Abstract
Growing evidence suggests that telomeres, non-coding DNA sequences that shorten with age and stress, are related in an undefined way to individual breeding performances and survival rates in several species. Short telomeres and elevated shortening rates are typically associated with life stress and low health. As such, telomeres could serve as an integrative proxy of individual quality, describing the overall biological state of an individual at a given age. Telomere length could be associated with the decline of an array of physiological traits in age-controlled individuals. Here, we investigated the links between individuals’ relative telomere length, breeding performance and various physiological (body condition, natural antibody levels) and life history (age, past breeding success) parameters in a long-lived seabird species, the king penguin Aptenodytes patagonicus. While we observed no link between relative telomere length and age, we found that birds with longer telomeres arrived earlier for breeding at the colony, and had higher breeding performances (i.e. the amount of time adults managed to maintain their chicks alive, and ultimately breeding success) than individuals with shorter telomeres. Further, we observed a positive correlation between telomere length and natural antibody levels. Taken together, our results add to the growing evidence that telomere length is likely to reflect individual quality difference in wild animal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In evolutionary ecology, one important objective is to understand the causes for variation in individual fitness. Recently, it has been suggested that telomeres may reflect reproductive performances (Bauch et al. 2013) and affect individual survival (Haussmann et al. 2005; Pauliny et al. 2006; Bize et al. 2009; Barrett et al. 2013; Boonekamp et al. 2014). Telomeres are non-coding, highly conserved, DNA sequences that cap and protect the genetic integrity of eukaryotic chromosomes (Blackburn 1991; Monaghan and Haussmann 2006). Those sequences shorten progressively through successive DNA replication cycles until they reach a critical length causing chromosome instability, cell senescence and ultimately cell death (Blackburn 2000). Although specific restoration mechanisms exist (for instance, the enzyme telomerase works to restore eroding telomeres; Haussmann et al. 2007), studies have shown that telomeres generally shorten as individuals age (Pauliny et al. 2006; Salomons et al. 2009; Heidinger et al. 2012; Barrett et al. 2013; Bauch et al. 2013; Boonekamp et al. 2014). In addition, even though telomere length is partly heritable (Slagboom et al. 1994; Reichert et al. 2015), the high inter-individual variability observed at birth (Okuda et al. 2002), and amongst individuals of the same age (Slagboom et al. 1994), suggests that telomere length is also determined by extrinsic environmental factors. For instance, oxidative damage is known to affect telomere length (von Zglinicki 2002) in both early life and adulthood (Epel et al. 2004; Valdes et al. 2005; Puterman et al. 2010; Blackburn and Epel 2012; Geiger et al. 2012; Boonekamp et al. 2014), and between-individual differences in telomere length as a possible consequence of early life stress can be conserved through age (Heidinger et al. 2012).
In wild animals, variation in telomere length may allow assessing individual quality (Wilson and Nussey 2010), i.e. inter-individual differences in the ability to cope with external and/or internal challenges that could be associated with variability in a suite of phenotypic traits (immunity, body condition, stress resilience, foraging performances) and linked to fitness. For instance, differences in individual telomere length and erosion rates have been linked to individual differences in breeding performances and breeding effort in species as different as dunlins Calidris alpina (Pauliny et al. 2006), leatherback turtles Dermochelys coriacea (Plot et al. 2012) or common terns Sterna hirundo (Bauch et al. 2013). Whereas mechanisms of cell senescence may naturally be associated with a decline in individual breeding performances, telomere length and shortening rates should also be concomitant with the deterioration of main organism’s functions (Hughes and Reynolds 2005). To date however, how inter-individual variation in telomere length relates to variability in physiological traits associated with individual performance (e.g. body condition, immunity) remains to be determined in natural conditions.
In the present study, we examined the links between relative telomere length, individual body condition and immunity in the king penguin, long-lived seabird breeding in subantarctic environmental conditions. In addition, we assessed the links between relative telomere length and fitness proxies (i.e. breeding initiation date, the amount of time parents maintained their chick alive, and ultimately breeding success in rearing chick until fledging) in those colonial breeders. Long-lived species, including seabirds, provide especially good models for investigating the relationship between telomere length and individual performances because it could be visible on a long time scale. As central place foragers, the energetic commitment to reproduction is generally high in seabirds (Lack 1968) and harsh conditions during breeding have been suggested to affect telomere shortening rates both in captive (Reichert et al. 2014a) and in the wild (Mizutani et al. 2013; Young et al. 2013). Here, in 50 adult king penguins of known age (5–9 years old) belonging to a population continuously monitored since 1998, we specifically tested whether telomere length was related to birds’ body condition, immune status and breeding performance, all measured in the same year. Importantly, the relatively narrow age range of birds we monitored allowed us to investigate the specific importance of telomere length rather than chronological age per se in accounting for individual variation in physiological and breeding parameters. We predicted that, if telomere length indeed reflects an individual’s capacity to cope with life stress, it should be related to among-individual variations in body condition, immunity and fitness. In addition, with the idea that individual quality is consistent to some extent (Wilson and Nussey 2010), we expect inter-individual differences in telomere length to also reflect inter-individual variations in past breeding history.
