Abstract
Echinoderms are the dominant megafaunal taxa in Antarctic and Subantarctic waters in terms of abundance and diversity, having a predominant role in structuring communities. The current study presents new data on the asteroids, holothuroids, and ophiuroids (three of the five extant classes of echinoderms) collected in seven scientific campaigns (1995–2012) from Bouvet Is., South Shetland Is., and the Eastern Weddell Sea, from a wide bathymetric range (0–1,525 m). Among the 316 echinoderms collected, we extended the bathymetric ranges of 15 species and expanded the geographic distribution of 36 of them. This novel dataset was analyzed together with previous reports in order to establish general patterns of geographic and bathymetric distribution in echinoderms of the Southern Ocean (SO). Nearly 57 % of the assembled-data species resulted endemic of the SO, although further taxonomic efforts in less accessible areas are needed. Interestingly, some islands presented high levels of species richness even comparable to large geographic areas. While generally exhibiting a wide range of eurybathy, there were differences in species composition across depths corresponding to sublittoral, upper and lower bathyal, and abyssal. Bathymetric distribution was analyzed considering biological aspects for each class. As expected, circumpolar trends were found, although hydrographic currents may be the cause of differences in species composition among SO areas. Our analyses suggest zoogeographic links between Antarctica and the adjacent ocean basins, being the Scotia Arc the most remarkable. This study contributes to the knowledge of large-scale diversity and distribution patterns in an Antarctic key group.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The separation of Antarctica from South America allowed the formation of the Antarctic Circumpolar Current (ACC) and the establishment of the Polar Front (PF). This thermal and hydrographic barrier hampers marine organisms’ dispersion from north to south and vice versa at the Southern Ocean (SO; Barker and Thomas 2004). Simultaneously, the PF promotes the dispersal of marine organisms—larvae or adults—from west to east around Antarctica (Fell 1962; Olbers et al. 2004), and the East Wind Drift along the Antarctic coast deeply affects the distribution of shelf fauna. The combination of geographic isolation and climate change has led to a rich marine Antarctic biota with high number of endemic taxa (Brandt and Gutt 2011). However, numerous species are also shared between the SO and the nearest geographic neighbors mainly due to their connections during the Cenozoic (Clarke et al. 2005). The Magellanic region, through the Drake Passage and Scotia Arc, especially acts as a potential faunal exchange pathway (Clarke et al. 2005; Brandt et al. 2007a). Despite the present day knowledge, essential baseline data on marine biodiversity and biogeography are still lacking for most regions of the SO (Kaiser et al. 2013). This is urgently required to identify biological responses to predicted environmental changes in Antarctica. Gutt et al. (2004) pointed out the need of comparative studies between Antarctic and South-American fauna to better understand species’ dispersion capabilities and the effect of isolation of populations on their distribution.
Diachronic anchor ice greatly influences Antarctic benthic community structure. Short-term seasonal and spatial variations from anchor and sea ice contribute to the patchiness of benthic communities in the Antarctic continental shelf (Raguá-Gil et al. 2004). At the same time, long-term glacial and interglacial cycles allowed allopatric speciation (Thatje et al. 2005), thus promoting diversification and wide bathymetric tolerances for several Antarctic taxa (Brandt et al. 2007a; Rogers 2007). Furthermore, due to a very deep continental shelf and a weakly stratified water column, circumantarctic distributions and broad depth ranges are also widespread characteristic features of marine Antarctic fauna (Brey et al. 1996; Soler i Membrives et al. 2009; Hemery et al. 2012). This suggests that the deep-sea fauna around Antarctica, largely consisting of taxa with high dispersal capabilities, may be related both to adjacent shelf communities and to deep-sea fauna from other oceans, being directly connected below 3,000 m (Brandt et al. 2007a, b; Pawlowski et al. 2007). In fact, differences in the reproductive mode might explain composition variations between sites and depths (Raguá-Gil et al. 2004). Thus, long-range dispersion by pelagic planktotrophic and lecithotrophic larvae facilitates the spreading of many species and increases their colonization capacity of highly disturbed habitats, contrary to brooding organisms that have lower dispersal capabilities (Shilling and Manahan 1994; Poulin et al. 2002).
Recently, total species richness of macrozoobenthic organisms inhabiting the Antarctic continental shelf has been estimated to comprise between 11,000 and 17,000 species, of which over 8,800 are presently known and described (Griffiths 2010; De Broyer et al. 2011). Antarctic benthic fauna is characterized by the lack of durophagous species either as competitors or as predators (Clarke et al. 2004). Thus, echinoderms are the dominant errant megafaunal taxa in the SO in terms of abundance and diversity and have a predominant role in structuring benthic communities (Dayton et al. 1974; Clarke and Johnston 2003; Chiantore et al. 2006). Around 10 % of the known Antarctic macrozoobenthic species are echinoderms, with Asteroidea (208 species; De Broyer et al. 2011), Holothuroidea (187 species; O’Loughlin et al. 2011), and Ophiuroidea (126 species; Stöhr et al. 2012), being the most speciose classes. Although echinoderm species richness is higher in the continental shelf, where dense communities of sessile suspension feeders and its wandering associated fauna dominate, they also show a high diversity along the slope and on the deep-sea plains (Billett et al. 2001; Aronson et al. 2007).
At the beginning of the twentieth century, the South Shetland Is. and the Weddell Sea echinoderm fauna were widely explored (e.g., Ludwig 1903; Vaney 1914; Koehler 1917). More recently, high species richness of asteroids, ophiuroids, and holothuroids has been found on a regular basis in these areas (Gutt 1990a, b; Gutt and Piepenburg 1991; Massin 1992a; Piepenburg et al. 1997; Presler and Figielska 1997; Manjón-Cabeza et al. 2001; Manjón-Cabeza and Ramos 2003). In addition, within the last decades, new collections and re-examinations of previously collected material have contributed to the description of new species (Carriol and Féral 1985; Gutt 1990a, b; Massin 1992a; Stampanato and Jangoux 1993; O’Loughlin 2002, 2009; Massin and Hétérier 2004; O’Loughlin and Ahearn 2008; Janosik and Halanych 2010). Other than the South Shetland Is. and the Weddell Sea, echinoderm fauna from Subantarctic areas such as the remote Bouvet Is. has been also surveyed within the last years (Arntz 2006). Interestingly, it appears to be that the major reason for the impoverished fauna occurring in the vicinities of Bouvet Is. is under-sampling rather than isolation or geological youth (Arntz et al. 2006). This island has been proposed as a missing link in the SO, connecting macrozoobenthic fauna with the adjacent Magellanic South America, the Antarctic Peninsula, and the high Antarctic Weddell Sea (Arntz et al. 2006; Gutt et al. 2006), although little is known about its echinoderm fauna.
