Abstract
Increases in populations of the two native higher plants that exist in the Antarctic have been thought to be caused by an improvement in their reproductive performance as a result of regional warming. Colobanthus quitensis dispersion by seeds and establishment are limited to years with sufficiently high summer temperatures. The main objective of this work was to evaluate the effect of increased growth temperature on the germination ability of C. quitensis. Individuals were collected from King George Island and grown at two different thermoperiods: 5/2 and 11/5 °C, day/night. Mature seeds produced under both growth temperatures were tested for viability and incubated under a range of temperatures from 5° to 35 °C. Seed viability was not significantly different between growth temperatures. Germination for C. quitensis was highly temperature dependent. Germination was modulated by temperatures over 10 °C. Seeds from 5/2 °C showed significant higher germination (>80 %) between 10 and 15 °C. Seed developed at 11/5 °C showed a broader range of temperature for germination between 10 and 25 °C. Higher growth temperature (11/5 °C) reduced the germination time significantly. Our results suggest that annual production of seeds leads to a high probability of germination at warmer environmental conditions because dormancy would be reduced at higher temperatures. Therefore, higher environmental temperatures, as those predicted to occur because of global warming, would increase the propagation of C. quitensis by germination.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One important feature for plant persistence in extreme environments is the ability to reproduce and to form sustainable populations. However, it has been observed that with the current climate warming, several species inhabiting cold habitats such as arctic and alpine areas have increased their reproductive performance by either increasing seed production or increasing seed germination, or both (Zhang et al. 2014).
Only two vascular plants naturally occur in the Antarctic: Deschampsia antarctica Desv. and Colobanthus quitensis (Kunt) Bartl. Studies evaluating the reproduction of these plants in different areas along their distribution indicate that both species show frequent flowering, although the production of ripe and viable seeds is sporadic and restricted only to favorable years with warmer summers (Holtom and Greene 1967; Longton and Holdgate 1967; Greene and Holtom 1971; Edwards 1974; Fowbert and Smith 1994). Thus, it has been suggested that temperature increases resulting from the global climate change could promote the regeneration of the Antarctic plants (Convey and Smith 2006). Indeed, the climatic amelioration experienced in the Antarctic in the last decades as a consequence of increases in temperature (Vaughan et al. 2003) has contributed to the expansion of both species along the Antarctic Peninsula (Smith 1994; Convey and Smith 2006).
While D. antarctica is able to colonize new localities by both vegetative dispersal and recruitment from naturally dispersed seeds (Edwards 1972), C. quitensis has to be dispersed by seeds (Fowbert and Smith 1994). Seeds are dispersed over long distances by winds, which are very common in the Antarctic Peninsula and adjacent islands, while short distance seed dispersal in this species is considered to rely on rain splash and water streams produced by snow melting (Edwards 1974). Seed bank formation under extremely adverse conditions could be critical for population persistence, where recruitment is limited to infrequent years with sufficiently high summer temperatures (Fowbert and Smith 1994; Grobe et al. 1997).
In C. quitensis, seed production seems to be significantly affected by summer air temperatures (Edwards 1974; Fowbert and Smith 1994). There is some evidence that for this species the optimum temperature for maximum germination is relatively high (Corner 1971). Corte (1961) observed higher germination at 9 °C than at 2 °C in seeds coming from the Danco Coast (64°10′S), while Corner (1971) with seeds from the Argentine Islands (65°15′S) obtained the highest germination rates at temperatures between 12° and 20 °C. Ruhland and Day (2001) reported that seed germination rates in C. quitensis were high at room temperature.
It is well known that the temperature experienced by mother plants during seed development and maturation may have important influences on the germinability of seeds (Gutterman 2000). In some species, for example, plants developed at higher temperatures produce seeds with higher germination capacity (Datta et al. 1972), while in others an inverse relation between maturation and germinability has been found (Keigley and Mullen 1986). Thus, the effect of temperature during seed development and the subsequent germination ability is not well understood. Studies evaluating the effect of maternal plants growing at different temperatures suggest that increases in temperature during seed development might decrease seed dormancy (Baskin and Baskin 1998), although the responses have not been clear enough in different species.