Materials and methods
Study species
This study was carried out in the king penguin colony of ‘La Grande Manchotière’, on Possession Island, Crozet Archipelago (46°24′S 51°45′E). During the incubation and chick-brooding phases of the 2009 breeding season, 50 known-aged king penguins (21 birds in incubation and 29 birds in brooding) were studied (n = 13, 26 and 11 for 5-, 8- and 9-year-old birds, respectively). These birds were part of a long-term monitoring programme started in 1998. They were implanted with subcutaneous passive transponder tags (0.8 g) when they were 10-month-old chicks, just before fledging (Gendner et al. 2005; Le Vaillant et al. accepted). At that time, a blood sample was also collected from each individual for subsequent sex determination (methods adapted from Griffiths et al. 1998). King penguins breed for the first time between 3 and 6 years old (Weimerskirch et al. 1992). Whereas, their longevity is unknown, in our database the oldest transponder-tagged individuals were implanted as breeding adults in 1991, and thus, they were more than 22 years old in 2009. Consequently, it is likely the studied birds represented first or middle-aged breeders and had no risk to be senescent. Indeed, we showed that the breeding success is comparable between 6 and 11 years old, while in 4- and 5-year-old birds it is significantly lower (Le Vaillant et al. accepted). Transponder-tagged birds were detected by an automatic identification system (Gendner et al. 2005) when they entered and exited the colony during the breeding season. By monitoring the duration tagged birds spent in the colony and at sea, the system enabled us to reconstruct the breeding pattern of each individual, and to determine the number of successful breeding attempts throughout its life (Descamps et al. 2002; Gauthier-Clerc et al. 2004; Saraux et al. 2011a). Past breeding success was determined as an index accounting for individual age, i.e. the residuals of a regression of the number of past breeding success on age (Lescroël et al. 2010). As the clutch size of king penguins is one and the annual reproductive success is relatively low (ca. 40 %; Saraux et al. 2011a), especially for young individuals (i.e. 5-year-old penguins; see Le Vaillant et al. accepted), we assessed breeding performances through two different metrics. First, breeding success was defined as a binary variable depending on whether the chick was fledged (success = 1) or not (success = 0). Second, assuming that birds maintaining their chick alive for longer periods are of higher quality, we defined breeding duration as the amount of time penguins maintained their offspring alive from laying to chick fledging or breeding failure. This allowed us to work on a continuous variable (range 22–254 days) providing a more sensitive analysis than the binomial one. However, the above assumption is only reasonable for failed breeders, and we fixed the duration of successful breeders to its mean (i.e. 368 days). This avoids making any assumption on successful breeder quality according to duration, but still indicates that successful breeders are of better quality than failed ones. From individual records of colony attendance, we extracted breeding arrival dates, the breeding date. In king penguins, breeding initiation spreads from October to late February (Barrat 1976). Late breeding has a negative impact on breeding success (Weimerskirch et al. 1992), as late birds having little time to raise the chick before the onset of winter (Weimerskirch et al. 1992; Stier et al. 2014).
In this study, all birds were captured when departing from the colony to feed at sea. This allowed minimizing the disturbance caused to the colony and to the breeding partner and/or chick. A blood sample was obtained (see analysis below), and we measured the body mass, flipper and bill length of each bird to produce an index of body condition (see Saraux et al. 2011b; Viblanc et al. 2012). Briefly, we used a principal component analysis of bill and flipper length to establish a structural size index (SSI). The first principal component of these two parameters explained 76 % of the variation. Body condition was then defined as the residuals of a regression of body mass on SSI.
Blood sample analysis
We used a heparinized syringe to collect 1 mL of blood from the marginal flipper vein of each bird departing for a foraging trip at sea. The blood was immediately centrifuged for 5 min at 6000 RPM, to separate red blood cells (RBC) from plasma, and stored at −20 °C. We extracted DNA from 5 µl of RBC using a commercial kit (DNeasy Blood and Tissue kit, Qiagen). We measured relative telomere length following the procedure of qPCR described by Criscuolo et al. (2009) and previously validated for king penguins (Geiger et al. 2012). A single control gene (Aptenodytes patagonicus zinc finger protein, NCBI accession number AF490194) was defined as a gene non-variable in copy numbers within our population (hereafter non-VCN: Smith et al. 2011). The primer sequences were defined by Primer 3 software as Royal 1: 5′-TACATGTGCCATGGTTTTGC-3′; Royal 2: 5′-AAGTGCTGCTCCCAAAGAAG-3′. Primer concentrations in the final mix were 200 mM for telomere length determination and 300 mM for the control gene. Telomere and control gene PCR conditions were as follows: 2 min at 95 °C followed by 40 cycles of 15 s at 95 °C, 30 s at 56 °C, 30 s at 72 °C and 60 s at 95 °C. We used 2.5 ng DNA per reaction and the BRYT Green® fluorescent probe (GoTaq®qPCR Master Mix, Promega, France). Amplification efficiency of the qPCR run was 105 % (telomere, T) and 100 % (non-VCN gene). Final calculation of telomere length (T/non-VCN ratio) was done following Pfaffl (2001) using the telomere and non-VCN-specific efficiencies of each plate and the qPCR cycle numbers proportional to the individual telomere length (Cq T) or to the number of copies of the non-VCN gene (Cq S). The final measure obtained was then expressed as measure of relative telomere length. Relative telomere lengths ranged between 0.10 and 1.78 T/non-VCN or T/S ratio. Measurements were run as duplicates on a single plate. Intra-assay variability (CV) was 2.11 ± 0.25 and 1.53 ± 0.19 % for the Cq T and Cq S values, respectively, and of 13.63 ± 1.16 % for the final T/S (N = 50 duplicates).