Bearing in mind the ecological importance of echinoderms as one of the major groups structuring the Antarctic and Subantarctic benthos, our aim was twofold: first, to enhance the present knowledge of Antarctic echinoderms species and their geographic and bathymetric distribution by identifying species from widely studied (Eastern Weddell Sea and South Shetland Is.) and poorly explored (Bouvet Is.) areas; second, to determine the bathymetric and geographic distributions of Antarctic echinoderms in the SO combining our data with all bibliographic resources available so far. Species composition of the areas studied was compared to the adjacent ocean basins and discussed. Asteroids, ophiuroids, and holothuroids were selected as target classes within echinoderms to address both objectives.
Materials and methods
Collection and identification of newly collected samples
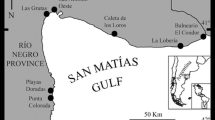
The study area comprised Bouvet Is., the South Shetland Is., and the Eastern Weddell Sea. Samples from Bouvet Is. and the Weddell Sea were collected during the Antarctic cruises ANT XV/3 (February 1998) and ANT XXI/2 (November 2003–January 2004) on board the R/V Polarstern (AWI, Bremerhaven, Germany) at 33 stations. Samples from the South Shetland Is. (mostly from the vicinities of Deception and Livingston Is.) were collected at eight stations on board the BIO-Hespérides in January 1995 and January 2006 during the BENTART and ECOQUIM-2 cruises, respectively. During the ACTIQUIM campaigns at Deception and Livingston Is. (2007–2008, 2008–2009, and 2010–2011), 18 stations were surveyed by SCUBA diving. Collection ranged from 0 to 1,525 m depth using Agassiz trawl, bottom trawl, epibenthic sledge, giant box corer, and Rauschert dredge in Bouvet Is. and the Weddell Sea, and Agassiz trawl and rock dredge in the South Shetland Is. (Table 1). In all cases, sampling was qualitative.
After photographing the living animals, they were preserved in 70 % ethanol for further taxonomic identification to the lowest possible taxonomical level (Table 2). Key references and synopses used for the identification within the different classes of echinoderms were: Ludwig (1903), Koehler (1917), Clark (1962, 1963), Clark and Downey (1992), Stampanato and Jangoux (1993), and Presler and Figielska (1997) for asteroids; Koehler (1917), Mortensen (1936), Fell (1961), and Madsen (1967) for ophiuroids; Théel (1886), Vaney (1914), Carriol and Féral (1985), Gutt (1990a, b), Massin (1992a, b, 2010), Massin and Hétérier (2004), O’Loughlin (2002, 2009), O’Loughlin and Ahearn (2005, 2008), Cross et al. (2009), O’Loughlin et al. (2009, 2011), O’Loughlin and VandenSpiegel (2010), O’Loughlin and Whitfield (2010), and O’Loughlin personal notes (unpublished data) for holothuroids. Crinoids and echinoids were not studied here due to the low number of samples collected during our surveys.
Assembled data
Our data (Table 2) were analyzed together with the Antarctic and Subantarctic echinoderm species list gathered from the available literature, the ‘Scientific Committee on Antarctic Research Marine Biodiversity Information Network’, and the SCAR’s Marine Biodiversity Information database (SCAR-MarBIN; http://www.scarmarbin.be/; De Broyer et al. 2012). Metadata were checked against the major world databases, World Ophiuroidea (Stöhr and O’Hara 2012), World Asteroidea (Mah 2009), and complemented with the Antarctic Marine Invertebrates of the NMNH/Smithsonian Institution Databases (Lemaitre et al. 2009). Global Biodiversity Information Facility (GBIF; http://www.gbif.org/) and Ocean Biogeographic Information Systems (OBIS; http://iobis.org/) databases were used together with information within keys to compile all metadata (Online Resources 1–3). Due to the heterogeneous nature of the compiled data and the assignment of the occurrences of the species to geographic areas (see below), sampling effort could not be calculated in this study.
Study area
Bathymetric and geographic metadata from other cruises reported in the literature and online databases together with our own new data were pooled and analyzed in order to evaluate the relationships between taxa in 20 areas of the SO (Fig. 1). Following several authors (Clarke and Johnston 2003; Barboza et al. 2011; O’Loughlin et al. 2011), Antarctica was divided into eight geographic areas: Antarctic Peninsula, Amundsen Sea, Bellingshausen Sea, Dumont D’Urville Sea (including Ballenny Is.), Enderby Plain, Prydz Bay, Ross Sea, and Weddell Sea. Also, eight island groups were considered: Bouvet Is., Heard and McDonald Is., Kerguelen Is., South Georgia Is., South Sandwich Is., South Shetland Is., South Orkney Is., and the Subantarctic Marion, Prince Edward, and Crozet Is. (these last three considered as a single group). Due to proximity to the study area, Australia, New Zealand (including Macquarie Is.), South Africa, and South America shared species distribution were also included in the analysis for comparison purposes.
Data analysis
Due to unequal sampling efforts (in both terms of regions surveyed and bathymetry) and use of heterogeneous gear to obtain all metadata included in the analysis, binary data (presence/absence) were chosen to construct the echinoderm data matrix. Echinoderm presence/absence was preferred rather than abundance because our study treated a large-scale area; therefore, habitat patchiness and/or heterogeneity would bias our results. We performed cluster and MDS analyses to examine the faunal patterns among the different areas and across depths. The widely used Bray-Curtis index was used to build the similarity matrix, being this index equivalent to the Sörensen index for presence/absence matrices (Clarke et al. 2006). Hierarchical clustering was obtained using the group linkage clustering technique to evaluate the similarities in species composition between regions. Depths were divided into 500-m categories, except for the bathymetric range of 0–100 m as it was statistically different to the closer ranges, and included into the MDS plot; less surveyed depths (>6,000 m) were not included in the MDS analysis presented here since their composition was remarkably different from the rest of bathymetric categories.
