Abstract
Sea-ice is a key physical driver of Antarctic marine ecosystems. Understanding ecological effects of sea-ice is particularly important given current and future climate change, but a major obstacle is the impracticality of manipulating sea-ice at a relevant scale. However, large-scale anomalous events, such as those occurring in Commonwealth Bay, East Antarctica, provide opportunities for natural experiments. Historically, katabatic winds have kept Commonwealth Bay ice-free for most of each year, but since 2010, a massive grounded iceberg has resulted in year-round sea-ice cover. We surveyed benthic communities in Commonwealth Bay approximately 3 years after continuous sea-ice cover began and found algal bed communities in severe decline. The majority (~75 %) of large macroalgae were decomposing, and the remainder were discoloured or bleached, while approximately 40 % of encrusting coralline algae were bleached. Accompanying this, the presence of invertebrates such as ophiuroids and polychaetes suggests that communities are in the early stages of transitioning to an invertebrate-dominated state. With a known start date, monitoring benthic communities in Commonwealth Bay will allow quantification of rates of benthic regime shifts in response to sea-ice cover, and improve understanding of the vulnerability of polar ecosystems to climate change.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sea-ice is a layer of ice several metres thick that forms on the surface of polar oceans each winter. On most polar coastlines, sea-ice “breaks out” during summer, as warmer temperatures weaken its structure, and winds fragment and transport it offshore. This creates a seasonal cycle of sea-ice presence and absence, which in turn causes the subtidal environment to alternate between two very different sets of conditions. Sea-ice affects many aspects of the marine environment, including light (Clark et al. 2013), disturbance (Gutt et al. 1996; Smale et al. 2008), and sedimentation (Dunbar et al. 1989), all which influence benthic communities on shallow seabeds. The seasonal extent and duration of sea-ice is, however, rapidly changing in the Arctic (Boé et al. 2009) and parts of Antarctica (Mayewski et al. 2009). Understanding the relationship between sea-ice and biota is critical to predicting ecological impacts of climate change.
The most obvious way in which sea-ice affects the shallow subtidal environment is by regulating light entering the water column. This determines the potential for photosynthesis (Aguilera et al. 1999), and thus the abundance of primary producers (Runcie and Riddle 2006). Areas where sea-ice breaks out in spring or early summer can receive enough light to support dense forests of macroalgae (Krause-Jensen et al. 2012), while areas covered by ice for most of the year often contain dark-adapted invertebrate communities (Johnston et al. 2007; Clark et al. 2013). Due to seasonal variability in sunlight at high latitudes, the amount of light reaching the seabed per year (annual light) is sensitive to the timing of sea-ice breakout during summer. Recent work has identified a “tipping-point” phenomenon in which benthic communities can experience regime shifts from invertebrate to algal-dominated states in response to relatively small changes in the timing of sea-ice breakout (Clark et al. 2013).
Besides light, sea-ice also regulates the degree of physical disturbance to the seabed (Barnes and Souster 2011). Physical disturbance to benthos under fast ice (sea-ice attached to land) is rare, because sea-ice limits the ability of icebergs to drift and scour the seabed. Iceberg scour is a major form of physical disturbance on polar coasts (Gutt 2001), and the frequency of scour events is proportional to the time an area is ice-free (Smale et al. 2008). The effects can be significant. The species diversity in benthic communities on the West Antarctic Peninsula, for instance, has been found to be positively related to time since iceberg scour (Brown et al. 2004), with concern that increased scouring frequency will decrease alpha diversity on some sections of coast (Smale et al. 2008). Sea-ice also influences turbidity and sedimentation by forming a solid barrier between ocean and atmosphere, and adds biogenic sedimentation in the form of detrital sea-ice algae and fauna (Dunbar et al. 1989).