A measure of innate immunity was obtained by determining plasmatic non-specific natural antibody (NAbs) levels (see below) using a hemagglutination test from 25 µl of plasma (Matson et al. 2005). Plasma NAbs are major humoral components of innate immunity (Palacios et al. 2009). The haemagglutination assay was carried out in 96-well plates using a small amount of plasma (50 µl) following Matson et al. (2005). Plasma was introduced into column 1 and serially diluted (1:2) with phosphate-buffered saline (PBS; Sigma-Aldrich, Lyon, France), from column 2 to column 11, with column 12 serving as a negative control (PBS only). A fixed amount of a sheep red blood cell (SRBC) suspension was added to each well, and the plate was then incubated for 90 min at 37 °C. The SRBCs were provided by the slaughterhouse (Haguenau, Alsace, France) under veterinarian authorization and conserved in 50 % Alsever’s solution (Sigma-Aldrich). The SRBCs were washed four times as previously described and re-suspended in 0.1 % PBS (Matson et al. 2005). The plate was tilted to an angle of 45° for 20 min at room temperature to enhance agglutination visualization. The intra-assay variability was 4.9 % (N = 5 duplicates). The agglutination titre (NAb score; i.e. SRBC concentration for which an agglutination reaction was observed) for each individual was scored blindly by the same experimenter (M.L.V.) and reflects levels of NAbs only. Nevertheless, because plasma samples were serially diluted, NAb scores do not directly reflect the plasma concentrations of NAbs.
Statistics
Statistical analyses were conducted using the R 2.15.2 statistical environment (R Development Core Team 2008). The link between NAbs, age, body condition and relative telomere length was asserted using linear models (LMs). Then, variation in arrival date was analysed using a LM with sex, age (as a covariate), relative telomere length, NAbs, body condition, past breeding success and two-way interactions with relative telomere length as independent variables. Non-significant effects were removed sequentially from the model. Finally, we investigated the relationship between relative telomere length, breeding duration (continuous variable; LM) and breeding success (binomial response; generalized linear model, GLM). Sex, age, relative telomere length, NAbs, body condition, past breeding success, arrival date and breeding stage (i.e. incubation vs. brooding; as no effect was found, we do not present it in results) were entered as independent variables, and all two-way interactions with relative telomere length were considered. Non-significant effects were removed sequentially from the model. The most appropriate model was selected using Akaike’s information criterion (AICc; AIC adjusted for small sample size). In general, the model with the lowest AICc was selected, except when ∆AICc < 2. In this case, AICc weights were examined as well as the number of parameters (the most parsimonious models were favoured to avoid overparametrization). Residual normality was asserted using Shapiro–Wilk’s normality test. Significant results for p < 0.05 are reported as mean ± SE.
Results
Individual and physiological parameters
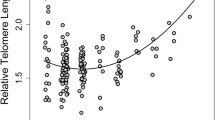
Relative telomere length and NAbs values were positively related (estimate ± SE = 0.11 ± 0.03; LM: F 1, 48 = 11.09, p = 0.002; N = 50 individuals; Fig. 1), suggesting that birds with higher natural antibody levels had longer telomeres. Relative telomere length and body condition were also positively related, though not significantly (estimate ± SE = 0.09 ± 0.05, LM: F 1, 48 = 3.50, p = 0.067). In contrast, we found no cross-sectional relationship between age and relative telomere length (estimate ± SE = 0.01 ± 0.03, LM: F 1, 48 = 0.12, p = 0.727). Finally, relative telomere length and past breeding success were not related (estimate ± SE = 0.30 ± 0.24, LM: F 1, 48 = 1.51, p = 0.226; N = 50 individuals).
Timing of arrival at the breeding site and breeding performances
Model selection retained relative telomere length, sex and age as important variable explaining bird arrival date at the colony for breeding (see online supplementary information Table S1). Relative telomere length had a significant negative effect on arrival date (LM: F 4, 45 = 6.259, p = 0.004; Fig. 2; Table 1), birds with longer telomeres arriving earlier at the colony. For a 10 % increase in relative telomere length, individuals arrived approximately 1 day earlier at the colony. Age also had a significant effect on arrival date (p = 0.002) and interacted significantly with relative telomere length (p = 0.007). Thus, the negative effect of telomere length on arrival date was more pronounced in young birds. In general, males arrived earlier (11.4 days on average) than females to breed (p = 0.030; see Table 1).
