Abstract
Plants have developed sophisticated and complex epigenetic regulation-based mechanisms to maintain stable growth and development under diverse environmental conditions. Histone deacetylases (HDACs) are important epigenetic regulators in eukaryotes that are involved in the deacetylation of lysine residues of histone H3 and H4 proteins. Plants have developed a unique HDAC family, HD2, in addition to the RPD3 and Sir2 families, which are also present in other eukaryotes. HD2s are well conserved plant-specific HDACs, which were first identified as nucleolar phosphoproteins in maize. The HD2 family plays important roles not only in fundamental developmental processes, including seed germination, root and leaf development, floral transition, and seed development but also in regulating plant responses to biotic and abiotic stresses. Some of the HD2 members coordinate with each other to function. The HD2 family proteins also show functional association with RPD3-type HDACs and other transcription factors as a part of repression complexes in gene regulatory networks involved in environmental stress responses. This review aims to analyse and summarise recent research progress in the HD2 family, and to describe their role in plant growth and development and in response to different environmental stresses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants, being sessile in nature, have developed sophisticated and complex mechanisms to respond to different developmental and environmental stress signals (Kapazoglou and Tsaftaris 2011). Epigenetic regulation plays an important role in various biological processes ranging from the maintenance of genome stability to developmental scheduling and regulating responses to various environmental stresses (Kim et al. 2015; Luo et al. 2012a, b, c; Pokholok et al. 2005; Wolffe 1998). Control of gene expression via epigenetic regulation is governed by DNA methylation and nucleosomal core histone modifications (Shinozaki and Yamaguchi-Shinozaki 2007; Urano et al. 2010). A basic nucleosome structure consists of an octamer of core histone proteins, including H2A, H2B, H3, and H4 (Zhou et al. 2013), with the N-terminal lysine residues (also called the histone tails) of histone H3 and histone H4 projected outward. These tails can be subjected to several types of post-translational modifications, including acetylation, methylation, phosphorylation, ubiquitination, and sumoylation. These histone modifications act as a switch to turn gene expression ‘on’ or ‘off’ and, therefore, provide a flexible method of regulating gene expression in response to developmental and environmental signals (Kouzarides 2007; Kurdistani et al. 2004; Zhang and Reinberg 2001). Histone acetylation and deacetylation are important histone modifications and are catalysed by the enzymes called histone acetyltransferases (HATs) and histone deacetylases (HDACs), respectively. HATs are considered to be gene activators, as they transfer the acetyl group to lysine residues of core histone proteins, resulting in relaxed, transcriptionally active euchromatin and subsequent gene activation, whereas HDACs remove the acetyl groups from the lysine residues, resulting in a compact nucleosome structure, thereby leading to transcriptional repression of associated genes (Kuo et al. 1996; Shahbazian and Grunstein 2007; Zhang et al. 1998). HDACs and HATs often play roles as components of multiprotein and chromatin remodelling complexes (Ríos et al. 2007; Saez et al. 2008). Proper control of the acetylation status of histone lysine residues is crucial for the regulation of gene expression in eukaryotes (Pandey et al. 2002; Pfluger and Wagner 2007).
HDACs are classified into three different families, including the Reduced Potassium Deficiency 3 (RPD3) family, the silent induced regulator 2 (SIR2) family, and the histone deacetylase 2 (HD2) family (Kim et al. 2015; Luo et al. 2012a, b, c; Ma et al. 2013; Pandey et al. 2002). The former two families, RPD3 and SIR2, share sequence homology with yeast HDACs and are found in different eukaryotes, whereas the HD2 family shares no sequence homology to yeast HDACs and has only been identified in plants and green algae (Bourque et al. 2016; Ma et al. 2013). This plant-specific HD2 family is emerging as a crucial player in different aspects of plant growth and development and in response to different environmental stresses. This review will update the current knowledge of the HD2 family with special attention to its regulation in plant development and response to environmental stresses.
Evolution and molecular characteristics of the plant-specific HD2 family
The plant-specific HD2 family is thought to have evolved through multiple rounds of progressive genome duplication; this corresponds to the presence of at least two HD2 genes in each of the dicots (Bourque et al. 2016). Based on phylogenetic analysis of DNA and protein sequences of HD2s, Pandey et al. (2002) suggested that a single HD2 gene in the ancestors of monocots and dicots led to the development of all other HD2 genes in each of the monocot and dicot species. For example, Arabidopsis contains four HD2 family members, HD2A, HD2B, HD2C, and HD2D, which resulted from three successive rounds of genome duplication events (Bourque et al. 2016; Soltis et al. 2009). Sequence homology analysis of Arabidopsis HD2s based on DNA and protein sequences shows that the fourth member, HD2D, is a distantly related member of the HD2 family (Han et al. 2016a, b). The phylogenetic tree indicates the largest geetic distance between HD2D and other HD2 members, while HD2A and HD2B show the highest sequence homology (Fig. 1).
Phylogenetic analysis of HD2 family proteins in plants. Protein sequences of HD2s from A. thaliana, Glycine max (soybean), O. sativa (rice), S. lycopersicum (tomato), Hordeum vulgare (barley), and Zea mays (maize) were obtained from National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov/). Phylogenetic tree was constructed by Maximum Likelihood method (with bootstrap analysis of 1000 replicates and partial deletion parameters) using MEGA X software. The numbers at the nodes indicate the bootstrap values. Each name includes an accession number followed by a gene name
HD2s were first identified as nucleolar phosphoproteins in maize, where expression of HD2-type genes was associated with actively dividing embryonic cells (Lusser et al. 1997). The HD2 family was later identified in various plant species, with numbers varying in different plant species. Four members were identified in Arabidopsis and maize, six in soybean and tobacco, two in rice and barley, and three in tomato (Bourque et al. 2016; Dangl et al. 2001; Demetriou, et al. 2009; Hollender and Liu 2008; Nicolas-Francès et al. 2018; Wu et al. 2003; Yang et al. 2018; Zhao et al. 2015a, b).
Based on phylogenetic analysis of at least 1205 HD2 sequences, Bourque et al. (2016) reported that the first four amino acids of the pentapeptide motif (MEFWG) in the N-terminal domain of HD2 proteins are fully conserved, whereas the fifth residue (G) is conserved in 96% of the sequences analysed. The pentapeptide MEFWG motif and histidine 25, surrounded by hydrophobic amino acids in the N-terminal domain, are fully conserved in Arabidopsis, maize, soybean, tobacco, and rice. The presence of these conserved residues along with aspartate/glutamate at position 69 is considered to be important for catalytic activity in gene-regulation mechanisms (Bourque et al. 2016; Dangl et al. 2001; Zhou et al. 2004). The length of almost all of the HD2s ranged from 232 to 384 amino acids. The differences in the lengths of HD2s are associated with the length of the highly variable central acidic domain (Bourque et al. 2016). Although HD2 proteins are regarded as gene repressors that act via deacetylation activity, the deacetylase domain of HD2 family proteins does not significantly match with any of the other HDAC family proteins, suggesting a specific role of the HD2 family in the biological processes in plants. The C-terminal domain of most HD2 proteins contains a putative C2H2-type (CxxC(x)12H(x)4H) zinc-finger (ZnF) motif, a unique structural feature of the plant-specific HD2 family (Fig. 2). The nature and locus of these ZnF residues suggest that they belong to the TFIIIA class of ZnF. ZnF is generally considered to be associated with DNA–protein interactions because of the presence of the conserved motif, QALGGH. Because HD2 proteins lack this conserved motif and appear to have no other DNA-binding motifs, it is very likely that ZnF is associated with protein–protein interactions in gene regulatory networks (Bourque et al. 2016; Dangl et al. 2001; Mackay and Crossley 1998; Takatsuji 1998). The presence or absence of ZnF residue in HD2 proteins could possibly play an important role in implicating functional diversity, as suggested by Nicolas-Francès et al. (2018).