Results
New data
A total of 316 specimens were identified in this study, including asteroids, ophiuroids, and holothuroids (Table 2). Out of these, 32 asteroids (107 specimens) from four orders were identified to species level. The most represented asteroid families in number of species were Asteriidae and Odontasteridae (five species each), followed by Pterasteridae (four), Ganeriidae, Goniasteridae, and Solasteridae (three species each), Astropectinidae, Echinasteridae, Poranidae, and Stichasteridae (two species each), and finally Asterinidae (one). Thirteen ophiuroid species (53 specimens) from the two existing orders (Euryalida and Ophiurida) were identified. The greatest number of ophiuroid species was found within the Ophiuridae family (six species), and two different species were identified from each Gorgonocephalidae, Amphiuridae, and Ophiacanthidae families; Ophiodermatidae had only one species. Out of the 156 holothuroid specimens collected, 34 species from the six existing orders were found. The most speciose families were the dendrochirotid Cucumariidae (14) and Psolidae (nine) followed by the families Chiridotidae, Elpidiidae, and Synallactidae (three species each), while Ypsilothuriidae and Molpadiidae had only one species each.
Our data extended the bathymetric ranges of 15 species (10 Holothuroidea and 4 Asteroidea) and enlarged the geographic distribution of 36 species (19 Holothuroidea, 13 Asteroidea, and 4 Ophiuroidea; see Table 2). Our data expanded the bathymetric range of Diplasterias kerguelenensis to superficial waters (0 m), and four species of holothuroids down to 1,525 m (Paradota weddellensis, Peniagone vignioni, Protelpidia murrayi, Rhipidothuria racovitzai). Remarkably, although some species were found for the first time in Bouvet Is. (Cucamba psolidiformis, Cucumaria attenuata, Ophiacantha antarctica, Ophioplinthus gelida, Ophiura rouchi, Psolidium incubans, R. racovitzai), the Weddell Sea (Acodontaster elongatus, Perknaster fuscus, Psolidium whittakeri, Psolus paradubiosus, Pteraster rugatus, Pteraster stellifer), and the South Shetland Is. (Cladodactyla crocea, Crucella scotiae, D. kerguelenensis, Echinocucumis hispida, Psolus charcoti), they were previously recorded from the vicinities of these regions or they were considered as circumantarctic. The asteroid Solaster longoi and the holothuroid Trachythyone cynthiae, previously known only from Marion Is. group and Pridz Bay, respectively, have been reported for the first time in the Weddell Sea.
Assembled data
Species richness
To date, a total of 555 species of echinoderms (excluding echinoids and crinoids) have been recorded from Antarctic waters including our data and previous literature: 229 asteroids, 129 ophiuroids, and 197 holothuroids. The total number of species was slightly higher than those reported by recent studies (De Broyer et al. 2011; O’Loughlin et al. 2011; Stöhr et al. 2012) as we have also included here works with some species only identified to genus level. The regions with the highest species richness were as follows: South Shetland Is. (229), Antarctic Peninsula (211), Weddell Sea (201), South Orkney (184), South Georgia (182), and South Sandwich (180) islands (the last three being part of the Scotia Arc), and Ross Sea (176). Dumont D’Urville Sea (151) and Enderby Plain (134) had intermediate species richness, while Prydz Bay (112), Marion, Prince Edward and Crozet Is. (99), Bellingshausen Sea (96), Kerguelen Is. (80), Amundsen Sea (69), Heard Is. (66), and Bouvet Is. (52) presented the lowest species richness values (Fig. 2). Asteroids dominated at all regions except for the Amundsen Sea and the Scotia Arc, which are dominated by holothuroids and ophiuroids.
Endemic and shared species
Remarkable differences were found between classes and areas studied when considering endemic species in Antarctic regions (Fig. 3). More than half of the species of each class appeared to be endemic to the SO: 63 % (125 species) in holothuroids, 59 % (76 species) in ophiuroids, and ca. 50 % (113 species) in asteroids. The highest endemism rates were present in the Weddell Sea and the Marion Is. group for asteroids, the Antarctic Peninsula and the Dumont D’Urville Sea for ophiuroids, and the Scotia Arc and the Marion Is. group for holothuroids (Fig. 3). Among the shared species with other non-Antarctic geographic regions, 38 % appeared under 2,500 m. Antarctic fauna was more related to South America (36 % species similarity) in species composition than to New Zealand (13 %), Australia (9 %), or South Africa (7 %; see Online Resource 4).
Bathymetric ranges and distribution
As a general trend, species composition gradually changed across depths and was separated accordingly to the sublittoral, upper and lower bathyal, and upper and lower abyssal (Fig. 4a; Vinogradova 1997; Zezina 1997). However, asteroids appeared to be distributed in wider depth bands, since the distance between depth ranges in the MDS was less pronounced (Fig. 4b). Thus, asteroids, which have an 80 % of similarity, had four clusters of species restricted to the sublittoral and upper bathyal (0–1,000 m), lower bathyal (1,000–3,500 m), upper abyssal (3,500–5,500 m), and lower abyssal (>5,500 m). With a 60 % of similarity between ophiuroid samples, four different ophiuroid groups could be distinguished: sublittoral and upper bathyal (0–1,000 m), lower bathyal (1,000–2,500 m), upper abyssal (2,500–4,500 m), and lower abyssal (>4,500 m; Fig. 4c). Holothuroids had a distinct shallow-water fauna (0–100 m) with only species of the order Dendrochirotida (Neopsolidium convergens, Pseudocnus intermedia, Psolus granulosus, and Squamocnus spp.). Sea cucumber assemblages seemed to segregate in sublittoral and upper bathyal (100–1,000 m), lower bathyal (2,000–3,500 m), upper abyssal (3,500–5,000 m), and lower abyssal (>5,000 m; Fig. 4d). High species richness found between 100 and 500 m was the general tendency for the three echinoderm classes—with more than 65 % of species reported within this depth range—progressively decreasing with depth, although holothuroids had a less pronounced decrease in species composition until 3,000 m (Online Resource 5). Abyssal depths had less species richness for asteroids (20 %) and ophiuroids (10 %), which had only two and one species at these depths, respectively. Conversely, holothuroids had more than 30 % of species richness (eight species) restricted to abyssal depths.
