, no significant correlations were found between size and environmental conditions. As we mentioned, C. opilio larvae have an extended planktonic larval period and can be transported long-distances from parental populations, making it difficult to track the forcing environmental conditions involved in the megalopal phenotype (Parada et al. 2010).
Size variability has profound implications on larval behaviour, buoyancy, sinking rate, swimming efficiency, and defence against predation (Webb et al. 2006). Large-bodied larvae can benefit from the exploitation of a wider size food spectra and from a more efficient energy allocation (Anger 2001), but in contrast, they are disproportionally more vulnerable to visual predators, such as planktivorous fish (Brooks and Dodson 1965). On the contrary, large-bodied larvae also develop longer rostral, dorsal and lateral carapace spines, which were demonstrated to be prominent defensive structures to estuarine crab larvae, preventing the high predation risk by planktivorous fish in estuarine systems (Morgan 1990). Likely, the development of longer spines of C. opilio larvae at colder high latitudes could be a predatory-avoidance trait to minimize the higher risk of attacks by visual predators due to larger body size of larvae. Moreover, to distinguish the larvae of C. opilio from C. bairdi and other majoids, such as Hyas spp., several inconspicuous morphological characteristics must be checked. Therefore, many measurements (carapace length, rostral–dorsal length, and the posterolateral spine length of the third abdominal somite) were proposed as key characters for an easier identification of this larval group (Haynes 1973; Davidson and Chin 1991). Morphometric characters could be a useful tool for a quick discrimination of larvae from the same sample or oceanographic region, but in view of the environmental-mediated larval plasticity, body size measurements should be used with caution and always in combination with morphological characters for reliable identifications, as was previously suggested by Webb et al. (2006) and Korn et al. (2010).
Pelagic and benthic coupling
Bottom Trawls in 2008 showed adult decapods with higher CPUE were C. opilio, hermit crab, Hyas spp., and C. bairdi. These species, from a feeding ecology stand point, are generalist feeders as opposed to specialists (Wieczorek and Hooper 1995; Berge et al. 2009). Berge et al. (2009) found over the last century, in the North Atlantic Svalvard-area, that the marine ecosystem has undergone a shift from a community dominated by specialized predators to the current community characterized by generalistic species with wider habitat distribution. The authors related this phenomenon to environmental disturbance due to the warming trend in the area, which was also observed in other southern Atlantic areas and in the North Sea (Kirby and Beaugrand 2009; Lindley et al. 2010). It is likely that a similar transition in the community structure is already occurring in the Eastern Bering Sea and Chukchi Sea, but further studies are needed to confirm it.
Most of the benthic species ranged widely, indicating community similarities among areas. C. opilio is a commercially important species, very abundant from depths of 50–200 m on the continental shelf from the Bering, Chukchi, and Beaufort Seas and in the northwestern Atlantic, and an introduced population was recently discovered in the Barents Sea (Alvsvåg et al. 2009; Hardy et al. 2011; Rand and Logerwell 2011). In the southeastern Bering Sea, the lucrative snow crab fishery was declared overfished in 1999, after a large period of catch fluctuations since 1980 (NPFMC 2009). This area is the northern distribution limit of C. bairdi, where it overlaps with C. opilio (Incze et al. 1987). Our results confirmed that C. bairdi was only present in the south Bering Sea and, hence, its range was not extended northward, as was expected under the ongoing warming changes in the area. In fact, we did not find larvae of C. bairdi further than 57N, indicating that the larval distribution was strongly related to the adult populations. In addition, the warmer conditions in the Pacific Subarctic/Arctic seas seem to favour the growth and reproduction of C. opilio, which has driven a northward contraction of the distribution range (Parada et al. 2010; Rand and Logerwell 2011). Our results report lower abundance values of both adults and larvae of C. opilio in the SE Bering Sea, but we consider that the analysis of a longer time-series is needed to support the northern contraction of its populations.