Despite the importance of sea-ice cover to marine benthic ecosystems, practical constraints make it almost impossible to experimentally manipulate sea-ice cover at relevant spatial or temporal scales. Most prior work on the ecological effects of sea-ice duration on shallow benthos has been correlative, using spatial gradients (Krause-Jensen et al. 2012; Clark et al. 2013), or monitoring areas where the timing of sea-ice break-out has gradually changed over decades (Barnes and Souster 2011; Kortsch et al. 2012; Quartino et al. 2013). These studies have found links between sea-ice and benthic community composition, but their interpretations have been limited by their dependence on correlative data. However, a recent event in Commonwealth Bay, East Antarctica, has provided a natural experiment to assess the effects of sea-ice cover on marine benthic communities in one specific location.
Until recently, Commonwealth Bay—the location of Sir Douglas Mawson’s research station during the Australasian Antarctic Expedition (AAE) of 1911–1914—was rarely covered by sea-ice. A polynya (an area of continuous open water) existed adjacent to the shore, created by strong katabatic winds that travel off the continental ice cap and are funnelled into Cape Denison by local topography. Under normal circumstances, these winds transport surface ice offshore as quickly as it forms, and Mawson reported that during their 2-year stay, there were only 2 days per year in which sea-ice was stable enough to work upon (Mawson 1940). Cape Denison is officially the windiest place on earth at sea level, with an annual average daily maximum wind speed of 71 km per hour (Kidson 1946; Parish and Walker 2006).
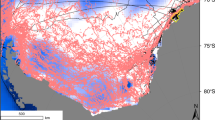
Sea-ice conditions changed dramatically in December 2010, when a giant iceberg (~100 km2 in area) named B09B became grounded in Commonwealth Bay (Shadwick et al. 2013) and has remained there since. B09B originated from the collapse of the Larsen B ice shelf in the Weddell Sea, which fragmented into massive icebergs that have drifted around the continent. The residence of B09B in Commonwealth Bay has prevented sea-ice on the landward side of the iceberg from being transported offshore, creating year-round sea-ice cover (Fig. 1a). This imposed continuous sea-ice cover over an area that was previously ice-free for most of each year, creating a natural experiment of the effects of sea-ice on local biota.
Maps of the study area. a Maximum fast ice extent (red line) of Geroge V Land from the mean 20-day MODIS imagery over Austral winter (June–August), 2000–2009 and 2011–2012. Cape Denison is marked with a star. MGT refers to Mertz Glacier Tongue and CB to Commonwealth Bay. B09B is a massive iceberg that became grounded in Commonwealth Bay in 2010. Adapted from Tamura et al. (2012). b Map of Cape Denison, Commonwealth Bay. Points indicate sampling sites in the present study and in Mawson’s 1912–1914 Australasian Antarctic Expedition. Inset map shows the location of Commonwealth Bay in Antarctica. (Color figure online)
We visited Commonwealth Bay in December 2013 and surveyed marine benthic communities at several sites around Cape Denison (Fig. 1b). We aimed to assess whether benthos in this region showed signs of change, since the onset of continuous sea-ice cover in 2010. In the absence of quantitative historical data, it is not possible to statistically compare current and past communities, but there are sources that qualitatively describe benthic communities prior to 2010. Marine biota was described by scientists during the Australasian Antarctic Expedition of 1912–1914 (Lucas 1919; Mawson 1940), and underwater photographs of the seabed were taken in January 2008 (Fig. 2a, b). Additionally, remnants of the previous ecosystem are still present, allowing inference of recent change.
Methods
Pre-B09B benthic communities
We sourced information on the historical state of benthic communities around Cape Denison, Commonwealth Bay, from reports produced by Sir Douglas Mawson’s Australian Antarctic Expedition (e.g. Lucas 1919; Mawson 1940). This expedition sampled five stations (Fig. 1b) over a 2-year period, from 1912 to 1913. Samples were collected with dredge hauls several times per year [see Mawson (1940) for methodological details]. In addition, we obtained underwater photographs taken at Boat Harbour (Fig. 1b) in January 2008 by Erwan Amice.