Geographic relationships
Cluster analyses suggested several regional groups with similar faunal composition (Fig. 5). The dendrogram obtained after pooling data of all classes established four distinct geographic zones with a similarity greater than 40 % in the echinoderm fauna (Fig. 5a): cluster 1, formed by the Scotia Arc Is., the Antarctic Peninsula, the Ross and Weddell seas, and the East Antarctic areas (Dumont D’Urville Sea, Enderby Plain, and Prydz Bay); cluster 2, formed by the remote Bouvet Is. alone, and related to cluster 1; cluster 3, formed by the Amundsen and Bellingshausen seas; and cluster 4, formed by the Subantarctic Heard Is., Kerguelen Is., and the Marion, Prince Edward, and Crozet Is. group. Bathymetric distributions of the species of each cluster are specified in Online Resources 1–3 (for Asteroidea, Ophiuroidea, and Holothuroidea, respectively). There were mild differences in the cluster analysis when treating echinoderm classes separately. The clusters observed for asteroids and ophiuroids were quite similar to those obtained for the whole echinoderm dataset, except for the case of Amundsen and Bellingshausen seas, which did not fall within the same cluster (Fig. 5b, c). As for holothuroids, areas from cluster 1 and cluster 3 were grouped together, while the East Antarctica areas were more related to areas from cluster 2; in addition, Marion, Prince Edward, and Crozet Is. were not grouped together with Heard Is. and Kerguelen Is. (Fig. 5d).
Hierarchical clustering (group average) of the echinoderm fauna analyzed from the Southern Ocean using the Bray-Curtis distance (assembled data). a The three classes grouped together, indicating the four clusters in different colors, b Asteroidea, c Ophiuroidea, and d Holothuroidea. In all cases, 40 % of similarity was chosen as a threshold to group regions. (Color figure online)
Discussion
Our work has contributed to expand the knowledge on echinoderm bathymetric and geographic distribution in the SO. Within the 79 species identified in our survey pertaining to the classes Asteroidea, Ophiuroidea, and Holothuroidea, the families Asteriidae, Odontasteridae, Ophiuridae, Cucumariidae, and Psolidae were the most speciose in Bouvet Is., the Eastern Weddell Sea, and the South Shetland Is. at shelf depths (0–800 m). Our data show that, even though these areas have been widely sampled through the last decades (Gutt 1990a, b; Gutt and Piepenburg 1991; Massin 1992a; Piepenburg et al. 1997; Presler and Figielska 1997; Manjón-Cabeza et al. 2001; Manjón-Cabeza and Ramos 2003; Arntz 2006; Arntz et al. 2006; Gutt et al. 2006), new species records are still being found. This is especially true for Bouvet Is. with seven echinoderm species recorded for the first time in this study. More importantly, the echinoderm diversity described so far in the SO is surely underrepresented since several newly recorded taxa still are currently undescribed (Kaiser et al. 2013). In addition, cryptic speciation may also cause underestimations of echinoderm diversity in the SO. As an example, two new sea star species of the well-known genus Odontaster have recently been described combining molecular and morphological analyses (Janosik and Halanych 2010). Interestingly, these new species occurred along the Antarctic Peninsula, perhaps one of the best-studied regions in the SO (Griffiths 2010). Indeed, integrative taxonomic approaches revealed that some species defined by morphological characters are in fact complexes of cryptic species (Rogers 2007; O’Loughlin et al. 2011). Thus, future work on the re-evaluation of identified sibling species will probably enrich the number of taxa in the SO.
Antarctic and Subantarctic regions presented general trends in species composition when treating metadata of all compiled species records. Species richness among classes was relatively high and similar between the well-studied areas, such as Scotia Sea Is., Weddell and Ross seas, Antarctic Peninsula, and adjacent islands, as seen in Griffiths (2010). In turn, when considering less-sampled areas, such as Amundsen and Bellingshausen seas, Bouvet Is. or the Kerguelen group, their number of species decreased, possibly due to their geographic isolation. Notice that asteroids were the most diverse class in the Marion group, with values similar to those of larger geographic areas (Fig. 2). Arntz (2006) suggested that Subantarctic islands may have served as refugia for benthic shallow-water organisms during Cenozoic glacial maxima. The current island patchiness and/or habitat heterogeneity may have allowed higher numbers of species with different ecological niches, leading to high degrees of endemism, something that has already been observed for holothuroids (Gutt 2007). In fact, asteroids and holothuroids showed the highest endemism values in the Marion Is. area (Fig. 3) possibly due to marked isolation of this Subantarctic area. Other regions with a high degree of endemism for holothuroids were Amundsen and Bellingshausen seas, while ophiuroids exhibited higher endemism in the East Antarctica and the Antarctic Peninsula, probably due to recent increase in sampling effort in these areas (Manjón-Cabeza and Ramos 2003; O’Loughlin et al. 2009). To achieve a better understanding on the echinoderm biodiversity in these areas, different sampling methods and greater collecting efforts are specially needed.