Schematic representation of plant-specific HD2 protein domains. This is a typical representation of Arabidopsis HD2 protein. Green box indicates a conserved region (MEFWG) in N-terminal domains of all HD2-type proteins, Red box represents Nuclear localization signal (NLS) and yellow box contains a putative C2H2-type ZnF motif (color figure online)
Biological functions of HD2s in plant growth and development
HD2 family in Arabidopsis: Plants undergo various physiological and molecular changes to respond to different internal and external signals (Liu, et al. 2014; Nakashima et al. 2009). Members of the HD2 family play a significant role in plant developmental processes. In Arabidopsis, HD2A, HD2B, and HD2C genes are predominantly expressed in primary leaves, embryonic tissues, flowers and siliques whereas the expression of HD2D, a distantly related HD2 family member, is observed mainly in stem and floral parts, with the lowest expression observed in the leaves and roots (Wu et al. 2000, 2003; Zhou et al. 2004). Different expression patterns of HD2D indicate that this gene may have different functions compared to other HD2 genes in Arabidopsis.
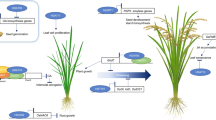
HD2s are involved in many plant development-related processes including vegetative growth, flowering and seed development in Arabidopsis (see Fig. 3 and Table 1). HD2A plays a role in the development of inflorescence stems in Arabidopsis. The hd2a mutants exhibited reduced height and thickness of the inflorescence stem. Transmission electron microscopy revealed that the size of individual fibre cells and tracheary elements was significantly decreased with increased cell wall thickness in the stem of hd2a mutants (Zhang et al. 2019). The expression of many secondary wall-related biosynthetic genes, including lignin and cellulose, was affected in the mutants, indicating the role of HD2A in the development of vascular tissues in the stem of Arabidopsis. Antisense silencing of HD2A in Arabidopsis results in aborted seed development with reduced siliques, whereas overexpression of HD2A causes delayed flowering and poor seed development. Expression of several seed development-responsible genes, including LEA, ABF3, and SMP, is repressed in the overexpression lines (Wu et al. 2000, 2003; Zhou et al. 2004).
The working model of HD2-type HDACs involved in different stages of Arabidopsis plant growth and development. a Role of HD2 family genes in germination: HD2A and HD2C work antagonistically to regulate the expression of germination related genes in the glucose-based germination pathway. HD2B regulates the expression of GA2ox2 (GA) which plays a role in seed germination. b Role of HD2 family genes in root development: HD2A and HD2B coordinate to repress the expression of GA2ox2 which negatively regulates the root development. HD2D is negative regulator of root development. c Role of HD2 family genes in leaf development: HD2A and HD2B interact with AS1/2 and repress the miRNA165/166 for normal leaf polarity and development. d Role of HD2 family genes in flowering initiation: HD2C interact with MRG1/2 resulting in the repression of FT gene leading to repress flowering initiation. Overexpression of HD2D repressed the flowering time related genes in the downstream of signaling pathway resulting in delayed flowering. e Role of HD2 family genes in seed development: HD2A is involved in regulation of seed developmental related genes in Arabidopsis. All arrow heads represent positive regulators, while all stop lines represent negative regulators associated with related pathways. Black rectangles indicate open reading frames (ORFs). Reports have shown functional redundancy of HD2C and HD2D in regulating flowering time (D, as indicated by black double arrowhead bracket with question mark). However, there is no direct evidence that these HD2 coordinate with each other or follow different pathways to accomplish similar function
Vegetative growth, including root, shoot, and leaf development, is strongly associated with the modification of histone acetylation. A significant decrease in Arabidopsis primary root growth was observed in hd2a/hd2b double mutants compared to hd2a and hd2b single mutants. Quantitative PCR analysis showed that the expression of HD2B was significantly increased in the hd2a single mutant line. This shows an additive effect of HD2A and HD2B in controlling root growth in single mutants (Li et al. 2017). The findings further showed that HD2B binds to the promoter region of GIBBERELLIN 2-OXIDASE2 (GA2ox2). Hyperacetylation of the lysine 9 residue of histone 3 (H3K9) at the locus of GA2ox2 was found in both the hd2b and hd2a/hd2b mutants. GA2ox2 overexpression lines showed the same decrease in root length as the double mutants, whereas knockout of GA2ox2 in the hd2a/hd2b double mutant line rescued root growth compared to wild-type (WT) (Li et al. 2017). This indicates that HD2A and HD2B coordinate to modulate the GA2ox2 pathway to regulate root growth in Arabidopsis.
Using genome-wide association mapping and transcriptome analysis, Yano et al. (2013) identified HD2B as a genetic factor related to seed dormancy in Arabidopsis. Expression of HD2B is significantly upregulated (> 10 folds) in seeds under cold and after ripening (dry storage of seeds), treatments which are adopted to break seed dormancy, suggesting that downregulation of HD2B may be required to maintain seed dormancy. Gibberellins (GA) are required in breaking seed dormancy and stimulating seed germination. Gibberellins level was significantly increased in HD2B transgenic lines. Transgenic seeds treated with an HDAC inhibitor, trichostatin A (TSA), showed reduced accumulation of endogenous GA in imbibed seeds. This indicates the role of HD2B in seed germination rather than seed dormancy (Colville, et al. 2011; Yano, et al. 2013). These reports do not precisely depict the role of HD2B in seed dormancy and germination, and further research is required in this regard.
Arabidopsis ASYMMETRIC LEAVES 1 and 2 (AS1, AS2) genes control leaf morphology. RNA interference (RNAi)-mediated simultaneous knockdown of HD2A and HD2B in an as2 mutant background caused an abnormal distribution of microRNA165/166 in leaves and an abaxial filamentous leaf phenotype. Further studies indicated that both HD2A and HD2B interact with AS1 and/or AS2 to mediate the expression and/or distribution of miRNA165/66 (Kidner and Martienssen 2004; Ueno et al. 2007), suggesting that HD2A and HD2B coordinate with AS1 and AS2 to play functional roles in establishing leaf morphology in Arabidopsis. The HD2s may coordinate with each other and with other proteins to play roles in mediating the expression of target genes to regulate plant developmental processes.