Post-B09B field sampling
We sampled benthic communities at Cape Denison in December 2013, almost 3 years after the arrival of B09B and the onset of continuous sea-ice cover. We chose nearshore sites because shallow areas were more likely former algal habitat (Mawson 1940). We surveyed the seabed at five sites (Fig. 1b; Table 1) by deploying a waterproof video camera through holes drilled in the sea-ice. At each site, we conducted three replicate drops, spaced 5 m apart. For each replicate, we drilled a hole in the sea-ice using a 16-cm ice corer and lowered a video camera apparatus through the hole using a rope. The camera apparatus consisted of rope running through a PVC pipe (20 mm diameter) with a GoPro Hero 2® camera attached to the end of the pipe facing downwards. A 1.5-kg lead weight was attached to the pipe, and a 12 cm length of wood was attached to the camera to prevent lens damage when reaching the seabed, and to provide scale. Once the apparatus made contact with the seabed, the rope was raised 1 m to film the seabed from a standard distance and thus cover the same area (2.25 × 1.25 m = 2.81 m2). We used the rope to measure depth, and from the ice-hole, recorded the thickness of the sea-ice and depth of snow (Table 1).
Image analysis
To quantify benthic communities, we extracted one still image from each video when the camera was stationary 1 m above the seabed. Estimates of percentage cover for each taxon were obtained by superimposing 50 randomly positioned points onto each image using the software Coral Point Count (CPCe) v.4.1 and recording the taxon under each point. Taxa were classified to the lowest possible taxonomic resolution. We differentiated between five conditions of macroalgae (in decreasing order of health): healthy, grazed, discoloured, bleached, and decomposing. Pink encrusting coralline algae were categorised as healthy (pink) or bleached (white). This visual classification has been demonstrated to reliably reflect condition of red and large brown macroalgae elsewhere (Campbell et al. 2011). We recorded the total number of solitary invertebrates in each still image and the maximum number of fish seen in each video (during the 2 min after the camera was positioned 1 m above the seabed) at any one time (MaxN).
Data analyses
Benthic community data were analysed for spatial variation using permutational multivariate analyses of variance (Anderson 2001) with the PERMANOVA+ add-on in PRIMER v6 (Anderson et al. 2007). Similarity matrices were based on Bray–Curtis distances of square root transformed percentage covers (community structure), or on the Jaccard similarity measure (presence/absence; community composition). Analyses used 9999 unrestricted permutations of raw data. Multivariate dispersion was compared across sites using PERMDISP in PRIMER v6 (Anderson et al. 2007).
Results
Pre-B09B benthic communities
Early records describe a benthos mostly composed of algae in the shallows and invertebrates at depth (Mawson 1940, Table 2). Algae reported in the area by Lucas (1919) are listed in Table 3. Algae in photographs taken in 2008 in Boat Harbour resemble Palmaria decipiens (red) and Monostroma hariotii (green) (Fig. 2).
Post-B09B benthic communities
In December 2013, benthic communities were dominated by ailing canopy-forming macroalgae (likely Himantothallus grandifolius), partially bleached pink encrusting coralline algae (Rhodophyta) and turfing algae (Fig. 3a). Invertebrates were moderately abundant at some sites (Fig. 3b), particularly brittle stars (Ophiuroids). Ice-fishes (Notothenioidei) were found at all sites and were responsive to the moving camera.
Community composition and abundance of taxa at each site. a Percentage cover of space-occupying taxa. b Boxplot showing counts of solitary taxa. The upper and lower edges (“hinges”) of boxes correspond to the first and third quartiles (the 25th and 75th percentiles). The upper whiskers extend from the hinge to the highest value that is within 1.5 × IQR of the hinge, where IQR is the inter-quartile range, or distance between the first and third quartiles. The lower whisker extends from the hinge to the lowest value within 1.5 × IQR of the hinge. Data beyond the end of the whiskers are outliers and plotted as points
Macroalgae were generally in very poor condition (Fig. 4). The majority (78 %) were classified as decomposing, while 20 % were discoloured and 2 % were bleached. No macroalgae that occurred under census points were considered healthy. Encrusting coralline algae showed less signs of stress than macroalgae: 56 % were considered healthy (i.e. retained pink colour) and 44 % were bleached.
