Abstract
The abundance and vertical distribution of zooplankton in the mesopelagic zone are important to better understand their role in carbon and energy transfer in the Southern Ocean ecosystem. In the austral summer of 2012/2013, in Prydz Bay, Antarctica, the vertical profiles of zooplankton community structures between 0 and 1500 m were investigated by multivariate analysis of samples collected using a Hydro-Bios MultiNet (200-µm mesh, 0.5 m2 mouth size). Four zooplankton communities belonging to distinct water strata were identified. Group 1 contained samples collected from the surface water strata (<100 m) of four shelf and neritic stations. Group 2 was composed of samples collected from the neritic and shelf regions (<500 m) and the upper layers (0–200 m) of the oceanic region. Group 3 mainly comprised samples collected from the mesopelagic and upper bathypelagic zones (200–1500 m) of shelf and oceanic stations north of the shelf break edge. Group 4 consisted of samples in the 1000–1500 m water stratum of three oceanic stations. The four groups differed more in animal abundance than in species composition. Similarity percentage analysis (SIMPER) showed that zooplankton communities in the upper depth strata (0–200 m) had higher abundance and more pronounced dissimilarity within samples than those below 200 m. A few species (Metridia gerlachei, Rhincalanus gigas, Alacia spp.) showed significant diel vertical migration based on quadratic regression analysis. Sampling depth was the strongest differentiating factor between samples. These results suggest that depth-related differences in environmental characteristics of water masses, such as temperature and salinity, may have the greatest effect upon community structure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Zooplankton vertical distribution profiles have been examined in different regions of the world’s oceans to study the impact of environmental factors on the patterns in these profiles, as well as the role of zooplankton in biogeochemical cycles (Schmidt et al. 2011; Schulz et al. 2012). In the Southern Ocean, euphausiids and copepods are critical components of both the abundance and biomass of the planktonic community and act as a link between primary production and higher trophic levels (Hunt et al. 2007). Much of the work on the vertical distribution of zooplankton has focused on krill and copepod species (Daly and Macaulay 1991; Schnack-Schiel and Hagen 1994; Atkinson 1998). A large number of researchers have also studied the vertical distribution of the entire zooplankton community (Hopkins 1985a; Atkinson and Peck 1988; Hopkins and Torres 1988; Ward et al. 2014). Environmental factors (temperature and salinity), food availability, lipid store biophysical properties, and diel and ontogenetic vertical migrations are considered to be the main factors influencing zooplankton vertical distribution patterns (Atkinson et al. 1992; Atkinson 1998; Brugnano et al. 2010; Pond and Tarling 2011).
Prydz Bay is the largest incursion into the East Antarctic land mass. Previous studies have identified that several water masses exist in this region during the summer: Antarctic Surface Water, Circumpolar Deep Water, Antarctic Bottom Water, Winter Water, and Shelf Water (Smith et al. 1984; Williams et al. 2010; Shi et al. 2013). Zooplankton community structures in the epipelagic zones, as well as interannual dynamics, have been systematically studied in this region (Hosie and Cochran 1994; Hosie et al. 1997, 2003; Swadling et al. 2010; Yang et al. 2011a). An oceanic community, a neritic community, and a krill-dominated community in a latitudinal distribution pattern have been identified (Hosie et al. 1997; Yang et al. 2011a). To date, most zooplankton community research in Prydz Bay has been limited to samples collected from 200 m to the surface water (epipelagic zones), whereas vertical profiles of zooplankton community structures in the mesopelagic and bathypelagic zones of Prydz Bay have been less reported (Hosie and Stolp 1989; Terazaki 1989). It is unclear whether the latitudinal distribution patterns of epipelagic zooplankton communities also exist in the mesopelagic and bathypelagic zones. Moreover, it is necessary to obtain more information about the abundance and vertical distribution of zooplankton in the mesopelagic zone to better understand their role in carbon and energy transfer in the Prydz Bay ecosystem.
To address this shortage of information, zooplankton samples from discrete depths throughout the water column to 1500 m were collected from 11 stations distributed in the oceanic, shelf, and neritic regions of Prydz Bay using a Hydro-Bios MultiNet during the austral summer of 2012/2013. The main objectives of this work are to describe the composition and vertical profile of zooplankton community structures relative to environmental factors, with emphasis on the vertical distribution and stage composition of dominant copepods.
