Abstract
In waters surrounding James Ross Island (JRI), which is generally inaccessible, high chlorophyll-a concentration [Chla] can often be detected during summer periods by ocean color imagery. The region is influenced by a retreating sea ice edge from Weddell Sea and freshwater runoff from JRI glaciers, factors that probably trigger phytoplankton growth. In this work, we relate phytoplankton composition and biomass [Chla and carbon] with environmental factors in two successive late summer periods, in 2008 (1–3 March) and 2009 (17–20 February). Remote sensing data were used to corroborate the findings during those few sampling days. High surface [Chla] patches were observed through both remote sensing and field data (up to 7.61 mg Chla m−3 in 2009), and associated with a relatively shallow upper mixed layer (UML) (19–109 m in 2008 and 16–74 m in 2009). Sea surface temperatures were lower in 2008 (−1.19 to −0.62 °C) than in 2009 (−0.87 to −0.46 °C). Sea ice coverage was greater in 2008 than in 2009 summer, implying an earlier sea ice retreat in the latter year, when higher average [Chla] was obtained from field samples (3.3 mg m−3, compared to 1.5 mg m−3 in 2008). The eastern side of JRI appeared to be relatively sheltered from the dominant pattern of large-scale westerly winds. Diatoms dominated the phytoplankton community, with presence of large diatom species (e.g., Odontella weissflogii) typical of an advanced sea ice melt condition. Those blooms were sustained by a shallow UML associated with relative shelter from winds, due to proximity with the island.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
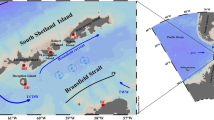
The eastern region of the tip of the Antarctic Peninsula (Fig. 1) is under the influence of the Weddell Sea and surface waters are typically covered with pack ice throughout the year, with a partially ice-free area present only during Austral summer (February–March) (Kang et al. 2001). High phytoplankton biomass has been observed in marginal ice zones of the Weddell Sea (Park et al. 1999), where high primary productivity rates are associated with shallow mixed layers, induced by low-salinity water from melted sea ice (Smith and Nelson 1990; Kang et al. 2001). The hydrographic and biological features of this region are dominated by meteorological processes, which control sea ice dynamics (e.g., formation, advection and melting) and upper ocean stratification (Robertson et al. 1998; Kang et al. 2001). Most of the James Ross Island (JRI) is associated with abundance of snow, with almost 80 % of the island being covered by glaciers, which can be subjected to micro-scale melting processes (Sone et al. 2007). During the summer months, meltwater inputs from both glaciers and JRI lakes (Hawes and Brazier 1991; Sone et al. 2007) and free and drifting icebergs have a strong influence on this coastal marine ecosystem, by changing the upper thermohaline properties of seawater (Pisarevskaya and Popov 1991) and by increasing the iron availability (Sedwick and DiTullio 1997; Moore and Doney 2006). However, data on phytoplankton dynamics from comprehensive field surveys are scarce in this region, mainly due to difficulty in accessing those coastal areas, as a result of constant sea ice coverage even during summer months (Kang et al. 2001).
The degree to which physical, chemical and biological factors limit phytoplankton growth in the Southern Ocean is not completely understood, and its relative abundance can vary with location, season, oceanic and local meteorological conditions (Lancelot et al. 1993; Montes-Hugo et al. 2009). For instance, sea ice dynamics is thought to play an important role on coastal phytoplankton communities of polar regions (Smith et al. 1998; Garibotti et al. 2003; Arrigo et al. 2008). Melting sea ice acts particularly as a source of essential micronutrients, including iron (Sedwick and DiTullio 1997; Moore and Doney 2006), and contributes to an increasing stratification of surface layers, providing excellent conditions for high primary productivity (Boyd 2002; Garibotti et al. 2003; Marrari et al. 2008). Sea ice may also accumulate iron from both atmospheric deposition of mineral dust (Edwards and Sedwick 2001) and sedimentary sources, when developing in coastal/shelf regions (Fitzwater et al. 2000; Sedwick et al. 2000; Grotti et al. 2005).
Changes in local environmental parameters in combination with early retreat of sea ice may cause shifts in the time scale of the seasonal succession of phytoplankton assemblages, with important ramifications on the food chain (Garibotti et al. 2003; Loeb et al. 2009). For instance, Montes-Hugo et al. (2009) have demonstrated, in the western continental shelf of the Antarctic Peninsula, two opposing trends in phytoplankton biomass over the past 20 years across a latitudinal gradient. An increasing trend in the southern region was accompanied by shifts in the community composition, with a greater fraction of large diatom cells, whereas in the northern region, a decrease in biomass was associated with a smaller fraction of diatoms. They also detected a decrease in the extent of summer sea ice in areas that were previously covered with sea ice for most of the year (Montes-Hugo et al. 2009).
Sea ice cover dynamics, such as earlier or later retreats, may not directly affect the abundance of phytoplankton cells in Antarctica, but can induce changes in composition of phytoplankton communities and cell size distribution, particularly in coastal regions (Garibotti et al. 2003; Annett et al. 2010). Diatoms have generally shown to be major contributors to autotrophic biomass around the Antarctic Peninsula, although other taxa such as Phaeocystis antarctica Karsten 1905 can play a major role in other Antarctic regions such as the Ross Sea (Arrigo and van Dijken 2004). Therefore, plankton composition studies are essential to identify specific attributes of key species and therefore determine their respective roles, both in biogeochemical cycles and in the pelagic food chain (Garibotti et al. 2003; Smetacek et al. 2004; Garibotti et al. 2005; Loeb et al. 2009).
Mendes et al. (2012) reported a broader spatial coverage study on phytoplankton dynamics around the Antarctica Peninsula during the same sampling period, including the vicinities of JRI, but with an emphasis on HPLC-derived pigment data. The main goal of the present study is to focus on the phytoplankton community and species composition of the distinctive blooms detected by ocean color images in the vicinities of JRI and associated environmental conditions. Physical, optical and biogeochemical properties were measured in the field during the late austral summer of two successive years in the region.