In agreement with other studies dealing with various invertebrate taxa, Antarctic echinoderms also exhibited a high range of eurybathy (Brey et al. 1996; Soler i Membrives et al. 2009; Figuerola et al. 2012). This seems to be explained by both the palaeoclimatic history of Antarctica and the current iceberg scour activity (Clarke et al. 2004; Thatje et al. 2005; Smale et al. 2008). It is hypothesized that Cenozoic glacial–interglacial cycles may have driven an environmental force toward the evolutionary trend of eurybathy in many Antarctic benthic invertebrates. During the extension of continental ice sheet, shelf fauna may have gone extinct or forced to go into deeper water refugia. Conversely, during the shelf ice retreats at the subsequent interglacial, the defaunated shelf could have been re-colonized by fauna from the slope (Clarke et al. 2004), deep sea, or shelters on the continental shelf (Thatje et al. 2005). In addition, eurybathic tendencies of the current benthic shelf fauna (to depths of 500 m) are reinforced by the erosive action of recurrent iceberg scouring (Smale et al. 2008). Our analysis showed that echinoderms were gradually distributed across depths. Bathymetric distribution in Ophiuroidea fitted with the depth limits suggested by Clarke and Johnston (2003), and also for other studies using other taxa (Piepenburg et al. 1997; Aldea et al. 2008; Figuerola et al. 2012). Thus, ophiuroid communities were distinguished in sublittoral and upper bathyal (0–1,000 m), lower bathyal (1,000–2,500 m), upper abyssal (2,500–4,500 m), and lower abyssal (>4,500 m). Asteroid communities from sublittoral and bathyal depths were similar in species composition, thus reinforcing the proposed tendency of eurybathy. This result might be influenced by the generalist and opportunistic feeding strategies observed for several species of this class (McClintock 1994). We distinguished a stenobathic shallow-water fauna for Holothuroidea, mainly characterized by the occurrence of suspension-feeding Dendrochirotida species. Generally, all classes decreased in species richness with depth probably due to a reduction in organic matter input, the main factor controlling Antarctic benthos (Arntz et al. 1994). However, holothuroid’s species richness decreased moderately when compared to asteroids and ophiuroids. The diversity of feeding strategies in this class (i.e., suspension-feeding dendrochirotids from shallow waters, deposit-feeding deep-sea elasipodid holothuroids) may use different food qualities of the suspended matter equally along water depth (Gutt and Piepenburg 1991; McClintock 1994). This might reflect the mild reduction in species richness across depths.
The cluster analysis suggested four groups or clusters of similar echinoderm faunal composition. Cluster 1, comprising Antarctic Peninsula, South Shetland Is., South Orkney Is., Weddell Sea, South Georgia Is., and South Sandwich Is., has been identified in previous studies on Echinodermata and other phyla, and the relationship between these areas might be influenced by the Weddell Gyre (Arntz et al. 2005; Barnes et al. 2009; Barboza et al. 2011; Figuerola et al. 2012). This clockwise current connects the Weddell Sea with the Scotia Arc through the Antarctic Peninsula (Orsi et al. 1993), allowing dispersion of echinoderm’s planktonic larvae or even epiplanktonic adults (Olbers et al. 2004). The rest of the areas in cluster 1 are located in the Eastern Antarctica (Dumont D’Urville Sea, Ross Sea, Enderby Plain, and Prydz Bay) and may be connected to the above-mentioned Weddell Gyre areas through the East Wind Drift (Brey et al. 1996; Olbers et al. 2004). Cluster 2 was only composed by Bouvet Is., a remote area probably also influenced by the Weddell Gyre, as previously reported for different taxa (Barnes 2005; Arntz et al. 2006; Gutt et al. 2006).
Amundsen and Bellingshausen seas comprised cluster 3 and were the areas with less species richness relative to their extension, which may in part be due to the comparatively less sampling effort conducted in these areas (Griffiths 2010). In fact, due to the relative ancient formation of both seas, a higher number of species, when compared to close seas, would have been expected (Thomson 2004). Nevertheless, Saiz et al. (2008) described low species richness in the Bellingshausen Sea and suggested that this impoverished fauna was related to low-food supply, a situation exacerbated by the influence of periodic physical disturbances (such as iceberg scour).
Finally, cluster 4 was composed by Heard and McDonald Is., Kerguelen Is., and the Marion, Prince Edward, and Crozet group, a series of Subantarctic islands located in the Southern Indian Ocean at the edge of the PF. Their species composition similarity might be explained by the effects of the ACC, which promotes the dispersal of marine organisms from west to east in a clockwise pattern, as it has already been suggested for echinoderms (Fell 1962) and other taxa (Barnes 2002; Raguá-Gil et al. 2004). The geographic proximity of Heard and Kerguelen Is. might also have an effect in their similar echinoderm fauna. In fact, they lay on the so-called Kerguelen Plateau (1,000–2,500 m deep), which has recently been proposed as a glacial refugium for echinoderm species (Hemery et al. 2012).
Comparing SO echinoderms with the adjacent ocean basins, South America was the basin that shared more species with all the areas considered in this study. In particular, the Scotia Sea shared the highest number of species, since its intermediate location represents a physical link between both the SO and South America (Barnes 2005; Kim and Thurber 2007). Their biogeographic similarities might be explained by geological history (both areas were connected during the Cenozoic) or by a two-way migration of both shallow-water and the deep ocean fauna. Turbulent flow structures, called eddies, have been also hypothesized as a mechanism for transport of bathyal organisms (to 1,000 m) from north to south of the ACC and vice versa (Clarke et al. 2005). Moreover, there is a global thermohaline circulation of Antarctic Bottom Water, which connects the abyssal Weddell Sea with the southwest Atlantic basin, allowing the dispersal of deep-sea organisms (Pawlowski et al. 2007). Other than South America, areas such as New Zealand, Australia, and South Africa harbor echinoderm species in common to the SO; this may also be explained by global patterns of deep-sea water circulation.
We firmly believe that our data input analyzed together with bibliographic datasets in this biogeographic study will serve to understand the dynamics of a key group structuring the Antarctic benthic fauna. However, the amount of new data reflects the need of more taxonomic and biogeographic studies in Antarctic and Subantarctic areas. Different sampling methods and an increase of survey efforts are specially needed in the less surveyed areas with supposedly low species richness (e.g., Amundsen Sea, Bellingshausen Sea, Bouvet Is.), while in higher-sampled areas major taxonomic effort is also necessary.