Current warming trends will generate higher summer temperatures and a longer growing season in the Maritime Antarctic, which could benefit seed germination in C. quitensis. However, to our knowledge, there is no information regarding the effect of growth temperature on the germination response to temperature in C. quitensis. Thus, our main objective in this study was to evaluate the effect of two growth temperatures (5/2 and 11/5 °C) on different aspects of seed germination of C. quitensis. We suggest that seeds produced under 11/5 °C (warming temperature) have a higher germination capacity compared to those produced at 5/2 °C, extending the range of optimum germination temperatures.
Materials and methods
Plant material and treatments
Colobanthus quitensis plants were collected in the vicinity of Arctowski Station in King George Island (62°09′S, 58°28′W) and transplanted to plastic pots. These plants were transported to facilities in the University of Concepción, Chile, and placed in growth chambers at ~300 μmol photons m−2 s−1, 18/6 h light/dark, and around 60 % RH. Plants were grown at two different temperatures: 5/2 and 11/5 °C (day/night). The lower thermoperiod (5/2 °C) intended to expose plants to temperatures similar to average temperatures during the Maritime Antarctic summer, while the higher thermoperiod (11/5 °C) covers the range of maximum temperatures that might be reached as a consequence of regional warming during the next century. Completely developed seeds produced under both growth temperatures were collected for test viability and germination.
Viability test
To assess the viability of seeds, we collected 50 seeds from each of five individuals at both growth thermoperiods. Seeds were placed in 1.5-ml plastic tubes containing ten seeds per tube. Seeds were moistened with distilled water for 24 h. Then, the seed coat was longitudinally cut to expose the embryo to a staining solution. We added 1 ml of 5-cyano-2,3-di-(p-tolyl) tetrazolium chloride 0.5 % and stored the seeds in darkness for 24 h. Finally, a red-stained embryo was considered as a viable seeds.
Seed germination test
Collected seeds of both growth thermoperiods (5/2 and 11/5 °C) were placed in petri dishes (20 seeds/dish) on a layer of filter paper moistened to saturation with distilled water and maintained this way throughout the experiments. Petri dishes were covered with Parafilm to minimize water losses. There were five replicates per treatment. Seeds were incubated in darkness in seven controlled growth chambers, each set up at a constant temperature (5°, 10°, 15°, 20°, 25°, 30°, and 35 °C). Temperature was also monitored by thermocouples installed inside the petri dishes. We considered a seed germinated when the radicle doubled the size of the seed. Germinated seeds were periodically counted under green light and subsequently removed from the petri dishes. The experiment lasted 32 days.
Data analysis
We determined the percentage of germination, time to germination (corresponding to the number of days needed for the first seed to germinate) and the Index of Germination (GI), corresponding to Σ Gt/Tt, with Gt being the number of seeds germinating on day t and Tt the day of seed germination.
To evaluate differences in viability, we used a Student's t test (P < 0.05). To determine the effect of temperature on germination in seeds of plants grown at 5/2° and 11/5 °C, we performed a two-way ANOVA (P < 0.05) where the factors were growth temperature × germination temperature. A posteriori Tukey test was used to determine differences between germination temperatures in both growth treatments (P < 0.05). Percentages of germination values were arcsine square root transformed before analysis to normalize the variance (Zar 1999). All the statistical analyses were performed with STATISTICA 6.0 software.
Results
Seed viability
Plants grown at 11/5 °C produced a similar number of viable seeds as plants grown at 5/2 °C, resulting in 96 ± 2.4 and 90 ± 5.4 % viability, respectively (P = 0.347). Similarly, the number of seeds per flower was not significantly affected by growth temperature (21.3 ± 4.7 and 20.6 ± 4.7 seeds for 5/2° and 11/5 °C, respectively).