Materials and methods
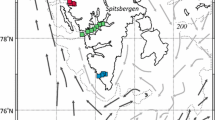
During the 29th Chinese National Antarctic Research Expedition (CHINARE) cruise, zooplankton were collected on board the R.V. Xuelong using a Hydro-Bios MultiNet (200-µm mesh, 0.5 m2 mouth size) at 11 stations in Prydz Bay, Antarctica, during the austral summer of 2012/2013 (Fig. 1). Sampling was conducted as the ship arrived at each station, irrespective of time of day. Stations were numbered in chronological order according to sampling date (Table 1). These 11 stations represent the oceanic region (stations 2, 3, 4, 8, and 9), shelf region (stations 10 and 11), and neritic region (stations 1, 5, 6, and 7) of Prydz Bay. Station 7 was located in the polynya region, where higher productivity is usually found (Arrigo and van Dijken 2003). The MultiNet was towed vertically using a winch with 3000 m of wire. At stations deeper than 2000 m (stations 2, 3, 4, 8, 9, and 10), samples were taken to depth of 1500 m (Table 1). The net could reach only 1600 m when the winch wire was paid out to lengths of 2900 m due to unfavorable field conditions. For stations 1, 5, 6, and 7 with depths less than 1000 m, the lowest depth of the fifth sample was set 150 m above the bottom to ensure that the net could reach the triggering depth but avoid touching the seafloor. Net intervals were designed as 0, 100, 200, 500, 1000, and 1500 m for the oceanic stations and 0, 50, 100, 200, 300, and 500 m for the neritic stations (Table 1). The MultiNet was equipped with a multiparameter probe system to simultaneously measure physicochemical and biological factors (temperature, salinity, and fluorescence). The chlorophyll a (chl a) data used in this study were derived from the calibrated fluorescence sensor. Zooplankton from five water strata at each station were collected (Table 1), and the samples were preserved in 5 % buffered formalin solution.
In the laboratory, large (total length >3 mm) macrozooplankton species were counted in each entire sample. For all other species, aliquots from 1/2 to 1/32 were counted. Aliquots were divided according to the numerical density of individuals using a Folsom plankton splitter. Subsamples with approximately 500 specimens were counted using a dissecting microscope (Nikon SMZ 745T).
Multivariate analyses were performed using the analysis of similarity (ANOSIM), similarity percentages analysis (SIMPER), and Biota-Environmental matching procedure (BIO-ENV) tools in the PRIMER software package (Plymouth, UK) version 6 (Clarke and Ainsworth 1993; Clarke and Gorley 2006). The analyses were similar to those outlined by Yang et al. (2011a). Zooplankton abundance data were fourth-root-transformed and subjected to q-type cluster analysis (samples or stations were arranged into groups) based on the Bray–Curtis dissimilarity index and group average linkage classification (Field et al. 1982). Nonmetric multidimensional scaling (NMDS) was also performed to replicate the station groupings produced by cluster analysis (Hunt et al. 2007). ANOSIM was used to test for differences between resultant groups. The clustered groups were then subjected to SIMPER routines to determine the species contribution to the similarity within and differences between groups. Meanwhile, ANOSIM analysis using sampling depth (0, 100, 200, 500, 1000, 1500 m) as a factor was also conducted to determine differences between samples collected from various depth strata.
A series of quadratic regression analyses of [ln(x + 1)-transformed] abundance of all species over different sampling depths (0–100, 100–200, 200–500, 0–200, 0–500 m) against sampling time was conducted to determine the impact of diel vertical migration of zooplankton caused by arbitrary sampling time on the data (Daase and Eiane 2007).
Indicator value (IndVal) analysis was applied to identify the indicator species of each station cluster (Dufrene and Legendre 1997). The IndVal method combines measures of group specificity (A ij ) and group fidelity (B ij ):
and
where N individuals ij is the mean number of individuals of species i in the sample of group j, while N individuals i is the sum of the mean numbers of individuals of species i over all groups. N samples ij is the number of samples in group j where species i is present, while N samples j is the number of samples in group j. Subsequently, the IndVal was calculated as
The values of A and B are multiplied because they represent independent information about species distribution and are multiplied by 100 to produce percentages. IndVal ≥ 25 % was selected as the cutoff point for an indicator taxon using this method, meaning that such a taxon was mainly present in ≥50 % of samples in a group and that its relative abundance in that group was mostly ≥50 %. Species with IndVal higher than 25 % in two or more groups were considered indicator species for these groups in the study (Table 2). Generally, the abundance of species with higher IndVal in one community was usually higher across the samples in the same group.
Species associations were investigated using inverse (r-type) analysis (grouping of species). To avoid the random association of rare, low-abundance species, 21 species that contributed >2 % to the intracommunity similarity on SIMPER analysis or having IndVal > 25 % were chosen. Most of these species satisfied the criterion for selection proposed by Field et al. (1982) of having more than an arbitrary percentage dominance (2 % in this study) at any one station.
The BIO-ENV procedure was used to estimate which set of environmental variables (temperature, salinity, chlorophyll a, and sampling depth) best explained the zooplankton community structure. BIO-ENV analysis is based on determining the Spearman’s rank correlation coefficient (ρw) between the biological and environmental similarity matrices. A value of ρw = 0 would imply no match between the two matrices, while a value of ρw = 1 means a perfect match (Clarke and Ainsworth 1993).