References
Aldea C, Olabarria C, Troncoso JS (2008) Bathymetric zonation and diversity gradient of gastropods and bivalves in West Antarctica from the South Shetland Islands to the Bellingshausen Sea. Deep Sea Res Part I Oceanogr Res Pap 55:350–368
Arntz WE (2006) Bouvet Island: a stepping stone in the Southern Ocean? Polar Biol 29:81–82
Arntz WE, Brey T, Gallardo A (1994) Antarctic zoobenthos. Oceanogr Mar Biol 32:241–304
Arntz WE, Thatje S, Gerdes D, Gili JM, Gutt J, Jacob UTE, Montiel A, Orejas C, Teixidó N (2005) The Antarctic-Magellan connection: macrobenthos ecology on the shelf and upper slope, a progress report. Sci Mar 69:237–269
Arntz WE, Thatje S, Linse K, Avila C, Ballesteros M, Barnes DKA, Cope T, Cristobo FJ, De Broyer C, Gutt J, Isla E, López-González P, Montiel A, Munilla T, Ramos-Esplá AA, Raupach M, Rauschert M, Rodríguez E, Teixidó N (2006) Missing link in the Southern Ocean: sampling the marine benthic fauna of remote Bouvet Island. Polar Biol 29:83–96
Aronson RB, Thatje S, Clarke A, Peck LS, Blake DB, Wilga CD, Seibel BA (2007) Climate change and invasibility of the Antarctic benthos. Annu Rev Ecol Evol Syst 38:129–154
Barboza CADM, Moura RB, Lanna AM, Oackes T, Campos LS (2011) Echinoderms as clues to Antarctic~South American connectivity. Oecol Aust 15:86–110
Barker PF, Thomas E (2004) Origin, signature and palaeoclimatic influence of the Antarctic Circumpolar Current. Earth Sci Rev 66:143–162
Barnes DK (2002) Biodiversity: invasions by marine life on plastic debris. Nature 416:808–809
Barnes DKA (2005) Changing chain: past, present and future of the Scotia Arc’s shallow benthic communities. Sci Mar 69:65–89
Barnes DKA, Kaiser S, Griffiths HJ, Linse K (2009) Marine, intertidal, freshwater and terrestrial biodiversity of an isolated polar archipelago. J Biogeogr 36:756–769
Billett DSM, Bett BJ, Rice AL, Thurston MH, Galéron J, Sibuet M, Wolff GA (2001) Long-term change in the megabenthos of the Porcupine Abyssal Plain (NE Atlantic). Prog Oceanogr 50:325–348
Brandt A, Gutt J (2011) Biodiversity of a unique environment: the Southern Ocean benthos shaped and threatened by climate change. In: Zachos FE, Habel JC (eds) Biodiversity hotspots. Springer, Berlin, pp 503–526
Brandt A, De Broyer C, De Mesel I, Ellingsen KE, Gooday AJ, Hilbig B, Linse K, Thomson MRA, Tyler PA (2007a) The biodiversity of the deep Southern Ocean benthos. Philos Trans R Soc B Biol Sci 362:39–66
Brandt A, Gooday AJ, Brandão SN, Brix S, Brökeland W, Cedhagen T, Choudhury M, Cornelius N, Danis B, De Mesel I, Diaz RJ, Gillan DC, Ebbe B, Howe JA, Janussen D, Kaiser S, Linse K, Malyutina M, Pawlowski J, Raupach M, Vanreusel A (2007b) First insights into the biodiversity and biogeography of the Southern Ocean deep sea. Nature 447:307–311
Brey T, Dahm C, Gorny M, Klages M, Stiller M, Arntz WE (1996) Do Antarctic benthic invertebrates show an extended level of eurybathy? Antarct Sci 8:3–6
Carriol RP, Féral J-P (1985) Réexamen de quelques Psolidae (Holothurioidea, Echinodermata) antarctiques et subantarctiques. Description de deux nouvelles espèces du genre Psolus. Bull Mus Nat Hist Nat 7:49–60
Chiantore M, Guidetti M, Cavallero M, De Domenico F, Albertelli G, Cattaneo-Vietti R (2006) Sea urchins, sea stars and brittle stars from Terra Nova Bay (Ross Sea, Antarctica). Polar Biol 29:467–475
Clark AM (1962) Asteroidea. Reports BANZ Antarct Res Exped 1929–1931 Ser B 9:1–104
Clark HES (1963) The fauna of the Ross Sea, Part 3, Asteroidea. NZ Oceanogr Inst Mem 151:1–84
Clark AM, Downey ME (1992) Starfishes of the Atlantic. Chapmann and Hall, London
Clarke A, Johnston NM (2003) Antarctic marine benthic diversity. Oceanogr Mar Biol 41:47–114
Clarke A, Aronson RB, Crame JA, Gili J-M, Blake DB (2004) Evolution and diversity of the benthic fauna of the Southern Ocean continental shelf. Antarct Sci 16:559–568
Clarke A, Barnes DKA, Hodgson DA (2005) How isolated is Antarctica? Trends Ecol Evol 20:1–3
Clarke KR, Somerfield P, Chapman MG (2006) On resemblance measures for ecological studies, including taxonomic dissimilarities and a zero-adjusted Bray-Curtis coefficient for denuded assemblages. J Exp Mar Bio Ecol 330:55–80
Cross IA, Gebruk A, Rogacheva A, Billett DSM (2009) Peniagone crozeti, a new species of elasipodid holothurian from abyssal depths off the Crozet Islands in the Southern Indian Ocean. Zootaxa 2096:484–488
Dayton PK, Robilliard GA, Paine RT, Dayton LB (1974) Biological accommodation in the benthic community at McMurdo Sound, Antarctica. Ecol Monogr 44:105–128
De Broyer C, Danis B, Editors with 64 S-MT (2011) How many species in the Southern Ocean? Towards a dynamic inventory of the Antarctic marine species. Deep Sea Res Part II 58:5–17
De Broyer C, Clarke A, Koubbi P, Pakhomov E, Scott F, Vanden Berghe E, Danis B (2012) Echinodermata. The SCAR–MarBIN Register of Antarctic Marine Species (RAMS). http://www.scarmarbin.be/scarramsabout.php
Fell HB (1961) New genera and species of ophiuroidea from Antarctica. Trans R Soc NZ 88:839–841
Fell HB (1962) West-Wind-Drift dispersal of echinoderms in the Southern hemisphere. Nature 193:759–761
