Abstract
Macroalgae, in particular kelps, produce a large amount of biomass in Kongsfjorden, which is to a great extent released into the water in an annual cycle. As an example, the brown alga Alaria esculenta loses its blade gradually, 3 ± 0.8 % of the blade area per day (August 2012), thereby adding to the pool of particulate organic matter (POM) in the fjord. Upon release small thallus pieces are “aging” in that they are prone to leaching and serving as substrate for microorganisms, thus turning into palatable food for suspension and bottom feeders. In order to define a macroalgal baseline for the Kongsfjorden food web, stable isotopes δ14C and δ15N were measured in individuals of A. esculenta, Saccharina latissima and Laminaria digitata directly sampled after collection and in artificially produced POM (aPOM) of A. esculenta that was allowed to age under experimental conditions. In aPOM from this species sampled in August 2012 the C/N ratios decreased between d1 and d8 of a 14-day culture period in parallel to the fading photosynthetic activity of the algal fragments as demonstrated by use of an Imaging-PAM. Microscopic observations of the aPOM in August 2012 and 2013 revealed the frequent occurrence of small brown algal endo- and epiphytes. First feeding experiments with Mysis oculata (Mysids) and Hiatella arctica (Bivalves) showed that these species can ingest macroalgal POM. The importance of kelp-derived POM for the food web is subject of the current research.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The impressively high macroalgal biomass produced in rocky shore subtidal areas around the world becomes regularly obvious on beaches in the aftermath of storms. The contribution of this primary production to the adjacent ecosystem has been studied in Antarctica (Dunton 2001) and the Southern Indian Ocean (Kaehler et al. 2000, 2006), along the Atlantic Ocean from South Africa (Bustamante and Branch 1996) via Brittany in France (Schaal et al. 2010, 2012; Leclerc et al. 2013a, b) to Scotland (Orr et al. 2014) and to Northern Norwegian kelp forests (Fredriksen 2003). At the Western side of the Atlantic in Nova Scotia, extensive research on kelp detritus was conducted by Krumhansl and Scheibling (2011, 2012). Macroalgal material was considered a substantial part of the detritus supporting the macrobenthic community of the cold Arctic Hornsund (Spitsbergen) by Sokołowski et al. (2014) and Renaud et al. (2015) provided ample evidence for the Arctic Isfjorden (Spitsbergen).
Direct consumption of fresh macroalgal thalli is a locally important phenomenon only, reported to comprise 30–40 % of the net primary production (Duarte and Cebrián 1996; Kelly et al. 2012) down to 10 % (Mann 1973, 1988 lit. therein). All studies cited so far have found kelp-derived detritus to heavily subsidize invertebrate benthic suspension feeders and/or bottom feeders at least at times of phytoplankton shortage. The extent of this subsidy can reach >90 % of their diet (Dunton 2001). These findings, obtained in the Atlantic and Southern Ocean, are contradicted by Page et al. (2008) and Yorke et al. (2013) for the Macrocystis dominated reefs of the Santa Barbara Channel at the Pacific Ocean. The authors assigned little use of kelp-derived material to suspension feeders, but noted consumption by benthic herbivores like sea urchins or probably detritivores. In a review Miller and Page (2012) underlined the importance of a better understanding of POM and its use by consumers. They requested the isotopic variability of phytoplankton sources (offshore and coastal) to be considered since that might, among other factors, have led to an overestimation of kelp particles as food for suspension or detritus feeders. They, moreover, suggested investigating feeding selectivity. Other studies in Pacific locations like the natural experiment conducted by Duggins et al. (1989) at the Aleutian Islands suggested a considerable role of kelp production for suspension feeders particularly in winter. At the North Alaskan Boulder Patch, Dunton and Schell (1987) found, likewise depending on season and phytoplankton availability, up to 50 % of body carbon derived from kelp detritus in a mysid crustacean that would link the food web to higher predators such as fish, birds and marine mammals.
The amount of kelp-derived detritus that becomes available for consumers varies with kelp species and hydrographic conditions (Krumhansl et al. 2014). The nutritional quality of this kelp-derived material, e.g., phlorotannin content and C:N ratio, seems to improve with thallus degradation (Duggins and Eckman 1997; Levinton et al. 2002; Norderhaug et al. 2003), but is also dependent on season (Norderhaug et al. 2006) and the macroalgal species (Dethier et al. 2014).
Food web studies are nowadays largely based on analyses of stable isotopes that need well-defined baselines (e.g., Renaud et al. 2011, 2015). Detritus collected in sediment traps is always a mixture and cannot be well differentiated for analysis. In order to assess the role of macroalgal detritus in the marine ecosystem of Kongsfjorden, a characterization of this potential baseline for isotope studies was necessary. A determination of the isotopic signatures (δ13C and δ15N) of freshly collected kelp species (Alaria esculenta, Saccharina latissima, Laminaria digitata) from Hansneset (Kongsfjorden, Spitsbergen) was taken as a first orientation and amended by experiments with artificially produced particulate organic matter (aPOM) of A. esculenta, one of the dominating kelp species at this site (Hop et al. 2012, Bartsch et al. 2015).
We tested the following hypotheses: (1) the relatively large macroalgal biomass enters the food web of Kongsfjorden as particulate organic matter, not only from disintegrating detached seaweeds but also derived by erosion from living macroalgae. (2) Small fragments of macroalgae floating in seawater eventually change their biochemical characteristics and turn from plant to substrate for microorganisms. (3) Aged macroalgal POM in suspension or sunk to the bottom can probably be utilized by some animals in Kongsfjorden as part of their diet. (4) Characterizing the signal of the detritus pool is important for all food web studies and the study of kelp-derived POM from Kongsfjorden may help to approach a better knowledge of the detritus baseline by defining one of its different components.