Flowering induction is specifically dependent on day length in Arabidopsis. FLOWERING LOCUS T (FT) is a florigen gene that promotes flowering in Arabidopsis under long-day conditions. Guo, et al. (2020) studied the epigenetic regulation mechanism of the FT gene in Arabidopsis and reported that HD2C is recruited to the locus of FT at dusk via an MRG1/2 dependent pathway to regulate flowering time under long-day conditions. HD2C binds with histone methylation readers MRG1/2 and deacetylates lysine 9, 23, 27 of histone H3 to repress FT gene expression and early flowering.
Colville et al. (2011) studied the role of HD2 genes during germination and early stages of seedling growth. The hd2a mutants were found to show increased germination, whereas hd2c mutants showed decreased germination on glucose-containing germination medium. However, HD2A/hd2c double mutants showed germination similar to that of WT. This suggests an interesting antagonistic function of HD2A and HD2C in regulating germination, thus fine-tuning the germination in double mutants.
Arabidopsis transgenic plants overexpressing HD2D showed delayed germination, decreased primary root length with more lateral roots, and a high root to shoot ratio compared to WT plants (Farhi et al. 2017; Han et al. 2016a, b). HD2D transgenic plants also showed a significant delay in flowering (Han et al. 2016a, b), whereas hd2d mutants showed an early flowering phenotype under both long- and short-day conditions (Farhi et al. 2017). Several flowering inducer genes, including SOC1, FUL, SEP3, and FT, were downregulated in HD2D transgenic plants, suggesting a role for the HD2D gene in regulating the network genes in plant transition from vegetative to reproductive stage. It is still not clear whether HD2C and HD2D coordinate to target a subset of genes involved in early flowering. The underlying mechanism of HD2A and HD2B in plant flowering is yet to be elucidated. These studies suggest that HD2s may work in a coordinated fashion to play multiple roles in regulating plant growth and development in Arabidopsis.
HD2 family in rice
Besides the model plant Arabidopsis, HD2s have also been studied in some crops, including rice, potato, and tomato (Zhao et al. 2015a, b). Studies in rice have demonstrated the involvement of HD2s in plant development and flowering time. Overexpression of HDT701 triggered early flowering in hybrid rice under long-day conditions compared to its parents (Oryza sativa ssp. indica and O. sativa ssp. japonica), which was probably owing to the resulting suppression of the over-dominant flowering time repressor gene Heading date 1 (Hd1), leading to early flowering. The expression of several other flowering-related genes was also affected in hybrid rice (Li et al. 2011). Another study reported that hdt701 rice mutants showed delayed flowering under both short-day and long-day conditions. Expression of the flowering suppressor genes Hd1 and IDS1, an upstream repressor of Ehd1, was significantly increased in hdt701 mutant lines. The ChIP assay revealed that HDT701 binds to IDS1 in the promoter region to inhibit its expression, leading to flowering induction (Cho et al. 2018). Knockdown of HDT702 also caused abnormal plant height with narrow leaves and stems (Hu et al. 2009). These data indicate that HD2s play important roles in regulating flowering time and plant development in rice.
HD2 family in potato and tomato
ScHD2a, an ortholog of Arabidopsis HD2A in potato (Solanum chacoense), showed elevated accumulation of transcripts in ovules after fertilisation (Lagacé et al. 2003), suggesting that this gene is important for normal seed development. RNAi-mediated silencing of the HD2-type SlHDT3 gene in tomato resulted in delayed fruit ripening and prolonged shelf life. RNAi-SlHDT3 plants showed lower ethylene content and reduced expression of ripening-associated genes, including E4, E8, RIN, Pti4, and LOXB. This finding suggests that the HD2-type SlHDT3 plays a role in the ethylene biosynthetic pathway and delays fruit ripening (Guo, et al. 2017).
HD2 family in woody plants
The role of the HD2 family in woody plants has not yet been widely studied. Recently, a study was performed on poplar (Populus trichocarpa) plants to investigate the functions of the HD2 family (Ma et al. 2020; Tong et al. 2018). Researchers cloned the HD2-type genes, PtHDT902 and PtHDT903, and investigated their expression, localisation, and functions in poplar. Both proteins were found to be localised to the nucleus. Overexpression of PtHDT902 in poplar and Arabidopsis caused increased gibberellin biosynthesis, with increased primary root length in Arabidopsis. Transgenic poplar showed hypersensitivity to salt stress with limited adventitious root formation. Decreased transcript levels of salt responsive genes including HIGH-AFFINITY K + TRANSPORTER 1 (HKT1) and GALACTINOL SYNTHASE 4 (GolS4) were observed in the transgenic poplar lines (Ma et al. 2020). This indicates that PtHDT902 plays a negative role in salt stress response in poplar. However, the specific role of HD2 in herbaceous and woody plants may be different and will, therefore, require further study.
Role as nucleolar phosphoproteins in ribosomal genes regulation
As typical nucleolar localising proteins, HD2-type HDACs are considered to play a role in the regulation of ribosomal genes (Kim et al. 2014; Lawrence et al. 2004; Lusser et al. 1997). Lawrence et al. (2004) studied the nucleolar dominance in A. suecica, an allotetraploid hybrid of A. thaliana and A. arenosa. The hybrid contains transcriptionally silenced rRNA genes inherited from A. thaliana and transcriptionally active rRNA genes inherited from A. arenosa (Chen et al. 1998). The A. suecica hybrid treated with TSA (HDAC inhibitor) showed re-activation of silenced A. thaliana- inherited rRNA genes and significantly upregulated the expression of A. arenosa-inherited rRNA genes. Interestingly, RNAi-mediated knockdown of HD2A in A. suecica resulted in the loss of H3K9 deacetylation, accompanied by loss of H3K9 dimethylation and de-repression of the rRNA genes inherited from A. thaliana. The nucleolar localisation of HD2A indicates that this HDAC may play a direct role in the rRNA gene silencing required for nucleolar dominance (Lawrence et al. 2004). Another report published by the same group in 2007 demonstrated that rRNA gene silencing-based nucleolar dominance was progressively established at the early postembryonic tissue developmental stage. RNAi-mediated knockdown of HD2A and HDA6 in A. suecica disrupted the condensation and repression of developmentally regulated nucleolus organiser regions (NORs) of A. thaliana-derived rRNA genes along with a decreased connection to histone 3, which is dimethylated at lysine 9 (H3K9me2) (Pontes et al. 2007). This finding suggests that both HDA6 and HD2A are necessary for establishing nucleolar dominance at the early stages of vegetative development. According to Pontes et al. (2007), both proteins localise to the nucleolus, which indicates that these proteins may interact and act directly on the target rRNA genes in the establishment of postembryonic nucleolar dominance in A. suecica.