Multivariate community structure varied significantly between sites (Table 4). There were, however, no significant differences in multivariate dispersion across sites (PERMDISP: pseudo-F 4,10 = 2.33, p = 0.24). When considering composition only, there was no significant variability (Table 4) nor differences in dispersion across sites (PERMDISP: pseudo-F 4,10 = 1.79, p = 0.41).
Discussion
Change in sea-ice cover has clearly impacted marine benthic communities at Cape Denison, Commonwealth Bay. After approximately 3 years of continuous sea-ice cover, large canopy-forming macroalgae that previously dominated nearshore seabed were in severe states of decline. Approximately three quarters of macroalgae censused were decomposing, while the remainder were bleached or discoloured. None of the macroalgae we observed were considered healthy. Encrusting coralline algae were bleached to a lesser extent (~40 % bleached), although this is not surprising given they have lower light requirements and often occur under macroalgae canopy (Irving et al. 2005) or sea-ice (Kühl et al. 2001). This ratio of bleaching is similar to that reported in response to canopy loss, in both polar (mean ± SE = 56.85 ± 8.43 %: Casey Station, Antarctica: Irving et al. 2005) and temperate (45.98 ± 5.91 % in southern Australia: Irving et al. 2004) studies. The ecosystem of 2013 contrasts with historical records of healthy macroalgae forests in the area. To our knowledge, there are no quantitative records of local benthos prior to the arrival of B09B, but observations from the original Australasian Antarctic Expedition report macroalgae in benthic grabs (Lucas 1919; Mawson 1940), and underwater photographs taken in January 2008 show seabed dominated by large amounts of healthy macroalgae. Moreover, the spatial dominance of remnant algal beds is indicative of former healthy macroalgae forests, similar to those found in other predominantly ice-free areas of shallow Antarctic coast (Johnston et al. 2007; Clark et al. 2013).
While algae showed signs of acute stress, invertebrates and ice-fishes were present at every site. Some invertebrate taxa, such as spirorbid polychaetes and some bryozoa, are commonly found on macroalgae and are visible in the pre-B09B photographs from 2008, but others are more typical of ice-covered ecosystems. For example, polychaete fanworms (Sabellidea) are common in ice-covered areas around Casey Station, East Antarctica (unpub. data), and ice-fishes are generally found beneath sea-ice (Fuiman et al. 2002). Detritivorous brittle stars (Ophiuridae) play an important role in nutrient cycling under sea-ice (Lohrer et al. 2013), and their abundance here may be linked to increased availability of detrital macroalgae. Herbivores, such as echinoderms that are often common in temperate and tropical algal beds and exert considerable grazing pressure (Harrold and Reed 1985), were present but in low abundance.
Polar macroalgae have evolved to withstand long periods of winter darkness, but cannot obtain their minimum light requirements when covered by sea-ice for several consecutive years. Benthic communities can experience regime shift in response to change in sea-ice cover, primarily through change in annual light (Clark et al. 2013). The poor condition of macroalgae in Commonwealth Bay, together with the occurrence of dark-adapted invertebrates and ice-fishes, suggests that benthic communities of Cape Denison are in the early stages of transition from algae- to invertebrate-dominated ecosystem states. Recent studies have documented ecosystem change in the opposite direction—i.e. increased algal cover with decreased sea-ice duration (Kortsch et al. 2012), but this is the first time a regime shift from algae to invertebrates has been observed with a known start date. Continued sampling at this and reference locations will allow the rate of transition between ecosystem states to be quantified.