Materials and methods
Study area
The study area comprised coastal waters near JRI, east of the tip of the Antarctic Peninsula, adjacent to deep waters of the northwestern Weddell Sea (Fig. 1). Surface waters in this area are typically covered with pack ice throughout the year, which makes this a region difficult to access (Kang et al. 2001). The cruises were conducted in 2008 (1–3 March, SOS-I cruise) and in 2009 (17–20 February, SOS-II cruise), hereafter named as late summer of 2008 and late summer of 2009, respectively. Given meteorological and ocean conditions across the region, logistical issues allowed to occupy 20 (2008) and 12 (2009) stations between 1- and 3-h intervals across the study area (Table 1). The sampling was part of the project “Southern Ocean Studies for Understanding Global Climate Issues (SOS-CLIMATE)”, on board R/V Ary Rongel, as a Brazilian contribution to the IV International Polar Year.
Oceanographic data
Vertical profiles of temperature, salinity, fluorescence (WET Labs ECO-AFL/FL ®) and beam attenuation at 660 nm (WET Labs C-star ®) were obtained with a Sea-Bird® CTD/Carrousel 911+ system. Temperature, salinity and pressure data were obtained from CTD downcast profiles, while stimulated fluorescence and beam attenuation data were obtained from upcast profiles. Seawater was sampled from surface and selected depths (Table 1) with 5-L Niskin bottles attached to the carrousel for biological analyses, performed later in the land laboratory. The C-Star calibration and data processing protocol was followed to calculate the beam attenuation coefficient (c), which was later corrected for pure seawater attenuation. The resulting attenuation coefficient is attributed mainly to particles (by absorption and/or scattering) and, to a lesser extent, to colored dissolved organic material—cDOM—(absorption). In order to eliminate calibration-related uncertainties, c was also corrected by subtracting values measured at ~2100–2200 m (particle-free depth), far away from the sea bed. This value was considered an offset constant and was applied to late summer of 2008 (0.04 m−1) and late summer of 2009 (0.17 m−1) data. These offset values were subtracted from each c profile value in both cruises. Moreover, in situ stimulated fluorescence was converted into [Chla] values through a linear correlation analysis, in order to relate to beam attenuation data.
Upper mixed layer (UML) depth
The UML was considered the depth at which potential density differed by 0.02 kg m−3 from mean potential density measured between 5 and 10 m depth, as it best represented that parameter during both cruises (modified from Mitchell and Holm-Hansen (1991) and Hewes et al. (2008), which adopted a change in density of 0.05 kg m−3 over 5-m intervals).
Wind and air temperature data
Daily data of surface wind speed and direction were retrieved from the radar scatterometer SeaWinds (www.ssmi.com/qscat) on board the QuikSCAT satellite. In order to verify a possible influence of wind forcing on the UML depth near JRI, three-day average wind fields were calculated for days preceding each sampling period, using a spatial grid resolution of 0.25° × 0.25°.
Average air temperature was calculated over 4 months (December to March) of each sampling year. Values were retrieved from the Meteorological Station database at the Marambio Argentine Base (Base AntárticaMarambio; available at www.wunderground.com), which is located on the southeastern side of JRI approximately 30 km from the southernmost stations in our sampling grid.
Satellite data
[Chla]s and sea ice concentrations were derived from satellite data within a region delimited by 64.5°S–63.2°S and 57.7°W–54°W. Due to cloud interference in daily images during the sampling periods, [Chla] was derived from monthly composites of MODIS-Aqua satellite images. Level 3 (L3) Standard Mapped Images were obtained from http://oceancolor.gsfc.nasa.gov at a 4-km resolution. Daily images of sea ice concentration, retrieved from the AMSR-E sensor (AQUA platform) with a spatial resolution of approximately 6 × 4 km at 89 GHz, were used to calculate monthly mean images for the study area. The investigated period spanned from January to March in both years (2008 and 2009). The Artist Sea Ice (ASI) algorithm was applied to retrieve sea ice concentration between 0 and 100 % (Spreen et al. 2008). Hemispherical (6.25 km grid) sea ice concentration (ASI algorithm) daily maps were obtained from the Institute of Environmental Physics, University of Bremen (www.iup.physik.uni-bremen.de). The AMSR-E-derived data were corrected for cloud contamination based on the 18/23/37 GHz channels (Spreen et al. 2008). In the monthly images (January–March), ice-edge pixels were defined as those where sea ice concentration was ≤15 % (Marrari et al. 2008; Spreen et al. 2008). Pixels above this limit were classified as sea ice pixels and, in this way, expressed the sea ice coverage of the study area. MODIS-Aqua data per se cannot be used to accurately discriminate sea ice from clouds. For this reason, the AMSR-E monthly images, which are corrected for cloud-related contamination, were used to identify the ice covered areas (black areas in Fig. 4) and cloud-covered pixels were identified from ocean color images (MODIS-Aqua) as white areas in Fig. 4.
In situ chlorophyll-a concentration ([Chla])
Discrete samples for [Chla] analyses were collected from several depths (0–200 m, selected based on the in situ fluorescence profile) at most stations (Table 1). Seawater samples (0.5–1 L) were filtered onto Whatman 25-mm GF/F filters, which were immediately stored in liquid nitrogen. In the laboratory, pigments were extracted with 2 mL of 95 % cold-buffered methanol (2 % ammonium acetate) for 30 min at −20 °C and stored in the dark. The samples were then sonicated (Bransonic, model 1210) and centrifuged. Extracts were filtered (Fluoropore PTFE filter membranes, 0.2-μm pore size) and immediately analyzed by high-performance liquid chromatography (HPLC). More details on pigment composition and concentration, as well as CHEMTAX analysis results for the JRI region, can be found in Mendes et al. (2012).
Phytoplankton community composition, abundance and carbon biomass
For phytoplankton identification and counting, only surface water samples were collected and preserved in amber glass flasks (~250 mL) with 2 % alkaline Lugol’s iodine solution. Settling chambers (50–100 mL settling volume) were inspected on an Axiovert 135 ZEISS inverted microscope (Sournia 1978; Utermöhl 1958) at 200×, 400×and 1000× magnifications, and cell identification was made according to specific literature (Hasle and Syvertsen 1996; Scott and Marchant 2005). Cells were counted by enumerating at least 300 individuals of the most frequent species (Lund et al. 1958). The abundance of each species (expressed in 104 cells L−1) was converted into biovolume estimates using two or three linear dimensions from captured images by a camera (Spot Insight QE) attached to the microscope or during observation. At least 30 specimens were randomly chosen for metrics of each species or major taxa, and biovolume was subsequently estimated using a geometric shape with the closest resemblance (Hillebrand et al. 1999). Cell carbon content (carbon biomass) was calculated by using different carbon-to-volume ratios: for diatoms and dinoflagellates, the formula pgC cell−1 = 0.109 × V 0.991 was applied, in accordance with Montagnes et al. (1994), and for all other algae groups, in accordance with Menden-Deuer and Lessard (2000), the formula pgC cell−1 = 0.216 × V 0.939was applied, where V is the biovolume estimate of each phytoplankton species in the respective group.