Chen et al. (2018) demonstrated that both HD2B and HD2C co-localise in the nucleolus, while HD2C interacts with HD2B and forms homo- and/or hetero-dimers. Genome-wide analysis revealed that HD2C interacts and represses the expression of important ribosome biogenesis-related genes in the nucleolus. Loss of function single or double mutants of HD2B and HD2C displayed abnormal accumulation of 18S pre-rRNA intermediates, further resulting in an aberrant developmental pleiotropy, involving short roots and narrow leaves (Chen et al. 2018). Ribosomal protein S6 (RPS6), which is also localised in the nucleolus, interacts with HD2B and plays a possible role in the transcriptional regulation of rRNA genes in Arabidopsis. Overexpression of both genes together in protoplasts resulted in a decreased accumulation of 18S pre-rRNA intermediates, triggering downregulation of some rRNA genes (Kim et al. 2014), suggesting that these genes may play a collective and direct role in ribosome biogenesis and ultimately in plant development.
Biological functions of HD2 family members in response to environmental stresses
The homeostatic external environment is a key factor regulating plant growth, reproduction, and survival. Plants facing an imbalanced external environment undergo a series of morphological, physiological, and molecular changes during growth (Mehrotra et al. 2014; Sah et al. 2016). In plants, gene regulatory networks related to environmental stress responses have been studied by analysing the different stress-responsive genes that are responsible for the synthesis of functional and regulatory proteins such as transcription factors (Fujita et al. 2011; Sah et al. 2016). Modifications in the histone acetylation status of stress-responsive genes caused by biotic and abiotic stresses are important underlying mechanisms of gene expression regulation, which have been investigated in many plant species. Environmental stresses can cause changes in the acetylation status of lysine 9 and 14 (K9, K14) at histone H3 and lysine 18 and 27 (K18, K27) at histone H4 of stress-responsive genes (Chen et al. 2010; Kim et al. 2015; Kuang et al. 2012; Luo et al. 2012a, b, c; Song et al. 2020), indicating that a large group of functionally related genes are regulated in a coordinated manner through histone modifications in different stress responses in plants. Yeast and animal-based studies have demonstrated that HDACs often play roles as components of multiprotein and chromatin remodelling complexes (Ríos et al. 2007; Saez et al. 2008).
Plants can withstand environmental stresses by modifying their behaviour and stimulating the production of stress hormones. The phytohormone, abscisic acid (ABA), plays an important role in regulating various developmental processes and adaptive stress responses and is produced in increased quantities by plants upon stimulation (Fujita et al. 2011). ABA is thought to regulate nearly 10% of protein-coding genes in Arabidopsis. The expression of ABA-responsive genes is regulated by a number of ABA-related transcription factors (Fujita et al. 2011). Many ABA-dependent and independent transcription factors are known to be regulated by HDACs (Glass and Rosenfeld 2000; Jepsen and Rosenfeld 2002). Reports have shown the involvement of RPD3-type HDACs in regulating plant responses to abiotic stresses in different species via ABA-dependent pathways (Kuang et al. 2012; Song et al. 2019). This section summarizes the recent research progress in the HD2 family in relation to their role in response to internal and external cues in different species.
Arabidopsis
HD2 family genes show differential expression patterns in response to various abiotic stresses (Ding et al. 2012; Grandperret et al. 2014; Hollender and Liu 2008; Kuang et al. 2012; Sridha and Wu 2006; Wu et al. 2003). Cold treatment of Arabidopsis plants caused the upregulation of many HDAC genes, including HD2-type HD2A, HD2B, and HD2C genes. Most of these genes showed gradual upregulation, indicating a corresponding response to cold stress (To et al. 2011). Contrary to cold response, expression of all HD2s was repressed by salt and ABA (Kuang et al. 2012; Luo et al. 2012a, b, c).
HOS15, a DDB1-CUL4 Associated factor (DCAF) protein, encodes one of 85 WD40-repeat proteins in Arabidopsis which directly interacts with specific targets by acting as a substrate receptor for its ubiquitination and subsequent degradation by the proteasome (Ali and Yun 2020; Fonseca and Rubio 2019). Zhu et al. (2008) reported the association of HOS15 with HD2C in histone modification in response to cold stress in Arabidopsis. The study demonstrated that HOS15 acts as a DCAF protein and interacts with and degrades the HD2C protein via ubiquitination and proteasome-mediated degradation, thus modulating the expression of cold-responsive (COR) genes under freezing stress in Arabidopsis (Park et al. 2018). Lim et al. (2020) studied the function of the HOS15 binding protein, POWERDRESS (PWR) in response to cold stress. This study showed that HOS15 interacts with HD2C with the help of PWR at the promoter region of COR genes, thus mediating the degradation of HD2C, leading to the upregulation of COR genes as a part of the cold stress response. The pwr mutants showed down-regulation of the COR genes with increased sensitivity to cold stress. This suggests that these three proteins associate together to build a histone-modifying complex of HOS15-PWR-HD2C and regulate the expression of COR genes in cold stress response.
Buszewicz et al. (2016) revealed HD2C as a positive regulator of the heat stress response. Upon heat exposure, HD2C transcript levels were significantly upregulated, which downregulated the expression of heat-responsive genes. The hd2c and brm (a subunit of the SWI/SNF complex) knockout mutants displayed growth-retarding phenotypes upon heat exposure for 24 h (Buszewicz et al. 2016). Transgenic plants overexpressing HD2C displayed an insensitive phenotype to exogenous applied ABA and showed tolerance to salt and drought stresses with decreased transpiration rates. Expression of ABA-responsive genes, including LATE EMBRYOGENESIS ABUNDANT PROTEIN (LEA)-class genes, RD29B and RAB18, was upregulated in HD2C overexpression lines (Kuang et al. 2012; Sridha and Wu 2006). Kuang et al. (2012) showed that HD2C interacts with the RPD3-type HDA6 protein and regulates plant responses to ABA and salt stresses. The level of gene activation mark histone H3K9K14ac in the promoter region of ABA-responsive genes, ABI1 and ABI2, was found to be higher in hda6/hd2c double mutant plants. The loss-of-function hd2c T-DNA insertion mutants displayed increased sensitivity to ABA during germination and showed decreased tolerance to abiotic stresses compared to WT (Kuang et al. 2012). These reports support the hypothesis that HD2C is involved in ABA pathways in mediating the plant stress response. Later, using a bimolecular fluorescence complementation assay, it was shown that HD2A and HD2D can also physically interact with HDA6. HD2 proteins were also shown to interact with HDA19 (Kuang, et al. 2012a; Luo et al. 2012b). Overexpression of HD2D in Arabidopsis also displayed enhanced tolerance to salt, drought and cold stress (Han et al. 2016a, b). Currently, it is not clear whether HD2C and HD2D coordinate to respond to these abiotic stresses. Both ABA and other stresses can induce changes in histone acetylation levels of some ABA and abiotic stress-responsive genes, suggesting that HDACs and some transcription factors work in co-ordination to regulate functionally related genes in abiotic stress responses. Further research is needed to reveal whether the induction of different HD2s are linked to each other and how ABA works in coordination with HD2s to mediate the expression of target genes in response to different abiotic stresses.