Materials and methods
Collection of algae
Thalli of the kelps A. esculenta, S. latissima and L. digitata were collected by scuba diving at 5 m depth at Hansneset, Kongsfjorden, Svalbard, in June 2010 and 2011 and in August 2012 and 2013. (78.9851°N, 11.9659°E. Decimal degrees for reference in http://toposvalbard.npolar.no/).
Test of blade erosion
Racks, described by Olischläger and Wiencke (2013), were deployed in the fjord at 78.9295°N 11.9224°E (http://toposvalbard.npolar.no/) in June 2010 and 2011 and in August 2012 and 2013. The racks were adjusted to 4 m water depth so that, due to stipe lengths, algal blades were floating at about 5 m depth. Twelve individual sporophytes of A. esculenta per rack were fastened at their stipes each with two silicon-coated cable ties, the holdfast serving as additional stopper. The silicon prevented damage of the stipes. Only one specimen was lost. Before and after deployment, every specimen’s blade length was measured from stipe/blade transition to the end of the blade. Since erosion at the tip of the blade counteracts meristematic growth at the base, the latter was assessed according to the well-established method of Mann (1973): a small hole was punched at 10 cm above the transition of stipe and blade at the beginning and the position of the hole re-measured at the end of the exposure at sea. Data were processed in GraphPad Prism Software, version 6.03. In 2012 and 2013 all A. esculenta were additionally photographed before and after exposure and their change in blade area measured by means of WINFOLIA software. There was no statistical correlation between the position of individual algae on the rack (ends vs. middle of the rack) and erosion rates, (data not shown).
Preparation of experimental POM
In August 2012 A. esculenta specimens from Hansneset had lost a considerable part of the blade already. To artificially produce particulate organic matter (subsequently called ‘aPOM’), 30 specimens with a mean blade length of 44.1 ± 11.7 cm were available. 240 disks (diameter 25 mm; 5 or 10 per alga) were punched between 10 and 18 cm from stipe/blade transition and evenly distributed into 5 batches of 7–8 g fresh weight. Batches were minced separately in a glass household blender (HR2084/00, Philips, Amsterdam, The Netherlands). The inset steel filter, a cylinder, diameter 6.6 cm, was used to reduce the available space between disks and cutting edges of the knives. Fragmentation was performed in three steps, each with addition of 100 ml of seawater simultaneously used to rinse the walls of the blender: 30 s in 100 ml; 60 s in 200 ml; 30 s in 300 ml. Particles were allowed to settle for 2–4 h, the supernatant was decanted and the remaining particles resuspended. The suspension, continuously stirred with a glass rod, was split to an equivalent of 1 g blotted algal tissue per glass beaker. Filtered seawater (GF/D 2.7 µm pore size) was added to make up 800 ml POM suspension per Fernbach culture flask, resulting in a relatively large seawater surface to facilitate gas exchange. The cultures were placed on Orbitron orbital shakers (INFORS HT, Bottmingen/Basel, Switzerland) and shaken slowly and continuously at 40 rpm, 4 °C and a photon fluence rate of 20–25 µmol m−2 s−1. Every second day, up to day 14, at least three replicate cultures were filtered onto pre-combusted and pre-weighed GF-C (1.2 µm) filters, shortly washed with deionized water and lyophilized for further analysis. Since environmental conditions were not well known, various data loggers were deployed at Hansneset for the first time from July to August 2012. At 5 m depth they recorded water temperatures between 3.5 and 6 °C and a photon fluence rate of 1.5–11 µmol m−2 s−1 below and 16–105 µmol m−2 s−1 above the algal canopy (Bartsch, unpublished data).
To investigate how long small fragments of algae in the aging cultures retain their unique capability of plants to perform photosynthesis, 3 ml subsamples were pipetted into small Petri dishes before filtering, dark adapted for 10 min, and placed under the Imaging-PAM (Walz, Effeltrich, Germany) for measurement of the maximum quantum yield, Fv/Fm. Filters covered with the retrieved aPOM were measured with the Imaging-PAM as well. Fv/Fm values were measured over an area of 0.53 cm2.
A comparison of measurements with the Imaging-PAM and the PAM 2100 (Walz, Effeltrich, Germany) showed higher fluorescence for the PAM 2100, because it penetrates to the lower cell layers and therefore measures higher fluorescence. In the case of Alaria aPOM surface measurement with the Imaging-PAM was sufficient, because light microscopy showed only one epidermal cell layer containing chloroplasts.
C/N ratio and content of soluble phlorotannins
Prior to analyses the lyophilized algal tissue was ground to a fine powder in a Micro-Dismembrator U (B. Braun Biotech International, Melsungen, Germany).
The relative contents of carbon and nitrogen were determined in the process of stable isotope analysis and the values converted to the atomic ratio.
Phlorotannin content was analyzed on freeze dried material, kept as dark as possible at all stages of analysis, applying the Folin–Ciocalteu assay (Folin and Ciocalteu 1927). Extraction of polyphenols was performed with 80 % cool (5 °C) methanol: 1.5 ml of methanol was pipetted onto an aliquot of approximately 10 mg sample in brown Eppendorf cups. 5 min of vortexing were followed by 1 h shaking at 5 °C in a Thermomixer (Comfort, Eppendorf, Wesseling, Germany) at 350 rpm and centrifuging at 4 °C at 2500×g for 5 min. The supernatant was decanted into 5-ml vials. The procedure was repeated five times, the supernatants pooled for each sample and stored at −80 °C until further processing.
Since measured phenolic contents were very low, the possibility that the polyphenols had been too much diluted was tested by a repetition on samples of the same origin with only two extraction steps. The values were slightly lower as would be expected, but confirmed the data shown in Fig. 4b.