Concerning breeding duration, the best model retained age, past breeding success and relative telomere length as important explanatory variables (see online supplementary information Table S2). Both age and past breeding success had significant positive effects on breeding duration considered as a continuous variable (LM: F 3, 46 = 13.67, p < 0.001 and p = 0.008, respectively; Table 2). Relative telomere length also had a significant positive effect on breeding duration (p = 0.041; Fig. 3), confirming the trend previously observed on the binary variable. Breeding success considered as binomial response was explained by age, past breeding success and relative telomere length (see online supplementary Table S3 for model selection). Age and past breeding success had significant positive effects on breeding success when it was considered as a binomial response (GLM: p < 0.001 and p < 0.001, respectively). Whereas we observed a positive trend of relative telomere length on breeding success (p = 0.063) and no significant effect of the breeding date (p = 0.210). The interaction between breeding date and relative telomere length had a positive effect on breeding success (p < 0.001), meaning that the effect of breeding date on breeding success is greater for individual with long telomeres.
Discussion
Controlling for age, we found that king penguins with longer telomeres arrived earlier in the colony to breed, tended to have higher breeding success, and that their chicks survived longer than individuals with shorter telomeres (viz. breeding duration was longer), suggesting a link between telomere length and fitness proxies. We also found that breeding performances (breeding duration and breeding success) were affected by past breeding success. Our results are consistent with several studies on long-lived species, younger breeders and/or breeders with lower quality generally performing less well in terms of breeding than older breeders and/or individuals of greater quality (in seabirds; DeForest and Gaston 1996; Ezard et al. 2007, in mammals; Moyes et al. 2009, 2011; Nisbet and Dann 2009; Froy et al. 2013). Not surprisingly breeding performances were also explained by breeding date initiation as previously reported in king penguin (Weimerskirch et al. 1992; Stier et al. 2014) and other seabird species (DeForest and Gaston 1996; Ezard et al. 2007; McCleery et al. 2008). The positive effect of age we observed on breeding duration (the fact that chicks survived longer for older-aged birds) might be explained by the fact that older and more experienced individual arrived earlier at the breeding colony, possibly being able to establish their breeding territory in higher-quality areas (Bried and Jouventin 2001).
Our results on the positive relationship between relative telomere length and natural antibody levels are novel. Immunity function is strongly related to survival (Møller and Saino 2004), and strong links have also been found between telomere length and individual survival (Bize et al. 2009; Salomons et al. 2009; Geiger et al. 2012; Barrett et al. 2013). Those results suggest that telomere length may have a role in mediating the observed effects of immune function on survival. In human, it has been shown that the rate of telomere length change in leukocyte predicts mortality (Epel et al. 2009). Moreover, telomere shortening is linked to cellular ageing (in vitro and in vivo, see Samassekou et al. 2010) and affected by both environmental and metabolic stress (Voillemot et al. 2012; Nettle et al. 2013; Boonekamp et al. 2014). Whereas positive links between telomere length and fitness are often documented (see references above), the contrary has also been reported. For instance, in a longitudinal study on common terns, Bauch et al. (2013) found a quadratic relationship between telomere loss and the number of raised chicks to day 10. In this case, individuals with the highest reproductive success until day 10 lost fewer telomere base pairs than less successful parents attending only one or two chicks in the nest (Bauch et al. 2013). In addition, individuals performing better over the long term were those with shorter telomeres (Bauch et al. 2013). How may such differences be conciliated? Actually, it is possible that those contrasting results reflect two sides of the same coin: (1) long telomeres could reflect higher resistance of good quality individuals to life stress, but at the same time (2) higher metabolic investments (e.g. into reproduction) from high-quality individuals may incur increased costs leading to higher rates of telomere loss (Bauch et al. 2013; Reichert et al. 2014a). In both cases, telomeres may reflect performances, but these studies make it clear that heterogeneity in individual traits in relation to species-specific life history characteristics is to be accounted for to fully understand how telomere length is interpretable. For instance, reproduction may effectively induce pervasive telomere loss (Reichert et al. 2014a; Sudyka et al. 2014), but high-quality individuals may tolerate such stress because of a higher ability to protect/restore their telomeres (Bauch et al. 2013) or to resist to the deleterious impact of accumulating senescent cells. Nevertheless, here, we found no link between relative telomere length and past breeding success. Further, it has been recently shown that stressful rearing conditions in early life (experimentally increased broods) enhances telomere loss in corvid chicks (Boonekamp et al. 2014). Therefore, whereas high-quality individuals may lose more telomeres during reproduction, they may also produce offspring with longer telomeres because they provide high-quality parental care. The relative consequences of those costs/benefits on fitness remain to be determined. Importantly, future research needs to pinpoint the mechanisms leading to variability in telomere length (e.g. energy vs. social stress) in adult king penguin. Indeed, heritability estimates of telomere length are relatively low in king penguins (Reichert et al. 2014a), suggesting that important effects of environmental stress on telomere dynamics in offspring should be considered. For instance, king penguin chicks reared under contrasting environmental conditions are known to differ in their phenotype (Stier et al. 2014), and chicks with longer telomeres have been found to survive better under harsh rearing conditions (reared late during breeding season; Stier et al. 2014).