Statistical analysis
In order to relate [Chla] to abiotic factors (salinity and c), Pearson-r parametric correlation coefficients were calculated at p < 0.05 statistical significance. Salinity was selected because it was considered a proxy of meltwater influence (from melting of sea ice and/or glaciers) and c was assumed to be mainly related to phytoplankton biomass and, to a lesser extent, to dissolved degradation products.
By examining the spatial distribution of [Chla] and salinity in the study region, two groups of samples could be recognized: low-salinity and high-[Chla] stations near JRI and high salinity and low-[Chla] stations further away from JRI. In order to objectively find a threshold to discriminate between those regions, we have applied a binary recursive partitioning (regression tree) analysis on salinity and [Chla] data using the software R 3.0.2 (R Development Core Team 2013) following Crawley (2007). The results pointed to the isohaline of 34.27 as a threshold between the two groups of stations: lower salinity–higher biomass (Group 1), comprising stations R101-R103, R106-R108, R111-R114, R116 and R117 (2008), and R201, R202, R204-R209 (2009); and higher salinity–lower biomass (Group 2), comprising stations R104, R105, R109, R110, R115 and R118-R120 (2008), and R203, R210-R212 (2009). Following that, the biological data were averaged for each group for further analysis.
Statistical analysis of phytoplankton composition was performed based on both cell abundance and carbon estimates of four major phytoplankton groups [nanoflagellates (<20 µm), dinoflagellates, diatoms and autotrophic ciliates. Differences between groups were evaluated by a Kruskal–Wallis one-way analysis of variance followed by Dunn’s method for pairwise multiple comparison procedures (Abdi 2007), as the necessary assumptions for parametric analysis were not achieved. With respect to the main phytoplankton taxa, mean relative contribution of cell abundance and carbon biomass estimates were calculated for Groups 1 and 2.
Results
Environmental features
T/S diagrams (Fig. 2a, b) for both cruises showed three different types of surface waters, hereafter named as (in colored dots): Weddell Sea Shelf Water (WSSW; yellow dots), Meltwater (blue dots) with lower salinities and Confined Water (red dots), which were classified according to the salinity levels in the surface layer (200 m). WSSW was characterized by potential temperatures >−1 °C and salinity <34.29. Meltwater was present near glaciers and characterized by potential temperatures between −1.2 and −0.7 °C and salinity <34.27. The so-called Confined Water was observed in the UML (about 100 m) exclusively within the Antarctic Sound (AS) and presented a narrow potential temperature range (−0.9 to −0.6 °C) and higher salinities (34.3–34.4).
Potential temperature and salinity diagram (T–S) for the study area during a 2008 and b 2009. The colored dots represent waters within the upper mixed layer. Meltwater (blue) was present at stations R113–R115 (2008) and R205–R207 (2009). Yellow dots represent the Weddell Sea Shelf Water (WSSW). Red dots show the Confined Water, nomenclature adopted in this work to the highest salinities found in surface layer of the study area. (Color figure online)
Differences in physical oceanographic data between both late summer periods (2008 and 2009) were observed from distributions of surface temperature, surface salinity and UML depth (Fig. 3). Higher surface temperatures were observed in 2009 (−0.87 to −0.46 °C) than in 2008 (−1.19 to −0.62 °C) (Fig. 3a, d). Furthermore, UML depth ranged from 19 to 109 m (16–74 m) in 2008 (2009), while the UML was >85 m in the Antarctic Sound in both years (Fig. 3c, f). On the other hand, surface salinity was very similar in both surveys (Fig. 3b, e).
The 3-day average satellite wind data (direction and speed) generally showed a reasonably similar pattern in both sampling periods (data not shown). Wind speeds attained 22 m s−1 in 2008 and 23 m s−1 in 2009, with a predominantly westerly direction. It is possible that JRI acted as a shelter from westerly winds, particularly surface waters east of the island.
Average air temperatures for the study area (December–March) were higher in the summer of 2008–2009 (6 °C) than in the summer of 2007–2008 (4.75 °C). This difference may suggest that there was a more extended sea ice melting area as well as a relatively higher contribution of melted glaciers near JRI in the summer of 2008–2009.
[Chla]s and sea ice cover
The monthly mean [Chla]s for January, February and March (2007–2008 and 2008–2009) in the study area showed prominent peaks in January and February of both 2008 and 2009 (Fig. 4a, b, d, e, respectively) with [Chla] values in 2009 almost twofold higher than in 2008. The average concentrations in January for 2008 and 2009 were 1.4 and 3.4 mg m3, respectively. The lower average in the first year can be associated with the extensive sea ice coverage during the 3-month period prior to the 2007–2008 summer bloom. Average values decreased in February (although a bloom can be observed south of the selected area in 2009) and March (Fig. 4). It is noteworthy that the sea ice area in 2009 (black pixels in Fig. 4d, e, f) is more restricted to the south (mainly south of 64.5°S) than in 2008 (black pixels in Fig. 4a–c). In general, the [Chla]s spatial distribution pattern within the study area for January–March 2008 indicated patches with moderate-biomass levels compared to higher-[Chla] patches within the same period in 2009.
MODIS-Aqua imagery of monthly mean [Chla] (color pallet), showing also the mean location of sea ice covered (black dotted pixels). Images refer to January–March 2008 (a, b, c) and January–March 2009 (d, e, f). The black rectangle indicates the approximate coverage of the study area. (Color figure online)
Phytoplankton abundance, biomass and optical features
The spatial patterns of fluorescence levels (Fig. 5a, d) and beam attenuation c (Fig. 5c, f) were similar to the [Chla] surface distribution (Fig. 5b, e), with the highest values (~6.2 mg m3) in the vicinities of JRI, particularly during late summer of 2009. A decrease in [Chla] and in fluorescence can be seen eastward from JRI (Fig. 5a, b, d, e) and was accompanied by an increase in salinity level (Fig. 3b, e). High surface [Chla] values were associated with low surface salinity, especially when in the presence of Meltwater (salinity <34.27).