Seed germination
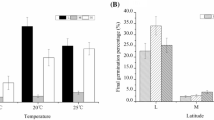
Germination of seeds of C. quitensis was affected by both the temperature experienced by mother plants (growth temperature) and the range of temperatures for germination (Table 1). For both growth thermoperiods, a significant cumulative germination started after 8 days, reaching the maximum percentages of germination after around 11 days (Fig. 1). Plants grown at 5/2 °C showed a higher percentage of cumulative germination of 96 and 82 % at 10 and 15 °C, respectively, for plants grown at 11/5 °C with a maximum of 72 % of germination after 11 days. For seeds developed at 11/5 °C, the germination was significantly increased (Fig. 2) from 20 to 25 °C compared to those from plants grown at 5/2 °C.
Effect of temperature on germination of C. quitensis seeds produced at two growth thermoperiods (5/2 and 11/5 °C). The cumulative germination percentages of C. quitensis seeds at different germination temperatures along the time of the experiment (32 days) are plotted (mean value ± standard error; n = 5). The asterisk indicates significant differences among germination temperatures at day 22 corresponding to the last day of germination for all the treatments
Seeds from 5/2 °C growth temperature showed the highest germination (>80 %) between 10° and 15 °C. Seed developed at 11/5 °C showed a broader range of temperature (10°–25 °C) for maximum germination than those developed at 5/2 (Fig. 2). Germination was the lowest at 5 °C (no germination indeed) and over 30 °C in plants grown at both growth thermoperiods.
In plants grown at 5/2 °C the germination time was significantly lower (P < 0.05) between 10 and 20 °C than at 5 °C (Table 2). Seeds of plants grown at 11/5 °C reduced their germination times significantly at 10–30 °C (Table 2). At higher temperature (30 and 35 °C), the germination time increased in both growth thermoperiods compared to that observed at 5 °C. The germination index (GI) was significantly different between growth temperatures at 20 and 25 °C (Table 2). Plants growing at 11/5 °C showed higher values of GI, which indicates that warmer temperatures improved the rate of germination, promoting that seeds germinate earlier and in a higher amount.
Discussion
Growth temperature has little effect on seed viability
The harsh weather of polar regions and high mountain habitats compromises the production of viable seeds during some growing seasons (Walck et al. 2011; Bernareggi et al. 2015). The impact of global warming on regeneration from seeds is of growing interest. It has been suggested that higher temperatures could improve seed viability and germination (Milbau et al. 2009). In a study testing the germinability of arctic plants in 13 species, they found high germination under warming temperatures, suggesting that climate warming is already increasing seed viability (Müller et al. 2011).
Seeds developed under both growth temperatures showed a high degree of viability (over 90 %). This agrees with Convey (1996), who showed that C. quitensis is able to produce flowers with viable seeds in most years in different sites throughout its Antarctic range. This species can even maintain a seed bank (McGraw and Day 1997; Ruhland and Day 2001) with viable seeds. Gielwanowska et al. (2011) collected seeds from Arctowski Antarctic Station during 2002; after 2 years of storage, seeds still showed about 80 % viable embryos. However, mature seeds are produced sporadically only in favorable years (Holtom and Greene 1967; Longton and Holdgate 1967; Greene and Holtom 1971; Edwards 1974; Fowbert and Smith 1994), which can indicate that warmer growing seasons could have a positive effect on seed development. Day et al. (1999) reported that warmer summers in Antarctica will favor the flowering development and production of heavier and more viable seeds. Laboratory assays cultivating plants of C. quitensis at different temperatures have shown that the number of seeds increased when plants are growing close to their optimum temperature (personal observation); however, the viability of seeds in this plant has not been tested. In our results, the absence of significant differences between both thermoperiods suggests that prolonged periods of warmer temperatures in the Antarctic Peninsula might not have a negative effect on seeds viability. Further studies evaluating the effect of warmer temperatures on seed production of flowers and seeds are necessary to evaluate this hypothesis.
Germination of seeds was affected by both temperature experienced by mother plants and temperature experienced by seeds
The highest germination percentages were obtained from plants grown at 5/2° compared to 11/5 °C (96 and 72 %, respectively). There is strong evidence that changing temperatures has a preconditioning effect on the production and germination of seeds, which corresponds to maternal influences through interaction with the environment (Roach and Wulff 1987). The ability of the maternal environment to affect the offspring phenotype through changes in gene expression may potentially allow progeny adaptations to the habitat conditions experienced by the maternal parent (Bernareggi et al. 2015). It is known that the effects of the maternal environment during seed development and maturation can affect various seed traits including germination and seedling growth (Mondoni et al. 2011; Bernareggi et al. 2015). The highest percentage of germination in seeds developed at lower temperatures suggests an adaptation of Antarctic species to germinate under low-temperate conditions. This strategy has also been reported for high-altitude species (e.g., Milbau et al. 2009) and in Arctic populations as an adaptation to the harsh climate (Graae et al. 2008).