Stable isotope composition
All determinations were performed on finely ground powder (Micro-Dismembrator, see above) of either lyophilized aPOM or lyophilized disks (diameter 25 mm) that had been punched out along algal blades of A. esculenta, S. latissima and L. digitata (n = 6 of each species). Values of disks punched out with a cork borer at <5 cm from stipe/blade transition (meristematic tissue), mid and end of blade were combined to get an average isotope value for each specimen.
Pilot experiments showed that acidification with HCl to remove CaCO3 did not change the carbon values, which would have been the case had there been any inorganic carbon present, but had an erratic effect on nitrogen values. Therefore, all data presented here were obtained without acidification of the samples.
Determination of stable isotope composition, 13C/12C for carbon and 15N/14N for nitrogen, was performed using an elemental analyzer (Flash EA 1112, Thermo Scientific, Milan, Italy) coupled with an isotope ratio mass spectrometer (Delta V Advantage with a Conflo IV interface, Thermo Scientific, Bremen, Germany) at the LIENSs stable isotope facility of the University of La Rochelle, France, for samples directly taken from the algae and at AWI Bremerhaven, Marine Chemistry, for aPOM. Results are expressed in the δ unit notation as deviations from standards (Vienna Pee Dee Belemnite for δ13C and N2 in air for δ15N) following the formula: δ13C or δ15N = [(R sample/R reference)−1] × 103, R being either 13C/12C or 15N/14N. Calibration was performed with reference materials [USGS-24 (LIENS), IAEA CH7 (AWI) and IAEA-CH6, −600 for carbon; IAEA-N1, −N2, (plus −N3, −600 at LIENS) for nitrogen]. Analytical precision was screened with an internal standard of acetanilide (Thermo Scientific) and was found for both isotopes to be <0.15 (LIENS) or <0.20 (AWI). Recent intercalibration between both laboratories showed very good comparability of results.
Light microscopy
The condition of aPOM tissue as well as the medium background was regularly monitored during the culture period. Every second day representative photographs were taken on a Leica DM2000 compound photomicroscope (Leica Microsystems, Wetzlar, Germany) or a Zeiss Axioskop 40 with Zeiss Axiocam ERC5 s (Zeiss, Göttingen, Germany).
Conduction of feeding experiments
Feeding experiments are considered preliminary, because the number of available consumers was limited to three specimens in the case of Mysis oculata (Mysidae) and the feeding period of Hiatella arctica (Bivalvia) was only 1 week.
Individuals of M. oculata (30–32 mm long) were placed separately in 100 ml aerated seawater in glass bowls (diameter 9 cm) and starved for 2 days. After water exchange they were fed ‘ad libitum’ with Alaria aPOM previously cultured for 7 days. After 2 days fecal strings were recovered from the bowls, squashed on a glass slide and examined microscopically.
With 2 days of starvation in advance, one side of each of 14 H. arctica (shell width 8.8–19 mm) was glued on a plastic button with Loctite 454 instantaneous gel glue (Henkel, Munich, Germany), which was reversibly mounted on perforated stainless steel stages hung into a mouth blown hatching jar. The jar was filled with 7 L of filtered seawater and Alaria aPOM, previously cultured for 7 days, was added ad libitum. The POM was kept suspended by aeration from the jar bottom. Light conditions were relatively dim but irregular in a shared 4–5 °C environmental room. After 1 week, with aPOM still visible in suspension, bivalves were dissected to remove their intestinal tract and the remains frozen for further analysis. Three of the dissected guts were squashed on a glass slide and viewed microscopically.
Statistics
Unless mentioned otherwise data are shown as mean and standard error of the mean. All statistical analyses were performed using the GraphPad Prism Software, version 6.03 (GraphPad Software, Inc., USA). Following a Bartlett’s or Brown-Forsythe test for variances, a one-way ANOVA with post hoc Tukey’s test was used to detect significant differences between samples of different years or culture days, respectively. In the case of stable isotope analysis, statistical tests were performed separately for each isotope.
Results
To assess the importance of algal material entering the water column by kelp blade erosion, we measured the loss of blade area during a period of 19 days in August 2012 (Fig. 1). During this period 25–77 % of the blade area had decayed and the eroded particles and fragments been released to the water column. The erosion of the blade area is also reflected in the low growth rate of the blade in August compared to June. Blade length measurements showed low growth rates in June and even negative values in August (Fig. 2). Meristematic growth was significantly higher in June compared to August (Fig. 2a). Meristematic growth was reduced in August and was accompanied by erosion of up to 76.7 % of the blade area (mean ± standard deviation (sd) 2012: 56.2 ± 15.4; 2013: 14.6 ± 8.8) within 19 days (Fig. 1). In June apical loss of blade tissue was more than compensated for by meristematic activity in all individuals, while in August meristematic growth could not replace blade erosion (Fig. 2b). The percentage blade loss in A. esculenta during August was independent of original blade length (Fig. 1). However, a comparison of two size classes in June (blade lengths 45–62 cm vs. 70–96 cm; data not shown) revealed that the smaller size class had a slight, but significantly (p < 0.05) higher relative growth rate in the meristem as well as in the overall blade length.
Artificially produced fractions of A. esculenta changed their characteristics in the course of 14 days of culture and macroscopically eventually resembled “marine snow”. Figure 3a illustrates relatively high chlorophyll fluorescence (Fv/Fm; color coded) of 3 ml subsamples on day 1 of culture and Fig. 3b relatively low Fv/Fm values determined on GF-filters that were used to collect the suspended aPOM on day 14. On day 6 of culture maximum quantum yield (Fv/Fm), an indication for photosynthetic activity of the algal fragments had decreased by about 50 % as shown in Fig. 3c. At the beginning of the experiment, values of Fv/Fm were in the range of corresponding values from intact algae at the same distance to the stipe/blade transition (mean ± sem, 0.520 ± 0.062, n = 14). Other parts of the blade showed different values.