Figuerola B, Monleón-Getino T, Ballesteros M, Avila C (2012) Spatial patterns and diversity of bryozoan communities from the Southern Ocean: South Shetland Islands, Bouvet Island and Eastern Weddell Sea. Syst Biodivers 10:109–123
Griffiths HJ (2010) Antarctic marine biodiversity—What do we know about the distribution of life in the Southern Ocean? PLoS One 5:e11683
Gutt J (1990a) New Antarctic holothurians (Echinodermata)—I. Five new species with four new genera of the order Dendrochirotida. Zool Scr 19:101–117
Gutt J (1990b) New Antarctic holothurians (Echinodermata)— II. Four species of the orders Aspidochirotida Elasipodida and Apodida. Zool Scr 19:119–127
Gutt J (2007) Antarctic macro-zoobenthic communities: a review and an ecological classification. Antarct Sci 19:165–182
Gutt J, Piepenburg D (1991) Dense aggregations of three deep-sea holothurians in the southern Weddell Sea, Antarctica. Mar Ecol Prog Ser 68:277–285
Gutt J, Sirenko BI, Smirnov IS, Arntz WE (2004) How many macrozoobenthic species might inhabit the Antarctic shelf? Antarct Sci 16:11–16
Gutt J, Fricke A, Teixidó N, Potthoff M, Arntz WE (2006) Mega-epibenthos at Bouvet Island (South Atlantic): a spatially isolated biodiversity hot spot on a tiny geological spot. Polar Biol 29:97–105
Hemery LG, Eléaume M, Roussel V, Améziane N, Gallut C, Steinke D, Cruaud C, Couloux A, Wilson NG (2012) Comprehensive sampling reveals circumpolarity and sympatry in seven mitochondrial lineages of the Southern Ocean crinoid species Promachocrinus kerguelensis (Echinodermata). Mol Ecol 21:2502–2518
Janosik AM, Halanych KM (2010) Unrecognized Antarctic biodiversity: a case study of the genus Odontaster (Odontasteridae; Asteroidea). Integr Comp Biol 50:981–992
Kaiser S, Brandão SN, Brix S, Barnes DKA, Bowden DA, Ingels J, Leese F, Schiaparelli S, Arango CP, Badhe R, Bax N, Blazewicz-Paszkowycz M, Brandt A, Brenke N, Catarino AI, David B, Ridder C, Dubois P, Ellingsen KE, Glover AG, Griffiths HJ, Gutt J, Halanych KM, Havermans C, Held C, Janussen D, Lörz AN, Pearce DA, Pierrat B, Riehl T, Rose A, Sands CJ, Soler-Membrives A, Schüller M, Strugnell JM, Vanreusel A, Veit-Köhler G, Wilson NG, Yasuhara M (2013) Patterns, processes and vulnerability of Southern Ocean benthos: a decadal leap in knowledge and understanding. Mar Biol 160:2295–2317
Kim S, Thurber A (2007) Comparison of seastar (Asteroidea) fauna across island groups of the Scotia Arc. Polar Biol 30:415–425
Koehler R (1917) Échinodermes (astéries, ophiures et echinides) recueillis par M. Rallier du Baty aux îles de Kerguelen en 1913–1914. Ann I Oceanogr Paris 7:1–87
Lemaitre R, Harasewych MG, Hammock J (2009) ANTIZ v 1.07: a database of Antarctic and Sub-Antarctic marine invertebrates. National Museum of Natural History, Smithsonian Institution. http://invertebrates.si.edu/ANTIZ
Ludwig H (1903) Seesterne. Résultats du Voyage de la S. Y. Belgica 1897–1898–1899. Rap Sci Zool R20:1–72
Madsen FJ (1967) Ophiuroidea. B.A.N.Z. Antarctic research expedition (1929–1931) under the command of sir Douglas Mawson. Rep Ser B 9:123–145
Mah CL (2009) World Asteroidea database. http://www.marinespecies.org/asteroidea
Manjón-Cabeza ME, Ramos A (2003) Ophiuroid community structure of the South Shetland Islands and Antarctic Peninsula region. Polar Biol 26:691–699
Manjón-Cabeza ME, Lirio Y, Ramos A (2001) Distribution of asteroid genera (Echinodermata) off South Shetland Islands and the Antarctic Peninsula. Bol Inst Esp Oceanogr 17:263–270
Massin C (1992a) Three new species of Dendrochirotida (Holothuroidea, Echinodermata) from the Weddell Sea (Antarctica). Bull Inst r Sci nat Belgique Biol 62:179–191
Massin C (1992b) Holothurians (Echinodermata) from Marion and Prince Edward Islands: new and little-known species. Zool Scr 21:311–324
Massin C (2010) On a small collection of Antarctic sea cucumbers (Echinodermata; Holothuroidea) from Léopold III Bay and vicinity. Bull Inst r Sci nat Belgique Biol 80:261–275
Massin C, Hétérier V (2004) On a new species of apodid, Taeniogyrus magnibaculus n. sp. (Echinodermata, Holothuroidea), from Antarctica, living on the spines of cidarid echinoids. Polar Biol 27:441–444
McClintock J (1994) Trophic biology of antarctic shallow-water echinoderms. Mar Ecol Prog Ser 111:191–202
Mortensen T (1936) Echinoidea and Ophiuroidea. Discovery Reports, Cambridge 12:199–348
O’Loughlin PM (2002) Report on selected species of BANZARE and ANARE holothuroidea, with reviews of Meseres Ludwig and Heterocucumis Panning (Echinodermata). Mem Mus Vic 59:297–325
O’Loughlin PM (2009) BANZARE holothuroids (Echinodermata: Holothuroidea). Zootaxa 2196:1–18
O’Loughlin PM, Ahearn C (2005) A review of pygal-furrowed Synallactidae (Echinodermata: Holothuroidea), with new species from the Antarctic, Atlantic and Pacific oceans. Mem Mus Vic 62:147–179
O’Loughlin PM, Ahearn C (2008) Antarctic and Sub-Antarctic species of Psolidium Ludwig (Echinodermata: Holothuroidea: Psolidae). Mem Mus Vic 65:23–42
O’Loughlin PM, Van Den Spiegel D (2010) A revision of Antarctic and some Indo-Pacific apodid sea cucumbers (Echinodermata: Holothuroidea: Apodida). Mem Mus Vic 67:61–95
O’Loughlin PM, Whitfield E (2010) New species of Psolus Oken from Antarctica (Echinodermata: Holothuroidea: Psolidae). Zootaxa 2528:61–68