As stated in the “Methods” section, two groups were identified, based upon [Chla] and salinity levels, and those could be separated by the 34.27 salinity threshold: lower salinity–higher biomass (Group 1) and a higher salinity–lower biomass (Group 2). Based on this criterion, phytoplankton composition data are presented for both groups (Table 2). Although some stations (R101, R102, R103, R108, R117, R202) could have been assigned to areas of mixing between Meltwater and WSSW, showing a slight increase in surface salinity (up to 34.29) and considerably high [Chla] (>1 mg m−3 for 2008, and >3 mg m−3 for 2009 data set), these stations were included in Table 2 within Group 1 data.
In general, minimum and maximum values of [Chla] were lower in late summer of 2008 than in late summer of 2009 (Table 2). In Group 1 (Group 2), [Chla] range was 1.05–4.50 (0.25–1.01) mg m−3 in 2008 and 2.99–7.61 (0.36–1.28) mg m−3 in 2009. In relation to phytoplankton composition and biomass, the entire area was dominated by diatoms, which comprised the bulk of phytoplankton carbon in the bloom areas around JRI. However, nanoflagellates (<20 µm) were numerically prominent in both sampling years. Their average cell abundance was statistically different from diatom cell abundance only in Group 1 in 2008 (H = 18.00, p = 0.0004, see more details in Table 2), although with a much smaller biomass. Dinoflagellates and the autotrophic ciliate Myrionecta rubra Lohmann 1908 were fairly noticeable in 2009. On average, diatom cell abundance was higher in Group 1 (61,181 cells L−1 in 2008 and 290,128 cells L−1 in 2009) than in Group 2 (4383 cells L−1 in 2008 and 23,701 cells L−1 in 2009), which reflected their carbon biomass estimates. Higher average values were found in Group 1 (161.8 µg L−1 in 2008 and 256.6 µg L−1 in 2009) than in Group 2 (17.5 µg L−1 in 2008 and 35.3 µg L−1 in 2009) (Table 2).
In general, the phytoplankton species composition showed no considerable differences between sampling years or between the two groups (Table 3). However, some particular features could be observed in the microscopic analysis. Many Chaetoceros species were identified, both from Phaeoceros (C. bulbosus (Ehrenberg) Heiden 1928, C. criophilus Castracane 1886, C. dichaeta Ehrenberg 1844, C. flexuosus Mangin 1915; with the latter two in very low concentration) and Hyalochaete (C. debilis Cleve 1894, C. neglectus Karsten 1905, C. tenuissimus Meunier 1913 that were assembled as C. spp. <10 µm) subgenera. Many small cell-sized diatom species (<20 µm of greatest axial linear dimension), including Chaetoceros Hyalochaete (13.30 × 104 cells L−1 on average and 11 % of mean relative contribution to total cell abundance) and unidentified centrics (5.50 × 104 cells L−1 on average and 5 % from the total cell abundance) were more abundant in Group 1, particularly in 2009 (Table 3). Conversely, large diatom cells of Thalassiosira spp. (spanning 20–100 µm in diameter), Odontella weissflogii (Janisch) Grunow 1884, Corethron pennatum Castracane 1886, Eucampia antarctica (Castracane) Mangin 1915 and unidentified centrics (diameter >100 µm) were the most important contributors to the phytoplankton community, in terms of carbon biomass, independent of area or sampling period (Table 3).
Relationship between [Chla] and environmental parameters
[Chla] was significantly correlated to salinity, best represented by a polynomial relationship (r 2 = 0.81, p < 0.05 in 2008 and r 2 = 0.66, p < 0.05 in 2009; see Fig. 6), but with some differences between the two sampling years. A more pronounced salinity gradient was seen in 2009.
The relationship between fluorescence intensity and extracted [Chla] (0–200 m) was significant on both cruises (r 2 = 0.77, n = 69, p < 0.001 in 2008 and r 2 = 0.83, n = 41, p < 0.001 in 2009) (Fig. 7a). Based on those relationships, values of fluorescence (relative units) were converted into [Chla], in order to relate to depth profiles of beam attenuation c within the UML. Higher correlations between fluorescence-derived [Chla] and c were found for Group 1 stations (black dots, Fig. 7b, c) close to JRI, both in 2008 (r 2 = 0.92) and in 2009 (r 2 = 0.76). This suggests that phytoplankton was a major component contributing to light attenuation in the lower-salinity–higher-biomass area, with the exception of stations R104 and R105, whose surface [Chla] was low (0.26 and 0.25 mg m−3, respectively) (see Fig. 7b). In Group 2, particularly around the AS in 2008, a high relationship (r 2 = 0.88) was found between the optical parameter and fluorescence-derived [Chla] (Fig. 7b, gray dots), but with a noticeably higher slope. In 2009, no relationship was found for the Group 2 (Fig. 7c, gray dots). In both cases, other optical components (such as detritus and non-algal particulate matter) were probably of greater significance to c. In summary, c was better associated with the phytoplankton biomass within the lower-salinity–higher-biomass area, i.e., within the vicinities of JRI.
Relationships between [Chla] (mg m−3) and fluorescence (Relative Unit—RU) for 2008 (black dots) and 2009 (gray dots) (a). Beam attenuation coefficient (660 nm) (m−1) versus fluorescence-derived [Chla] (relative unit—RU) is shown for 2008 (b) and 2009 (c). Black dots and lines indicate linear fitting regressions for majority of stations in Group 1 and gray dots and lines refer to most of stations in Group 2. Note that stations R104 (red dots) and R105 (light blue dots) were considered as exceptions within the Group 1, whose surface [Chla] were low (0.26 and 0.25 mg m−3, respectively). See text for statistical results. (Color figure online)
Discussion
In situ environmental features
Over the sampled coastal and shelf sites on both cruises, surface layers were clearly influenced by horizontal mixing between water masses from the Weddell Sea (WSSW, corresponding to the High Salinity Shelf Water) and freshwater input (Meltwater) derived from nearby glaciers, establishing a salinity gradient eastward of the JRI. Other studies have observed this pattern in the region (Piatkowski 1989; Gordon et al. 2000; von Gyldenfeldt et al. 2002). The northwestward flow of WSSW, which enters the Bransfield Strait around the tip of the Antarctic Peninsula and the AS (Piatkowski 1989; Gordon et al. 2000), could be an important source of micronutrients (particularly iron). This would fertilize surface waters of the eastern portion of the Antarctic Peninsula (Sañudo-Wilhelmy et al. 2002), providing optimal conditions for phytoplankton growth.