Alaria esculenta. a 3 ml aPOM in Imaging-PAM on day 1 of culture. Color-coded picture at relatively high Fv/Fm. b Filter covered with aPOM, day 14 of culture. Color-coded picture at relatively low Fv/Fm. Circles delineate areas of integrated measurement of fluorescence. c Course of photosynthetic quantum yield with culture time, showing means and standard error of the mean (n = 3 or 4)
To assess the nutritional value and palatability of aging Alaria-aPOM, C/N ratio and phlorotannin content were determined. As Fig. 4a shows, the C/N ratio follows an exponential curve with slower change after day 6 when photosynthetic activity has practically ceased.
Alaria esculenta. aPOM a Carbon/Nitrogen ratio with aging culture (individual values; n = 3; Nonlinear regression according to Akaike’s criterion: y = 16.85−0.1752x + 14.88; R 2 = 0.911). b Phlorotannin concentrations in aging culture (individual data points, mean ± standard deviation; n = 3–4). C Phlorotannin content from same blade area prior to mincing (control; day 0; n = 4), D from obviously decaying parts of the blade (day 0; n = 2)
Fresh blade tissue from the same blade region as the aPOM material, directly freeze dried, had a phlorotannin content of 1.57 ± 0.62 % dw (mean ± SD; % dry weight;) (Fig. 4b, C; n = 4), while the phlorotannin content of Alaria-aPOM was below 1 % of dry weight, even on the first day of POM culture (Fig. 4b) and decreasing to the minimum level of about 0.24 % dw toward day 6. Two pieces of visibly decaying blade areas had an intermediate phlorotannin content of 0.746 and 0.527 % dw (Fig. 4b, D).
In an attempt to characterize the isotopic position of A. esculenta within the kelp population of Kongsfjorden, samples of S. latissima and L. digitata were analyzed in addition to A. esculenta. In this case isotopic data of blade material from meristem, mid blade and decaying tip were integrated. Table 1 shows that the three large kelp species form a solid baseline with no significant difference in δ15N. Contrastingly, all three are significantly different in their δ13C values, even though there is considerable variation in carbon fractionation within blades of the same species (Buchholz C, unpublished data).
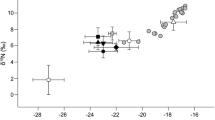
The kelp with the thinnest blade, A. esculenta, was chosen for POM experiments. In the mixture of blade tissue from 30 specimens minced to artificial POM, stable isotope composition was found to change during aging in culture (Fig. 5). In the beginning, values resemble those from fresh tissue. Later, following culture day 6, the samples became enriched with heavier nitrogen as well as with heavier carbon isotopes. There are no significant differences between culture days 2 and 4 and 6–14. Considering the low number of n = 3, the significant difference (p = 0.03) in δ13C between day 1 and day 2 is probably not realistic.
During cultivation of Alaria aPOM, random subsamples were regularly examined by light microscopy with and without phase contrast: Even after 14 days of culture without exchange of the initially filtered seawater, the number of visible bacteria was very limited. They were only found at the violated fringes of algal particles, while the background between particles remained clear. At least two species of flagellates (cell bodies mostly spherical, diameter approx. 5 µm or elongate approx. 3.2 µm long with 2 heteromorph flagella) were more conspicuous and their number increased during culture. Diatoms and cyanobacteria were observed in low numbers, while ciliates and ameba were missing. A. esculenta blade material from August 2012 as well as August 2013 was characterized by many tufts of small brown epi- or endophytic algae altering the surface of their hosts. Most of them were Laminariocolax aecidioides (Rosenvinge) (Fig. 6a, b) and other members of the order Ectocarpales, e.g., Ectocarpus spp. and Pylaiella spp. (Fredriksen et al. 2014). In addition, various morphs of filamentous, probably also brown algae, were observed. Contrastingly, blade material from June 2011, prepared the same way was not contaminated by epi- and endophytes and had a typical and largely uniform A. esculenta aspect concerning size and shape of cells and chloroplasts (Fig. 6c).
Typical microscopic view of Alaria esculenta blade particles from aPOM: a, b in August, c in June. Laminariocolax aecidioides thalli and hairs on Alaria particles a at small magnification, b at higher magnification. One of the typical very numerous epi- and endophytic tufts found in August 2012 as well as August 2013. Several other filamentous epiphytic species were less conspicuous. c Typical aspect of “clean” Alaria blade epidermis as found in June 2010 and June 2011
The palatability of aPOM was tested in feeding experiments with the mysid M. oculata and the bivalve H. arctica. The mysids had almost completely processed the offered aPOM into fecal strings after 2 days already. Feces of Hiatella could not be distinguished in the present setup, but the intake of algal particles could be shown after 1 week. Both animals ingested A. esculenta aPOM, since more or less intact algal particles could be found in the feces of the mysid and the small intestine of the bivalve (Fig. 7).
Discussion
Even without great storms that can result in dislodgement of whole kelp thalli (Dexter and Scheibling 2012) that will subsequently be disintegrating, macroalgal detritus is continually produced in A. esculenta via blade erosion (Figs. 1, 2; see also Mann 1973, 1988). The amount produced varies between individuals and species, with season and environmental conditions of the particular year. Nevertheless, every year the complete blade material is eventually released into the water column. While complete kelp thalli, that are regularly found by divers in sea floor depressions, can take a long time to disintegrate at low temperatures (Zielinski 1981; Brouwer 1996), smaller fragments with a relatively larger surface area will be subjected to a faster aging process: They become light-weight and more easily re-suspended and therefore available as potential food items for suspension feeders and bottom feeders (Krumhansl and Scheibling 2012; Leclerc et al. 2013a).