Results
Environmental conditions
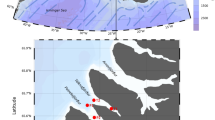
The ice had mainly retreated at all stations before our sampling. In general agreement with previously reported hydrographic studies of Prydz Bay (Smith et al. 1984; Nunes Vaz and Lennon 1996; Williams et al. 2010; Shi et al. 2013), four principal water masses in the shelf and oceanic regions were found (Fig. 2a). Summer Surface Water (SSW) with relatively high temperature and low salinity existed in the surface layer at all stations (Fig. 2b, c). SSW is mainly formed by ice melting and solar heating. Underneath the SSW of the shelf and neritic region, Shelf Water (SW) characterized by colder temperature (near freezing point) and salinity ranging from 34.4 to 34.6 was found (Fig. 2b, c). Relatively warm (near 1 °C) and salty (S > 34.5) Circumpolar Deep Water (CDW) was found at oceanic stations (Fig. 2). CDW can upwell to nearly 200 m in oceanic regions (Fig. 2b, c). Cold and salty Winter Water (WW), formed by wintertime convection, was found above the CDW, approximately above 200 m.
Dominant species
Different vertical profiles of abundance of the dominant species are given in Online Resource 1. The numerical abundances of the species were highly variable but were mainly concentrated in the upper layers and decreased with depth (Online Resource 1).
Calanoides acutus abundance was maximum in the 0–100 m layer but sharply decreased below this (Online Resource 1a). The late copepodite stages (CIV and CV) and females comprised the majority of the population, and the early copepodite stages (CI–CIII) were mainly found in the 0–200 m water strata (Online Resource 1a). Calanus propinquus showed a distribution pattern similar to C. acutus, with higher abundances in the epipelagic zones (Fig. 2b). However, the C. propinquus population was much younger and dominated by the early copepodite stages (CI–CIII), which accounted for more than 50 % of the population in most samples of the upper 500-m stratum (Online Resource 1b). In contrast to C. acutus and C. propinquus, the vertical profile of Metridia gerlachei abundance showed a deeper distribution and peaked in the upper mesopelagic layer at 200–500 m (Online Resource 1c). The abundance of M. gerlachei was higher in the neritic region than the oceanic and shelf regions (Online Resource 1c). Rhincalanus gigas was almost entirely distributed in oceanic regions, and the bulk of the population was composed of the late copepodite stages CIV–CV and females (Online Resource 1d).
Euphausia superba was mainly distributed in the upper 100 m, and the population was mainly composed of juveniles and adults below 200 m (Online Resource 1e). E. crystallorophias was only found in the neritic regions (Online Resource 1f). The population of E. crystallorophias was dominated by the late furcilia stages FIV–VI, juveniles, and adults in the upper 200 m, while the early calyptopis stages CI–CIII made a large contribution to the population structure of the 200–500-m strata (Online Resource 1f). The population structure of Thysanoessa macrura showed significant regional variation in the upper 100-m strata, with the young stages CI–CIII, FI–FIII, juveniles, and adults being mainly distributed in the oceanic, neritic, and shelf regions, respectively (Online Resource 1g).
Although undersampled using the 200-µm mesh net in this study, the copepods Oithona similis, O. frigida, Oncaea curvata, and Triconia antarctica and Ctenocalanus citer were the most dominant zooplankton species (Table 2). The abundance of O. similis, O. curvata, and C. citer was higher in the neritic regions than in the shelf and oceanic regions in the same water strata, while O. frigida showed higher abundance in the shelf and oceanic regions (Online Resource 1).
Community structure
Four distinct communities were identified by cluster analysis, each one mainly identifying a different depth layer (Fig. 3). ANOSIM results indicated that the four clusters were significantly different at p < 0.05. The upper epipelagic water strata (0–50 or 0–100 m) samples of stations 1, 4, 5, and 6 were inconsistent with other samples and were placed into one group (Fig. 3). This group was typified by moderate abundance (mean 27,020 ind 1000 m−3) and mainly included the early copepodites CI–III of C. propinquus and the small copepods O. similis, O. curvata, T. antarctica, and C. citer. Group 2 mainly comprised zooplankton from the upper water strata (Fig. 3). This group also showed clear horizontal zonation and could be divided into two subgroups: subgroup 2a, which was mainly composed of samples collected from the neritic stations, and subgroup 2b, which comprised the zooplankton from the shelf and oceanic regions (Fig. 3). The mean abundance of group 2a was the highest (88,220 ind 1000 m−3), and the indicator species were E. crystallorophias, M. gerlachei, and the small abundant copepods C. citer, O. similis, O. curvata, T. antarctica, and Scolecithricella minor (Table 2). Group 2b also showed relatively high abundance (50,350 ind 1000 m−3), and the indicator species were T. macrura, C. acutus, C. propinquus, M. gerlachei, R. gigas, Paraeuchaeta antarctica, Eukrohnia hamata, Haloptilus ocellatus, Heterorhabdus austrinus, Alacia spp., and small copepods such as O. similis, O. frigida, and C. citer (Table 2). Group 3 mainly contained samples collected from the mesopelagic and upper bathypelagic zones (200–1500 m) of shelf and oceanic stations north of the shelf break edge (Fig. 3). The average abundance (13,410 ind 1000 m−3) was lower than that recorded in group 1 or 2. Group 3 was characterized by high proportions of Aetideopsis minor, Bathycalanus richardi, Megacalanus princeps, the late copepodite stages of C. acutus and R. gigas, P. antarctica, M. gerlachei, Eukrohnia hamate, Alacia spp., and the small copepods O. similis, O. frigida, O. curvata, T. antarctica, and C. citer (Table 2). Group 4 comprised samples in the upper bathypelagic zone (1000–1500 m) of three oceanic stations (3, 8, and 9; Fig. 3); the average abundance was the lowest (316 ind 1000 m−3), and the assemblage was dominated by B. richardi and two small copepods, O. curvata and O. similis (Table 2).