In this work, higher sea surface temperatures were found in late summer of 2009 than in that of 2008, probably causing an earlier sea ice melting in 2009 (see Figs. 2, 4) and this receding process may act as a Fe source (Moore and Doney 2006). Furthermore, melting glaciers from JRI and other neighboring glaciers may have caused freshwater runoff, carrying dissolved terrigenous trace metals, including iron, into coastal waters, which subsequently stimulated higher levels of phytoplankton biomass in late summer of 2009. On the other hand, the Confined Water mass observed only in the upper mixed layer (about 100 m) of the AS was probably advected from the region east of JRI, under influence of the WSSW mass (von Gyldenfeldt et al. 2002).
The lower surface salinities observed in 2009 were the main signature of the Meltwater mass reflecting on a shallower UML depth close to JRI. By contrast, higher salinities, coupled with deeper UML (around 100 m), were a typical structure observed in the AS. Those scenarios have been described in other studies from several Antarctic sites (Ducklow et al. 2007; Hewes 2009; Montes-Hugo et al. 2009; Mendes et al. 2012) and are shown to affect the dynamics of phytoplankton communities, i.e., an elevated autotrophic biomass in shallow UML, as opposed to low-biomass conditions in deep UML regions.
Based on the salinity gradient eastward from JRI, a strong association of low-salinity waters with high phytoplankton biomass was determined in the region. Significant inverse relationships were observed between phytoplankton biomass and salinity (r 2 = 0.81 in 2008 and r 2 = 0.66 in 2009). During both periods, high biomass levels (>1 mg m−3) were associated with salinities lower than 34.3, characterized as lower-salinity–higher-biomass areas in the vicinities of JRI. Hewes (2009) reported a unimodal pattern of [Chla] across salinity gradients in waters surrounding the South Shetland Islands in the western Antarctic Peninsula. It was suggested that phytoplankton biomass would be primarily controlled by UML depth at high salinities and by iron at low salinities, with optimal conditions at intermediate salinity values around 34 (Hewes 2009). Therefore, we reinforce the association between low salinities and high phytoplankton biomass, particularly in late summer of 2009, when a similar polynomial relationship (see Fig. 6) (y = −7.2x 2 + 465.9x − 7503.8, n = 40) to that in Hewes (2009)’s (y = −6.87x 2 + 468.77x − 7994.2, r 2 = 0.54, n = 164) was found. During both study periods, wind speed patterns were similar (around 23 m s−1). A predominantly easterly direction suggests that sampling stations near JRI were on a relatively sheltered position, allowing the maintenance of a shallow UML depth. Other studies have emphasized the Island’s effect on phytoplankton biomass levels, depending on location around the island, i.e., the wind-sheltered side can promote stable water column conditions that result in growth and accumulation of phytoplankton (Vernet et al. 2008; Montes-Hugo et al. 2009). The lowest UML depth values in 2009 (minimum of 16 m) may be a clear signal of glacial ice melting in decreasing surface salinity and the establishment of a very shallow UML, in combination with adequate wind conditions.
Growth season of phytoplankton from remote sensing data
Previous studies on phytoplankton biomass distribution in the northeastern Antarctic Peninsula have commonly described that ice-edge blooms tend to move progressively southward and intensify during December–February, when the highest [Chla] values (>4 mg m−3) would be attained (Holm-Hansen et al. 2004). Based on the monthly [Chla]s composites (Fig. 4), the phytoplankton blooms became more prominent southwards, from January to March, and strikingly evident in the summer of 2009. This is probably associated with a seasonal retraction of sea ice (see gray areas in Fig. 4), favoring an ice-edge bloom near 65°S in February 2009. A study based on Environmental Satellite (Envisat) Advanced Synthetic Aperture Radar (ASAR) images, from 1989 to 2010, showed an extensive ice melting area from high freshwater discharges from glaciers (Costi 2011, unpublished data) in the northern area of the Peninsula’s tip. The freshwater discharge varied from 0.49 Gigatons (from December of 2007 to February of 2008) to 1.69 Gigatons (from December of 2008 to February of 2009). In the summer of 2008–2009, a higher freshwater discharge before or during the study period was probably an important factor for the development of a stronger phytoplankton bloom than during the summer of 2007–2008. At the same time, a higher average air temperature, by 1.25 °C comparing to the subsequent year, probably contributed to higher glacier melting rates and freshwater discharge, as stated by Costi (2011 and unpublished data). These environmental conditions must have favored input of micronutrient and a shallow UML also provided adequate conditions to phytoplankton growth near JRI.
Both the magnitude and extension of glacial retreat (up to 3.79 km2 year−1) near JRI have recently been interpreted as a direct consequence of rapidly changing climate across the Antarctic Peninsula region, which significantly affects the accumulation and ablation of ice caps (Rau et al. 2004). Data remain scarce regarding the implications of those environmental shifts on cascading effects over the biota in the Southern Ocean, particularly on phytoplankton communities. For instance, based on remote sensing information, a remarkable increase in [Chla]s (1998–2006) was found, compared with more recent (1978–1986) data set, south of 63°S in the western continental shelf of the Antarctic Peninsula (Montes-Hugo et al. 2009). This increasing trend was primarily attributed to rapid regional climate change, with the southern sector (>64°S) showing increased phytoplankton biomass due to shallower UML, a longer sea ice season and a weaker influence of wind on UML depth and, therefore, on mean light levels (Montes-Hugo et al. 2009 and references therein). Taking this into account, it could be suggested that the eastern sector of the Antarctic Peninsula would be displaying similar climate changing effects on the phytoplankton community.
With respect to [Chla] satellite image analysis, it was not possible to determine the relative contribution of either glacial or sea ice melting processes in JRI surrounding waters on the marked phytoplankton blooms observed during the sampling periods. Remote sensing retrieved [Chla] has already shown some limitations, particularly regarding missing values due to cloud-covered pixels (Holm-Hansen et al. 2004). Therefore, we can suggest that during the study periods, particularly in 2009, the eastern side of the Antarctic Peninsula’s tip was exposed to warmer summer period (inferred from the average temperature of 6 °C compared to 4.75 °C for 2008 summer), which may have contributed to stronger sea ice melting, creating both greater exposed ocean areas and the strengthening the water column stratification, favoring primary productivity. Nonetheless, this scenario could be interpreted as an example of annual variations (Marrari et al. 2008) associated with climate change in the region.