The spatial match–mismatch between adult population and larval distribution in the region results from the reproductive output of adult females and larval transport (Incze et al. 1987; Parada et al. 2010). In our case, we observed a clear association between the larval and adult distribution in most of the decapods (see Fig. 8a, b). This, along with the absence of C. bairdi larvae in St. Lawrence Island or Chukchi Sea, suggests that during the summers of 2007 and 2008, the retention processes may maintain the larval pool near the adult populations. Mesoscale circulation features (such as internal waves, tidal fronts, and eddies), coupled with vertical and horizontal larval swimming behaviours, may lead to the ecologically scaled retention of larvae relatively close to the source populations (Cowen et al. 2000). Moreover, larval retention can promote higher recruitment rates, because the parental habitat is normally favourable for the larval settlement (Pechenik 1999). In addition, Parada et al. (2010) applied biophysical modelling to estimate the larval transport and connectivity of C. opilio and observed that most of the larval pool that originated in the eastern Bering Sea is retained near there, but some larvae are transported northward, crossing the Bering Strait. Recently, using population genetics, Albrecht et al. (2014) showed a single homogenized C. opilio stock exists throughout Alaskan waters. Therefore, it seems that the few larvae transported long-distances northward are enough to maintain a gen flow among populations and connectivity (Parada et al. 2010). Larvae of C. opilio have a long planktonic duration that lasts 3–5 months and consists of two zoeal stages and one megalopa stage (Yamamoto et al. 2014). This relatively long planktonic phase, in combination with the current circulation, may facilitate eventual long-distance dispersal between widely distributed populations (Shanks and Eckert 2005). In this sense, ocean currents in this region are relative strong and dominated by a northward flow from the Gulf of Alaska to the Chukchi Sea through the Bering Sea water masses: the inshore Alaskan Coastal Water (ACW) flow, the offshore Anadyr Water (AW) flow, and the Bering Shelf Water (BSW) flow between them, centred around St. Lawrence Island (Coachman and Charnell 1979; Coachman 1986) (Fig. 1d). Unfortunately, our sampling was not designed to study physical processes related with mesoscale circulation, but it is a key question to understand larval transport and connectivity. Therefore, we encourage the scientific community to implement this analysis for future expeditions to the Pacific Subarctic/Arctic sector.
Future prospects
We recommend future monitoring studies on benthic adults, collected by trawling, for a more detailed identification, and catch processing onboard to provide data on the size, sex, and maturity of individuals. Using the Hokkaido University zooplankton long-term time-series in the Bering Sea, we are extending the temporal period to track alterations in the decapod larvae community related with climate changes and fisheries. For that, it would be interesting the use of the NOAA-NMFS Eastern Bering Sea Bottom Trawl Survey results (http://www.afsc.noaa.gov/Kodiak/shellfish/crabebs/crabsurvey.htm) to link the larval results with adult population dynamics. However, to detect northern distribution shifts of subarctic species in relation to the current and future conditions in the Arctic, more accurate larval descriptions are needed. It is relatively easy to identify the most abundant larvae using the available larval descriptions, but it is rather complicated to classify the less common species, such as species belonging to the families Hippolytidae or Crangonidae, which are diverse in the northeast Pacific and have the potential to move northward to higher latitudes. In the case of the commercially important shrimps of the family Pandalidae, the larval descriptions are dated and do not follow the current standards proposed by Clark et al. (1998). In addition, taxonomists and meroplankton ecologists benefit from having access to English versions of current and past Japanese and Russian larval keys. Therefore, we encourage the scientific community to make efforts in this matter, describing in detail the larval development of crustacean decapods obtained in the laboratory from ovigerous females.
References