Species assemblages
To identify assemblages of species with similar vertical and spatial distribution patterns, 21 taxa identified by SIMPER analysis as being principally responsible for community similarities or having IndVal >25 % in the four community groups were subjected to r-type cluster analysis (Fig. 4). The three krill species, E. superba, E. crystallorophias, and T. macrura, were mainly divided from other species due to their patchy distribution. Moreover, the MultiNet could not collect krill effectively compared with copepods. Excluding the krill species, four main zooplankton assemblages were identified (Fig. 4). Species in assemblage A were abundant in zooplankton community groups 1, 2, and 3, especially group 2a (Table 2). Species in assemblage B were mainly concentrated in groups 2 and 3 (Table 2). C. acutus, P. antarctica, and O. frigida occurred most frequently in community group 2, while the other three species were found in high abundance in community group 3 (Table 2). Assemblage C included two copepods, H. ocellatus and H. austrinus, indicator species of group 2b (Table 2). Species in assemblage D were widely distributed throughout groups 3 and 4 (Table 2).
Factors affecting community structure
Cluster analysis based on the Bray–Curtis dissimilarity index showed that sampling depth was by far the strongest differentiating factor between samples (Fig. 3). The results of BIO-ENV analysis (Table 3) also identified sampling depth as the largest single contributor to the zooplankton community (ρ = 0.619), followed by salinity, temperature, and chl a (ρ = 0.456, ρ = 0.398, and ρ = 0.297). Sampling depth and chl a gave the best rank correlation coefficient (ρ = 0.678).
ANOSIM analysis using sampling time (day or night) as a factor showed a value of R = 0.022 (p > 0.05), while the larger magnitude of R (R = 0.74, p < 0.001) for sampling depth indicated that sampling time had slight influence on the cluster analysis compared with water stratum.
Over different sample depths (0–100, 100–200, 200–500, 0–200, 0–500 m), significant results (p < 0.05) for quadratic regression analysis of [ln(x + 1)-transformed] species abundance against sampling time were found for R. gigas and Alacia spp. in the 0–100 m water strata and for M. gerlachei in the 200–500 m water strata. No significant relationship was found for the other cases (p > 0.05).
Discussion
Community structure
All species examined in this study are common in the Southern Ocean, and most have a wide distribution range. Similar to zooplankton vertical structure research conducted in the polar ocean (Kosobokova and Hirche 2000; Hunt and Hosie 2003; Kosobokova and Hopcroft 2010; literature in Table 4), communities were also broadly associated with different depth layers in this study (Fig. 3). Differences in vertical community structure were largely attributable to variation in species abundance rather than variation in species composition per se (Table 2). Zooplankton could benefit from aggregating at particular depths to reduce intra- and interspecific competition and decrease predation risk (Schulz et al. 2012). Most species have characteristic but wide vertical distribution ranges, inhabiting two or more of the defined water masses. This was demonstrated by the r-type cluster analysis, in which most species failed to cluster at distinct water strata (Fig. 4). Kosobokova and Hopcroft (2010) also found a similar phenomenon in the Arctic Ocean, and suggested that species composition between layers was determined gradually rather than abruptly. Another explanation is that the towing depth strata in our study may include different water masses to varying degree. The main sampling strata design could not determine to which water masses the majority of species were confined. It has been suggested that the warm nutrient-rich upper CDW may contribute to a favorable zooplankton environment in Marguerite Bay, Western Antarctic Peninsula (Marrari et al. 2011). Many mesopelagic species, such as A. minor, B. richardi, and M. princeps, with high abundances in the 500–1000 m layer also occupy waters both above and below this layer (data not shown). Therefore, it is not surprising that the zooplankton from water strata C (200–500 m), D (500–1000 m), and E (1000–1500 m) of most stations clustered together (Fig. 3). It should be noted that the deep-water samples (1000–1500 m) from the three northernmost stations (3, 8, and 9) showed the lowest abundances (Table 2). The Antarctic Circumpolar Current (ACC) at these stations at 1000–1500 m had stronger flow than at other oceanic stations (unpublished data). Further field sampling should be conducted in the future to determine whether the relatively stronger flow has a negative effect on zooplankton distribution. Upwelling in the CDW cannot reach the shelf region in Prydz Bay (Fig. 2; Shi et al. 2013). Accordingly, zooplankton from 200 to 500 m of the shelf and neritic region did not cluster together with those collected in the same stratum of the oceanic region (Fig. 3). Affected by air–sea (summer heating, wind) and sea–ice interactions, the water masses above 200 m were complex and highly variable (Williams et al. 2010; Michels et al. 2012), while the water masses at mid-water depth showed relatively stable temperature and salinity (Fig. 2). Correspondingly, the dissimilarity of zooplankton community was more pronounced in the upper strata, while the assemblages of zooplankton were more similar in the deeper strata (Fig. 3). Less pronounced differences between deeper zooplankton clusters compared with epipelagic clusters were also reported in the northern Mid-Atlantic Ridge, the Arctic’s Canada Basin, and the 80°W sector of the Southern Ocean west of the Drake Passage (Hosia et al. 2008; Kosobokova and Hopcroft 2010; Ward et al. 2014). Zooplankton communities in the epipelagic layer and especially the surface layer are highly dynamic in relation to environmental forces, such as global warming and changing ice conditions, and more attention should be paid to understand the susceptibility of Antarctic pelagic ecosystems (Flores et al. 2014).