Buszewicz et al. (2016) performed GFP-binding affinity chromatography to precipitate interacting proteins of HD2C and found that HD2C was bound to HD2A and BRM-containing SWI/SNF chromatin remodelling complexes. This suggests the functional association of HD2C with HD2A, indicating that some HD2s may work together in a close relationship with gene regulation activity. Functional association within HD2 family members as well as with RPD3-type HDACs as part of repression complexes may be critical for regulating gene expression through histone modifications.
Arabidopsis DNA METHYL TRANSFERASE 2 (AtDNMT2) has been found to be involved in regulating gene expression (Cao and Jacobsen 2002). DNMT2, which is responsible for the methylation of DNA or RNA in Arabidopsis, is dependent on the activity of the HD2 family. A region of the N-terminal domain of AtDNMT2 showed physical interaction with HD2A, HD2B, and HD2C, but not with HD2D. A significant decrease in the repression activity of AtDNMT2 was observed in the hd2c mutant line (Song et al. 2010). Since HD2s are involved in ABA and abiotic stress responses, the relationship between DNMT2 and HD2s indicates an interplay between DNA methyltransferases and histone deacetylases in response to abiotic stresses.
Regarding biotic stress response in Arabidopsis, pathogen-associated molecular patterns (PAMPs), also called microbial-associated molecular patterns (MAMPs) are major regulators of plant innate immunity against plant pathogens and introduce mega-scale changes in gene expression patterns (Asai et al. 2002; Ausubel 2005). MPK3 is a plant mitogen-activated protein (MAP) kinase, activated during PAMP-triggered MAPK signalling pathways upon recognition of plant pathogens (Gao et al. 2008; Meng and Zhang 2013). It is believed that PAMPS initiate systematic acquired resistance (SAR) against a broad spectrum of pathogens in plants by inducing localised programmed cell death (PCD), as a hypersensitive response (HR) to confine the pathogenic infection (Ma and Berkowitz 2007). Latrasse et al. (2017) reported that the MAP kinase MPK3 directly interacts with and phosphorylates HD2B during PAMP-triggered immunity, where MPK3 directly employs HD2B in the reprogramming of defence-related gene expression by modulating acetylation of lysine 9 of histone 3 (H3K9) to mediate innate immunity in Arabidopsis. They also reported that HD2B relocalises to the nucleus from the nucleolus upon recognition of bacterial flagellin fragment flg22, thus inducing global H3K9 histone deacetylation to mediate the expression of bacterial defence-related genes in Arabidopsis.
Rice and barley
Overexpression of HDT701 in rice displayed decreased acetylation levels at H4K5K16 (histone H4, lysine K5, and K16), and showed hypersensitivity to different rice pathogens, including Magnaporthe oryzae and Xanthomonas oryzae. Knockdown of HDT701 showed an opposite response with increased expression of defence-related genes and PAMPs, as well as hyposensitivity to rice pathogens, suggesting HDT701 as a negative regulator of innate immunity. Using the ChIP assay, Ding et al. (2012) also found that HDT701 can directly interact with a subset of defence pathway genes including MAPK5 and MAPK6, and transcription factors such as WRKY71 and WRKY53 to regulate their expression. This suggests that HDT701 downregulates defence-related genes, thus negatively regulating plant innate immunity. Although, studies on the involvement of the HD2 family in biotic stress responses are limited, studies on Arabidopsis and rice provide evidences that the HD2 family is involved in the biotic stress responses, and functions by coordinating with defence pathway-related genes. Further research on different species is required to further validate these findings.
HDT701 showed stable expression throughout the plant life cycle. HDT701 transgenic rice plants showed hypersensitivity to salt, drought, and ABA stress during germination, however, and hyposensitivity to these stresses at the seedling stage (Zhao et al. 2015a, b). In contrast, hdt701 mutant seedlings displayed increased sensitivity to both salt and drought stresses (Wai and An 2018). These mutants showed a dramatic increase in expression levels of WRKY45, an upstream regulator of the ABA-related genes, SNAC1 and NCED4. Expression of the HD2-type genes; HDAC2-1 and HDAC2-2 in barley and HDT701 and HDT702 in rice, was significantly upregulated upon exogenous application of jasmonic acid (JA) and salicylic acid (SA), whereas rice HD2 gene expression was decreased in response to ABA exposure (Demetriou et al. 2009; Fu et al. 2007). Barley HD2-type genes showed a divergent response to ABA treatment. This finding suggests that the rice HD2 gene, HDT701, may be involved in ABA-related pathways to mediate plant stress response.
Tobacco: Bourque et al. (2011) studied the tobacco HD2-type proteins NtHD2A and NtHD2B. Exposure of tobacco plant to cryptogein, an elicitin family protein, secreted by oomycete Phytophthora cryptogea caused the phosphorylation of both HD2-type HDACs. The expression of these two genes was found to decrease significantly upon exposure to cryptogein, suggesting that cryptogein is an inhibitor of HD2s, thus leading to cryptogein-induced programmed cell death in tobacco (Bourque et al. 2011). HD2-type proteins containing the ZnF motif in Arabidopsis (AtHD2A/AtHD2C) and tobacco (NtHD2A/NtHD2B) showed similar germination responses to salt stress compared to non-ZnF HD2 proteins (NtHD2C, AtHD2B/AtHD2D) (Nicolas-Francès et al. 2018; Sridha and Wu 2006), indicating that HD2 proteins containing ZnF motifs may have functional redundancy compared to non-ZnF HD2 proteins.
Longan fruit: Kuang et al. (2012) demonstrated that the HD2-type gene, DlHD2, from longan fruit interacts with the ETHYLENE-RESPONSIVE FACTOR 1 (DIERF1) transcription repressor and regulates the expression of genes involved in longan fruit senescence. The DiHD2 and DIERF1 genes showed upregulation of their transcript levels in relation to longan fruit senescence stored under different temperature conditions. PtHDT902 overexpressing poplar plants demonstrated hypersensitivity to salt stress. Expression of PtHDT903 was downregulated under salt stress and upregulated under cold stress (Ma et al. 2020; Tong et al. 2018). These studies show the versatile functionality that the HD2 family plays in different species in different aspects of plant development and response to environmental stress.
HD2s show functional diversity in terms of their role in plant developmental processes and biotic and abiotic stress responses. HD2 family members may not be redundant proteins and can be functionally coordinative or complementary. However, these HD2s may follow different pathways to accomplish common functions. Taken together, these studies provide significant evidence that the HD2 family plays a role in different biological processes to address the plant’s internal and external challenges.