Albrecht GT, Hundertmark KJ, Valentin AE, Hardy SM (2014) Panmixia in Alaskan populations of the snow crab Chionoecetes opilio (Malacostraca: Decapoda) in the Bering, Chukchi, and Beaufort Seas. J Crust Biol 34:31–39
Alvsvåg J, Agnalt AL, Jørstad K (2009) Evidence for a permanent establishment of the snow crab (Chionoecetes opilio) in the Barents Sea. Biol Invas 11:587–595
Anger K (2001) The Biology of Decapod Crustacean Larvae. Crustacean Issues, vol 14. A. A. Balkema, Rotterdam
Armstrong DA, Incze LS, Wencker DL, Armstrong JL (1981) Distribution and abundance of decapod crustacean larvae in the southeastern Bering Sea with emphasis on commercial species. Final Report Outer Continental Shelf Environmental Assessment Program Research Unit 609. University of Washington School of Fisheries, Seattle
Arrigo KR, van Dijken G, Pabi S (2008) Impact of a shrinking Arctic ice cover on marine primary production. Geophys Res Lett 35:L19603
Berge J, Renaud PE, Eiane K, Gulliksen B, Cottier FR, Varpe Ø, Brattegard T (2009) Changes in the decapod fauna of an Arctic fjord during the last 100 years (1908–2007). Polar Biol 32:953–961
Brandão MC, Freire AS (2015) Large-scale spatial variability of decapod and stomatopod larvae along the South Brazil Shelf. Cont Shelf Res 107:11–23
Brooks JL, Dodson SI (1965) Predation, body size, and composition of plankton. Science 150:28–35
Clark PF, Calazans DK, Pohle GW (1998) Accuracy and standardization of brachyuran larval descriptions. Invertebr Reprod Dev 33:127–144
Coachman LK (1986) Circulation water masses, and fluxes on the southeastern Bering Sea shelf. Cont Shelf Res 5:23–108
Coachman LK, Charnell RL (1979) On lateral water mass interaction: a case study, Bristol Bay, Alaska. J Phys Oceanogr 9:278–297
Cowen RK, Sponaugle S (2009) Larval dispersal and marine population connectivity. Annu Rev Marine Sci 1:443–466
Cowen RK, Lwiza KM, Sponaugle S, Paris CB, Olson DB (2000) Connectivity of marine populations: open or closed? Science 287:857–859
Davidson KG, Chin EA (1991) A comparison of the taxonomic characteristics and duration of the laboratory reared larvae of snow crabs, Chionoecetes opilio (O. fabricius) and toad crabs, Hyas sp. from Atlantic Canada. Aquat Sci 1762:1–21
De Grave S, Pentcheff ND, Ahyong ST et al. (2009) A classification of living and fossil genera of decapod crustaceans. Raff Bull Zool 21:1–109
Dew CB (2008) Red king crab mating success, sex ratio, spatial distribution, and abundance estimates as artifacts of survey timing in Bristol Bay, Alaska. North Am J Fish 28:1618–1637
Eisner L, Hillgruber N, Martinson E, Maselko J (2013) Pelagic fish and zooplankton species assemblages in relation to water mass characteristics in the northern Bering and southeast Chukchi seas. Polar Biol 36:87–113
Epelbaum AB, Borisov RR, Kovatcheva NP (2006) Early development of the red king crab Paralithodes camtschaticus from the Barents Sea reared under laboratory conditions: morphology and behaviour. J Mar Biol Ass UK 86:317–333
Fisher JL (2006) Seasonal timing and duration of brachyuran larvae in a high-latitude fjord. Mar Ecol Prog Ser 323:213–222
Giménez L (2010) Relationships between habitat conditions, larval traits, and juvenile performance in a marine invertebrate. Ecology 91:1401–1413
González-Ortegón E, Giménez L (2014) Environmentally mediated phenotypic links and performance in larvae of a marine invertebrate. Mar Ecol Prog Ser 502:185–195
Grebmeier JM (2012) Shifting patterns of life in the Pacific Arctic and Sub-Arctic Seas. Annu Rev Marine Sci 4:63–78
Grebmeier JM, Overland JE, Moore SE, Farley EV, Carmack EC, Cooper L WK, Frey E, Helle JH, McLaughlin FA, NcNutt SL (2006) A major ecosystem shift in the northern Bering Sea. Science 311:1461–1464
Grebmeier JM, Moore SE, Overland JE, Frey KE, Gradinger R (2010) Biological response to recent Pacific Arctic sea ice retreats. EOS 91:161–63
Hadfield MG, Strathmann MF (1996) Variability, flexibility and plasticity in life histories of marine invertebrates. Oceanol Acta 19:323–334
Hardy SM, Lindgren M, Konakanchi H, Huettmann F (2011) Predicting the distribution and ecological niche of unexploited snow crab (Chionoecetes opilio) populations in Alaskan waters: a first open-access ensemble model. Integr Comp Biol 51:608–622
Haynes E (1973) Descriptions of prezoeae and stage I zoeae of Chionoecetes bairdi and C. opilio (Oxyrhyncha, Oregoninae). Fish Bull 71:769–775