With respect to defining the zooplankton community structure in the epipelagic zone, the neritic and oceanic communities were also clearly divided in this study (Fig. 3), in agreement with numerous studies conducted in Prydz Bay and other regions of the Southern Ocean (Hosie et al. 1997, 2000; Chiba et al. 2001; Hunt et al. 2007). The continental shelf edge has usually been identified as the main boundary between these two communities in Prydz Bay (Hosie and Cochran 1994; Yang et al. 2011a). The geographic distinction of the community structure may be due in part to the different indicator species of each community (such as E. crystallorophias for neritic group 2a, R. gigas, H. ocellatus, and H. austrinus for oceanic group 2b; Table 2), as well as the higher abundances found in the neritic regions. Compared with a 6-year dataset for zooplankton in Prydz Bay collected with a 330-µm mesh NORPAC net (Yang et al. 2011a), the abundance of oceanic community group 2b (50,350 ind 1000 m−3) was similar to that of previous works, while the abundance of neritic community group 2a (88,220 ind 1000 m−3) was lower in this study than in previous studies. A latent-heat-type coastal polynya formed by substantial katabatic wind activity from the Amery Ice Shelf (Williams et al. 2007) often appears in Prydz Bay. The phytoplankton biomass of the neritic regions was usually higher than that in the oceanic regions (Fig. 3b; Arrigo and van Dijken 2003; Yang et al. 2011a). In this study, station 7, located in the polynya region, showed high chl a concentration. The other stations, 1, 5, and 6, in group 2a were located in the shelf region and had lower zooplankton densities.
Diel vertical migration (DVM) is a common behavior of many Southern Ocean copepod species (Atkinson et al. 1992; Lopez and Huntley 1995; Hernandez-Leon et al. 2001; Hosie et al. 2003; Hunt and Hosie 2003, 2005). Thus, the observed vertical differences in species composition and abundance may be influenced by DVM of different zooplankton species. However, based on the series of quadratic regression analyses, no significant relationship between sampling time and abundance was found for most species. These results indicate that, despite the arbitrary differences in sampling time, DVM did not severely affect the observed distributional patterns of community structure between depth layers in this study. In the future, sampling should be adjusted according to hydrography and should be performed at similar times to reduce potential bias introduced by diel vertical migration of zooplankton.
Dominant species
The large copepods C. acutus, C. propinquus, M. gerlachei, and R. gigas are known to account for a large proportion of the total zooplankton abundance in Prydz Bay and other parts of the Southern Ocean (Hopkins and Torres 1988; Schnack-Schiel and Hagen 1994; Hosie et al. 1997; Schnack-Schiel et al. 2008; Yang et al. 2011a). The success of these numerically dominant species is likely due to their different life strategies (Atkinson 1998). C. acutus was considered herbivorous in summer and in diapause at depth in winter, and C. propinquus and M. gerlachei, omnivorous and less reliant on depth diapause during their life cycles, while R. gigas showed an intermediate life strategy (Atkinson 1998).
The vertical patterns in the abundance and population structure of these copepods are mainly in agreement with those of previous research (Schnack-Schiel et al. 1991; Schnack-Schiel and Hagen 1994; Yang et al. 2011b). The young stages of herbivorous C. acutus in this study, mainly concentrated in the upper layers (Online Resource 1a), may have found smaller food at shallower depths (Laakmann et al. 2009). The small proportion of the late copepodite stages (CIV and CV) and females distributed below 500 m (Online Resource 1a) may indicate that winter descent for a percentage of the C. acutus population had already started (Marin 1988; Schnack-Schiel et al. 1991). The dominance of the early stages (CI and CII) of C. propinquus and M. gerlachei in the surface water (Online Resource 1b, c) corroborates the results from other regions of the Southern Ocean and indicates that spawning of these two species may have still been underway (Schnack-Schiel and Hagen 1994). It should be noted that the diel vertical migration of M. gerlachei in the 200–500 m layer is significant (p < 0.05), which can also be observed in the high standard error of the mean in the total abundance of M. gerlachei in Online Resources 1 and 2. Atkinson (1998) reported that egg laying of R. gigas peaked prior to December and occurred later in the season within the Scotia Sea. The lack of offspring of R. gigas in this study (Online Resources 1 and 2) may indicate that main reproduction had finished long before our sampling.
