Abstract
The ivory gull (Pagophila eburnea) is a high-Arctic species considered endangered in most parts of its breeding range. Ivory gulls must cope with not only the reduction in sea ice cover triggered by climate change but also increasing contaminant loads due to changes in global contaminant pathways and the release of previously stored pollutants from melting snow and ice. This top predator may be affected by biomagnification processes of a variety of compounds with concentrations dramatically increasing from water to higher trophic levels. The objective of this study was therefore to assess the contaminant bioaccumulation of this species in four colonies located on Barentsøya, Svalbard, in link with its trophic behaviour. To that end, contaminants, including organochlorines (OCs), brominated flame retardants (BFRs), and perfluorinated alkyl substances (PFASs), were determined in the blood (plasma and whole blood) of ivory gulls sampled over several years. Carbon- and nitrogen-stable isotopes were also determined in different tissues (feathers, plasma and red blood cells, or whole blood) to infer the trophic level (δ15N) and feeding habitat (δ13C) during both the breeding and moulting periods. The most quantitatively abundant contaminants found in the ivory gull were p,p′-DDE (dichlorodiphenyldichloroethylene), ΣPCB (polychlorobiphenyl), and PFOS (perfluorooctane sulphonate). Several compounds including most of the PFASs, trans-nonachlor, cis-nonachlor, and BDE-28 were correlated with nitrogen values. This study highlighted variability in trophic behaviour among individuals during the breeding and the moulting periods. Overall, similar feeding habitats and strategies were used between breeding sites which was echoed by similar contaminant levels.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Arctic region is currently undergoing dramatic environmental changes, with temperature increasing twice as fast as the global average and sea ice extent reaching a record minimum in 2012 with a reduction of 45 % compared with the 1979–2010 climatology (Lei et al. 2015). It is predicted that climate change will result in profound modifications of the Arctic marine food web and contaminant pathways in this region. The main reasons involved are temperature increase, more low-pressure activity, increased river discharges and run-off from glaciers, and accelerated rate of sea ice melting (ACIA 2004; Hoegh-Guldberg and Bruno 2010). These climate-related variables may therefore influence the transport and thus the bioavailability of contaminants to Arctic biota (Macdonald et al. 2005).
The Arctic acts as a sink for contaminants produced in industrialized parts of the word and transported northward by air, sea, and water masses (de Wit et al. 2004). Amongst contaminants, persistent organic pollutants of anthropogenic origin have especially been found in Arctic biota for decades (Bourne and Bogan 1972; Gabrielsen et al. 1995). Recently, perfluorinated alkyl substances (PFASs) and brominated flame retardants (BFRs) also became widespread in the Arctic environment (Butt et al. 2010; de Wit et al. 2010). In particular, polybrominated diphenyl ethers (PBDE) constitute one of the main classes of brominated compounds used as flame-retardant additives that can be found in ecosystems and taxa (Renner 2000; Covaci et al. 2011). Due to their propensity for persistence and bioaccumulation in wildlife, PFASs and BFRs have become of global environmental concern in addition to legacy organochlorine (OC) compounds.
Among wild populations, seabirds are particularly vulnerable to contaminants because they are relatively long-lived species that bioaccumulate contaminants throughout their life. Moreover, most seabirds are predators that feed at the top of the food web (Hobson et al. 2002). They are thus affected by biomagnification processes of a variety of compounds (e.g. PFAS, polychlorinated biphenyl ‘PCB’) with concentrations dramatically increasing from water to higher trophic levels. Strong sub-lethal and/or lethal concentrations and adverse effects were observed at the individual (Dawson 2000) and population (Goutte et al. 2014a, b) levels. These adverse effects include endocrine disruption, impairment of the immune system, oxidative stress, or bone metabolism impairments (Verreault et al. 2004; Bourgeon et al. 2012; Pellerin Plourde et al. 2013).
In aquatic systems, food constitutes the major exposure route of seabirds to contaminants. One of the main goals in ecotoxicology is therefore to investigate more precisely the trophic ecology of species to better understand exposure pathways and explain contaminant concentrations in animal populations (Roscales et al. 2010; Leat et al. 2013). Stable isotopes of carbon and nitrogen are useful tools to examine trophic relationships and/or origins of prey to elucidate broad‐scale, inter‐ and intraspecific dietary patterns, and so to determine whether differences in foraging strategies explain variations in contaminant uptake (Jardine et al. 2006). The basic isotopic concept is that an animal’s chemical composition is directly influenced by what it consumes. Consumers are enriched in 15N relative to their food and consequently, stable nitrogen isotope measurements (δ15N) serve as indicators of a consumer trophic position. By contrast, stable carbon ratios (δ13C) vary little along the food chain, and in the marine environment, δ13C values are mainly used to indicate the foraging habitats of predators (e.g. benthic versus pelagic habitats, inshore versus offshore; Hobson 1995).
The ivory gull (Pagophila eburnea) is a high-Arctic species that frequents ice-filled waters throughout the year. The main wintering grounds identified for northeast Atlantic populations (north Greenland, Svalbard and Franz Josef Land) of this long-distant migrant species are in south-east Greenland, along the Labrador sea ice edge and in the Bering Strait region (Gilg et al. 2010; Spencer et al. 2014). Adults have only a single moult per cycle, a strategy unique among gulls (Howell 2001). Their prebasic moult starts in March and April and is mostly completed before the pre-laying to hatching period (mid-June to early August). The primary moult is then suspended at this time, and the moult of outermost primaries is concluded in late August or September. Consequently, moulting and breeding greatly overlap in the ivory gull, presumably an adaptation to the constraints of its high-latitude winter range and the brief Arctic summer (Howell 2001). The ivory gull is considered endangered in most parts of its breeding range, especially in Canada, where studies documented an 80 % decline in numbers since the 1980′s (Gilchrist and Mallory 2005). Nevertheless, it is one of the world’s most poorly known seabird species even though its high position in the marine food web makes it particularly sensitive to contaminant exposure (Karnovsky et al. 2009). Previous studies demonstrated that this free-ranging Arctic species is exposed to high contaminant concentrations along its distribution range in the Canadian, Norwegian, and Russian Arctic (Braune et al. 2007; Miljeteig et al. 2009; Lucia et al. 2015). It is thought that some ivory gulls specialize on scavenging remains of polar bear kills, whereas others feed mainly on polar cod (Boreogadus saida) and crustaceans associated with the ice edge (Divoky 1976; Mehlum 1990; Mehlum and Gabrielsen 1993; Karnovsky et al. 2009). Overall, climate-related perturbations (e.g. reduction in sea ice cover, modification of the Arctic marine food web, modification of pollutant bioavailability) are expected to profoundly impact the trophic behaviour of the ivory gull and potentially increase its contaminant exposure.