It has been reported that several species grown at higher temperatures produce seeds with higher germination than those produced at low temperatures (see list of references in Baskin and Baskin 1998). In our study, seeds produced under warmer conditions were able to extend their range of temperature for germination at 10 °C. This expansion in the germination range is indicative of release of dormancy, which has been described for winter annual species (Probert 2000). The effects of high temperature are often rapid and include a positive interaction between high temperature and control of dormancy by other factors (Probert and Smith 1986). Temperature affects germination in three primary ways: moisture, hormone production, and enzyme activity. In most species, an increase in temperature alone may have a positive impact (Dove 2010). Higher germination temperature (25–35 °C) significantly decreased (P < 0.05) the time of germination of plants developed at 11/5 °C. Adapting the timing of germination to a new environmental condition may also depend on maternal effects. Warmer climate could modify soil conditions (e.g., temperature), influencing seed preservation and the chance of seed survival. The effect of seed resistance to warmer temperatures driven by the maternal effects could buffer seeds against such thermal variations in their environment (Bernareggi et al. 2015). In this context, maternal-induced novel phenotypes will provide fundamental support in the changing environment, to safegard the progeny (Nicotra et al. 2010).
Germination range extension under warmer temperatures
Seeds developed at two growth temperatures (5/2° and 11/5 °C) showed the highest germination between 10 and 25 °C (Fig. 2). At 5 °C, none of the seeds germinated, even though this temperature corresponds to the average temperature at which this species grows in Antarctica during the summer season (field observations). Similarly, the tundra species Colobanthus crassifolius did not have germination 5 °C. However, if the temperature fluctuated between 5° and 18 °C, the seeds germinated (Billings and Mooney 1968). Apparently, temperature fluctuations such as those experienced in the field during the growing season are an essential requirement to promote germination in this species. It has been suggested that low germination percentages under some temperature regimes could be due to significant degrees of secondary dormancy. In this case, seeds are released from the plant in a nondormant state, but become dormant if the conditions for germination are unfavorable (Baskin and Baskin 1998). This absence of germination at low temperature could be related to low temperature-induced dormancy. Seed dormancy has been argued to be a mechanism by which plants have adapted to an unpredictable environment (Baskin and Baskin 1998). Gielwanowska et al. (2011) suggest that both polar vascular plants living in the Antarctic enter a deep secondary dormancy, probably after winter, which requires a certain variable temperature, light, or nitrate concentration to break dormancy. Our results suggest that C. quitensis's germination has a strong temperature dependence with a window of germination modulated by temperatures over 10 °C; therefore, higher environmental temperatures, as are predicted to occur because of global warming, would be beneficial to improve propagation by germination in this species. This agrees with other authors, who report that establishment of this species is sporadic and only occurs during sunnier summers when temperatures are warm enough to generate germination (Smith 2003; Gielwanowska et al. 2011). Nonetheless, temperatures over 30 °C significantly reduced the rate of germination in this species. Thermodormancy at high temperature has also been reported in seeds of Lectuca sativa (Reynolds and Thompson 1971) and Avena sativa (Poljakoff-Mayber et al. 1990), which can become dormant at temperatures higher than the maximum for germination. Induction of thermodormancy at high temperatures appears to be a species-specific characteristic (Reynolds and Thompson 1971). C. quitensis decreased its germination percent at 30 °C. The mechanisms for avoiding germination under high temperatures are probably related to its adaptation to grow in a harsh cold environment.