Concluding remarks
It is well established that the modification of histone acetylation levels plays an important role in plant development and response to environmental stresses. The existence of another HDAC family in addition to the RPD3 and SIR2 families put forward an interesting question as to why evolution provided the plants with the third plant specific HD2 family. HDACs, in general, are recruited to target areas to modify the chromatin structure at the given loci to inhibit gene transcription. HD2, being a unique HDAC family in plants, plays critical roles not only in fundamental developmental processes, including seed germination, root and leaf development, floral transition, and seed development, but also in regulating plant responses against biotic and abiotic stresses (Table 1). Members of the HD2 family have shown functional associations with RPD3-type HDACs, and many other genes as part of repression complexes in gene regulatory networks (Fig. 4). Studies in mutants and overexpression lines of HD2C and HD2D have shown that these HD2s show similar responses towards different abiotic stresses, suggesting that HD2s may follow different pathways to accomplish the same functions. HD2s, which are involved in plant developmental processes and stress responses, show functional diversity, indicating that these may not be redundant proteins, but are functionally either independent or coordinative and/or complement. Unravelling the upstream signalling pathways that are responsible for the induction and biological functions of the HD2 family and downstream target genes and gene regulatory networks offers an important perspective, and could contribute to the understanding of the mechanisms by which the HD2 family regulates plant developmental processes and stress responses.
Relation and network of HD2 family proteins in Arabidopsis based on current knowledge. HD2 proteins associated with other HDACs (green) and non-HDAC proteins (yellow) are involved in plant growth and stress response in Arabidopsis. Double head arrows between HD2s and other proteins indicate physical interaction. a Physical interaction of HD2A with three HDAC proteins (including HD2C of HD2 family) and two non-HDAC proteins. b Physical interaction of HD2B with one HDAC protein (HD2C) of HD2 family and four non-HDAC proteins. c Physical interaction of HD2C with four HDAC proteins (including HD2A and HD2B of HD2 family) of and three non-HDAC proteins. d Physical interaction of HD2D with two HDAC proteins (belonging to RPD3 family) (color figure online)
Author contributions statement
MT and LT conceived the idea and planned the manuscript. MT performed the literature search and data analysis and wrote the manuscript. MT and LT critically edited the manuscript.
References
Ali A, Yun D-J (2020) Arabidopsis HOS15 is a multifunctional protein that negatively regulate ABA-signaling and drought stress. Plant Biotechnol Rep. https://doi.org/10.1007/s11816-020-00600-1
Asai T, Tena G, Plotnikova J, Willmann MR, Chiu W-L, Gomez-Gomez L, Boller T, Ausubel FM, Sheen JJN (2002) MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415:977–983
Ausubel F (2005) Are innate immune signaling pathways in plants and animals conserved? Nat Immunol 6:973–979
Bourque S, Dutartre A, Hammoudi V, Blanc S, Dahan J, Jeandroz S, Pichereaux C, Rossignol M, Wendehenne D (2011) Type-2 histone deacetylases as new regulators of elicitor-induced cell death in plants. New Phytol 192:127–139
Bourque S, Jeandroz S, Grandperret V, Lehotai N, Aime S, Soltis DE, Miles N, Melkonian M, Deyholos M, Leebens-Mack J (2016) The evolution of HD2 proteins in green plants. Trends Plant Sci 21:1008–1016
Buszewicz D, Archacki R, Palusiński A, Kotliński M, Fogtman A, Iwanicka-Nowicka R, Sosnowska K, Kuciński J, Pupel P, Olędzki J (2016) HD2C histone deacetylase and a SWI/SNF chromatin remodelling complex interact and both are involved in mediating the heat stress response in Arabidopsis. Plant, Cell Environ 39:2108–2122
Cao X, Jacobsen SE (2002) Role of the Arabidopsis DRM methyltransferases in de novo DNA methylation and gene silencing. Curr Biol 12:1138–1144
Chen ZJ, Comai L, Pikaard CS (1998) Gene dosage and stochastic effects determine the severity and direction of uniparental ribosomal RNA gene silencing (nucleolar dominance) in Arabidopsis allopolyploids. Proc Natl Acad Sci 95:14891–14896
Chen L-T, Luo M, Wang Y-Y, Wu K (2010) Involvement of Arabidopsi s histone deacetylase HDA6 in ABA and salt stress response. J Exp Bot 61:3345–3353
Chen X, Lu L, Qian S, Scalf M, Smith LM, Zhong X (2018) Canonical and noncanonical actions of Arabidopsis histone deacetylases in ribosomal RNA processing. Plant Cell 30:134–152
Cho L-H, Yoon J, Wai AH, An G (2018) Histone deacetylase 701 (HDT701) induces flowering in rice by modulating expression of OsIDS1. Mol Cells 41:665
Colville A, Alhattab R, Hu M, Labbé H, Xing T, Miki B (2011) Role of HD2 genes in seed germination and early seedling growth in Arabidopsis. Plant Cell Rep 30:1969
Dangl M, Brosch G, Haas H, Loidl P, Lusser A (2001) Comparative analysis of HD2 type histone deacetylases in higher plants. Planta 213:280–285
Demetriou K, Kapazoglou A, Tondelli A, Francia E, Stanca MA, Bladenopoulos K, Tsaftaris AS (2009) Epigenetic chromatin modifiers in barley: I. Cloning, mapping and expression analysis of the plant specific HD2 family of histone deacetylases from barley, during seed development and after hormonal treatment. Physiol Plant 136:358–368
Ding B, del Rosario BM, Ning Y, Meyers BC, Wang G-L (2012) HDT701, a histone H4 deacetylase, negatively regulates plant innate immunity by modulating histone H4 acetylation of defense-related genes in rice. Plant Cell 24:3783–3794
Farhi J, Tian G, Fang H, Maxwell D, Xing T, Tian L, behavior, (2017) Histone deacetylase HD2D is involved in regulating plant development and flowering time in Arabidopsis. Plant Signal 12:e1300742
Fonseca S, Rubio V (2019) Arabidopsis CRL4 complexes: surveying chromatin states and gene expression. Front Plant Sci 10:1095
Fu W, Wu K, Duan J (2007) Sequence and expression analysis of histone deacetylases in rice. Biochem Biophys Res Commun 356:843–850
Fujita Y, Fujita M, Shinozaki K, Yamaguchi-Shinozaki K (2011) ABA-mediated transcriptional regulation in response to osmotic stress in plants. J Plant Res 124:509–525