Diatom assemblages: sea ice and optical-related features
The environmental features mentioned above favored the dominance of diatoms as major contributors to the surface [Chla], especially for the lower-salinity–higher-biomass area. This was in contrast to what was observed in other sectors around the Antarctic Peninsula, such as the offshore Weddell Sea and Drake Passage, where small phytoflagellates such as Phaeocystis antarctica Karsten 1905 and cryptophytes were predominant during the same sampling periods (Mendes et al. 2012, 2013). Based on HPLC/CHEMTAX data, fucoxanthin/diatoms attained more than 90 % of [Chla] in most sampling stations, showing their predominance in biomass near JRI (Mendes et al. 2012). The present work presented complementary data on detailed species composition of the phytoplankton community, showing that large diatoms species dominated phytoplankton biomass, with important contribution of dinoflagellates on those recurrent phytoplankton blooms. There were no striking differences in phytoplankton composition between Groups 1 and 2, or between both years. As expected, several tiny (<20 µm) diatom species/taxa were primarily important in cell numbers, while larger cells attained a much greater carbon biomass. Although there are relatively limited data on phytoplankton community near JRI, Olguín and Alder (2011) described cell abundance (447,075–777,213 cells L−1) and carbon biomass (117.96–156.03 µg C L−1) with a great contribution (from 72 to 85 %) of the small cell-sized Chaetoceros tortissimus Gran 1900 near JRI. Interestingly, they pointed out that the 2002 summer (January–March) was characterized by an anomalously low sea-ice coverage, corresponding to a “warm year” (Olguín and Alder 2011 and references therein). Similar cell abundances and carbon biomass were found in our work in 2009 (see Table 2), which can also be considered an anomalously “warm year.”
Several Chaetoceros species were identified in this work, including C. tortissimus in both late summer periods (Table 3). The presence of this diatom species, as well as others identified here, can help estimate the timing of sampling within the seasonal progression of diatoms, since at some Antarctic sites, even with marked interannual variation, it is possible to identify early, middle and late stages of the blooms, based on occurrence of certain species (Denis et al. 2006; Annett et al. 2010). For instance, high cell concentration of C. Hyalochaete spp. (as C. neglectus) in early summer has been related to an early phase of primary production, following sea ice retreat processes (Ligowski et al. 1992; Garibotti et al. 2005; Buffen et al. 2007), whereas the large cell-sized Odontella weissflogii has been considered as a typical species of mid-season assemblages, well adapted to stratified conditions (Gomi et al. 2005) and still associated with sea ice (Palmisano and Garrison 1993). The similar-shaped centrics Proboscia spp. and Rhizosolenia antennata (Ehrenberg) N. E. Brown 1920 have normally been found in late diatom assemblages (Stickley et al. 2005; Annett et al. 2010) and also indicate, along with many larger Thalassiosiraspp., open ocean conditions (Olguín and Alder 2011). Thus, we can suggest that diatom assemblages in this work represented an admixture of species related to recent melting sea ice (such as Chaetoceros spp., small Minidiscus-resembling unidentified centrics) and some species more associated with advanced sea ice retreat, stratified conditions (specially by Odontella weissflogii) and a cluster of Thalassiosira spp. and Corethron pennatum, more related to open ocean conditions. Finally, it was noteworthy that Rhizosolenia spp., assuming their prominent growth period in late summer season, was marginally important in the higher-salinity–lower-biomass area (Group 2) in late summer of 2009 (0.05 µg C L−1 on average, but with mean relative contribution <1 % to total carbon biomass (see Table 3).
The relationship between [Chla] and the attenuation coefficient c was notably different for Group 2 stations, with most stations close to the AS, as compared to the remaining stations in both study years (see gray dots in Fig. 7). Low [Chla] (average ~0.63 mg m−3) of Group 2, associated with relatively high c values, could indicate that other suspended particulate material, as well as colored dissolved organic matter (cDOM) derived from phytoplankton metabolism, would have contributed to light attenuation within that area, particularly the AS in 2009. It is known that different particle compositions and/or size distribution of phytoplankton communities (not notably seen in our study) can reflect in a degraded relationship between c and phytoplankton biomass (Behrenfeld and Boss 2006), as was the case in 2009. In summary, we can state that c and [Chla] were tightly coupled (r 2 ranging 0.87–0.96) close to JRI only, demonstrating that both indices would be reliable tracks of changes in phytoplankton biomass.
Although phytoplankton data were not collected during the entire warm growth season in the eastern section of the Antarctic Peninsula’s tip, this work provides new environmental and biological data from this almost inaccessible coastal region. The upper waters close to JRI were clearly characterized by a diatom-dominated phytoplankton community governed by water column features, e.g., relatively low salinity and shallow UML, sea ice and glacial melting processes. Regarding retreating processes of sea ice, it has been observed that both differences in timing and type of sea ice breakout, either as persistent patchy cover or showing a rapid shift to ice-free conditions, were coupled with high variability in phytoplankton assemblages and seasonal succession (Annett et al. 2010). Those spatial and temporal variations impose the need for further comprehensive studies that confirm any recurrent patterns in response to sea ice and glacial melting and explore the relationship between sea ice breakout/retreat and species assemblages in this region.
Conclusions
In this paper, we have presented a comprehensive study of phytoplankton communities over the eastern part of the Antarctic Peninsula’s tip, close to James Ross Island, where ice melting processes have driven recurrent phytoplankton blooms during two late summer seasons. Both remote sensing (January–March, 2008 and 2009) and in situ data of 2008 and 2009 late summer seasons showed that there is a tight relationship between the extent of sea ice and phytoplankton biomass levels in the east of the Antarctic Peninsula, specifically around JRI.
Relatively weaker winds due to sheltering by JRI and melting waters contributed to micronutrient availability and maintaining a stratified water column, which provided favorable conditions for phytoplankton growth and development. Two distinct groups of stations were identified based on relation salinity–[Chla]: Group 1 comprising sites close to JRI, associated with lower salinity and higher [Chla], and Group 2 comprising sites with higher-salinity and lower-[Chla] levels.
There were no noticeable differences in the composition of phytoplankton species between 2008 and 2009, or across the entire study region, with a clear mixture of diatom species, typical of ice-related processes. Results of optical measurements showed a significant relationship between light attenuation coefficient (660 nm) and [Chla], particularly at sites close to JRI, suggesting that phytoplankton was the main component influencing the attenuation of light in most of the study areas.