Haynes E (1976) Description of zoeae of coonstripe shrimp, Pandalus hypsinotus, reared in the laboratory. Fish Bull 74:323–342
Haynes E (1978) Description of larvae of the humpy shrimp, Pandalus goniurus, reared in situ in Kachemak Bay, Alaska. Fish Bull 76:457–465
Haynes E (1979) Description of larvae of the northern shrimp, Pandalus borealis, reared in situ in Kachemak Bay, Alaska. Fish Bull 77:157–173
Haynes E (1980) Larval morphology of Pandalus tridens and a summary of the principal morphological characteristics of North Pacific pandalid shrimp larvae. Fish Bull 77:625–640
Haynes E (1981) Description of stage II zoeae of snow crab, Chionoecetes bairdi, (Oxyrhyncha, Majidae) from plankton of Lower Cook Inlet, Alaska. Fish Bull 79:177–182
Haynes E (1985) Morphological development, identification, and biology of larvae of Pandalidae, Hippolytidae, and Crangonidae (Crustacea, Decapoda) of the northern north Pacific Ocean. Fish Bull 83:253–288
Incze L, Armstrong DA, Smith S (1987) Abundance of larval tanner crabs (Chionoecetes spp.) in relation to adult females and regional oceanography of the southeastern Bering Sea. Can J Fish Aquat Sci 44:1143–1156
Kirby RR, Beaugrand GG (2009) Trophic amplification of climate warming. Proc R Soc B 276:4095–103
Korn OM, Kornienko ES, Scherbakova NV (2010) A key for the identification of larvae of brachyuran and anomuran crabs in spring plankton of Peter the Great Bay, Sea of Japan. Rus Journ. Mar Biol 36:373–382
Kornienko ES, Korn OM (2009) Illustrated key for the identification of brachyuran zoeal stages (Crustacea: Decapoda) in the plankton of Peter the Great Bay (Sea of Japan). J Mar Biol Ass UK 89:379–386
Kurata H (1964a) Larvae of decapod Crustacea of Hokkaido. 3. Pandalidae. Bull Hokkaido Reg Fish Lab 12:1–15
Kurata H (1964b) Larvae of decapod Crustacea of Hokkaido. 4. Crangonidae and Glyphocrangonidae. Bull Hokkaido Reg Fish Lab 28:35–50
Kurata H (1964c) Larvae of decapod Crustacea of Hokkaido. 5. Paguridae (Anomura) Bull Hokkaido Reg Fish Lab 29:24–15
Kurata H (1964d) Larvae of Decapoda Crustacea of Hokkaido. 6. Lithodidae (Anomura), Bull Hokkaido Reg Fish Res Lab 29:49–65
Lindley JA, Beaugrand G, Luczak C, Dewarumez J-M, Kirby RR (2010) Warm-water decapods and the trophic amplification of climate in the North Sea. Biol Lett 6:773–776
Loher T, Armstrong DA (2005) Historical changes in the abundance and distribution of ovigerous red king crabs (Paralithodes camtschaticus) in Bristol Bay (Alaska), and potential relationship with bottom temperature. Fish Oceanogr 14:292–306
Marco-Herrero E, Rodríguez A, Cuesta JA (2012) Morphology of the larval stages of Macropodia czernjawskii (Brandt, 1880) (Decapoda, Brachyura, Inachidae) reared in the laboratory. Zootaxa 3338:33–48
Matsuno K, Yamaguchi A, Hirawake T, Imai I (2011) Year-to-year changes of the mesozooplankton community in the Chukchi Sea during summers of 1991, 1992 and 2007, 2008. Pol Biol 34:1349–60
Matsuno K, Landeira JM, Yamaguchi A, Hirawake T, Kikuchi T (2016) Spatial and geographical changes in mesozooplankton community through Bering and Chukchi Seas during summers of 2007 and 2008. Polar Sci 10:335–345
Metaxas A, Young CM (1998) Responses of echinoid larvae to food patches of different algal densities. Mar Biol 130:433–445
Morgan SG (1990) Impact of planktivorous fishes on dispersal, hatching, and morphology of estuarine crab larvae. Ecology 71:1639–1652
Morgan SG, Fisher JL, Miller SH, McAfee ST, Largier JL (2009) Nearshore larval retention in a region of strong upwelling and recruitment limitation. Ecology 90(12):3489–3502
Motoda S (1957) North Pacific standard plankton net. Inf Bull Planktol Jpn 4:13–15
Motoda S (1959) Devices of simple plankton apparatus. Mem Fac Fish Hokkaido Univ 7:73–94
North Pacific Fishery Management Council (NPFMC) (2009) Fishery Management Plan for Bering Sea/Aleutian Islands King and Tanner Crabs. North Pacific Fishery Management Council, Anchorage, p 228
Ohashi R, Yamaguchi A, Matsuno K, Saito R, Yamada N, Iijima A, Shiga N, Imai I (2013) Interannual changes in the zooplankton community structure on the southeastern Bering Sea shelf during summers of 1994–2009. Deep Sea Res II 94:44–56
Otto RS, MacIntosh, RA, Cummiskey PA (1990) Fecundity and other reproductive parameters of female red king crab (Paralithodes camtschatica) in Bristol Bay and Norton Sound, Alaska. In: B. Melteff (ed.), Proceedings of the International Symposium on King and Tanner Crabs, November 28–30, 1989. University of Alaska Sea Grant Program, Alaska, pp 65–90