Blade growth and erosion
As was shown in our field experiment under the relatively gentle conditions of floating in the water column, not being abraded on hard bottom by wave action nor being shaded by other algae, A. esculenta blades eroded to different extents both in spring and late summer (Fig. 2). Relative growth rates would have to be the same for meristematic growth (Fig. 2a) and total blade length (Fig. 2b), if all tissue was retained. Yet this is not the case: Growth rates of phylloid lengths are considerably lower than meristematic ones even in June, the spring period of most intensive gain of thallus tissue. In late summer, August 2012 and 2013 the reduced meristematic growth could not compensate erosion and relative growth rates became negative. The loss of blade area not only at the algal apex but also far down the lateral parts of the blades was often conspicuous (Fig. 1). The differences in mean area loss between August 2012 and August 2013 are probably due to variations in the environmental conditions of the year: Mean sea surface temperatures in the area, e.g., had fallen from the maximum in June/July one more degree centigrade in August 2012 compared to August 2013 (data from NASA GES DISC; Time series Area Statistics: http://giovanni.sci.gsfc.nasa.gov/giovanni/). Moreover, the experimental algae in 2013 were larger and had a less worn appearance at the start of the experiment (3.08.2013) than those of 2012 (13.08.2012). Two weeks later in 2013 they were probably in a comparable state. By winter, all blade tissue except for meristems is usually returned to the water column and becomes detritus as could already be anticipated from thalli cautiously brought to the laboratory and carefully re-immersed in water: in late August the blade fringes dissociated into pieces of various sizes. The data obtained here on blade formation and erosion complement the available data on the phenology of A. esculenta (Olischläger and Wiencke 2013). Concerning seasonal differences in growth rates, Kain (1989) calculated approx. 95 % of change between seasons (percentage of the maximum) for Alaria marginata at 50°N and temperatures of approx. 5 °C. Alaria esculenta collected near Dunstaffnage in NW Scotland contained 8 % less Alginate in July than in March (Schiener et al. 2015), which may also play a role in Alaria specimens getting more brittle and more disposed to erosion toward autumn.
Photosynthetic properties of experimental POM from A. esculenta
The question of how long algal pieces released into the water can still be considered functionally algal tissue and not exclusively substrate for microorganisms was tackled with artificially produced Alaria particulate organic matter (aPOM). The functioning of the photosystem II (PSII) was tested at different days of culture before the respective particles were retrieved on pre-combusted GF-C filters. An Imaging-PAM (Walz) was used for the first time on a suspension of small algal pieces. Measurements of Fv/Fm, the maximum quantum yield of photosystem II, helped as a proxy to assess photosynthetic activity, even on small 3 ml subsamples of the cultures. Likewise and somewhat easier this could be measured on the filters, even though they had been shortly rinsed with deionized water (Fig. 3).
Fv/Fm values are not necessarily the same over the whole extension of a blade (own observations, unpublished): Undisturbed meristem and adjacent young tissue had a relatively high Fv/Fm value of 0.7, while meristematic tissue shredded to POM fell into the range of shortly cultured aPOM. The starting values of Alaria aPOM corresponded to values measured in intact algae at the same distance from the meristem. After 6 days of culture the particles had obviously lost their autotrophic characteristics and became substrate to be remineralized by microorganisms. Culture conditions possibly accelerated this kind of decay, which in the field with more water exchange and natural light cycles may proceed more slowly. Season and thallus age at the beginning of the experiment are expected to also play a role in the speed of decay. Further experiments will be necessary to define these factors.
Food quality of A. esculenta aPOM
In some other areas of the ocean sea urchins, snails and crustaceans are major consumers of large quantities of fresh seaweeds. However, this is not true for the Arctic Kongsfjorden (Wessels et al. 2006, Wesławski, pers. comm.). It may be different with degraded algal material: Following the loss of photosynthetic activity, the ratio of carbon to nitrogen (C/N) became smaller (Fig. 4a) and reached a value below 20 at day 8 of culture. C/N values of about 17 (Russel-Hunter 1970, cited by Norderhaug et al. 2003) have been considered the upper limit of palatability for potential consumers of macroalgal POM. Later experiments demonstrated that neither C/N ratios >25 nor very low ones (<4) were beneficial for amphipod growth (Norderhaug et al. 2003, 2006). The C/N ratios obtained in Alaria aPOM seem to reach acceptable values after 6–8 days, when colonization with microbes has commenced. If carbon and nitrogen contents are considered separately (sem1), it becomes obvious that not only nitrogen is increasing but also carbon decreasing during culture. This may be due to a gradual degradation of carbon-rich polysaccharides like alginic acid and laminarin from the broken up cells at the fragment fringes. Loss of tannins is probably less important, because of their small quantity (algaeBASE http://seaweed.ie/nutrition/index.php).
Yet the phlorotannin content of macroalgal detritus is the second factor commonly observed as a measure for palatability. Unlike Duggins and Eckman (1997) and Norderhaug et al. (2003) Sosik and Simenstad (2013) found that phlorotannin content of kelps such as Agarum fimbriatum and Saccharina subsimplex did not decrease during decomposition and attributed that to their use of whole blades as opposed to small or pulverized kelp fragments of the previous publications. For Laminaria hyperborea, a kelp characterized by relatively low phlorotannin levels, a content of <0.5 % dw was reported after 3 days leaching in seawater (Norderhaug et al. 2006). The authors did not consider that of much importance for palatability. The values of aPOM reported in Fig. 4b were at a low level even on day 1 of culture and sank within 6–8 days to the probably negligible 0.2 % dw. Generally, phlorotannin levels vary not only with species but also in different tissues of the same species and as a function of environmental conditions as described by Van Alstyne et al. (1999). The values the latter authors found in the genus Alaria are well comparable to the values for larger pieces (disks) of A. esculenta blades reported here (Fig. 4b, C). The intermediate values of visibly decaying blade areas (Fig. 4b, D) indicate that the experimental POM preparation might have been relatively close to natural macroalgal tissue in the process of being released from the blade to become POM. The decrease to the very low phlorotannin content in aPOM (Fig. 4b) is probably due to a loss of physodes during the mincing process itself, when cell walls are destroyed and to subsequent leaching from the ruptured cells. That is in accordance with experiments by Duggins and Eckman (1997), who found polyphenol content of A. fimbriatum and Laminaria groenlandica to be lowered by 50 % 24 h after grinding.