Consequently, the aim of this study was to better understand the contaminant exposure and foraging strategies of the ivory gull considering the lack of data for this vulnerable seabird. Our objective was to describe contaminant exposure and trophic behaviour of four ivory gull colonies located on Barentsøya, Svalbard, to finally investigate their relationship. Contaminants, encompassing a number of OCs, BFRs, and PFASs, were determined in the blood (plasma and whole blood) of ivory gulls sampled over 4 years. Carbon- and nitrogen-stable isotopes were also determined in different tissues, including feathers and blood (plasma and red blood cells, or whole blood depending on the year) to investigate the foraging ecology during both the breeding and the moulting periods. The stable isotope method is based on time‐integrated assimilated food. Hence, the isotopic value of blood and feathers is representative of the isotopic niche of seabirds during the weeks preceding sampling and the moulting period, respectively.
Materials and methods
Sample collection and preparation
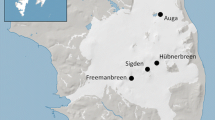
Ivory gulls were sampled for blood and ventral feathers between 2011 and 2014 in four breeding colonies (Auga, Freemanbreen, Hübnerbreen, Sigden, Fig. 1) on Barentsøya, Svalbard, and along the ice edge in Spring (April). When possible, both tissues were sampled on each bird (53 % of the samples). Moreover, different individuals were sampled among years. Birds were caught by the use of a single-catch closing net trap (spring trap) baited with a small piece of seal blubber. When it was possible, age and sex were determined, as well as morphometric measurements (e.g. mass, bill length, bill height, head + bill, wing length). Overall, whole blood was sampled for isotope analyses in 2011 (n = 6), 2012 (n = 5) and 2013 (n = 9), whereas plasma and red blood cells (erythrocytes) were obtained after centrifugation in the field (4000 rpm, 10 min) in 2013 (n = 38) and 2014 (n = 38). Ventral feathers were sampled in 2011 (n = 19), 2012 (n = 40), 2013 (n = 46), and 2014 (n = 34). Over the years, plasma and red blood cells were sampled in four colonies: Auga (n = 5), Freemanbreen (n = 27), Hübnerbreen (n = 19), and Sigden (n = 25), whereas whole blood was sampled in three colonies: Auga (n = 1), Freemanbreen (n = 8), and Hübnerbreen (n = 5), and the ice edge (n = 6), and feathers, in four colonies, Auga (n = 5), Freemanbreen (n = 71), Hübnerbreen (n = 30), Sigden (n = 25), and the ice edge (n = 8; Online Resource 1).
Of those samples, blood volumes were sufficient to analyse contaminants such as PFASs in plasma samples in 2013 (n = 38) and 2014 (n = 21), and whole blood in 2011 (n = 4), 2012 (n = 5) and 2013 (n = 1). Organochlorines and BFRs were analysed on fewer samples due to the higher volume required for the extraction protocol. Therefore, plasma samples were available in 2013 (n = 30) and 2014 (n = 9), and whole blood in 2011 (n = 4) and 2012 (n = 2). Over the years, PFASs were analysed in plasma samples from Auga (n = 5), Freemanbreen (n = 26), Hübnerbreen (n = 18), and Sigden (n = 10), and in whole blood samples from Auga (n = 1), Freemanbreen (n = 5), and on the ice edge (n = 4). Organochlorines and BFRs were analysed in plasma samples from Auga (n = 2), Freemanbreen (n = 17), Hübnerbreen (n = 13), and Sigden (n = 7), and in whole blood samples from Freemanbreen (n = 2) and along the ice edge (n = 4; Online Resource 2). This difference in sampling method (whole blood versus plasma) is explained by the late capacity to centrifuge blood on a remote fieldwork on Barentsøya. Both tissues are useful for studying different contaminants. The use of whole blood has been recommended when analysing OCs and BFRs to avoid loss of contaminants present in the cellular fraction (Volz et al. 2001; Leslie et al. 2007). Serum or plasma can be used for analysis of many contaminants but it is notable that concentrations of some poly- and perfluoroalkyl substances (PFASs) in whole blood are approximately half those in serum or plasma because of the volume displacement of cellular components, which do not appear to function as a sorbent for these substances (Ehresman et al. 2007). All the blood samples, including plasmas and red blood cells, were kept frozen at −20 °C until further analyses. Ventral feathers were washed to remove oil and dirt in a chloroform–methanol solution (2:1) in an ultrasonic bath for 2 min. Afterward, they were rinsed in two consecutive pure methanol baths for a few seconds and dried at 40 °C for 48 h.
Nitrogen- and carbon-stable isotope analysis
Depending on birds and years, stable isotopes were determined in ventral feathers and either on the whole blood or on the plasma and red blood cells (see above). Analyses were performed at the UMR 7266 CNRS-Université de La Rochelle in France. Cleaned feathers of ivory gulls were chopped using surgical stainless-steel scissors and accurately weighed (±0.001 mg) to a range between 0.1 and 0.4 mg. Blood samples were freeze-dried, grounded, and then weighed with the same accuracy and in the same range of masses. All samples were placed in tin capsules for carbon- and nitrogen-stable isotope analysis and analysed using an elemental analyser (Flash EA 1112 fitted with a “Zero Blank” option, Thermo Scientific, Milan, Italy) coupled to an isotope ratio mass spectrometer (Delta V Advantage, Conflo IV interface, Smart EA option, Thermo Scientific, Bremen, Germany). The results are reported in δ unit notation (expressed in per mil relative to standards: Vienna Pee Dee Belemnite for δ13C and N2 in air for δ15N). Within-run replicates of internal standards (acetanilide) were run, and values were on average −27 ± 0.03 ‰ and 1 ± 0.05 ‰ for δ13C and δ15N, respectively.