The overwhelming numerical dominance of small copepods, such as C. citer, O. frigida, O. similis, T. antarctica, and O. curvata, in the upper and mesopelagic zone (Online Resource 1) is similar to that previously reported in the Southern Ocean (Errhif et al. 1997; Dubischar et al. 2002; Hunt and Hosie 2006; Schnack-Schiel et al. 2008; Ward et al. 2014). However, these small species are too small to be retained by the 200-µm mesh, and the net used in this study would have lost over 90 % of them (Metz 1995; Smith et al. 1998; Dubischar et al. 2002; Jonasdottir et al. 2013).
Eukrohnia hamata, Alacia spp., P. antarctica, O. frigida, A. minor, B. richardi, and M. princeps are mainly omnivorous or even carnivorous (Hopkins 1985b; Albers et al. 1996; Blachowiak-Samolyk and Angel 2007; Ikeda et al. 2006; Laakmann and Auel 2010). The deeper distribution trend of these species is likely due to their consumption of microzooplankton as important prey items, as indicated by isotope δ 15N values (Ikeda et al. 2006; Laakmann and Auel 2010).
In conclusion, the general results of this research reveal some associations between vertical zooplankton community structure and depth-related environmental factors. Nevertheless, the vertical zooplankton community structure and individual distribution patterns discussed in this study may be regarded only as preliminary findings for Prydz Bay. More frequent sampling according to each specific water mass and better depth resolution are necessary to systematically analyze the zooplankton distribution with respect to different hydrological and biotic factors. Significant interannual variation in summer zooplankton community structure has been reported based on samples collected in the epipelagic zones (0–200 m) of Prydz Bay (Yang et al. 2011a). Additionally, forthcoming expeditions should conduct greater sampling for zooplankton in the mesopelagic and bathypelagic zones. Additionally, food supply and feeding strategy studies of zooplankton from different water strata, especially at greater depths, may shed new light on vertical distribution patterns and the role that zooplankton plays in the biogeochemical cycles and trophodynamic processes of Prydz Bay.
References
Albers C, Kattner G, Hagen W (1996) The composition of wax esters, triacylglycerols and phospholipids in Arctic and Antarctic copepods: evidence of energetic adaptations. Mar Chem 55:347–358
Arrigo KR, Van Dijken GL (2003) Phytoplankton dynamics within 37 Antarctic coastal polynya systems. J Geophys Res 108:3271
Atkinson A (1998) Life cycle strategies of epipelagic copepods in the Southern Ocean. J Mar Syst 15:289–311
Atkinson A, Peck JM (1988) A summer-winter comparison of zooplankton in the oceanic area around South Georgia. Polar Biol 8:463–473
Atkinson A, Sinclair JD (2000) Zonal distribution and seasonal vertical migration of copepod assemblages in the Scotia Sea. Polar Biol 23:46–58
Atkinson A, Ward P, Williams R, Poulet SA (1992) Feeding rates and diel vertical migration of copepods near South Georgia: comparison of shelf and oceanic sites. Mar Biol 114:49–56
Blachowiak-Samolyk K, Angel MV (2007) A year round comparative study on the population structure of pelagic ostracods in Admiralty Bay (Southern Ocean). Hydrobiologia 585:67–77
Brugnano C, Bergamasco A, Granata A, Guglielmo L, Zagami G (2010) Spatial distribution and community structure of copepods in a central Mediterranean key region (Egadi Islands–Sicily Channel). J Mar Syst 81:312–322
Chiba S, Ishimaru T, Hosie GW, Fukuchi M (2001) Spatio-temporal variability of zooplankton community structure off east Antarctica (90 to 160°E). Mar Ecol Prog Ser 216:95–108
Clarke KR, Ainsworth M (1993) A method of linking multivariate community structure to environmental variables. Mar Ecol Prog Ser 92:205–219
Clarke KR, Gorley RN (2006) Primer v6: user manual/tutorial. PRIMER-E, Plymouth
Daase M, Eiane K (2007) Mesozooplankton distribution in northern Svalbard waters in relation to hydrography. Polar Biol 30:969–981
Daly KL, Macaulay MC (1991) Influence of physical and biological mesoscale dynamics on the seasonal distribution and behavior of Euphausia superba in the Antarctic marginal ice zone. Mar Ecol Prog Ser 79:37–66
Dubischar CD, Lopes RM, Bathmann UV (2002) High summer abundances of small pelagic copepods at the Antarctic Polar Front—implications for ecosystem dynamics. Deep-Sea Res II 45:3871–3887
Dufrene M, Legendre P (1997) Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol Monogr 67:345–366