Gao M, Liu J, Bi D, Zhang Z, Cheng F, Chen S, Zhang Y (2008) MEKK1, MKK1/MKK2 and MPK4 function together in a mitogen-activated protein kinase cascade to regulate innate immunity in plants. Cell Res 18:1190–1198
Glass CK, Rosenfeld MG (2000) The coregulator exchange in transcriptional functions of nuclear receptors. Genes Develop 14:121–141
Grandperret V, Nicolas-Francès V, Wendehenne D, Bourque S (2014) Type-II histone deacetylases: elusive plant nuclear signal transducers. Plant Cell Environm 37:1259–1269
Guo J-E, Hu Z, Li F, Zhang L, Yu X, Tang B, Chen G (2017) Silencing of histone deacetylase SlHDT3 delays fruit ripening and suppresses carotenoid accumulation in tomato. Plant Sci 265:29–38
Guo Z, Li Z, Liu Y, An Z, Peng M, Shen WH, Dong A, Yu Y (2020) MRG1/2 histone methylation readers and HD2C histone deacetylase associate in repression of the florigen gene FT to set a proper flowering time in response to day-length changes. New Phytol. https://doi.org/10.1111/nph.16616
Han Z, Yu H, Zhao Z, Hunter D, Luo X, Duan J, Tian L (2016a) AtHD2D gene plays a role in plant growth, development, and response to abiotic stresses in Arabidopsis thaliana. Front Plant Sci 7:310
Han Z, Yu H, Zhao Z, Hunter D, Luo X, Duan J, Tian L (2016) AtHD2D Gene Plays a Role in Plant Growth, Development, and Response to Abiotic Stresses in Arabidopsis. Front Plant Sci. https://doi.org/10.3389/fpls.2016.00310
Hollender C, Liu Z (2008) Histone deacetylase genes in Arabidopsis development. J Integr Plant Biol 50:875–885
Hu Y, Qin F, Huang L, Sun Q, Li C, Zhao Y, Zhou D-X (2009) Rice histone deacetylase genes display specific expression patterns and developmental functions. Biochem Biophys Res Commun 388:266–271
Jepsen K, Rosenfeld MG (2002) Biological roles and mechanistic actions of co-repressor complexes. J Cell Sci 115:689–698
Kapazoglou A, Tsaftaris A (2011) Epigenetic chromatin regulators as mediators of abiotic stress responses in cereals. Abiotic Stress Plants Mechan Adap. https://doi.org/10.5772/36025
Kidner CA, Martienssen RA (2004) Spatially restricted microRNA directs leaf polarity through ARGONAUTE1. Nature 428:81–84
Kim Y-K, Kim S, Shin Y-j, Hur Y-S, Kim W-Y, Lee M-S, Cheon C-I, Verma DPS (2014) Ribosomal protein S6, a target of rapamycin, is involved in the regulation of rRNA genes by possible epigenetic changes in Arabidopsis. J Biol Chem 289:3901–3912
Kim J-M, Sasaki T, Ueda M, Sako K, Seki M (2015) Chromatin changes in response to drought, salinity, heat, and cold stresses in plants. Front Plant Sci 6:114
Kouzarides T (2007) Chromatin modifications and their function. Cell 128:693–705
Kuang J-f, Chen J-y, Luo M, Wu K-q, Sun W, Jiang Y-m, Lu W-j (2012) Histone deacetylase HD2 interacts with ERF1 and is involved in longan fruit senescence. J Exp Bot 63:441–454
Kuo M-H, Brownell JE, Sobel RE, Ranalli TA, Cook RG, Edmondson DG, Roth SY, Allis CD (1996) Transcription-linked acetylation by Gcn5p of histones H3 and H4 at specific lysines. Nature 383:269–272
Kurdistani SK, Tavazoie S, Grunstein M (2004) Mapping global histone acetylation patterns to gene expression. Cell 117:721–733
Lagacé M, Chantha S-C, Major G, Matton DP (2003) Fertilization induces strong accumulation of a histone deacetylase (HD2) and of other chromatin-remodeling proteins in restricted areas of the ovules. Plant Mol Biol 53:759–769
Latrasse D, Jégu T, Li H, de Zelicourt A, Raynaud C, Legras S, Gust A, Samajova O, Veluchamy A, Rayapuram N (2017) MAPK-triggered chromatin reprogramming by histone deacetylase in plant innate immunity. Genome Biol 18:131
Lawrence RJ, Earley K, Pontes O, Silva M, Chen ZJ, Neves N, Viegas W, Pikaard CS (2004) A concerted DNA methylation/histone methylation switch regulates rRNA gene dosage control and nucleolar dominance. Mol Cell 13:599–609
Li C, Huang L, Xu C, Zhao Y, Zhou D-X (2011) Altered levels of histone deacetylase OsHDT1 affect differential gene expression patterns in hybrid rice. PLoS One. https://doi.org/10.1371/journal.pone.0021789
Li H, Torres-Garcia J, Latrasse D, Benhamed M, Schilderink S, Zhou W, Kulikova O, Hirt H, Bisselinga T (2017) Plant-specific histone deacetylases HDT1/2 regulate gibberellin 2-oxidase2 expression to control arabidopsis root meristem cell number. Plant Cell 29:2183–2196
Lim CJ, Park J, Shen M, Park HJ, Cheong MS, Park KS, Baek D, Bae MJ, Ali A, Jan M (2020) The histone-modifying complex PWR/HOS15/HD2C epigenetically regulates cold tolerance. Plant Physiol 184:1097–1111
Liu X, Yang S, Zhao M, Luo M, Yu C-W, Chen C-Y, Tai R, Wu K (2014) Transcriptional repression by histone deacetylases in plants. Mol Plant 7:764–772
Luo M, Wang Y-Y, Liu X, Yang S, Wu K (2012) HD2 proteins interact with RPD3-type histone deacetylases. Plant Signal Behav 7:608–610
Luo M, Wang Y-Y, Liu X, Yang S, Lu Q, Cui Y, Wu K (2012) HD2C interacts with HDA6 and is involved in ABA and salt stress response in Arabidopsis. J Exp Bot 63:3297–3306
Luo M, Liu X, Singh P, Cui Y, Zimmerli L, Wu K (2012) Chromatin modifications and remodeling in plant abiotic stress responses. Biochimica et Biophysica Acta Mechan 1819:129–136
Lusser A, Brosch G, Loidl A, Haas H, Loidl P (1997) Identification of maize histone deacetylase HD2 as an acidic nucleolar phosphoprotein. Science 277:88–91
Ma W, Berkowitz GA (2007) The grateful dead: calcium and cell death in plant innate immunity. Cell Microbiol 9:2571–2585
Ma X, Lv S, Zhang C, Yang C (2013) Histone deacetylases and their functions in plants. Plant Cell Rep 32:465–478
Ma X, Liang X, Lv S, Guan T, Jiang T, Cheng Y (2020) Histone deacetylase gene PtHDT902 modifies adventitious root formation and negatively regulates salt stress tolerance in poplar. Plant Sci 290:110301
Mackay JP, Crossley M (1998) Zinc fingers are sticking together. Trends Biochem Sci 23:1–4
Mehrotra R, Bhalothia P, Bansal P, Basantani MK, Bharti V, Mehrotra S (2014) Abscisic acid and abiotic stress tolerance–Different tiers of regulation. J Plant Physiol 171:486–496
Meng X, Zhang S (2013) MAPK cascades in plant disease resistance signaling. Annu Rev Phytopathol 51:245–266
Nakashima K, Ito Y, Yamaguchi-Shinozaki K (2009) Transcriptional regulatory networks in response to abiotic stresses in Arabidopsis and grasses. Plant Physiol 149:88–95