One hypothesis linking telomere length to fitness is slowly emerging from the medical studies conducted by Blasco and collaborators. Using telomerase gene therapy, these authors induced the restoration of short telomeres in mice by activating telomerase (de Jesus et al. 2011), thereby improving mouse health and lifespan (de Jesus et al. 2012). Those results suggest that long telomeres do not simply reflect proper organism functioning, but also mechanistically underlie it in a certain way. In line with this, we recently found that experimentally activating telomerase led to longer telomeres and faster feather regeneration in birds (Reichert et al. 2014b). Those studies urgently call for further experiments to assess the causal mechanisms by which telomere length affects fitness. Nevertheless, while telomere length may predict mortality, the association diminishes with age (Boonekamp et al. 2013), possibly explained by the fact that telomere length is a measure of somatic redundancy, rather than a marker of biological age per se. It is difficult to conclude on the absence of a link between age and relative telomere length in king penguins given the small differences in age classes (5, 8 and 9 years old) of the present study. In addition, if selective disappearance occurred in our population (i.e. individuals with short telomeres disappearing earlier from the population leading to an over-representation of individuals with long telomeres at higher ages), this could mask within-individual relationships between telomere length and age (van de Pol and Verhulst 2006). Somatic redundancy stipulates that an organism consists of multiple elements that can replace each other, the decay of the last element causing organism death (Gavrilov and Gavrilova 2001). If applicable to telomeres, this idea opens fascinating perspectives for the study of long-lived seabirds using longitudinal studies.
Whereas our measure of telomere length was obtained from a single tissue (red blood cells), previous studies have generally found strong correlation between telomere lengths measured in different tissues of the same organism (Takubo et al. 2002; Daniali et al. 2013; Reichert et al. 2013). As a consequence, telomere lengths measured in one tissue are likely representative of that found in the entire body. In addition, inter-individual variability in telomere attrition rates has been shown to be stable through life at least in mice (Daniali et al. 2013) and captive short-lived birds (Heidinger et al. 2012). This suggests that inter-individual variability in telomere length may reflect overall organism functioning. In line with this idea, we found that individuals with shorter telomeres also had lower natural antibody levels, suggesting the lower physiological quality of these individuals. For instance, short telomere T lymphocytes have a reduced ability to respond to pathogens that requires cell proliferation (reviewed in Weng 2012). Since we measured telomere length in red blood cells, our data rather related to the myeloid lineage cell (e.g. macrophages, basophils) than the lymphoid-derived cells (e.g. lymphocytes). It follows that innate immune response may be weaker in penguins exhibiting red blood cells with short telomeres. Such a deterioration of the innate immune system may increase the general cost of body maintenance during reproduction, an energetically demanding period during which more energy is allocated to recover from infection when the innate shield is overwhelmed (Ardia 2005; Hanssen et al. 2005). The rational of this is that by keeping pathogens away based on a low-cost innate barrier, penguins save energy for their reproduction (Roitt et al. 2001; Bourgeon et al. 2007). Yet, red blood cell telomere length could mirror critical trade-offs between reproduction and self-maintenance in king penguins, a possibility that necessitates further investigation given the complexity of innate versus acquired immunity interactions and of the reality of their respective energy or non-energy costs (Lochmiller and Deerenberg 2000; Verhulst et al. 2005).
In conclusion, our study adds to the growing literature suggesting that telomere length may serve as an integrative proxy of individual quality, reflecting both physiological constraints and achieved fitness. The next step is to investigate the links between telomere length and other important components of fitness, such as foraging performances, to better understand how telomeres relate to differences in individual quality within a population; a question particularly suitable in the king penguin, a deep-diving bird continually facing dramatic foraging choices over his 1-year-long breeding period. In addition, whether telomeres provide a sufficiently stable proxy to assess inter-individual differences in performance levels over long time frames in king penguins, remains to be determined (e.g. Heidinger et al. 2012).