A. esculenta: isotope composition
As a first assessment and before we could investigate the isotope composition of particles of all major kelps, we tried to get an idea of the isotopic position of A. esculenta in the Kongsfjord kelp community from complete blades of two additional major kelp species, namely L. digitata, and S. latissima. Data are listed in Table 1. Pooled material along the blade gradient showed that in terms of nitrogen composition, all three kelps fall in the same range and therefore constitute a good first baseline for studies of trophic levels in the system at least at Hansneset. Carbon composition is significantly different between the three species despite of all intra-specific variability. Since the algae were collected from the same location (Hansneset) and the same depth (5 m), a species-specific carbon metabolism could be responsible. Enrichment in heavier carbon isotopes corresponds to increasing blade thickness between the three species. Their growth strategies being comparable, they nevertheless have a slightly different phenology, which may also explain some different carbon composition.
The second step was to test whether eroded kelp particles maintain the same isotopic characteristics as fresh algal tissue. As soon as macroalgal particles have entered the water column sinking eventually to the bottom, probably being re-suspended at times, it is difficult to identify these particles. Kaehler et al. (2006) examined water samples and dredged material under a light microscope and defined “angular fragments” as Macrocystis laevis fragments by means of exclusion of other possibilities. Usually, it is almost impossible to sort out and further characterize macroalgal particles from the material retrieved in sediment traps. To our knowledge only Fischer and Wiencke (1992) managed to identify fragments of brown algae in microscopical analyses of sediment samples. Our experiment with artificial POM was supposed to help filling the gap, since the contribution of macroalgal detritus to mixed diets is widely discussed (see above), but this part of the baseline in food web studies is not yet known well enough (see also Renaud et al. 2011, 2015).
With the experience of great intraspecific variation (see also Fredriksen 2003), great care was taken to keep the starting material for POM culture as homogeneous as possible (see also “Material & methods” section): During our culture of A. esculenta artificial POM isotopic composition changed from less to more enriched in heavier isotopes in δ13C as well as in δ15N. Longer culture time means increasing decomposition of the algal tissue and a similar phenomenon was already found by Fischer and Wiencke (1992) for Antarctic Phaeophyceae that had less negative δ13C values in decomposed parts of the blade. The two groups of data in Fig. 5 correspond to diminishing photosynthetic quantum yield and may, like the C/N data, be an indication of different organisms taking over (Goecke et al. 2010; Wahl et al. 2012). This requires further investigation by microbiologists, since in contrast to studies of North Sea kelps (L. digitata, S. latissima), (C Buchholz unpublished) no large increase in bacterial numbers could be observed.
Comparing the isotope composition of Alaria aPOM in the course of culture to the freshly excised blade tissue of the other species (Table 1; Fig. 5) reveals that δ13C of POM is approaching the “fresh” values of S. latissima and L. digitata, while δ15N becomes distinctly higher, most likely due to the above mentioned microbial takeover. With the importance of macroalgal biomass probably increasing for Arctic benthic consumers (McMeans et al. 2013), it will be necessary to identify the characteristics of aging POM separately for each kelp species to know whether they can be summarized as typically “aged kelp detritus”.
Light microscopic observations on A. esculenta
The only visibly increasing numbers of microbes on the aPOM were some species of flagellates. The situation may be different in unfiltered seawater, where microbes are probably transferred from “marine snow” to algal particles they accidentally contact. In our setup, microorganisms apart from bacteria must have been introduced into the system with the algal tissue, since the seawater used for culture was filtered. The three washing steps of the individual tissue disks before mincing will certainly not have cleaned them completely from all microbes.
The frequent occurrence of small brown algal endo- and/or epiphytes in August (Fig. 6) suggests to consider A. esculenta an Alaria-biocenosis that is eventually fractioned and decomposed. The relative portion of algal particles shifts from Alaria to other smaller algal species thus probably changing the biochemical properties of the potential food for consumers.
First feeding experiments
Feeding experiments with the crustacean M. oculata and the bivalve H. arctica showed both organisms to readily ingest Alaria aPOM (Fig. 7), while it is not yet known to what extent they metabolize it. More extensive feeding experiments, offering aPOM made from A. esculenta, must be conducted to eventually show the effect of a known macroalgal diet on the isotopic composition in mysids, amphipods, and bivalves as potential primary consumers. The results of this study will be incorporated in the food web model presently elaborated by Paar et al. (personal communication). Dethier et al. (2014) tested assimilation efficiency and several biochemical factors of two kelp species with clearly different polyphenolic characteristics. Acceptance by and nutritional value for consumers varied dependent on biochemical composition of the algae and their degree of aging as well as whether the consumer was an isopod, copepod or sea urchin. To get a good idea of which macroalgal species in which state of decay makes proper food for suspension or bottom feeding consumers, it can be expected that the main species of macroalgae found in a certain area will have to be tested individually for their food quality.