Quantifying rates of change in ecosystem state following increased sea-ice cover is important for two reasons. First, it provides estimates of ecological change in regions where sea-ice is increasing in extent or duration due to climate (Bintanja et al. 2013), or in places where iceberg groundings cause anomalous sea-ice retention. Glacial discharge is accelerating in both East (Miles et al. 2013) and West Antarctica (Rignot et al. 2004; Pritchard and Vaughan 2007), creating more icebergs, and reduced duration of seasonal fast ice will provide more grounding opportunities (Smale et al. 2008). It is important to note that regime shifts due to sudden change in sea-ice cover (e.g. due to iceberg grounding) may differ in some respects from those induced by gradual climate-related change. Under gradual change, species will have more time to adapt and may experience different interactions with the environment and one another at various stages in the transition. Nonetheless, scenarios of sudden change provide information in far shorter timeframes and give valuable insight into what to expect from gradual change.
Second, this information can be used to estimate the rate at which invertebrates can recolonise an area, following local extinction due to sea-ice loss. Ecological effects of sea-ice loss have received more attention than effects of sea-ice expansion, due to rapid reduction in seasonal sea-ice duration in the Arctic (Boé et al. 2009) and some areas of Antarctica (Barnes and Souster 2011; Parkinson 2004). Polar benthic ecosystems have responded to sea-ice loss (Kortsch et al. 2012), and the mechanism behind this response has been described (Clark et al. 2013), but a key unknown is the potential for recolonisation if former sea-ice conditions return. Data from Commonwealth Bay suggest that ecosystem change begins within 3 years from the onset of sea-ice cover and that sabellid fanworms and brittle stars are amongst the first invertebrates to colonise and/or increase in abundance.
The rate of transition between ecosystem states is dependent upon (1) the magnitude of environmental stress on incumbent populations, (2) the rate of immigration of replacement species, and (3) interactions between these and ecological processes such as competition, predation, and herbivory (Scheffer and Carpenter 2003). At least two algal species found in Commonwealth Bay (Iridaea cordata and Phyllophora antarctica) are chemically defended against herbivory (Amsler and McClintock 1998), but stress such as low light can increase susceptibility to herbivores (Wahl and Hay 1995). Macroalgae stressed by low light may also be more prone to microbial diseases (Campbell et al. 2014) or fouling by epibiota (Marzinelli et al. 2011), accelerating their decline. We observed low densities of herbivores compared to kelp forests in temperate and tropical systems, which may explain the relatively slow rate of change. Ecological processes in Antarctica are notoriously slow (Barnes 1996; Stanwell-Smith and Barnes 1997), and if herbivory is low then microbial degradation may be the primary biological driver of change. Given the very low temperatures, microbial degradation is also likely to be slow. Ongoing sampling for years or decades may be required to understand the full trajectory of ecological change and determine the point at which ecosystem state stabilises relative to other areas regularly covered by sea-ice.
Once stabilised, resultant community composition will be shaped by the dispersal limitations of potential immigrants. Considerable spatial heterogeneity exists in shallow flora and fauna around Antarctica (Gutt et al. 2013b), which likely reflects dispersal barriers between shallow-water populations. Approximately 86 % of the Antarctic coast is glaciated or ice-shelf cliff (Gutt et al. 2013a), so shallow benthos is rare and highly fragmented (Clark et al. 2015). Mawson (1940) reported a diversity of invertebrates in grab samples in Commonwealth Bay (Table 2), even at shallow depths when a polynya existed, and these taxa are likely to encroach shoreward and increase in abundance with persistent sea-ice cover. However, it is unclear whether invertebrates from other regions might reach Commonwealth Bay to form communities that resemble those under more permanent ice (e.g. Filinger et al. 2013). Alternatively, invertebrates could arrive from deeper habitat, where benthos is aphotic independently of sea-ice. However, while deep-water populations are less fragmented and potentially closer, they do face a bathymetric barrier that may prevent colonisation (Brey et al. 1996).