O’Loughlin PM, Manjón-Cabeza ME, Ruiz FM (2009) Antarctic holothuroids from the Bellingshausen Sea, with descriptions of new species (Echinodermata: Holothuroidea). Zootaxa 2016:1–16
O’Loughlin PM, Paulay G, Davey N, Michonneau F (2011) The Antarctic region as a marine biodiversity hotspot for echinoderms: diversity and diversification of sea cucumbers. Deep Sea Res Part II Top Stud Oceanogr 58:264–275
Olbers D, Borowski D, Völker C, Wölff J-O (2004) The dynamical balance, transport and circulation of the Antarctic Circumpolar Current. Antarct Sci 16:439–470
Orsi AH, Nowlin WD Jr, Whitworth T III (1993) On the circulation and stratification of the Weddell Gyre. Deep Sea Res Part I Oceanogr Res Pap 40:169–203
Pawlowski J, Fahrni J, Lecroq B, Longet D, Cornelius N, Excoffier L, Cedhagen T, Gooday AJ (2007) Bipolar gene flow in deep-sea benthic foraminifera. Mol Ecol 16:4089–4096
Piepenburg D, Vo J, Gutt J (1997) Assemblages of sea stars (Echinodermata: Asteroidea) and brittle stars (Echinodermata: Ophiuroidea) in the Weddell Sea (Antarctica) and off Northeast Greenland (Arctic): a comparison of diversity and abundance. Polar Biol 17:305–322
Poulin E, Palma AT, Jean-Pierre F, Féral J-P (2002) Evolutionary versus ecological success in Antarctic benthic invertebrates. Trends Ecol Evol 17:218–222
Presler P, Figielska E (1997) New data on the Asteroidea of Admiralty Bay, King George Island, South Shetland Islands. Pol Polar Res 18:107–117
Raguá-Gil JM, Gutt J, Clarke A, Arntz WE (2004) Antarctic shallow-water mega-epibenthos: shaped by circumpolar dispersion or local conditions? Mar Biol 144:829–839
Rogers AD (2007) Evolution and biodiversity of Antarctic organisms: a molecular perspective. Philos Trans R Soc Lond B Biol Sci 362:2191–2214
Saiz JI, García FJ, Manjón-Cabeza ME, Parapar J, Peña-Cantero A, Saucède T, Troncoso JS, Ramos A (2008) Community structure and spatial distribution of benthic fauna in the Bellingshausen Sea (West Antarctica). Polar Biol 31:735–743
Shilling FM, Manahan DT (1994) Energy metabolism and amino acid transport during early development of Antarctic and temperate echinoderms. Biol Bull 187:398–407
Smale DA, Brown KM, Barnes DKA, Fraser KPP, Clarke A (2008) Ice scour disturbance in Antarctic waters. Science 321:371
Soler i Membrives A, Turpaeva E, Munilla T (2009) Pycnogonids of the Eastern Weddell Sea (Antarctica), with remarks on their bathymetric distribution. Polar Biol 32:1389–1397
Stampanato S, Jangoux M (1993) Les astérides (Echinodermata) de la Baie Breid (Côte de la Princesse Ragnhild, quartier Enderby, Antarctique), avec la description d’une nouvelle espèce de Solaster. Bull Inst r Sci nat Belgique Biol 63:175–184
Stöhr S, O’Hara T (2012) World Ophiuroidea database. http://www.marinespecies.org/ophiuroidea
Stöhr S, O’Hara TD, Thuy B (2012) Global diversity of brittle stars (Echinodermata: Ophiuroidea). PLoS One 7:e31940
Thatje S, Hillenbrand C-D, Larter R (2005) On the origin of Antarctic marine benthic community structure. Trends Ecol Evol 20:534–540
Théel H (1886) Report on the Holothurioidea dredged by H.M.S. Challenger during the years 1873–1876. Part II. Rep Sci Results Voyag Chall Zool 14:1–290
Thomson MRA (2004) Geological and palaeoenvironmental history of the Scotia Sea region as a basis for biological interpretation. Deep Sea Res Part II Top Stud Oceanogr 51:1467–1487
Vaney C (1914) Holothuries. Deuxième Expédition Antarctique Française (1908-1910) commandée par le Dr Jean Charcot. Sciences Naturelles: Documents Scientifiques. Masson et Cie, Paris, pp 1–54
Vinogradova NG (1997) Zoogeography of the abyssal and hadal zones. Adv Mar Biol 32:325–387
Zezina ON (1997) Biogeography of the Bathyal Zone. Adv Mar Biol 32:389–426
Acknowledgments
The authors wish to thank Prof M. O’Loughlin (Museum Victoria, Australia) for his help in the identification of holothuroid species. Special thanks are given to M. Ballesteros, J. Cristobo, L. Núñez-Pons, and J. Vázquez for laboratory and field support. Thanks are also due to the Unidad de Tecnología Marina (CSIC), as well as the “Bentart”, the BIO-Las Palmas, the BIO-Hespérides, and the “Gabriel de Castilla” Spanish Antarctic Base crews for providing logistic support during the ECOQUIM-2 cruise. Thanks are due to Prof W. Arntz and the R/V Polarstern crew during the ANT XV/3 and XXI/2 Antarctic cruises. Thanks are also given to I. Afán and D. Aragonés (LAST-EBD-CSIC) for helping with map design. We also thank the support and valuable comments of A. Riesgo and J. Giménez and the helpful comments of three anonymous referees. We thank the editor, Dr. D. Piepenburg, for his patience and support along the revision of this manuscript. Funding was provided by the Spanish Government through the ECOQUIM and ACTIQUIM Projects (REN2003-00545, REN2002-12006E ANT, CGL2004-03356/ANT, CGL2007-65453, and CTM2010-17415/ANT).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Moles, J., Figuerola, B., Campanyà-Llovet, N. et al. Distribution patterns in Antarctic and Subantarctic echinoderms. Polar Biol 38, 799–813 (2015). https://doi.org/10.1007/s00300-014-1640-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-014-1640-5