Related to the initially formulated hypotheses, we can summarize: (1) particles are released into the water column by distal erosion from intact A. esculenta, even in the period of intensive growth (June). The erosion rate increased considerably in August so that the blades should be reduced to the meristem before winter. (2) In a laboratory culture simulating the fate of algal particles in the water column, artificial POM made from Alaria blades lost its photosynthetic activity after 6 days in culture, reached a C/N value of reasonable food quality after 8 days at which time they also had a negligible phlorotannin content. A. esculenta artificial POM isotopic composition changed from less to more enriched in heavier isotopes in δ13C as well as in δ15N between day 4 and day 6 of culture. (3) Two potential consumers of macroalgal POM, a mysid and a bivalve, ingested offered aPOM cultured for 7 days. Whether they can metabolize macroalgal material will have to be shown in more extensive experiments. (4) A first attempt to establish an isotopic baseline for food web studies showed that three kelp species (A. esculenta, S. latissima and L. digitata) collected at the same location showed a comparable nitrogen composition, while carbon composition was distinctly different. To establish trophic levels in the food web, the finding that δ15N was considerably higher in aged aPOM than in fresh material calls for a more detailed investigation of associated microorganisms and a comparison of aPOM of the different macroalgal species.
References
Bartsch I, Paar M, Fredriksen S, Schwanitz M, Daniel C, Wiencke C (2015) Changes in kelp forest biomass and depth distribution in Kongsfjorden (Spitsbergen) between 1996–1998 and 2012–2014 reflect Arctic warming. Polar Biol (in review)
Brouwer PEM (1996) Decomposition in situ of the sublittoral Antarctic macroalga Desmarestia anceps montagne. Polar Biol 16:129–137
Bustamante RH, Branch GM (1996) The dependence of intertidal consumers on kelp-derived organic matter on the west coast of South Africa. J Exp Mar Biol Ecol 196:1–28
Dethier MN, Brown AS, Burgess S, Eisenlord ME, Galloway AWE, Kimber J, Lowe AT, O’Neil CM, Raymond WW, Sosik EA, Duggins DO (2014) Degrading detritus: changes in food quality of aging kelp tissue varies with species. J Exp Mar Biol Ecol 460:72–79
Dexter KF, Scheibling RE (2012) Hurricane-mediated defoliation of kelp beds and pulsed delivery of kelp detritus to offshore sedimentary habitats. Mar Ecol Prog Ser 455:51–64
Duarte CM, Cebrián J (1996) The fate of marine autotrophic production. Limnol Oceanogr 41:1758–1766
Duggins DO, Eckman JE (1997) Is kelp detritus a good food for suspension feeders? Effects of kelp species, age and secondary metabolites. Mar Biol 128:489–495
Duggins DO, Simenstad CA, Estes JA (1989) Magnification of secondary production by kelp detritus in coastal marine ecosystems. Science 245:170–173
Dunton KH (2001) δ13C and δ15N measurements of antarctic peninsula fauna: trophic relationships and assimilation of benthic seaweeds. Am Zool 41:99–112
Dunton KH, Schell DM (1987) Dependence of consumers on macroalgal carbon (Laminaria solidungula) in an arctic kelp community. δ13C evidence. Mar Biol 93:615–625
Fischer G, Wiencke C (1992) Stable carbon isotope composition, depth distribution and fate of macroalgae from the Antarctic Peninsula region. Polar Biol 12:341–348
Folin O, Ciocalteu V (1927) On tyrosine and tryptophane determinations in proteins. J Biol Chem 73:627–650
Fredriksen S (2003) Food web studies in a Norwegian kelp forest based on stable isotope (δ13C and δ15N) analysis. Mar Ecol Prog Ser 260:71–81
Fredriksen S, Bartsch I, Wiencke C (2014) New additions to the benthic marine flora of Kongsfjorden, western Svalbard, and comparison between 1996/1998 and 2012/2013. Bot Mar 57:203–216
Goecke F, Labes A, Wiese J, Imhoff JF (2010) Chemical interactions between marine macroalgae and bacteria. Mar Ecol Prog Ser 409:267–299
Hop H, Wiencke C, Vögele B, Kovaltchouk N (2012) Species composition, zonation, and biomass of marine benthic macroalgae in Kongsfjorden, Svalbard. Bot Mar 55:399–414
Kaehler S, Pakhomov EA, McQuaid CD (2000) Trophic structure of the marine food web at the Prince Edward Islands (Southern Ocean) determined by δ13C and δ15N analysis. Mar Ecol Prog Ser 208:13–20
Kaehler S, Pakhomov EA, Kalin RM, Davis S (2006) Trophic importance of kelp-derived suspended particulate matter in a through-flow sub-Antarctic system. Mar Ecol Prog Ser 316:17–22
Kain JM (1989) The seasons in the subtidal. Br Phycol J 24:203–215
Kelly JR, Krumhansl KA, Scheibling RE (2012) Drift algal subsidies to sea urchins in low-productivity habitats. Mar Ecol Prog Ser 452:145–157
Krumhansl KA, Scheibling RE (2011) Detrital production in Nova Scotian kelp beds: patterns and processes. Mar Ecol Prog Ser 421:67–82
Krumhansl KA, Scheibling RE (2012) Production and fate of kelp detritus. Mar Ecol Prog Ser 467:281–302
Krumhansl KA, Lauzon-Guay JS, Scheibling RE (2014) Modeling effects of climate change and phase shifts on detrital production of a kelp bed. Ecology 95:763–774
Leclerc JC, Riera P, Leroux C, Lévêque L, Laurans M, Schaal G, Davoult D (2013a) Trophic significance of kelps in kelp communities in Brittany (France) inferred from isotopic comparisons. Mar Biol 160:3249–3258
Leclerc JC, Riera P, Leroux C, Lévêque L, Davoult D (2013b) Temporal variation in organic matter supply in kelp forests: linking structure to trophic functioning. Mar Ecol Prog Ser 494:87–105