In conclusion, in C. quitensis a high percentage of seed viability and maintenance of dormancy at low temperature appear to be adaptation features that allow the seed to survive under the extreme conditions of its Polar habitats. Germination in Polar regions only occurs when there is a guarantee for further growth and development. Warming in the Maritime Antarctic could extend the range of temperatures for germination in this species. Since the frequency of days with environmental temperatures over 10 °C will most likely increase because of regional warming, we postulate that germination in C. quitensis will increase. This positive effect of warming temperatures should be reflected in larger plants with higher fitness (increase in the number of flowers per plant) and an increase in the range of temperatures at which germination occurs.
References
Baskin CC, Baskin JM (1998) Seeds: ecology, biogeography, and evolution of dormancy and germination. Academic Press, New York
Bernareggi G, Carbognani M, Petragglia A, Mondoni A (2015) Climate warming could increase seed longevity of alpine snowbed plants. Alp Bot 125:69–78
Billings WD, Mooney HA (1968) The ecology of arctic and alpine plants. Biol Rev 43:481–529
Convey P (1996) The influence of environmental characteristics on the life history attributes of Antarctic terrestrial biota. Biol Rev 71:191–225
Convey P, Smith RIL (2006) Responses of terrestrial Antarctic ecosystems to climate change. Plant Ecol 182:1–10
Corner RWM (1971) Studies in Colobanthus quitensis (Kunth) Bartl. and Deschampsia antarctica Desv. IV: distribution and reproductive performance in the Argentine islands. Br Antarct Surv Bull 26:41–50
Corte A (1961) Fertilidad de las semillas en fanerogamas que crecen en Cabo Primavera (Costa de Danco), Peninsula Antartica. (Seed fertility in phanerogams growing at Spring Cape [Danco Coast, Antarctic Peninsula]). Contrib Inst Antart Argent 65:1–16
Datta SC, Evenari M, Gutterman Y (1972) Photoperiodic and temperature responses of plants derived from the various heteroblastic caryopses of Aegilops ovata L. J Indian Bot Soc 50:546–559
Day TA, Ruhland CT, Grobe CW, Xiong F (1999) Growth and reproduction of Antarctic vascular plants in response to warming and UV radiation reductions in the field. Oecologia 119:24–35
Dove N (2010) The effect of increasing temperature on germination of native plant species in the north woods region. University of Vermont, Thesis
Edwards JA (1972) Studies in Colobanthus quitensis (Kunth) Bartl. and Deschampsia antarctica Desv: V: distribution, ecology and vegetative performance on Signy Island. Br Antarct Surv Bull 28:11–28
Edwards JA (1974) Studies in Colobanthus quitensis (Kunt) Bartl. and Deschampsia Antarctica Desv.: VI: reproductive performance on Signy Island. Br Antarct Surv Bull 39:67–86
Fowbert JA, Smith RI (1994) Rapid population increases in native vascular plants in the Argentine Islands, Antarctic Peninsula. Arct Alp Res 26:290–296
Gielwanowska I, Bochenek A, Gojlo E, Görecki R, Kellmann W, Pastorczyk M, Szczula E (2011) Biology of generative reproduction of Colobanthus quitensis (Kunth) Bartl. from King George Island, South Shetland Islands. Polish Polar Research 32:139–155
Graae BJ, Alsos IG, Ejrnaes R (2008) The impact of temperature regimes on development, dormancy breaking and germination of dwarf shrub seeds from arctic, alpine and boreal sites. Plant Ecol 198:275–284
Greene DM, Holtom A (1971) Studies in Colobanthus quitensis (Kuhnt) Bartl. and Deschampsia antarctica Desv. III: distribution, habitats and performance in the Antarctic botanical zone. Br Antarct Surv Bull 26:1–29
Grobe CW, Ruhland CT, Day TA (1997) A new population of Colobanthus quitensis near Arthur Harbor, Antarctica: correlating recruitment with warmer summer temperatures. Arct Alp Res 29:217–221
Gutterman Y (2000) Maternal effects on seeds during development. In: Fenner M (ed) Seeds: the ecology of regeneration in plant communities, 2nd edn. CABI Publishing, Wallingford, pp 59–84