Determination of OCs and BFRs
OCs and BFRs were analysed from the plasma of 32 birds in 2013 and nine birds in 2014. The whole blood of four birds in 2011 and two birds in 2012 was also analysed. Analyses were performed at the Norwegian Institute for Air Research (NILU) in Tromsø, and included organochlorine pesticides (o,p′ DDT, p,p′ DDT, o,p′ DDE, p,p′ DDE, o,p′ DDD, p,p′ DDD, HCB, trans-, cis-chlordane, oxychlordane, trans-, cis-nonachlor and mirex) and 12 PCB congeners (PCB-28, -52, -99, -101, -105, -118, -138, -153, -180, -183, -187 and -194). BFRs encompassed eight PBDE congeners (BDE-28, -47, -99, -100, -138, -153, -154 and -183), BATE (2-bromoallyl-2,4,6-tribromophenyl ether), TBPA (tetrabromophthalic anhydride), PBT (pentabromotoluene), PBEB (pentabromoethylbenzene), DPTE (2,3-dibromopropyl- 2,4,6- tribromophenyl ether), HBB (hexabromobenzene), BTBPE (1,2-Bis (2,4,6-tribromophenoxy) ethane), BEHTBP (Bis (2-ethylhexyl) tetrabromophthalate), EHTeBB (2-ethylhexyl- 2,3,4,5-tetrabromobenzoate). Internal standard solutions (13C-labelled compounds from Cambridge Isotope Laboratories: Woburn, MA, USA) were added to a blood sample of 0.7–1.5 ml. The sample was extracted twice with 6 ml of n-hexane, after denaturation with ethanol and a saturated solution of ammonium sulphate in water. Matrix removal on florisil columns, separation on an Agilent Technology 7890 GC (gas chromatography) and detection on an Agilent Technology 5975C MSD (mass spectrometry) were performed as described by Herzke et al. (2009). The limit of detection (LoD) was threefold the signal-to-noise ratio for the analysed BFRs and OCs and ranged between 0.6 and 14,596 pg g−1 wet weight (wet wt) for HCB and TBPA, respectively. For validation of the results, blanks (clean and empty glass tubes treated like a sample) were run for every 10 samples, while standard reference material (1589a human serum from NIST) was also run for every 10 samples. Blank samples were below the detection limit for most reported analytes, and results from samples were deemed acceptable.
Determination of PFASs
Twenty PFASs were analysed at NILU, encompassing 6:2 FTS, 8:2 FTS, PFBS (perfluorobutane sulphonate), PFHxS (perfluorohexane sulphonate), PFHpS (perfluoroheptane sulphonate), PFOS (perfluorooctane sulphonate), PFDcS (perfluorodecane sulphonate), PFOSA (perfluorooctane sulphonamide), PFBA (perfluorobutanoate), PFPeA (perfluoropentanoate), PFHxA (perfluorohexanoate), PFHpA (perfluoroheptanoate), PFOA (perfluorooctanoate), PFNA (perfluorononanoate), PFDA (perfluorodecanoate), PFUnDA (perfluoroundecanoate), PFDoDA (perfluorododecanoate), PFTriA (perfluorotridecanoate), PFTeA (perfluorotetradecanoate), and PFHxDA (perfluorohexadecanoate).
Prior to analysis, samples were extracted and prepared as described previously (Powley et al. 2005). A sample volume of 0.2 mL of plasma or whole blood was spiked with 20 µL of the internal standard, a 13C perfluorinated compound mix, and subsequently extracted with 1 mL methanol in three consecutive 10 min ultrasonic treatments. After centrifugation, 0.8 mL of the supernatant solution was added to 25 mg ENVI-Carb and 50 μL glacial acetic acid and vortexed thoroughly. After additional centrifugation at 10,000 rpm for 10 min, 0.5 mL of the solution was transferred to a vial and 2040 pg of 3,7-br-PFDA as a recovery standard was added. Prior to measurement, 50 µL of a 2 mM aqueous ammonium acetate solution was added to the same volume of samples.
All standards were purchased as crystalline substances from Wellington with purities greater than 95 %. Instrumental measurement was carried out according to Hanssen et al. (2013).
Recoveries of the mass-labelled internal standards were 89 ± 12 % for 13C PFHxS, 87 ± 14 % for 13C PFOS, 96 ± 11 % for 13C PFOA, 113 ± 31 % for 13C PFNA, 104 ± 14 % for 13C PFDA, 108 ± 14 % for 13C PFUnDA, 126 ± 22 % for 13C PFDoDA, and 136 ± 29 % for 13C PFTeA.
Data treatment and statistical analyses
Several compounds that were detected or analysed in less than 60 % of the samples were excluded from statistical analyses including o,p′ DDT, p,p′ DDT, o,p′ DDE, o,p′ DDD, p,p′ DDD, cis-chlordane, PCB-152, BDE-138, BATE, TBPA, PBT, PBEB, DPTE, BTBPE, BEHTBP, EHTeBB, 6:2 FTS, 8:2 FTS, PFBS, PFHpS, PFDcS, PFOSA, PFBA, PFPeA, PFHxA, PFHpA, PFOA, PFHxDA. Values below the quantification limit were taken into account in calculation of the means by assigning those values one-half of the detection limit for the given contaminant (e.g. a value <0.02 was reported as 0.01 ng g−1 wet wt). Contaminant concentrations are expressed in ng g−1 wet wt.
As normality and homogeneity of variance were not achieved despite log10(x + 1) transformation (Cochran C test), nonparametric analysis of variance (Kruskal–Wallis and Mann–Whitney U test) was applied to assess differences in contaminant concentrations and stable isotope values for each parameter (colony, year, sex). Moreover, the Spearman test was applied to study correlations between parameters (contaminant concentrations and stable isotope values).
Results
Isotopic values depending on the colony, year, and sex of birds
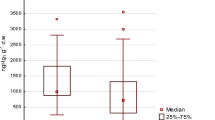
Trophic positions and foraging habitats of the ivory gull, assessed respectively through the determination of δ15N and δ13C, were investigated in feathers, whole blood, plasma, and red blood cells (Fig. 2). Figure 2 shows the mean isotopic values of the tissues studied. Individual variability was observed for each tissue. Stable carbon isotope values of feathers were especially variable, ranging between −20.8 and −17.5 ‰. Stable nitrogen isotope values in the plasma also ranged widely, varying between 13.0 and 16.3 ‰.