Algae do not have deep-water refuge like invertebrates, so their ability to recolonise relies exclusively on connectivity between shallow populations. Shallow rocky benthos occurs near Commonwealth Bay at the Stillwell Islands, although this area is also experiencing sea-ice build-up. Connectivity between shallow Antarctic benthic populations is poorly understood (but see Baird et al. 2011), but even if connectivity exists, other factors might impede the re-establishment of macroalgae (Campana et al. 2009; Zacher et al. 2009). For example, cleared space can be rapidly colonised by algal turfs (Connell 2007), which can inhibit macroalgae from recolonizing even once the initial disturbance is removed (Gorman and Connell 2009). The restoration of macroalgae forests therefore depends on propagule supply, and biological and environmental conditions post-disturbance (Campana et al. 2009; Zacher et al. 2009), potentially resulting in new ecosystem states with unique composition and function.
In conclusion, the grounding of B09B in Commonwealth Bay and consequential sea-ice retention has presented a rare opportunity to quantify the effect of sea-ice cover on shallow benthic communities. With a known start date, this event will provide estimates of both the rate of transition from algal- to invertebrate-dominated states in areas where sea-ice is expanding, and the potential for recovery in areas experiencing sea-ice loss. After approximately 3 years, macroalgae are in poor condition and invertebrates have begun colonisation, and these trends are likely to continue if sea-ice cover remains constant. Commonwealth Bay is also likely to experience a short-term increase in organic matter due to algal decay and a long-term reduction in the production of macroalgae fuelling the food web. Further studies that examine the spatial extent of macroalgae in the area prior to the decline, and effects of organics matter on other trophic levels, are needed to understand how the sea-ice will impact the marine ecosystem more generally.
References
Aguilera J, Karsten U, Lippert H, Vogele B, Philipp E, Hanelt D, Wiencke C (1999) Effects of solar radiation on growth, photosynthesis and respiration of marine macroalgae. Mar Ecol Prog Ser 191:109–119
Mayewski PA et al (2009) State of the Antarctic and Southern Ocean climate system. Rev Geophys 47:RG1003
Amsler CD, McClintock JB (1998) Chemical defence against herbivory in the Antarctic marine macroalgae Iridaea cordata and Phyllophora antarctica (Rhodophyceae). J Phycol 34:35–59
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46. doi:10.1111/j.1442-9993.2001.01070.pp.x
Anderson MJ, Gorley RN, Clarke KR (2007) Permanova + for primer: guide to software and statistical methods. Primer-E, Plymouth
Baird HP, Miller KJ, Stark JS (2011) Evidence of hidden biodiversity, ongoing speciation and diverse patterns of genetic structure in giant Antarctic amphipods. Mol Ecol 20:3439–3454
Barnes DKA (1996) Low levels of colonisation in Antarctica: the role of bryozoans in early community development. In: Gordon DP, Smith AM, Grant-Mackie JA (eds) Bryozoans in space and time. National Institute of Water and Atmospheric Research, Wellington, p 442
Barnes DKA, Souster T (2011) Reduced survival of Anarctic benthos linked to climate-induced iceberg scouring. Nat Clim Change 1:365–368
Bintanja R, van Oldenborgh GJ, Drijfhout SS, Wouters B, Katsman CA (2013) Important role for ocean warming and increased ice-shelf melt in Antarctic sea-ice expansion. Nat Geosci 6:376–379
Boé J, Hall A, Qu X (2009) September sea-ice cover in the Arctic Ocean projected to vanish by 2100. Nat Geosci 2:341