Errhif A, Razouls C, Mayzaud P (1997) Composition and community structure of pelagic copepods in the Indian sector of the Antarctic Ocean during the end of the austral summer. Polar Biol 17:418–430
Field JG, Clarke KG, Warwick RM (1982) A practical strategy for analyzing multispecies distribution patterns. Mar Ecol Prog Ser 8:37–52
Flores H, Hunt BPV, Kruse S, Pakhomov EA, Siegel V, van Franeker JA, Strass V, Van de Putte AP, Meesters EHWG, Bathmann U (2014) Seasonal changes in the vertical distribution and community structure of Antarctic macrozooplankton and micronekton. Deep-Sea Res I 84:127–141
Hernandez-Leon S, Portillo-Hahnefeld AP, Almeida C, Becognee P, Moreno I (2001) Diel feeding behaviour of krill in the Gerlache Strait, Antarctica. Mar Ecol Prog Ser 223:235–242
Hopkins TL (1985a) The zooplankton community of Croker Passage, Antarctic Peninsula. Polar Biol 4:161–170
Hopkins TL (1985b) Food web of an Antarctic midwater ecosystem. Mar Biol 89:197–212
Hopkins TL, Torres JJ (1988) The zooplankton community in the vicinity of the ice edge, western Weddell Sea, March 1986. Polar Biol 9:79–87
Hosia A, Stemmann L, Youngbluth M (2008) Distribution of net-collected planktonic cnidarians along the northern Mid-Atlantic Ridge and their associations with the main water masses. Deep-Sea Res II 55:106–118
Hosie GW, Cochran TG (1994) Mesoscale distribution patterns of macrozooplankton communities in Prydz Bay, Antarctica—January to February 1991. Mar Ecol Prog Ser 106:21–39
Hosie GW, Stolp M (1989) Krill and zooplankton in the western Prydz Bay region, September–November 1985. Proc NIPR Symp Polar Biol 2:34–45
Hosie GW, Cochran TG, Pauly T, Beaumont KL, Wright SW, Kitchener JA (1997) Zooplankton community structure of Prydz Bay, Antarctic, January–February 1993. Proc NIPP Symp Polar Biol 10:90–133
Hosie GW, Schultz MB, Kitchener JA, Cochran TG, Richards K (2000) Macrozooplankton community structure off East Antarctica (80–150°E) during the Austral summer of 1995/1996. Deep-Sea Res II 47:2437–3463
Hosie GW, Fukuchi M, Kawaguchi S (2003) Development of the Southern Ocean Continuous Plankton Recorder Survey. Prog Oceanogr 58:263–283
Hunt BPV, Hosie GW (2003) The Continuous Plankton Recorder in the Southern Ocean: a comparative analysis of zooplankton communities sampled by the CPR and vertical net hauls along 140°E. J Plankton Res 25:1–19
Hunt BPV, Hosie GW (2005) Zonal structure of zooplankton communities in the Southern Ocean South of Australia: results from a 2150 km continuous plankton recorder transect. Deep-Sea Res I 52:1241–1271
Hunt BPV, Hosie GW (2006) The seasonal succession of zooplankton in the Southern Ocean south of Australia, part I: the seasonal ice zone. Deep-Sea Res I 53:1182–1202
Hunt BPV, Pakhomov EA, Trotsenko BG (2007) The macrozooplankton of the Cosmonaut Sea, east Antarctica (30°E–60°E), 1987–1990. Deep-Sea Res I 54:1042–1069
Ikeda T, Yamaguchi A, Matsuishi T (2006) Chemical composition and energy content of deep-sea calanoid copepods in the Western North Pacific Ocean. Deep-Sea Res I 53:1791–1809
Jonasdottir SH, Nielsen TG, Borg CMA, Moller EF, Jakobsen HH, Satapoomin S (2013) Biological oceanography across the Southern Indian Ocean—basin scale trends in the zooplankton community. Deep-Sea Res I 75:16–27
Kosobokova K, Hirche HJ (2000) Zooplankton distribution across the Lomonosov Ridge, Arctic Ocean: species inventory, biomass and vertical structure. Deep-Sea Res I 47:2029–2060
Kosobokova K, Hopcroft RR (2010) Diversity and vertical distribution of mesozooplankton in the Arctic’s Canada Basin. Deep-Sea Res II 57:96–110
Laakmann S, Auel H (2010) Longitudinal and vertical trends in stable isotope signatures (δ13C and δ15N) of omnivorous and carnivorous copepods across the South Atlantic Ocean. Mar Biol 157:463–471
Laakmann S, Stumpp M, Auel H (2009) Vertical distribution and dietary preferences of deep-sea copepods (Euchaetidae and Aetieidae; Calanoida) in the vicinity of the Antarctic Polar Front. Polar Biol 32:679–689
Lopez MDG, Huntley ME (1995) Feeding and diel vertical migration cycles of Metridia gerlachei (Giesbrecht) in coastal waters of the Antarctic Peninsula. Polar Biol 15:21–30
Marin V (1988) Qualitative models of the life cycles of Calanoides acutus, Calanus propinquus, and Rhincalanus gigas. Polar Biol 8:439–446
Marrari M, Daly KL, Timonin A, Semenova T (2011) The zooplankton of Marguerite Bay, western Antarctic Peninsula—part II: vertical distributions and habitat partitioning. Deep-Sea Res II 58:1614–1629