Stable isotope values were compared between colonies to identify any diverging trends in trophic behaviour between birds breeding on Barentsøya. Birds from the Sigden colony showed significant differences in their stable isotope values compared to birds coming from other colonies. Figure 3 presents red blood cell mean values for all colonies, all years consolidated. Birds sampled at Sigden displayed significantly lower δ13C values than birds from Freemanbreen and Hübnerbreen colonies, as well as lower δ15N values than individuals from Hübnerbreen (δ13C: Sigden vs Freemanbreen p = 0.005, Sigden vs Hübnerbreen p < 0.0001; δ15N: Sigden vs Hübnerbreen p = 0.043). However, birds coming from Sigden were only sampled in 2014. To exclude year as confounding factor, stable isotope values were compared between colonies sampled in 2014 only. However, only Freemanbreen and Sigden could be compared because of low number of birds sampled in other colonies. The same trend was consequently observed that year. Sigden birds displayed lower stable isotope values than birds from Freemanbreen (δ13C: p = 0.024; δ15N: p = 0.031). Furthermore, plasma δ13C values were also significantly different between both colonies in 2014 (Sigden: −21.3 ± 0.1 ‰, Freemanbreen: −21.7 ± 0.1 ‰; p = 0.038). Similarly, sex could be considered as a confounding factor that may be responsible for differences observed between colonies. Unfortunately, it was not possible to sex all individuals used to study differences between colonies, especially in 2014. All the birds from Sigden were sampled that year, and only 7 individuals were sexed (F = 4, M = 3). However, when available, the number of males and females was balanced between colonies. For example, in 2013, numbers between males and females, if not equal, were in the same range (Freemanbreen: M = 12, F = 5; Auga: M = 1, F = 2; Hubnerbreen: M = 10, F = 6). Consequently, the sex of birds was most likely not responsible for differences observed between colonies. For whole blood, differences appeared between birds sampled on the ice edge and those from Freemanbreen. Ivory gulls from the ice edge had significantly lower δ15N values (14.2 ± 0.3 ‰) than those from Freemanbreen (14.9 ± 0.2 ‰), Kruskal–Wallis test, p = 0.045). However, birds from the ice edge and Freemanbreen were sampled on different years, 2011 and 2012–2013, respectively. It is therefore difficult to know whether this discrepancy is rather due to the year instead of the sampling location. In addition, feather values were similar between colonies for δ15N and δ13C.
The difference in stable isotope values between sampling years was investigated in feathers, plasma and red blood cells. The influence of year was not studied in whole blood because of the few available samples for this tissue. Overall, no differences were observed for feathers and plasma. Conversely, δ13C values in red blood cells were significantly higher in 2013 (−20.6 ± 0.1 ‰) compared to 2014 (−21.0 ± 0.1 ‰; p < 0.0001, Mann–Whitney U test).
Finally, the influence of sex on stable isotope values was investigated. The only significant difference was between δ13C values of male (−20.6 ± 0.04 ‰, n = 29) and females (−20.8 ± 0.07 ‰, n = 16) in red blood cells (p = 0.044, Mann–Whitney U test).
Influence of the time, location, and sex on contaminant concentrations
Mean concentrations of PFASs, OCs and BFRs in plasma and whole blood of ivory gulls are presented in Tables 1 and 2 for each sampling year. Firstly, the influence of time, therefore year, was investigated independently of colonies for each tissue. For PFASs, no significant differences were observed between sampling years for both tissues (Table 1). Plasma concentrations of BDE-100 and BDE-154 were, however, significantly higher in 2013 compared to 2014 (BDE-100: p = 0.002, BDE-154: p = 0.044), whereas whole blood concentrations were similar between years.
The influence of sampling location (colony) was consequently investigated, independently of the year and the sex of individuals. In plasma, discrepancies appeared for PFASs. Gulls captured at Auga displayed significantly lower PFOS, PFUnDA and PFDoDA concentrations than birds from Freemanbreen (PFOS: 14.9 ± 1.2 and 35.8 ± 4.0, PFUnDA: 7.2 ± 0.9 and 14.4 ± 1.3, PFDoDA: 1.33 ± 0.16 and 2.66 ± 0.24 ng g−1 wet wt for Auga and Freemanbreen, respectively; p < 0.05, Kruskal–Wallis test; Online Resource 3). To exclude sex as confounding factor, PFAS concentrations were compared on females only. Similar differences occurred between Auga and Freemanbreen for PFOS, PFUnDA, and PFDoDA (p < 0.05). Unfortunately, not enough samples were available in Auga to test for year as confounding factor. Organochlorine and BFR concentrations were not different between colonies for all the tissues studied.
Because of the potential maternal transfer of contaminants to eggs, sex-dependent differences in contaminant concentrations were also examined. The influence of this parameter appeared important for PFAS accumulation. Plasmatic concentrations of males (n = 28) were higher for all PFASs than females (n = 15), with the exception of PFNA and PFHxS (p < 0.05, Mann–Whitney U test). In contrast, males (n = 22) displayed lower HCB concentrations in the plasma than females (n = 11; 7.19 ± 0.75 vs 9.62 ± 0.39 ng g−1 wet wt; p = 0.012, Mann–Whitney U test).
The relationship between contaminants and body mass of birds was investigated. Plasma concentrations of PFASs were positively correlated with the body mass of birds (r ranged between 0.27 for PFHxS and 0.51 for PFDA). Simultaneously, HCB, PCB-118, and BDE-99 plasma concentrations were negatively correlated with the body mass of ivory gulls (r = −0.42, −0.32 and −0.48, respectively; Spearman test, p < 0.05).
Relationship between trophic position, feeding habitat and contaminant bioaccumulation
All colonies and years combined, PFAS concentrations were significantly correlated with δ15N values in the plasma (r comprised between 0.37 for PFOS and 0.52 for PFTeA; p < 0.05, Spearman test). Only PFHxS and PFNA concentrations were not significantly correlated with stable nitrogen isotope values. In contrast, δ13C values were not correlated with PFAS concentrations. The same trend was observed in whole blood. No correlations were observed between PFAS concentrations and δ13C values, whereas δ15N values were significantly correlated with most of the PFASs, with the exception of PFHxS and PFNA (r comprised between 0.66 for PFUnDA and 0.80 for PFTriA; p < 0.05, Spearman test).
For OCs and BFRs, δ15N values in plasma samples were positively correlated with trans-nonachlor (r = 0.41), cis-nonachlor (r = 0.32), and BDE-28 (r = 0.78), whereas δ13C values were only correlated with trans-nonachlor (r = 0.35; Fig. 4).
Discussion
We investigated the contaminant bioaccumulation in one of the most poorly researched seabirds in the Northern hemisphere, the ivory gull, and the relationship with its trophic habits. The trophic diet of this species has been previously described in Svalbard (Mehlum and Gabrielsen 1993) and other Arctic areas such as the Barents Sea (Mehlum 1990), the Chukchi Sea (Divoky 1976), and in northern Baffin Bay (Karnovsky et al. 2009). However, the large sample size of two noninvasive measures of long-term diet (e.g. blood and feathers) in our study provides an excellent opportunity to examine the relationship between contaminants and trophic ecology of ivory gulls, over several years and sampling locations on Barentsøya, Svalbard.