Brey T, Dahm C, Gorny M, Klages M, Stiller M, Arntz WE (1996) Do Antarctic benthic invertebrates show an extended level of eurybathy? Antarct Sci 8(1):3–6
Brown KM, Fraser KPP, Barnes DKA, Peck LS (2004) Links between the structure of an Antarctic shallow-water community and ice-scour frequency. Oecologia 141:121–129
Campana GL, Zacher K, Fricke A, Molis M, Wulff A, Quartino ML, Wiencke C (2009) Drivers of colonization and succession in polar benthic macro- and microalgal communities. Bot Mar 52:655–667
Campbell AH, Harder T, Nielsen S, Kjelleberg S, Steinberg PD (2011) Climate change and disease: bleaching of a chemically defended seaweed. Glob Change Biol 17:2958–2970
Campbell AH, Verges A, Steinberg PD (2014) Demographic consequences of disease in a habitat-forming seaweed and impacts on interactions between natural enemies. Ecology 95:142–152
Clark GF, Stark JS, Johnston EL, Runcie JW, Goldsworthy PM, Raymond B, Riddle MJ (2013) Light-driven tipping points in polar ecosystems. Glob Change Biol 19:3749–3761
Clark GF, Raymond B, Riddle MJ, Stark JS, Johnston EL (2015) Vulnerability of Antarctic shallow invertebrate-dominated ecosystem. Austral Ecol. doi:10.1111/aec.12237
Connell SD (2007) Water quality and the loss of coral reefs and kelp forests: alternative states and the influence of fishing. In: Connell SD, Gillanders BM (eds) Marine ecology. Oxford University Press, Melbourne
Dunbar RB, Anderson JB, Stockton WL (1989) Biogenic sedimentation in McMurdo Sound, Antarctica. Mar Geol 85:155–179
Filinger L, Janussen D, Lundalv T, Richter C (2013) Rapid glass sponge expansion after climate-induced Antarctic ice shelf collapse. Curr Biol 23:1330–1334
Fuiman L, Davis R, Williams T (2002) Behavior of midwater fishes under the Antarctic ice: observations by a predator. Mar Biol 140:815–822. doi:10.1007/s00227-001-0752-y
Gorman D, Connell SD (2009) Recovering subtidal forests in human-dominated landscapes. J Appl Ecol 46:1258–1265
Gutt J (2001) On the direct impact of ice on marine benthic communities, a review. Polar Biol 24:553–564
Gutt J, Starmans A, Dieckmann G (1996) Impact of iceberg scouring on polar benthic habitats. Mar Ecol Prog Ser 137:311–316
Gutt J, Barnes DKA, Lockhart SJ, van de Putte A (2013a) Antarctic macrobenthic communities: a compilation of circumpolar information. Nat Conserv 4:1–13
Gutt J, Griffiths HJ, Jones CD (2013b) Circumpolar overview and spatial heterogeneity of Antarctic macrobenthic communities. Mar Biodivers 43:481–487
Harrold C, Reed DC (1985) Food availability, sea urchin grazing, and kelp forest community structure. Ecology 66:1160–1169
Irving AD, Connell SD, Elsdon TS (2004) Effects of kelp canopies on bleaching and photosynthetic activity of encrusting coralline algae. J Exp Mar Biol Ecol 310:1–12
Irving AD, Connell SD, Johnston EL, Pile AJ, Gillanders BM (2005) The response of encrusting coralline algae to canopy loss: an independent test of predictions on an Antarctic coast. Mar Biol 147:1075–1083
Johnston EL, Connell SD, Irving AD, Pile AJ, Gillanders BM (2007) Antarctic patterns of shallow subtidal habitat and inhabitants in Wilke’s Land. Polar Biol 30:781–788
Kidson E (1946) Meteorology. Discussions of Observations at Adélie Land, Queen Mary Land and Macquarie Island. Sydney
Kortsch S, Primicerio R, Beuchel F, Renaud PE, Rodrigues J, Jørgen Lønne O, Gulliksen B (2012) Climate-driven regime shifts in Arctic marine benthos. Proc Natl Acad Sci. doi:10.1073/pnas.1207509109
Krause-Jensen D et al (2012) Seasonal sea ice cover as principal driver of spatial and temporal variation in depth extension and annual production of kelp in Greenland. Glob Change Biol. doi:10.1111/j.1365-2486.2012.02765.x