References
Ardia DR (2005) Individual quality mediates trade-offs between reproductive effort and immune function in tree swallows. J Anim Ecol 74:517–524
Barrat A (1976) Quelques aspects de la biologie et de l’écologie du Manchot royal Aptenodytes patagonicus des Iles Crozet. Com Natl Fr Rech Antarct 40:107–147
Barrett ELB, Burke TA, Hammers M, Komdeur J, Richardson DS (2013) Telomere length and dynamics predict mortality in a wild longitudinal study. Mol Ecol 22:249–259
Bauch C, Becker PH, Verhulst S (2013) Telomere length reflects phenotypic quality and costs of reproduction in a long-lived seabird. Proc R Soc B 280:20122540
Bize P, Criscuolo F, Metcalfe NB, Nasir L, Monaghan P (2009) Telomere dynamics rather than age predict life expectancy in the wild. Proc R Soc B 276:1679–1683
Blackburn EH (1991) Structure and function of telomeres. Nature 350:569–573
Blackburn EH (2000) Telomere states and cell fates. Nature 408:53–56
Blackburn EH, Epel ES (2012) Too toxic to ignore. Nature 490:169–171
Boonekamp JJ, Simons MJP, Hemerik L, Verhust S (2013) Telomere length behaves as biomarker of somatic redundancy rather than biological age. Aging Cell 12(2):330–332
Boonekamp JJ, Mulder E, Salomons HM, Dijkstra C, Verhulst S (2014) Nestling telomere shortening, but not telomere length, reflects developmental stress and predicts survival in wild birds. Proc R Soc B 281:20133287
Bourgeon S, Raclot T, Le Maho Y, Ricquier D, Criscuolo F (2007) Innate immunity, assessed by plasma NO measurements, is not suppressed during the incubation fast in eiders. Dev Comp Immunol 31:720–728
Bried J, Jouventin P (2001) The king penguin Aptenodytes patagonicus, a non-nesting bird which selects its breeding habitat. Ibis 143:670–673
Criscuolo F, Bize P, Nasir L, Metcalfe NB, Foote CG, Griffiths K, Gault EA, Monaghan P (2009) Real-time quantitative PCR assay for measurement of avian telomeres. J Avian Biol 40:342–347
Daniali L, Benetos A, Susser E, Kark JD, Labat C, Kimura M, Desai K, Granick M, Aviv A (2013) Telomeres shorten at equivalent rates in somatic tissues of adults. Nat Commun 5:1597
de Jesus BB, Schneeberger K, Vera E, Tejera A, Harley CB, Blasco MA (2011) The telomerase activator TA-65 elongates short telomeres and increases health span of adult/old mice without increasing cancer incidence. Aging Cell 10:604–621
de Jesus BB, Vera E, Schneeberger K, Tejera AM, Ayuso E, Bosch F, Blasco MA (2012) Telomerase gene therapy in adult and old mice delays aging and increases longevity without increasing cancer. EMBO Mol Med 4:691–704
DeForest LN, Gaston AJ (1996) The effect of age on timing of breeding and reproductive success in the thick-billed Murre. Ecology 77(5):1501–1511
Descamps S, Gauthier-Clerc M, Gender J-P, Le Maho Y (2002) The annual breeding cycle of unbanded Aptenodytes patagonicus on Possession Island (Crozet). Avian Sci 2:1–12
Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM (2004) Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci USA 101:17312–17315
Epel ES, Merkin SS, Cawthon R, Blackburn EH, Adler NE, Pletcher MJ, Seeman TE (2009) The rate of leukocyte telomere shortening predicts mortality from cardiovascular disease in elderly men. Aging 1:81–88
Ezard THG, Becker PH, Coulson T (2007) Correlations between age, phenotype, and individual contribution to population growth in common terns. Ecology 88:2496–2504
Froy H, Phillips RA, Wood AG, Nussey DH, Lewis S (2013) Age-related variation in reproductive traits in the wandering albatross: evidence for terminal improvement following senescence. Ecol Lett 16:642–649
Gauthier-Clerc M, Gendner J-P, Ribic CA, Fraser WR, Woehler EJ, Descamps S, Gilly C, Le Bohec C, Le Maho Y (2004) Long-term effects of flipper bands on penguins. Proc R Soc B 271:S423–S426
Gavrilov LA, Gavrilova NS (2001) The reliability theory of aging and longevity. J Theor Biol 213:527–545
Geiger S, Le Vaillant M, Lebard T, Reichert S, Stier A, Le Maho Y, Criscuolo F (2012) Catching-up but telomere loss: opening the black box of growth and ageing trade-off in wild king penguin chicks. Mol Ecol 21:1500–1510
Gendner J-P, Gauthier-Clerc M, Le Bohec C, Descamps S, Le Maho Y (2005) New application for transponders in studying penguins. J Field Ornithol 76:138–142
Griffiths R, Double MC, Orr K, Dawson RJG (1998) A DNA test to sex most birds. Mol Ecol 7:1071–1075
Hanssen SA, Hasselquist D, Folstad I, Erikstad KE (2005) Cost of reproduction in a long-lived bird: incubation effort reduces immune function and future reproduction. Proc R Soc B 272:1039–1046