Contaminant bioaccumulation in the ivory gull
Results in this study are similar to previously reported results in ivory gull eggs where high concentrations were reached for OCs, especially p,p′-DDE and PCBs (Braune et al. 2007; Miljeteig et al. 2009; Lucia et al. 2015). Previous experimental studies in birds have demonstrated that exposure to various OCs, such as PCB, DDE and mirex, affect parental behaviour. This has often been attributed to endocrine disruption or neurological disorders (McArthur et al. 1983; Peakall 1985; Keith and Mitchell 1993). Bustnes et al. (2001) demonstrated that plasma PCB concentrations in glaucous gulls (Larus hyperboreus) nesting on Bjørnøya, Svalbard, were positively correlated with the proportion of time birds were absent from the nest with concentrations ranging from 52 to 1079 ng g−1 wet wt. This study included eight PCB congeners. With 11 congeners in the present study, concentrations ranged between 134 and 1597 ng g−1 wet wt, which could imply potential reproductive disruptions for the ivory gull.
Perfluorinated organic compounds have been reported in tissues of Arctic biota (Butt et al. 2010). The most predominant PFAS in this study was PFOS. This result was similar to previous reports for Canadian (Martin et al. 2004; Tomy et al. 2004) and Norwegian Arctic avian wildlife (Verreault et al. 2005; Lucia et al. 2015). Levels observed in plasma samples of ivory gulls were below or of the same order of magnitude than those reported for the glaucous gull, an apex scavenger-predator seabird breeding in the Norwegian Arctic (Verreault et al. 2005). Perfluorooctane sulphonate levels for the glaucous gull ranged between 48 and 349 ng g−1 wet wt, whereas concentrations in this study ranged between 4 and 90 ng g−1 wet wt. Conversely, PFNA concentrations were of the same order of magnitude for both species, with glaucous gull concentrations ranging between below the detection limit (<2.33) and 6.33 ng g−1 wet wt and ivory gull concentrations ranging between 0.15 and 5.94 ng g−1 wet wt. Potential detrimental effects of PFOS include a competitive interaction with thyroid hormone thyroxin (T4) for binding sites on albumin and changes in hormone homeostasis (Jones et al. 2003; Thibodeaux et al. 2003). However, predominant, it seems unlikely that PFOS alone would contribute to measurable adverse effects in the ivory gull. Nevertheless, the cocktail of accumulated PFASs may trigger potential additive and synergetic impacts detrimental to this species. Simultaneously in this study, PFAS concentrations in the plasma were positively correlated with the body mass of birds. This observation may be partially linked to the structural similarity between PFASs and fatty acids (Vanden Heuvel et al. 2006). However, since blood concentrations only show a snapshot of the recent exposure of the investigated organisms, the causality might be more complex. This relationship between PFAS concentrations and the body mass could have several origins. For example, individuals with higher body mass may be more efficient in catching certain prey with a higher PFAS load and/or more food in general, or alternatively, be characterized by a slower metabolism and therefore a slower distribution of PFAS to the other compartments of the body. At the same time, sex-related differences appeared between males and females, the latter displaying lower concentrations for most of the PFASs. A previous study on mallards (Anas platyrhynchos) exposed chronically to a PFOS contaminated feed demonstrated sex-dependent differences in serum PFOS concentrations where males had approximately 5 to 6 times the concentrations observed in females (Newsted et al. 2007). This study clearly demonstrated the maternal transfer of this compound to eggs. Moreover, similar to PFOS, long-chain PFAS (PFTriA, PFTeA, and PFPeDA) also showed a tendency towards high accumulation in eggs of common guillemots (Uria aalge) from the Baltic Sea (Holmström and Berger 2008). Lower PFAS concentrations in ivory gull females consequently draw attention to the potential importance of PFAS maternal transfer to the eggs at the time of their formation as a mechanism of elimination.
Polybrominated diphenyl ethers concentrations were relatively low in the ivory gull ranging between 0.67 and 7.56 ng g−1 wet wt in the plasma. As a comparison, sum PBDE concentrations of the glaucous gull in the Norwegian Arctic ranged from 2.49 to 54.5 ng g−1 wet wt in the plasma (Verreault et al. 2007). However, this previous study included 38 congeners which could imply that similar levels may be reached in the ivory gull if more congeners were taken into account. Apart from BDEs, HBB was the only other BFR that was detected in the present study. Historically, HBB was widely used in Japan as an additive to paper, plastic, and electronic goods. However, the use of this substance has been reduced by Japan (350 tons in 2001; Watanabe and Sakai 2003), and this compound is not currently produced in Europe. The low levels observed could suggest a very low background in the food web of the ivory gull and the Arctic region in general. Overall, it is difficult to draw conclusions on the potential effects of contaminants in this study. Some compounds appeared to be linked to lower body masses of the birds (e.g. HCB, PCB-118, and BDE-99), but this result may also be linked to the bird’s reproductive status at the time of the sampling.
The present study covered 4 years of observations which provided very valuable information for a number of chemical compounds. However, there were few differences between sampling years and no clear patterns were observed. For example, PFAS levels did not differ significantly between 2011 and 2013, even if this lapse of time is relatively small. Contaminant concentrations seem rather to follow episodic events of contamination in Svalbard. Nevertheless, the sampling period still remains short in this study. It appears therefore fundamental to sustain the sampling effort over several years and tissues to properly evaluate the possible contamination patterns resulting from global change in the Arctic.
Relationship between trophic position, feeding habitat and contaminant bioaccumulation
In this study, the trophic level and feeding habitats of birds, measured through δ15N and δ 13C values, were lower compared to a study using feather data from museum specimens from the Canadian Arctic and western Greenland over a 130 year period (Bond et al. 2015). Isotope values in the present study were variable, both in blood and feathers. Assuming that birds from different colonies or origins forage in food webs with similar isotopic baseline, this result indicates individual differences in trophic behaviour during the breeding (blood) and moulting (feathers) periods, respectively. The importance of polar cod to the diet of ivory gulls has been previously documented (Divoky 1976; Mehlum 1990; Mehlum and Gabrielsen 1993; Karnovsky et al. 2009). Nevertheless, individuals also feed on other fish species such as Atlantic cod (Gadus morhua) and coalfish (Pollachius virens), and amphipods (Mehlum and Gabrielsen 1993). Some ivory gulls are also known to specialize on scavenging remains of predator kills (e.g. polar bears). Ivory gulls may therefore feed on preys situated at different trophic levels (Wassmann et al. 2006). This overall variability in stable isotope values among birds can also be explained by differences in foraging strategies between breeding and nonbreeding birds. Birds were often captured at the edge of the colonies to avoid any disturbances at the nest. Consequently, some sampled individuals could have been failed breeders which may result in differences in e.g. diet, fasting status, body mass, and overall biology and ecology. Goutte et al. (2014c) demonstrated differences in foraging strategies between breeders and nonbreeding birds in an Arctic population of black-legged kittiwakes (Rissa tridactyla). Nonbreeders were potentially less constrained by energetic costs and time, and therefore displayed a higher foraging range than breeders. The same tendency was demonstrated for earlier breeders which tended to forage at farther distances than kittiwakes breeding later in the season. The same patterns could apply to the ivory gull.