Levinton JS, Ward JE, Shumway SE (2002) Feeding responses of the bivalves Crassostrea gigas and Mytilus trossulus to chemical composition of fresh and aged kelp detritus. Mar Biol 141:367–376
Mann KH (1973) Seaweeds: their productivity and strategy for growth. Science 182:975–981
Mann KH (1988) Production and use of detritus in various freshwater, estuarine, and coastal marine ecosystems. Limnol Oceanogr 33:910–930
McMeans BC, Rooney N, Arts MT, Fisk AT (2013) Food web structure of a coastal Arctic marine ecosystem and implications for stability. Mar Ecol Prog Ser 482:17–28
Miller RJ, Page HM (2012) Kelp as a trophic resource for marine suspension feeders: a review of isotope-based evidence. Mar Biol 159:1391–1402
Norderhaug KM, Fredriksen S, Nygaard K (2003) Trophic importance of Laminaria hyperborea to kelp forest consumers and the importance of bacterial degradation to food quality. Mar Ecol Prog Ser 255:135–144
Norderhaug KM, Nygaard K, Fredriksen S (2006) Importance of phlorotannin content and C:N ratio of Laminaria hyperborea in determining its palatability as food for consumers. Mar Biol Res 2:367–371
Norwegian Polar Institute, Map of Svalbard http://toposvalbard.npolar.no/?lang=en
Olischläger M, Wiencke C (2013) Seasonal fertility and combined effects of temperature and UV-radiation on Alaria esculenta and Laminaria digitata (Phaeophyceae) from Spitsbergen. Polar Biol 36:1019–1029
Orr KK, Wilding TA, Horstmeyer L, Weigl S, Heymans JJ (2014) Detached macroalgae: its importance to inshore sandy beach fauna. Estuar Coast Shelf Sci 150:125–135
Page HM, Reed DC, Brzezinski MA, Melak JM, Dugan JE (2008) Assessing the importance of land and marine sources of organic matter to kelp forest food webs. Mar Ecol Prog Ser 360:47–62
Renaud PE, Tessmann M, Evenset A, Christensen GN (2011) Benthic food-web structure of an Arctic fjord (Kongsfjorden, Svalbard). Mar Biol Res 7:13–26
Renaud PE, Løkken TS, Jørgensen LL, Berge J, Johnson BJ (2015) Macroalgal detritus and food-web subsidies along an Arctic fjord depth-gradient. Front Mar Sci 2:31. doi:10.3389/fmars.2015.00031
Schaal G, Riera P, Leroux C (2010) Trophic ecology in a Northern Brittany (Batz Island, France) kelp (Laminaria digitata) forest, as investigated through stable isotopes and chemical assays. J Sea Res 63:24–35
Schaal G, Riera P, Leroux C (2012) Food web structure within kelp holdfasts (Laminaria): a stable isotope study. Mar Ecol 33:370–376
Schiener P, Black KD, Stanley MS, Green DH (2015) The seasonal variation in the chemical composition of the kelp species Laminaria digitata, Laminaria hyperborea, Saccharina latissima and Alaria esculenta. J Appl Phycol 27:363–373
Sokołowski A, Szczepańska A, Richard P, Kędra M, Wołowicz M, Węsławski JM (2014) Trophic structure of the macrobenthic community of Hornsund, Spitsbergen, based on the determination of stable carbon and nitrogen isotopic signatures. Polar Biol 37:1247–1260
Sosik EA, Simenstad CA (2013) Isotopic evidence and consequences of the role of microbes in macroalgae detritus-based food webs. Mar Ecol Prog Ser 494:107–119
Van Alstyne KL, McCarthy JJ III, Hustead CL, Kearns LJ (1999) Phlorotannin allocation among tissues of northeastern Pacific kelps and rockweeds. J Phycol 35:483–492
Wahl M, Goecke F, Labes A, Dobretsov S, Weinberger F (2012) The second skin: ecological role of epibiotic biofilms on marine organisms. Front Microbiol 3:292. doi:10.3389/fmicb.2012.00292
Wessels H, Hagen W, Molis M, Wiencke C, Karsten U (2006) Intra- and interspecific differences in palatability of Arctic macroalgae from Kongsfjorden (Spitsbergen) for two benthic sympatric invertebrates. J Exp Mar Biol Ecol 329:20–33
Yorke CE, Miller RJ, Page HM, Reed DC (2013) Importance of kelp detritus as a component of suspended particulate organic matter in giant kelp Macrocystis pyrifera forests. Mar Ecol Prog Ser 493:113–125
Zielinski K (1981) Benthic macroalgae of Admiralty Bay (King George Island, South Shetland Islands) and circulation of algal matter between the water and the shore. Pol Polar Res 2:71–94
Acknowledgments
This research was performed at the Ny Alesund International Research and Monitoring Facility on Spitsbergen (Svalbard) as part of the KOL 06 long term project. We are grateful for the logistic support from all AWIPEV teams. We wish to thank Max Schwanitz and several AWI diving teams for reliably supplying us with macroalgae. Martin Graeve and Benoit Lebreton deserve thanks for analyzing stable isotopes and for the additional effort of intercalibration. Ines Stuhldreier and Ulrike Dietrich were dedicated and cheerful student helpers at Ny Alesund. Claudia Daniel was a great relief with all the tedious laboratory work. Michael Greenacre, Paul Renaud and two more anonymous reviewers helped to improve the manuscript. We highly appreciate the time they invested and their good advice!
Author information
Authors and Affiliations
Corresponding author
Additional information
This article belongs to the special issue on the “Kongsfjorden ecosystem—new views after more than a decade of research”, coordinated by Christian Wiencke and Haakon Hop.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Buchholz, C.M., Wiencke, C. Working on a baseline for the Kongsfjorden food web: production and properties of macroalgal particulate organic matter (POM). Polar Biol 39, 2053–2064 (2016). https://doi.org/10.1007/s00300-015-1828-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-015-1828-3