Haussmann MF, Winkler DW, Vleck CM (2005) Longer telomeres associated with higher survival in birds. Biol Lett 1:212–214
Haussmann MF, Winkler DW, Huntington CE, Nisbet ICT (2007) Telomerase activity is maintained throughout the lifespan of long-lived birds. Exp Gerontol 42:610–618
Heidinger BJ, Blount JD, Boner W, Griffith K, Metcalfe NB, Monaghan P (2012) Telomere length in early life predicts lifespan. Proc Natl Acad Sci USA 109:1743–1748
Hughes KA, Reynolds RM (2005) Evolutionary and mechanistic theories of aging. Annu Rev Entomol 50:421–445
Lack DC (1968) Ecological adaptations for breeding in birds. Methuen, London
Le Vaillant M, Wilson RP, Kato A, Saraux C, Hanuise N, Prud’Homme O, Le Maho Y, Le Bohec C, Ropert-Coudert Y King penguins adjust their diving behaviour with age. J Exp Biol 215:3685–3692 (accepted)
Lescroël A, Ballard G, Toniolo V, Barton KJ, Wilson RP, Lyver PO, Ainley DG (2010) Working less to gain more: when breeding quality relates to foraging efficiency. Ecology 91:2044–2055
Lochmiller RL, Deerenberg C (2000) Trade-offs in evolutionary immunology: just what is the cost of immunity? Oïkos 88:87–98
Matson KD, Ricklefs RE, Klasing KC (2005) A hemolysis-hemagglutination assay for characterizing constitutive innate humoral immunity in wild and domestic birds. Dev Comp Immunol 29:275–286
McCleery RH, Perrins CM, Sheldon BC, Charmantier A (2008) Age-specific reproduction in a long-lived species: the combined effects of senescence and individual quality. Proc R Soc B 275:963–970
Mizutani M, Tomita N, Niizuma Y, Yoda K (2013) Environmental perturbations influence telomere dynamics in long-lived birds in their natural habitat. Biol Lett 9:20130511
Møller AP, Saino N (2004) Immune response and survival. Oïkos 104:299–304
Monaghan PH, Haussmann MF (2006) Do telomere dynamics link lifestyle and lifespan? Trends Ecol Evol 21:47–53
Moyes K, Morgan BJT, Donald A, Morris A, Morris SJ, Clutton-Brock TH, Coulson T (2009) Exploring individual quality in a wild population of red deer. J Anim Ecol 78:406–413
Moyes K, Morgan B, Morris A, Clutton-Brock T, Coulson T (2011) Individual differences in reproductive costs examined using multi-state methods. J Anim Ecol 80:456–465
Nettle D, Monaghan P, Boner W, Gillespie R, Bateson M (2013) Bottom of the heap: having heavier competitors accelerates early-life telomere loss in the European starling, Sturnus vulgaris. PLoS One 8:e83617
Nisbet ICT, Dann P (2009) Reproductive performance of little penguins Eudyptula minor in relation to year, age, pair-bond duration, breeding date and individual quality. J Avian Biol 40:296–308
Okuda K, Bardeguez A, Gardner JP, Rodriguez P, Ganesh V, Kimura M, Skurnick J, Awad G, Aviv A (2002) Telomere length in the newborn. Pedriatr Res 52:377–381
Palacios MG, Cunnick JE, Vleck D, Vleck CM (2009) Ontogeny of innate and adaptive immune defense components in free-living tree swallows, Tachycineta bicolor. Dev Comp Immunol 33:456–463
Pauliny A, Wagner RH, Augustin J, Szep T, Blomqvist D (2006) Age-independent telomere length predicts fitness in two bird species. Mol Ecol 15:1681–1687
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:2003–2007
Plot V, Criscuolo F, Zahn S, Georges J-Y (2012) Telomeres, age and reproduction in a long-lived reptile. PLoS One 7:e40855
Puterman E, Lin J, Blackburn E, O’Donovan A, Adler N, Epel E (2010) The power of exercise: buffering the effect of chronic stress on telomere length. PLoS One 5:e10837
R Development Core Team (2008) R: A language and environment for statistical computing [Internet]. Vienna (Austria): R Foundation for Statistical Computing. http://www.R-project.org
Reichert S, Criscuolo F, Verinaud E, Zahn S, Massemin S (2013) Telomere length correlations among somatic tissues in adult zebra finches. PLoS One 8:e81496
Reichert S, Stier A, Zahn S, Arrivé M, Bize P, Massemin S, Criscuolo F (2014a) Increased brood size leads to persistent eroded telomeres. Front Ecol Evol 2:1–11
Reichert S, Bize P, Arrivé M, Zahn S, Massemin S, Criscuolo F (2014b) Experimental increase in telomere length leads to faster feather regeneration. Exp Gerontol 52:36–38
Reichert S, Rojas ER, Zahn S, Robin JP, Criscuolo F, Massemin S (2015) Maternal telomere length inheritance in the king penguin. Heredity 114:10–16
Roitt I, Brostoff J, Male D (2001) Immunology. Mosby-Harcourt Publishers, London
Salomons HM, Mulder GA, van de Zande L, Haussmann MF, Linskens MHK, Verhulst S (2009) Telomere shortening and survival in free-living corvids. Proc R Soc B 276:3157–3165