This overall variability in stable isotope values among birds is accompanied by an overlap in stable isotope values among colonies. Ivory gulls from different breeding sites demonstrated similar feeding habitats and strategies. Only birds from one colony, Sigden, displayed lower δ15N and δ 13C values in their red blood cells compared to the rest of the colonies. This result suggests a diet in this colony originating from a more offshore and lower trophic level in the month prior to the blood sampling (Hobson et al. 1994). Diet-switching experiments on birds have indeed demonstrated that the half-life of stable isotopes in red blood cells was approximately 1 month (half-life = 29.8 days), versus 2–3 days in the plasma fraction (half-life = 2.9 days; Hobson and Clark 1993). Nevertheless, this observation was not echoed in plasma samples where isotopic values were not significantly different between colonies. It appears rather unlikely that birds from Sigden had an entirely different trophic behaviour compared to birds from other colonies. This divergence in isotopic values for this colony could reflect a possible shift in trophic behaviour during a special event (e.g. climatic event triggering poor sea ice conditions) or a specific food source only exploited by these birds at a certain period in time. The same overlap between colonies was observed for feathers thus demonstrating similar trophic behaviour during the moulting period.
The homogeneity in feeding behaviours between different colonies is reflected by the absence of differences in contaminant concentrations. Only the Auga colony displayed lower plasma PFAS concentrations (e.g. PFOS, PFUnDA, and PFDoDA) than birds from Freemanbreen. This discrepancy could be related to several factors including the fewer number of individuals sampled in Auga compared to Freemanbreen (n = 5 and 27, respectively). It is, however, difficult to draw exact conclusions about the origin of this difference since isotopic values in the plasma were not different between those colonies. This discrepancy may be the result of several factors including contaminant exposure of birds on their wintering and breeding grounds, the local diet, and the remobilization of contaminants from internal tissues (e.g. liver) in association with the reproductive status of individuals. Despite being amphiphilic, PFASs demonstrate bioaccumulation tendencies due to their ability to bind to proteins. Perfluorinated alkyl substances accumulated in the liver can for example be transferred to the forming egg as a protein-PFOS complex (Holmström and Berger 2008). During fasting, PFAS associated with proteins may also easily be remobilized in the blood system of birds. As specified above, birds sampled could have been failed breeders, influencing therefore their overall biology, ecology, and contaminant exposure. The rate of failed breeders may have been especially higher at Auga where predation on eggs is often high (personal observation).
Stable isotope analysis has become a powerful tool to study dietary exposure and biomagnification of contaminants in free-ranging animal populations (Jardine et al. 2006). In this study, nitrogen values in the blood of birds were positively correlated to most of the PFASs. This result is in line with previous findings about the biomagnification potential of these compounds in the Barents Sea food web (Haukås et al. 2007). Organochlorines and BFRs are also known to biomagnify along the food chain making species at high trophic levels such as the ivory gull, more vulnerable to contaminant exposure through their diet (Hop et al. 2002; Borgå et al. 2004; Braune et al. 2007). In this study, trans-nonachlor, cis-nonachlor, and BDE-28 were linked to nitrogen values which denote the increase of those contaminants along the Arctic food web. Alternatively, trans-nonachlor was the only compound clearly correlated with carbon values in birds, demonstrating therefore that ivory gulls with the most enriched δ13C values probably feeding on more inshore prey have a higher tendency to bioaccumulate this persistent pollutant.
Conclusion
The most quantitatively abundant contaminants found in the ivory gull were p,p′-DDE, ΣPCB and PFOS. Overall, OC, BFR and PFAS concentrations did not suggest direct lethal risk from these compounds. Nevertheless, their potential synergistic or additive effects warrant monitoring. This study also highlighted a possible individual variability in trophic behaviour during the breeding and moulting periods, respectively. Overall, ivory gulls from different breeding sites used similar feeding habitats and strategies which were echoed by similar contaminant levels. Results for several compounds, including most of the PFASs, trans-nonachlor, cis-nonachlor, and BDE-28, are in line with previous reports about their biomagnification potential. Finally, several factors (time, colony, sex) can influence the contaminant bioaccumulation and the trophic behaviour of ivory gulls. However, this study does not allow for a full quantification of their impact due to the lack of data available on this remote species. Further research is therefore needed to better understand the contaminant pressure and foraging strategies of the ivory gull through, for example, investigation of movement patterns.