Kühl M, Glud RN, Borum J, Roberts R, Rysgaard S (2001) Photosynthetic performance of surface-associated algae below sea ice as measured with a pulse-amplitude-modulated (PAM) fluorometer and O2 microsensors. Mar Ecol Prog Ser 223:1–14
Lohrer AM, Cummings VJ, Thrush S (2013) Altered sea ice thickness and permanance affects benthic ecosystem functioning in coastal Antarctica. Ecosystems 16:224–236
Lucas AHS (1919) The Algae of Commonwealth Bay. Sydney, Australia
Marzinelli EM, Underwood AJ, Coleman RA (2011) Modified habitats influence kelp epibiota via direct and indirect effects. PLoS ONE 6:e21936
Mawson D (1940) Marine biological programmme and other zoological and botanical activities, vol 2. University of Adelaide, Sydney, Australia
Miles BWJ, Stokes CR, Vieli A, Cox NJ (2013) Rapid, climate-driven changes in outlet glaciers on the Pacific coast of East Antarctica. Nature 500:563–567. doi:10.1038/nature12382
Parish TR, Walker R (2006) A re-examination of the winds of Adélie Land, Antarctica. Aust Meteorol Mag 55:105–117
Parkinson CL (2004) Southern Ocean sea ice and its wider linkages: insights revealed from models and observations. Antarct Sci 16:387–400
Pritchard HD, Vaughan DG (2007) Widespread acceleration of tidewater glaciers on the Antarctic Peninsula. J Geophys Res 112: F03S29. doi:10.1029/2006JF000597
Quartino ML, Deregibus D, Campana GL, Latorre GEJ, Momo FR (2013) Evidence of macroalgal colonization on newly ice-free areas following glacial retreat in potter cove (South Shetland Islands), Antarctica. PLoS ONE 8:e58223. doi:10.1371/journal.pone.0058223
Rignot E, Casassa G, Gogineni P, Krabill W, Rivera A, Thomas R (2004) Accelerated ice discharge from the Antarctic Peninsula following the collapse of Larsen B ice shelf. Geophys Res Lett. doi:10.1029/2004GL020697
Runcie JW, Riddle MJ (2006) Photosynthesis of marine macroalgae in ice-covered and ice-free environments in East Antarctica. Eur J Phycol 41:223–233
Scheffer M, Carpenter S (2003) Catastrophic regime shifts in ecosystems: linking theory to observation. Trends Ecol Evol 18:648–656
Shadwick EH et al (2013) Glacier tongue calving reduced water formation and enhanced carbon uptake. Geophys Res Lett 40:904–909
Smale DA, Brown KM, Barnes DKA, Fraser KPP, Clarke A (2008) Ice scour disturbance in Antarctic waters. Science 321:371
Stanwell-Smith D, Barnes DKA (1997) Benthic community development in Antarctica: recruitment and growth on settlement panels at Signy Island. J Exp Mar Biol Ecol 212:61–79
Tamura T, Williams GD, Fraser AD, Ohshima KI (2012) Potential regime shift in decreased sea ice production after the Mertz Glacier calving. Nature Comms 3:826. doi:10.1038/ncomms1820
Wahl M, Hay ME (1995) Associational resistance and shared doom—effects of epibiosis on herbivory. Oecologia 102:329–340
Zacher K, Rautenberger R, Hanelt D, Wulff A, Wiencke C (2009) The abiotic environment of polar marine benthic algae. Bot Mar 52:483–490
Acknowledgments
We thank members of the Australasian Antarctic Expedition 2013–2014 (www.spiritofmawson.com) for their support of this study. GFC was supported by a UNSW Strategic Research Grant awarded to ELJ. EMM thanks SIMS and Director PD Steinberg for additional support. Work was conducted under Antarctic Treaty (Environment Protection) permit number ATEP 13-14-AAE 2013.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Clark, G.F., Marzinelli, E.M., Fogwill, C. et al. Effects of sea-ice cover on marine benthic communities: a natural experiment in Commonwealth Bay, East Antarctica. Polar Biol 38, 1213–1222 (2015). https://doi.org/10.1007/s00300-015-1688-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-015-1688-x