References
Abdi H (2007) The Bonferonni and Šidák corrections for multiple comparisons. In: Salkind NJ (ed) Encyclopedia of measurement and statistics. Sage, Thousand Oaks (CA), pp 103–107
Annett AL, Carson DS, Crosta X, Clarker A, Ganeshram RS (2010) Seasonal progression of diatom assemblages in surface waters of Ryder Bay, Antarctica. Polar Biol 33:13–29
Arrigo KR, van Dijken GL (2004) Annual changes in sea-ice, chlorophyll a, and primary production in the Ross Sea, Antarctica. Deep Sea Res II 51:117–138
Arrigo KR, van Dijken GL, Bushinsky S (2008) Primary production in the Southern Ocean, 1997–2006. J Geophys Res 113(C08004):1–27
Behrenfeld MJ, Boss E (2006) Beam attenuation and chlorophyll concentration as alternative optical indices of phytoplankton biomass. J Marine Res 64:431–451
Boyd PW (2002) Environmental factors controlling phytoplankton processes in the Southern Ocean. J Phycol 38:844–861
Buffen A, Leventer A, Rubin A, Hutchins T (2007) Diatom assemblages in surface sediments of the northwestern Weddell Sea, Antarctic Peninsula. Mar Micropaleontol 62:7–30
Costi J (2011) Estimativa do derretimento e descarga de água na porção norte da Península Antártica. Dissertation, Federal University of Rio Grande do Sul
Crawley MJ (2007) Tree models. In: Crawley MJ (ed) The R book, 2nd edn. Wiley, West Sussex, pp 685–700
Denis D, Crosta X, Zaragosi S, Romero O, Martin B, Mas V (2006) Seasonal and subseasonal climate changes recorded in laminated diatom ooze sediments, Adélie Land, East Antarctica. Holocene 16(8):1137–1147
Ducklow HW, Baker K, Martinson DG, Quetin LB, Ross RM, Smith RC, Stammerjohn SE, Vernet M, Fraser W (2007) Marine pelagic ecosystems: the West Antarctic Peninsula. Phil Trans R Soc B 362:67–94
Edwards R, Sedwick P (2001) Iron in East Antarctic snow: implications for atmospheric iron deposition and algal production in Antarctic waters. Geophys Res Lett 28:3907–3910
Fitzwater SE, Johnson KS, Gordon RM, Coale KH Jr, Smith WO (2000) Trace metal concentrations in the Ross Sea and their relationship with nutrients and phytoplankton growth. Deep Sea Res II 47:3159–3179
Garibotti IA, Vernet M, Ferrario ME, Smith RC, Ross RM, Quetin LB (2003) Phytoplankton spatial distribution patterns along the western Antarctic Peninsula (Southern Ocean). Mar Ecol Prog Ser 261:21–39
Garibotti IA, Vernet M, Smith RC, Ferrario ME (2005) Interannual variability in the distribution of the phytoplankton standing stock across the seasonal sea-ice zone west of the Antarctic Peninsula. J Plankton Res 27(8):825–843
Gomi Y, Umeda H, Fukuchi M, Taniguchi A (2005) Diatom assemblages in the surface water of the Indian Sector of the Antarctic Surface Water in summer of 1999/2000. Polar Biosci 18:1–15
Gordon AL, Mensch M, Dong Z, Smethie WM, de Bettencour J (2000) Deep and bottom water of the Bransfield Strait eastern and central basins. J GeophysRes 105:11337–11346
Grotti M, Soggia F, Ianni C, Frache R (2005) Trace metals distributions in coastal sea ice of Terra Nova Bay, Ross Sea, Antarctica. Antarct Sci 17(2):289–300
Hasle GR, Syvertsen EE (1996) Marine diatoms. In: Tomas CR (ed) Identifying marine diatoms and dinoflagellates. Academic Press Inc., London, pp 5–385
Hawes I, Brazier P (1991) Freshwater stream ecosystems of James Ross Island, Antarctica. Antarct Sci 3:265–271
Hewes CD (2009) Cell size of Antarctic phytoplankton as a biogeochemical condition. Antarct Sci 21:457–470
Hewes CD, Reiss CS, Kahru M, Mitchell BG, Holm-Hansen O (2008) Control of phytoplankton biomass by dilution and mixed layer depth in the western Weddell-Scotia Confluence. Mar Ecol Prog Ser 366:15–29
Hillebrand H, Dürselen CD, Kirschtel D, Pollingher U, Zohary T (1999) Biovolume calculation for pelagic and benthic microalgae. J Phycol 35:403–424
Holm-Hansen O, Kahru M, Hewes CD, Kawaguchi S, Kameda T, Sushin VA, Krasovski I, Priddle J, Korb R, Hewitt RP, Mitchell BG (2004) Temporal and spatial distribution of chlorophyll-a in surface waters of the Scotia Sea as determined by both shipboard measurements and satellite data. Deep Sea Res II 51:1323–1331
Kang S, Kang J, Lee S, Chung KH, Kim D, Park MG (2001) Antarctic phytoplankton assemblages in the marginal ice zone of the northwestern Weddell Sea. J Plankton Res 23:333–352
Lancelot C, Mathot S, Veth C, de Baar H (1993) Factors controlling phytoplankton ice-edge blooms in the marginal ice-zone of the northwestern Weddell Sea during sea ice retreat 1988: field observations and mathematical modelling. Polar Biol 13(06):377–387
Ligowski R, Godlewski M, Lukowski A (1992) Sea ice diatoms and ice edge planktonic diatoms at the northern limit of the Weddell Sea pack ice. Proc NIPR Symp Polar Biol 5:9–20
Loeb V, Hofmann EE, Klinck JM, Holm-Hansen O, White WB (2009) ENSO and variability of the Antarctic Peninsula pelagic marine ecosystem. Antarct Sci 21:135–148
Lund JWG, Kipling C, Le Cren ED (1958) The inverted microscope method of estimating algal numbers and the statistical basis of estimations by counting. Hydrobiologica 11:143–170
Marrari M, Daly KL, Hu C (2008) Spatial and temporal variability of SeaWiFS chlorophyll a distributions west of Antarctic Peninsula: implications for krill production. Deep Sea Res II 55:377–392
Menden-Deuer S, Lessard EJ (2000) Carbon to volume relationships for dinoflagellates, diatoms, and other protist plankton. Limnol Oceanogr 45:569–579
Mendes CRB, Souza MS, Garcia VMT, Leal MC, Brotas V, Garcia CAE (2012) Dynamics of phytoplankton communities during late summer around the tip of the Antarctic Peninsula. Deep-Sea Res I 65:1–14