References
ACIA (2004) Impacts of a warming arctic: arctic climate impact assessment. Cambridge University Press, Cambridge
Bond AL, Hobson KA, Branfireun BA (2015) Rapidly increasing methyl mercury in endangered Ivory Gull (Pagophila eburnea) feathers over a 130 year record. Proc R Soc B 282:20150032
Borgå K, Fisk AT, Hoekstra PF, Muir DCG (2004) Biological and chemical factors of importance in the bioaccumulation and trophic transfer of persistent organochlorine contaminants in Arctic marine food webs. Environ Toxicol Chem 23:2367–2385
Bourgeon S, Leat EHK, Magnusdóttir E, Fisk AT, Furness RW, Strøm H, Hanssen SA, Petersen Æ, Olafsdòttir K, Borgå K, Gabrielsen GW, Bustnes JO (2012) Individual variation in biomarkers of health: influence of persistent organic pollutants in Great Skuas (Stercorarius skua) breeding at different geographical locations. Environ Res 118:31–39
Bourne WRP, Bogan JA (1972) Polychlorinated biphenyls in North Atlantic seabirds. Mar Pollut Bull 3:171–175
Braune BM, Mallory ML, Gilchrist HG, Letcher RJ, Drouillard KG (2007) Levels and trends of organochlorines and brominated flame retardants in Ivory Gull eggs from the Canadian Arctic, 1976 to 2004. Sci Total Environ 378:403–417
Bustnes JO, Bakken V, Erikstad KE, Mehlum F, Skaare JU (2001) Patterns of incubation and nest-site attentiveness in relation to organochlorine (PCB) contamination in Glaucous Gulls. J Appl Ecol 38:791–801
Butt CM, Berger U, Bossi R, Tomy GT (2010) Levels and trends of poly- and perfluorinated compounds in the arctic environment. Sci Total Environ 408:2936–2965
Covaci A, Harrad S, Abdallah MAE, Ali N, Law RJ, Herzke D, de Wit CA (2011) Novel brominated flame retardants: a review of their analysis, environmental fate and behavior. Environ Int 37:532–556
Dawson A (2000) Mechanisms of endocrine disruption with particular reference to occurrence in avian wildlife: a review. Ecotoxicology 9:59–69
de Wit CA, Fisk A, Hobbs K, Muir D, Gabrielsen G, Kallenborn R, Krahn M, Norstrom R, Skaare J (2004) AMAP Assessment 2002: Persistent Organic Pollutants in the Arctic. Arctic Monitoring and Assessment Program, Oslo
de Wit CA, Herzke D, Vorkamp K (2010) Brominated flame retardants in the Arctic environment-Trends and new candidates. Sci Total Environ 408:2885–2918
Divoky GJ (1976) The pelagic feeding habits of Ivory and Ross’ Gulls. Condor 78:85–90
Ehresman DJ, Froehlich JW, Olsen GW, Chang SC, Butenhoff JL (2007) Comparison of human whole blood, plasma, and serum matrices for the determination of perfluorooctanesulfonate (PFOS), perfluorooctanoate (PFOA), and other fluorochemicals. Environ Res 103:176–184
Gabrielsen GW, Skaare JU, Polder A, Bakken V (1995) Chlorinated hydrocarbons in Glaucous Gulls (Larus hyperboreus) in the southern part of Svalbard. Sci Total Environ 160–161:337–346
Gilchrist GH, Mallory ML (2005) Declines in abundance and distribution of the Ivory Gull (Pagophila eburnea) in Arctic Canada. Biol Conversat 121:303–309
Gilg O, Strøm H, Aebischer A, Gavrilo MV, Volkov AE, Miljeteig C, Sabard B (2010) Post-breeding movements of northeast Atlantic Ivory Gull Pagophila eburnea populations. J Avian Biol 41:532–542
Goutte A, Barbraud C, Meillère A, Carravieri A, Bustamante P, Labadie P, Budzinski H, Delord K, Cherel Y, Weimeskirch H, Chastel O (2014a) Demographic consequences of heavy metals and persistent organic pollutants in a vulnerable long-lived bird, the Wandering Albatross. Proc R Soc B 281:20133313
Goutte A, Bustamante P, Barbraud C, Delord K, Weimeskirch H, Chastel O (2014b) Demographic responses to mercury exposure in two closely-related Antarctic top predators. Ecology 95:1075–1086
Goutte A, Angelier F, Bech C, Clément-Chastel C, Dell’Omo G, Gabrielsen GW, Lendvai AZ, Børge M, Noreen E, Pinaud D, Tartu S, Chastel O (2014c) Annual variation in the timing of breeding, pre-breeding foraging areas and corticosterone levels in an Arctic population of Black-legged Kittiwakes. Mar Ecol Prog Ser 496:233–247
Hanssen L (2013) Partition of perfluoroalkyl substances (PFASs) in whole blood and plasma, assessed in maternal and umbilical cord samples from inhabitants of arctic Russia and Uzbekistan. Sci Total Environ 447:430–437
Haukås M, Berger U, Hop H, Gulliksen B, Gabrielsen GW (2007) Bioaccumulation of per- and polyfluorinated alkyl substances (PFAS) in selected species from the Barents Sea food web. Environ Pollut 148:360–371
Herzke D, Nygård T, Berger U, Huber S, Røv N (2009) Perfluorinated and other persistent halogenated organic compounds in European Shag (Phalacrocorax aristotelis) and Common Eider (Somateria mollissima) from Norway: a suburban to remote pollutant gradient. Sci Total Environ 408:340–348
Hobson KA (1995) Reconstructing avian diets using stable-carbon and nitrogen isotope analysis of egg components: patterns of isotopic fractionation and turnover. Condor 97:752–762
Hobson KA, Clark RG (1993) Turnover of d13C in cellular and plasma fractions of blood: implications for non-destructive sampling in avian dietary studies. Auk 110:638–641
Hobson KA, Piatt JF, Pitocchelli J (1994) Using stable isotopes to determine seabird trophic relationships. J Anim Ecol 63:786–798
Hobson KA, Fisk A, Karnovsky N, Holst M, Gagnon JM, Fortier M (2002) A stable isotope (δ13C, δ15N) model for the North Water food web: implications for evaluating trophodynamics and the flow of energy and contaminants. Deep-Sea Res Part II 49:5131–5150
Hoegh-Guldberg O, Bruno JF (2010) The impact of climate change on the world’s marine ecosystems. Science 328:1523–1528
Holmström KE, Berger U (2008) Tissue distribution of perfluorinated surfactants in Common Guillemot (Uria aalge) from the Baltic Sea. Environ Sci Technol 42:5879–5884
Hop H, Borgå K, Gabrielson GW, Kleivane L, Skaare JU (2002) Food web magnification of persistent organic pollutants in poikilotherms and homeotherms from the Barents Sea. Environ Sci Technol 36:2589–2597
Howell SNG (2001) Molt of the Ivory Gull. Waterbirds 24:438–442
Jardine TD, Kidd KA, Fisk AT (2006) Applications, considerations, and sources of uncertainty when using stable isotope analysis in ecotoxicology. Environ Sci Technol 40:7501–7511
Jones PD, Hu W, de Coen W, Newsted JL, Giesy JP (2003) Binding of perfluorinated fatty acids to serum proteins. Environ Toxicol Chem 22:2639–2649
Karnovsky NJ, Hobson KA, Brown ZW, Hunt GL Jr (2009) Distribution and diet of Ivory Gulls (Pagophila eburnea) in the North Water Polynya. Arctic 62:65–74