Holtom A, Greene SW (1967) The growth and reproduction of Antarctic flowering plants. Philos Trans R Soc: Lond, Ser B 252:323–337
Keigley PJ, Mullen RE (1986) Changes in soybean seed quality from high temperatures during seed fill and maturation. Crop Sci 26:1212–1216
Longton RE, Holdgate MW (1967) Temperature relationships of Antarctic vegetation. In: Smith JE (organ.) A discussion on the terrestrial Antarctic ecosystem. Philos Trans R Soc vol 252, pp 323–337
McGraw JB, Day TA (1997) Size and characteristics of a natural seed bank in Antarctic. Arct Alp Res 29:213–216
Milbau A, Graae BJ, Shectsova A, Nijs I (2009) Effects of a warmer climate on seed germination in the subarctic. Ann Bot 104:287–296
Mondoni A, Probert RJ, Rossi G, Vegini E, Hay FR (2011) Seeds of alpine plants are short-lived: implications for long-term conservation. Ann Bot 107:171–179
Müller E, Cooper EJ, Alsos IG (2011) Germinability of Arctic plants is high in perceived optimal conditions but low in the field. Bot Botanique 89:337–348
Nicotra AB, Atkin OK, Bonser SP, Davidson AM, Finnegan EJ, Mathesius U, Poot P, Purugganan MD, Richards CL, Valladares F, van Kleunen M (2010) Plant phenotypic plasticity in a changing climate. Trends Plant Sci 12:684–692
Poljakoff-Mayber A, Corbineau F, Côme D (1990) A possible mechanism of high temperature dormancy regulation in seeds of Avena sativa L. (cv. Moyencourt). Plant Growth Regul 9:147–156
Probert RJ (2000) The role of temperature in the regulation of seed dormancy and germination. In: Fenner M (ed) Seeds: the ecology of regeneration in plant communities. CAB International, Wallingford, pp 261–292
Probert RJ, Smith RD (1986) The joint action of phytochrome and alternating temperatures in the control of seed germination in Dactylis glomerata. Physiol Plant 67:299–304
Reynolds T, Thompson PA (1971) Characterization of the high temperature inhibition of germination of Lettuce (Lactuca sativa). Physiol Plant 24:544–547
Roach DA, Wulff RD (1987) Maternal effects in plants. Annu Rev Ecol Evol Syst 522:209–235
Ruhland CT, Day TA (2001) Size and longevity of seed banks in Antarctica and the influence of ultraviolet-B radiation on survivorship, growth and pigment concentrations of Colobanthus quitensis seedlings. Environ Exp Bot 45:143–154
Smith RIL (1994) Vascular plants as bioindicators of regional warming in the Antarctic. Oecologia 99:322–328
Smith RIL (2003) The enigma of Colobanthus quitensis and Deschampsia antarctica in Antarctica. In: Huiskes AHL, Gieskes WWC, Rozema J, Schorno RML, van der Vies SM, Wolff WJ (eds) Antarctic biology in a global context. Backhuys, Leiden, pp 234–239
Vaughan DG, Marshall GJ, Connolley WM, Parkinson C, Mulvaney R, Hodgson DA, King JC, Pudsey CJ, Turner J (2003) Recent rapid regional climate warming on the Antarctic Peninsula. Clim Change 60:243–274
Walck JL, Hidayati SN, Dixon KW, Thompson K, Poschlod P (2011) Climate change and plant regeneration from seed. Glob Change Biol 17:2145–2161
Zar JH (1999) Biostatistical analysis, 4th edn. Prentice-Hall, Upper Saddle River
Zhang YF, Wang C, Tian SL, Lu JQ (2014) Dispersal and hoarding of sympatric forest seeds by rodents in a temperate forest from northern China. iForest 7:70–74
Acknowledgments
The authors thank Projects CONICYT-PIA ART 1102 and Fondecyt 11130332, for funding our field and laboratory work and Instituto Antártico Chileno (INACH) for logistic support in Antarctica and the necessary permits to enter and collect plants in SPA 128.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sanhueza, C., Vallejos, V., Cavieres, L.A. et al. Growing temperature affects seed germination of the antarctic plant Colobanthus quitensis (Kunth) Bartl (Caryophyllaceae). Polar Biol 40, 449–455 (2017). https://doi.org/10.1007/s00300-016-1972-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-016-1972-4