Metz C (1995) Seasonal variation in the distribution and abundance of Oithona and Oncaea species (Copepoda, Crustacea) in the southeastern Weddell Sea, Antarctica. Polar Biol 15:187–194
Michels J, Schnack-Schiel SB, Pasternak A, Mizdalski E, Isla E, Gerdes D (2012) Abundance, population structure and vertical distribution of dominant calanoid copepods on the eastern Weddell Sea shelf during a spring phytoplankton bloom. Polar Biol 35:369–386
Nunes Vaz RA, Lennon GW (1996) Physical oceanography of the Prydz Bay region of Antarctic waters. Deep-Sea Res I 43:603–641
Pond DW, Tarling GA (2011) Phase transitions of wax esters adjust buoyancy in diapausing Calanoides acutus. Limnol Oceanogr 56:1310–1318
Schmidt K, Atkinson A, Steigenberger S, Fielding S, Lindsay MCM, Pond DW, Tarling GA, Klevjer TA, Allen CS, Nicol S, Achterberg EP (2011) Seabed foraging by Antarctic krill: implications for stock assessment, bentho-pelagic coupling, and the vertical transfer of iron. Limnol Oceanogr 56:1411–1428
Schnack-Schiel SB, Hagen W (1994) Life cycle strategies and seasonal variations in distribution and population structure of four dominant calanoid copepod species in the eastern Weddell Sea, Antarctica. J Plankton Res 16:1543–1566
Schnack-Schiel SB, Hagen W, Mizdalski E (1991) A seasonal comparison of Calanoides acutus and Calanus propinquus (Copepoda: Calanoida) in the southeastern Weddell Sea, Antarctica. Mar Ecol Prog Ser 70:17–27
Schnack-Schiel SB, Michels J, Mizdalski E, Schodlok MP, Schroder M (2008) Composition and community structure of zooplankton in the sea ice-covered western Weddell Sea in spring 2004—with emphasis on calanoid copepods. Deep-Sea Res II 55:1040–1055
Schulz J, Peck MA, Barz K, Schmidt JO, Hansen FC, Peters J, Renz J, Dickmann M, Mohrholz V, Dutz J, Hirche HJ (2012) Spatial and temporal habitat partitioning by zooplankton in the Bornholm Basin (central Baltic Sea). Prog Oceanogr 107:3–30
Shi JX, Dong ZQ, Chen HX (2013) Progress of Chinese research in physical oceanography of the Southern Ocean. Adv Polar Sci 24(2):86–97
Smith NR, Dong ZQ, Kerry KR, Wright S (1984) Water masses and circulation in the region of Prydz Bay, Antarctica. Deep-Sea Res 31:1121–1147
Smith S, Roman M, Prusova I, Wishner K, Gowing M, Codispoti LA, Barber R, Marra J, Flagg C (1998) Seasonal response of zooplankton to monsoonal reversals in the Arabian Sea. Deep-Sea Res II 45:2369–2403
Swadling KM, Kawaguchi S, Hosie GW (2010) Antarctic mesozooplankton community structure during BROKE-West (30°E–80°E). Deep-Sea Res II 57:887–904
Terazaki M (1989) Distribution of chaetognaths in the Australian sector of the Southern Ocean during the BIOMASS SIBEX cruise (KH-83-4). Proc NIPR Symp Polar Biol 2:51–60
Ward P, Tarling GA, Thorpe SE (2014) Mesozooplankton in the Southern Ocean: spatial and temporal patterns from Discovery Investigations. Prog Oceanogr 120:305–319
Williams WJ, Carmack EC, Ingram RG (2007) Physical oceanography of polynyas. In: Smith WO Jr, Barber DG (eds) Polynyas: windows to the world. Elsevier, Amsterdam, pp 55–86
Williams GD, Nicol S, Aoki S, Meijers AJS, Bindoff NL, Iijima Y, Marsland SJ, Klocker A (2010) Surface oceanography of BROKE-West, along the Antarctic margin of the south-west Indian Ocean (30–80°E). Deep-Sea Res II 57:738–757
Yang G, Li CL, Sun S (2011a) Inter-annual variation in summer zooplankton community structure in Prydz Bay, Antarctica, from 1999 to 2006. Polar Biol 34:921–932
Yang G, Li CL, Sun S (2011b) Population dynamics of four dominant copepods in Prydz Bay, Antarctica, during austral summer from 1999 to 2006. Chin J Oceanol Limnol 29:1065–1074
Acknowledgments
We would like to thank the crew of R.V. Xuelong for their assistance in the field. We are grateful to the Polar Biology Repository of the Marine Biological Museum of the Chinese Academy of Sciences (MBMCAS) for providing samples. We would like to thank Jiuxin Shi for help with water mass analysis. We thank Yong Jiang for assistance with NMDS analysis. This research was supported by polar project of SOA, China (CHINARE2015-01-05-02, CHINARE2016-01-05-02 and CHINARE2016-04-01-05).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, G., Li, C., Wang, Y. et al. Vertical profiles of zooplankton community structure in Prydz Bay, Antarctica, during the austral summer of 2012/2013. Polar Biol 40, 1101–1114 (2017). https://doi.org/10.1007/s00300-016-2037-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-016-2037-4