Keith JO, Mitchell CA (1993) Effects of DDE and food stress on reproduction and body condition of ringed turtle doves. Arch Environ Contam Toxicol 25:192–203
Leat EHK, Bourgeon S, Magnusdottir E, Gabrielsen GW, Grecian WJ, Hanssen SA, Olafsdottir K, Petersen A, Phillips RA, Strøm H, Ellis S, Fisk AT, Bustnes JO, Furness RW, Borgå K (2013) Influence of wintering area on persistent organic pollutants in a breeding migratory seabird. Mar Ecol Prog Ser 491:277–293
Lei R, Xie H, Wang J, Leppäranta M, Jónsdóttir I, Zhang Z (2015) Changes in sea ice conditions along the Arctic Northeast Passage from 1979 to 2012. Cold Reg Sci Technol 119:132–144
Leslie HA, Thomsen C, Brandsma S, Van Velzen M, Leonards PEG, de Boer J (2007) Decabromodiphenylether in human whole blood and serum. Proceedings of the BFR, Amsterdam
Lucia M, Verboven N, Strøm H, Miljeteig C, Gavrilo MV, Braune BM, Boertmann D, Gabrielsen GW (2015) Circumpolar contamination in eggs of the high-arctic Ivory Gull Pagophila eburnea. Environ Toxicol Chem 34:1552–1561
Macdonald RW, Harner T, Fyfe J (2005) Recent climate change in the Arctic and its impact on contaminant pathways and interpretation of temporal trend data. Sci Total Environ 342:5–86
Martin JW, Smithwick MM, Braune BM, Hoekstra PF, Muir DCG, Mabury SA (2004) Identification of long-chain perfluorinated acids in biota from the Canadian Arctic. Environ Sci Technol 38:373–380
McArthur MLB, Fox GA, Peakall DB, Philogéne BJR (1983) Ecological significance of behavioral and hormonal abnormalities in breeding ring doves fed an organochlorine chemical mixture. Arch Environ Contam Toxicol 12:343–353
Mehlum F (1990) Seabird distribution in the northern Barents Sea marginal ice-zone during late summer. Polar Res 8:61–65
Mehlum F, Gabrielsen GW (1993) The diet of High-Arctic seabirds in coastal and ice-covered, pelagic areas near the Svalbard archipelago. Polar Res 12:1–20
Miljeteig C, Strøm H, Gavrilo MV, Volkov A, Jenssen BM, Gabrielsen GW (2009) High levels of contaminants in Ivory Gull Pagophila eburnea eggs from the Russian and Norwegian Arctic. Environ Sci Technol 43:5521–5528
Newsted JL, Coady KK, Beach SA, Butenhoff JL, Gallagher S, Giesy JP (2007) Effects of perfluorooctane sulfonate on Mallard and Northern Bobwhite Quail exposed chronically via the diet. Environ Toxicol Pharmacol 23:1–9
Peakall DB (1985) Behavioral responses of birds to pesticides and other contaminants. Residue Rev 96:45–77
Pellerin Plourde S, Moreau R, Letcher RJ, Verreault J (2013) Is the bone tissue of Ring-billed Gulls breeding in a pollution hotspot in the St. Lawrence River, Canada, impacted by halogenated flame retardant exposure? Chemosphere 93:2333–2340
Powley CR, George SW, Ryan TW, Buck RC (2005) Matrix effect-free analytical methods for determination of perfluorinated carboxylic acids in environmental matrixes. Anal Chem 77:6353–6358
Renner R (2000) What fate for bromimated fire retardants? Environ Sci Technol 34:222A–226A
Roscales JL, Muñoz-Arnanz J, González-Solis J, Jiménez B (2010) Geographical PCB and DDT patterns in Shearwaters (Calonectris sp.) breeding across the NE Atlantic and the Mediterranean archipelagos. Environ Sci Technol 44:2328–2334
Spencer NC, Gilchrist HG, Mallory ML (2014) Annual Movement Patterns of Endangered Ivory Gulls: the Importance of Sea Ice. PLoS ONE 9:e115231
Thibodeaux JR, Hanson RG, Rogers JM, Grey BE, Barbee BD, Richards JH, Butenhoff JL, Stevenson LA, Lau C (2003) Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. I: maternal and prenatal evaluations. J Toxicol Sci 74:369–381
Tomy GT, Budakowsky W, Halldorson T, Helm PA, Stern GA, Friesen K, Pepper K, Tittlemier SA, Fisk AT (2004) Fluorinated organic compounds in an eastern Arctic marine food web. Environ Sci Technol 38:6475–6481
Vanden Heuvel JP, Thompson JT, Frame SR, Gillies PJ (2006) Differential activation of nuclear receptors by perfluorinated fatty acid analogs and natural fatty acids: a comparison of human, mouse, and rat peroxisome proliferator-activated receptor-α, -β, and - γ, liver X receptor-β, and retinoid X receptor-α. Toxicol Sci 92:476–489
Verreault J, Skaare JU, Jenssen BM, Gabrielsen GW (2004) Effects of organochlorine contaminants on thyroid hormone levels in Arctic breeding Glaucous Gulls, Larus hyperboreus. Environ Health Perspect 112:532–537
Verreault J, Houde M, Gabrielsen GW, Berger U, Haukås M, Letcher RJ, Muir DCG (2005) Perfluorinated alkyl substances in plasma, liver, brain, and eggs of Glaucous Gulls (Larus hyperboreus) from the Norwegian Arctic. Environ Sci Technol 39:7439–7445
Verreault J, Gebbink WA, Gauthier LT, Gabrielsen GW, Letcher RJ (2007) Brominated flame retardants in Glaucous Gulls form the Norwegian Arctic: more than just an issue of polybrominated diphenyl ethers. Environ Sci Technol 41:4925–4931
Volz SA, Johnston JJ, Griffin DL (2001) Solid phase extraction gas chromatography/electron capture detector method for the determination of organochlorine pesticides in wildlife whole blood. J Agric Food Chem 49:2741–2745
Wassmann P, Reigstad M, Haug T, Rudels B, Carroll ML, Hop H, Gabrielsen GW, Falk-Petersen S, Denisenko SG, Arashkevich E, Slagstad D, Pavlova O (2006) Food web and carbon flux in the Barents Sea. Prog Oceanogr 71:232–287
Watanabe I, Sakai S (2003) Environmental release and behavior of brominated flame retardants. Environ Int 29:665–682
Acknowledgments
The authors wish to thank the European Commission for its financial support through a Marie Curie fellowship to M. Lucia, as well as Gael Guillou from the “plateforme analyses isotopiques” (UMR LIENSs) for technical support during stable isotope analyses. We thank Linda Hanssen and Arntraut Götsch (NILU) for their assitance with the chemical analyses. We also thank Birgit Braune for her help with English. The fieldwork was supported by the Norwegian Polar Institute’s centre for Ice, Climate and Ecosystems (ICE) and the SEAPOP program (seapop.no). Thanks to Vidar Bakken, Audun Igesund, Cecilie Miljeteig, Knut Olsen, Maria Gavrilo, Olivier Gilg and Odd Kindberg for help in the field.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lucia, M., Strøm, H., Bustamante, P. et al. Contamination of ivory gulls (Pagophila eburnea) at four colonies in Svalbard in relation to their trophic behaviour. Polar Biol 40, 917–929 (2017). https://doi.org/10.1007/s00300-016-2018-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-016-2018-7