Mendes CRB, Tavano VM, Leal MC, Souza MS, Brotas V, Garcia CAE (2013) Shifts in the dominance between diatoms and cryptophytes during three late summers in the Bransfield Strait (Antarctic Peninsula). Polar Biol. doi:10.1007/s00300-12-1282-4
Mitchell BG, Holm-Hansen O (1991) Observations and modeling of the Antarctic phytoplankton crop in relation to mixing depth. Deep-Sea Res 38:981–1007
Montagnes DJS, Berges JA, Harrison PJ, Taylor FJR (1994) Estimating carbon, nitrogen, protein, and chlorophyll a from volume in marine phytoplankton. Limnol Oceanogr 39:1044–1060
Montes-Hugo M, Doney SC, Ducklow HW, Fraser W, Martinson D, Stammerjhn SE, Schofield O (2009) Recent changes in phytoplankton communities associated with rapid regional climate change along the Western Antarctic Peninsula. Science 323:1470–1473
Moore JK, Doney SC (2006) Remote sensing observations of ocean physical and biological properties in the region of the Southern Ocean Iron Experiment (SOFeX). J Geophys Res 111:C06026. doi:10.1029/2005JC003289
Olguín HF, Alder VA (2011) Species composition and biogeography of diatoms in antarctic and subantarctic (Argentine shelf) waters (37–76°S). Deep Sea Res II 58:139–152
Palmisano AC, Garrison DL (1993) Microorganisms in Antarctic sea ice. In: Friedmann EI (ed) Antarctic microbiology. Wiley, New York, pp 167–218
Park MG, Yang SR, Kang S, Chung KH, Shim JH (1999) Phytoplankton biomass and primary production in the marginal ice zone of the northwestern Weddell Sea during austral summer. Polar Biol 21:251–261
Piatkowski U (1989) Macroplankton communities in Antarctic surface waters: spatial changes related to hydrography. Mar Ecol Prog Ser 55:251–259
Pisarevskaya LG, Popov IK (1991) Free-drifting icebergs and thermohaline structure. Glaciers-Ocean-Atmosphere Interactions (Proceedings of the International Symposium, St. Petersburg, September 1990). IAHS Publ 208:447–454
R Development Core Team (2013) R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. Available at http://www.R-project.org/. Version: 3.0.2
Rau F, Mauz F, De Angelis H, Jaña R, Neto JA, Skvarca P, Vogt S, Saurer H, Gossmann H (2004) Variations of glaciers frontal positions on the northern Antarctic Peninsula. Ann Glaciol 39:525–530
Robertson RA, Padman L, Egbert GD (1998) Tides in the Weddell Sea. In: Jacobs SS, Weiss RF (eds) Ocean, Ice and Atmosphere interactions at the Antarctic continental margin. Antarctic Res Ser, AGU, Washington, 75:341–369 doi: 10.1029/AR075p0341
Sañudo-Wilhelmy SA, Olsen KA, Scelfo JM, Foster TD, Flegal AR (2002) Trace metal distributions off the Antarctic Peninsula in the Weddell Sea. Mar Chem 77:157–170
Scott FJ, Marchant HJ (2005) Antarctic Marine Protists. Australian Biological Resources Study and Australian Antarctic Division, Canberra 572p
Sedwick PN, DiTullio GR (1997) Regulation of algal blooms in Antarctic shelf waters by the release of iron from melting sea ice. Geophys Res Lett 24:2515–2518
Sedwick PN, DiTullio GR, Mackey DJ (2000) Iron and manganese in the Ross Sea, Antarctica: seasonal iron limitation in Antarctic shelf waters. J Geophys Res 105:11321–11336
Smetacek V, Assmy P, Henjes J (2004) The role of grazing in structuring Southern Ocean pelagic ecosystems and biogeochemical cycles. Antarct Sci 16(4):541–558
Smith WO Jr, Nelson DM (1990) Phytoplankton growth and new production in the Weddell Sea marginal ice zone in the austral spring and autumn. Limnol Oceanogr 35:809–821
Smith RC, Baker KS, Vernet M (1998) Seasonal and interannual variability of phytoplankton biomass west of the Antarctic Peninsula. J Marine Syst 17:229–243
Sone T, Fukui K, Strelin JA, Torielli CA, Mori J (2007) Glacier lake outburst flood on James Ross Island, Antarctic Peninsula region. Pol Polar Res 28:3–12
Sournia A (1978) Phytoplankton Manual. UNESCO, Paris
Spreen G, Kaleschke L, Heygster G (2008) Sea ice remote sensing using AMSR-E 89 GHz channels. J Geophys Res. doi:10.1029/2005JC003384
Stickley CE, Pike J, Leventer A, Dunbar R, Domack EW, Brachfeld S, Manley P, McClennan C (2005) Deglacial ocean and climate seasonality in laminated diatom sediments, Mc. Robertson Shelf. Antarctica. Palaeogeogr Palaeoecol 227:290–310
Utermöhl H (1958) Zur Vervollkommnung der quantitativen Phytoplankton-Methodik. Mitt Internationale Ver Theoretische und Angewandte Limnologie 9:1–38
Vernet M, Martinson D, Iannuzzi R, Stammerjohn S, Kozlowski W, Sines K, Smith R, Garibotti I (2008) Primary production within the sea-ice zone west of the Antarctic Peninsula: I-Sea ice, summer mixed layer, and irradiance. Deep Sea Res II 55:2068–2085
Von Gyldenfeldt A-B, Fahrbach E, García MA, Schröder M (2002) Flow variability at the tip of the Antarctic Peninsula. Deep Sea Res II 49:4743–4766
Acknowledgments
This project is part of GOAL (Group of High Latitude Oceanography) and was supported by funding from CNPq (Brazilian National Council on Research and Development), MMA (Ministry of Environment) and the Brazilian Antarctic Program (PROANTAR). The authors would like to thank the Brazilian Ministry of Science and Technology (MCT) and the crew of the Brazilian Navy research ship “AryRongel” for their assistance and for contributions with all cruise activities. We are very grateful to Rafael Mendes for HPLC analysis. A.M.S. Detoni and M.S. de Souza were funded by CAPES Foundation. M.S. de Souza was granted by CNPq and is now receiving a research fellowship from CAPES.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Detoni, A.M.S., de Souza, M.S., Garcia, C.A.E. et al. Environmental conditions during phytoplankton blooms in the vicinity of James Ross Island, east of the Antarctic Peninsula. Polar Biol 38, 1111–1127 (2015). https://doi.org/10.1007/s00300-015-1670-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-015-1670-7