Samassekou O, Gadji M, Drouin R, Yan J (2010) Sizing the ends: normal length of human telomeres. Ann Anat 192:284–291
Saraux C, Le Bohec C, Durant JM, Viblanc VA, Gauthier M, Beaune D, Park Y-H, Yoccoz NG, Stenseth NC, Le Maho Y (2011a) Reliability of flipper-banded penguins as indicators of climate change. Nature 469:203–206
Saraux C, Viblanc VA, Hanuise N, Le Maho Y, Le Bohec C (2011b) Effects of individual pre-fledging traits and environmental conditions on return patterns in juvenile king penguins. PLoS One 6:e20407
Slagboom PE, Droog S, Boomsma DI (1994) Genetic determination of telomere size in humans: a twin study of three age groups. Am J Hum Genet 55:876–882
Smith S, Turbill C, Penn DJ (2011) Chasing telomeres, not red herring, in evolutionary ecology. Heredity 107:372–373
Stier A, Viblanc VA, Massemin-Challet S, Handrich Y, Zahn S, Rojas ER, Saraux C, Le Vaillant M, Prud’homme O, Grosbellet E, Robin J-P, Bize P, Criscuolo F (2014) Starting with a handicap: phenotypic differences between early- and late-born king penguin chicks and their survival correlates. Funct Ecol 28:601–611
Sudyka J, Arct A, Drobniak S, Dubiec A, Gustafsson L, Cichoń M (2014) Experimentally increased reproductive effort alters telomere length in the blue tit (Cyanistes caeruleus). J Evol Biol 27:2258–2264
Takubo K, Izumiyama-Shimomura N, Honma N, Sawabe M, Arai T, Kato M, Oshimura M, Nakamura KI (2002) Telomere lengths are characteristic in each human individual. Exp Gerontol 37:523–531
Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas L, Aviv A, Spector TD (2005) Obesity, cigarette smoking, and telomere length in women. Lancet 366:662–664
van de Pol M, Verhulst S (2006) Age-dependent traits: a new statistical model to separate within- and between-individual effects. Am Nat 167:766–773
Verhulst S, Riedstra B, Wiersma P (2005) Brood size and immunity costs in zebra finches Taeniopygia guttata. J Avian Biol 36:22–30
Viblanc VA, Bize P, Criscuolo F, Le Vaillant M, Saraux C, Pardonnet S, Gineste B, Kauffmann M, Prud’homme O, Handrich Y, Massemin S, Groscolas R, Robin JP (2012) Body girth as an alternative to body mass for establishing body condition indexes in field studies: a validation in the king penguin. Physiol Biochem Zool 85:533–542
Voillemot M, Hine K, Zahn S, Criscuolo F, Gustafsson L, Doligez B, Bize P (2012) Effects of brood size manipulation and common origin on phenotype and telomere length in nestling collared flycatchers. BMC Ecol 12:1–8
Von Zglinicki T (2002) Oxidative stress shortens telomeres. Trends Biochem Sci 27:339–344
Weimerskirch H, Stahl JC, Jouventin P (1992) The breeding biology and population dynamics of King Penguin Aptenodytes patagonicus on the Crozet Islands. Ibis 134:107–117
Weng NP (2012) Telomeres and immune competency. Curr Opin Immunol 24:470–475
Wilson AJ, Nussey DH (2010) What is individual quality? An evolutionary perspective. TREE 25:207–214
Young RC, Kitaysky AS, Haussmann MF, Descamps S, Orben RA, Elliott KH, Gaston AJ (2013) Age, sex and telomere dynamics in a long-lived seabird with males-biased parental care. PLoS One 8(9):e74931
Acknowledgments
Authors declare no conflict of interests. We are grateful to O. Prud’Homme for his help in the field, to S. Massemin-Challet and S. Zahn for their help in some sample analyses and to H. Gachot-Neveu for sexing birds. We thank all the volunteers who tagged penguins over the years. We are especially grateful to three anonymous reviewers provided constructive comments on the paper. This work was supported by the Institut Polaire Français Paul-Emile Victor (IPEV Prog. 137), the Terres Australes et Antarctiques Françaises (TAAF), the Centre National de la Recherche Scientifique (Programme Zone Atelier de Recherches sur l’Environnement Antarctique et Subantarctique), the French National Research Agency (ANR) 'PICASO' grant (ANR-2010-BLAN-1728-01), the Fondation Prince Albert II de Monaco http://www.fpa2 and the Fondation des Treilles (to M.L.V.), the AXA Research Fund (to V.A.V.), and a Marie Curie Intra European Fellowship (FP7-PEOPLE-IEF-2008, European Commission; project no. 235962, to C.L.B.).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Le Vaillant, M., Viblanc, V.A., Saraux, C. et al. Telomere length reflects individual quality in free-living adult king penguins. Polar Biol 38, 2059–2067 (2015). https://doi.org/10.1007/s00300-015-1766-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-015-1766-0