Parada C, Armstrong DA, Ernst B, Hinckley S, Orensanz JM (2010) Spatial dynamics of snow crab (Chionoecetes opilio) in the eastern Bering Sea-Putting together the pieces of the puzzle. Bull Mar Sci 86:413–437
Park W, Shirley TC (2005) Diel vertical migration and seasonal timing of the larvae of three sympatric cancrid crabs, Cancer spp., in southeastern Alaska. Estuaries 28:266–273
Pechenik JA (1999) On the advantages and disadvantages of larval stages in benthic marine invertebrate life cycles. Mar Ecol Prog Ser 177:269–297
Pineda J, Hare J, Sponaugle S (2007) Larval transport and dispersal in the coastal ocean and consequences for population connectivity. Oceanography 20:22–39
Poole RLA (1966) Description of laboratory-reared zoeae of Cancer magister Dana, and megalopae taken under natural conditions (Decapoda, Brachyura). Crustaceana 11:83–97
Rand KM, Logerwell EA (2011) The first demersal trawl survey of benthic fish and invertebrates in the Beaufort Sea since the late 1970s. Polar Biol 34:475
Sato T, Suzuki H (2010) Female size as a determinant of larval size, weight, and survival period in the coconut crab, Birgus latro. J Crust Biol 30:624–628
Shanks AL, Eckert GL (2005) Population persistence of California current fishes and benthic crustaceans: a marine drift paradox. Ecol Monograph 75:505–524
Shannon CE, Weaver W (1949) The Mathematical Theory of Communications. University of Illinois Press, Illinois
Shirley SM, Shirley TC (1989) Interannual variability in density, timing and survival of Alaskan red king crab Paralithodes camtschatica larvae. Mar Ecol Prog Ser 54:51–59
Shirley SM, Shirley TC, Rice SD (1987) Latitudinal variation in the Dungeness crab, Cancer magister: zoeal morphology explained by incubation temperature. Mar Biol 95:371–376
Stabeno PJ, Kachel NB, Moore SE, Napp JM, Sigler M, Yamaguchi A, Zerbini AN (2012) Comparison of warm and cold years on the southeastern Bering Sea shelf and some implications for the ecosystem. Deep-Sea Res II(65–70):31–45
Starr M, Himmelman J, Therriault J (1990) Direct coupling of marine invertebrate spawning with phytoplankton blooms. Science 247:1071–1074
Stevens BG, Swiney KM (2007) Hatch timing, incubation period, and reproductive cycle for captive primiparous and multiparous red king crabs Paralithodes camtschaticus. J Crust Biol 27:37–48
Webb JB, Eckert GL, Shirley TC, Tamone SL (2006) Changes in zoeae of the snow crab, Chionoecetes opilio, with variation in incubation temperature. J Exp Mar Biol Ecol 339:96–103
Weems J, Mueter F, Pinchuk A (2016) Cold year crab larvae in the southern Chukchi Sea: variation in biogeographic abundance relative to water masses in 2012. Alaska Marine Science Symposium (AMSS). 25–29 January 2016, Anchorage, Alaska
Wieczorek SK, Hooper RG (1995) Relationship between diet and food availability in the snow crab Chionoecetes opilio (O. Fabricius) in Bonne Bay, Newfoundland. J Crust Biol 15:236–247
Wolotira R, Munk E, Bowerman JH (1984) Seasonal distribution and abundance of decapod larvae for the kodiak island region. NWAFC Processed Report 84–01
Yamamoto T, Yamada T, Fujimoto H, Hamasaki K (2014) Effects of temperature on snow crab (Chionoecetes opilio) larval survival and development under laboratory conditions. J Shellfish Res 33:19–24
Acknowledgements
We are grateful to the captain, officers, and crews of the T/S Oshoro-Maru for their help in sample collection. Special thanks to Prof. Yasuzumi Fujimori, Hokkaido University for his advices using the bottom trawl data. This study was supported by the Green Network of Excellence Program’s (GRENE Program) Arctic Climate Change Research Project: ‘Rapid Change of the Arctic Climate System and its Global Influences’. This work was a part of the Arctic Challenge for Sustainability (ArCS) Project. This study was also supported by a Grant-in-Aid for Scientific Research (A) (24248032) to AY, a Grant-in-Aid for Scientific Research on Innovative Areas (24110005) to AY from the JSPS and by postdoctoral fellowships (PE14055, P16401) to JML from JSPS.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Landeira, J.M., Matsuno, K., Yamaguchi, A. et al. Abundance, development stage, and size of decapod larvae through the Bering and Chukchi Seas during summer. Polar Biol 40, 1805–1819 (2017). https://doi.org/10.1007/s00300-017-2103-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-017-2103-6