Nicolas-Francès V, Grandperret V, Liegard B, Jeandroz S, Vasselon D, Aimé S, Klinguer A, Lamotte O, Julio E, de Borne FD (2018) Evolutionary diversification of type-2 HDAC structure, function and regulation in Nicotiana tabacum. Plant Sci 269:66–74
Pandey R, MuÈller A, Napoli CA, Selinger DA, Pikaard CS, Richards EJ, Bender J, Mount DW, Jorgensen RA (2002) Analysis of histone acetyltransferase and histone deacetylase families of Arabidopsis thaliana suggests functional diversification of chromatin modification among multicellular eukaryotes. Nucleic Acids Res 30:5036–5055
Park J, Lim CJ, Shen M, Park HJ, Cha J-Y, Iniesto E, Rubio V, Mengiste T, Zhu J-K, Bressan RA (2018) Epigenetic switch from repressive to permissive chromatin in response to cold stress. Proc Natl Acad Sci 115:E5400–E5409
Pfluger J, Wagner D (2007) Histone modifications and dynamic regulation of genome accessibility in plants. Curr Opin Plant Biol 10:645–652
Pokholok DK, Harbison CT, Levine S, Cole M, Hannett NM, Lee TI, Bell GW, Walker K, Rolfe PA, Herbolsheimer E (2005) Genome-wide map of nucleosome acetylation and methylation in yeast. Cell 122:517–527
Pontes O, Lawrence RJ, Silva M, Preuss S, Costa-Nunes P, Earley K, Neves N, Viegas W, Pikaard CS (2007) Postembryonic establishment of megabase-scale gene silencing in nucleolar dominance. PloS one. https://doi.org/10.1371/journal.pone.0001157
Ríos G, Gagete AP, Castillo J, Berbel A, Franco L, Rodrigo MI (2007) Abscisic acid and desiccation-dependent expression of a novel putative SNF5-type chromatin-remodeling gene in Pisum sativum. Plant Physiol Biochem 45:427–435
Saez A, Rodrigues A, Santiago J, Rubio S, Rodriguez PL (2008) HAB1–SWI3B interaction reveals a link between abscisic acid signaling and putative SWI/SNF chromatin-remodeling complexes in Arabidopsis. Plant Cell 20:2972–2988
Sah SK, Reddy KR, Li J (2016) Abscisic acid and abiotic stress tolerance in crop plants. Front Plant Sci 7:571
Shahbazian MD, Grunstein M (2007) Functions of site-specific histone acetylation and deacetylation. Annu Rev Biochem 76:75–100
Shinozaki K, Yamaguchi-Shinozaki K (2007) Gene networks involved in drought stress response and tolerance. J Exp Bot 58:221–227
Soltis DE, Albert VA, Leebens-Mack J, Bell CD, Paterson AH, Zheng C, Sankoff D, de Pamphilis CW, Wall PK, Soltis PS (2009) Polyploidy and angiosperm diversification. Am J Bot 96:336–348
Song Y, Wu K, Dhaubhadel S, An L, Tian L (2010) Arabidopsis DNA methyltransferase AtDNMT2 associates with histone deacetylase AtHD2s activity. Biochem Biophys Res Commun 396:187–192
Song J, Henry HA, Tian L (2019) Brachypodium histone deacetylase BdHD1 positively regulates ABA and drought stress responses. Plant Sci 283:355–365
Song J, Henry H, Tian L (2020) Drought-inducible changes in the histone modification H3K9ac are associated with drought-responsive gene expression in Brachypodium distachyon. Plant Biol 22:433–440
Sridha S, Wu K (2006) Identification of AtHD2C as a novel regulator of abscisic acid responses in Arabidopsis. Plant J 46:124–133
Takatsuji H (1998) Zinc-finger transcription factors in plants 54:582–596
To TK, Nakaminami K, Kim J-M, Morosawa T, Ishida J, Tanaka M, Yokoyama S, Shinozaki K, Seki M (2011) Arabidopsis HDA6 is required for freezing tolerance. Biochem Biophys Res Commun 406:414–419
Tong B, Xia D, Lv S, Ma X (2018) Cloning and expression analysis of PtHDT903, a HD2-type histone deacetylase gene in Populus trichocarpa. Biotechnol Biotechnol Equipm 32:1098–1104
Ueno Y, Ishikawa T, Watanabe K, Terakura S, Iwakawa H, Okada K, Machida C, Machida Y (2007) Histone deacetylases and ASYMMETRIC LEAVES2 are involved in the establishment of polarity in leaves of Arabidopsis. Plant Cell 19:445–457
Urano K, Kurihara Y, Seki M, Shinozaki K (2010) ‘Omics’ analyses of regulatory networks in plant abiotic stress responses. Curr Opin Plant Biol 13:132–138
Wai AH, An G (2018) Histone Deacetylase 701 enhances abiotic stress resistance in rice at the seedling stage by suppressing expression of OsWRKY45. In. 2nd Myanmar-Korea conference on Useful Plants, Dagon University
Wolffe AP (1998) Packaging principle: how DNA methylation and histone acetylation control the transcriptional activity of chromatin. J Exp Zool 282:239–244
Wu K, Tian L, Malik K, Brown D, Miki B (2000) Functional analysis of HD2 histone deacetylase homologues in Arabidopsis thaliana. Plant J 22:19–27
Wu K, Tian L, Zhou C, Brown D, Miki B (2003) Repression of gene expression by Arabidopsis HD2 histone deacetylases. Plant J 34:241–247
Yang C, Shen W, Chen H, Chu L, Xu Y, Zhou X, Liu C, Chen C, Zeng J, Liu J (2018) Characterization and subcellular localization of histone deacetylases and their roles in response to abiotic stresses in soybean. BMC Plant Biol 18:1–13
Yano R, Takebayashi Y, Nambara E, Kamiya Y, Seo M (2013) Combining association mapping and transcriptomics identify HD2B histone deacetylase as a genetic factor associated with seed dormancy in Arabidopsis thaliana. Plant J 74:815–828
Zhang Y, Reinberg D (2001) Transcription regulation by histone methylation: interplay between different covalent modifications of the core histone tails. Genes Develop 15:2343–2360
Zhang W, Bone JR, Edmondson DG, Turner BM, Roth SY (1998) Essential and redundant functions of histone acetylation revealed by mutation of target lysines and loss of the Gcn5p acetyltransferase. EMBO J 17:3155–3167
Zhang Y, Yin B, Zhang J, Cheng Z, Liu Y, Wang B, Guo X, Liu X, Liu D, Li H (2019) Histone Deacetylase HDT1 is Involved in Stem Vascular Development in Arabidopsis. Int J Mol Sci 20:3452
Zhao L, Lu J, Zhang J, Wu P-Y, Yang S, Wu K (2015) Identification and characterization of histone deacetylases in tomato (Solanum lycopersicum). Front Plant Sci 5:760
Zhao J, Zhang J, Zhang W, Wu K, Zheng F, Tian L, Liu X, Duan J (2015) Expression and functional analysis of the plant-specific histone deacetylase HDT701 in rice. Front Plant Sci 5:764
Zhou C, Labbe H, Sridha S, Wang L, Tian L, Latoszek-Green M, Yang Z, Brown D, Miki B, Wu K (2004) Expression and function of HD2-type histone deacetylases in Arabidopsis development. Plant J 38:715–724
Zhou B-R, Feng H, Kato H, Dai L, Yang Y, Zhou Y, Bai Y (2013) Structural insights into the histone H1-nucleosome complex. Proc Natl Acad Sci 110:19390–19395
Zhu J, Jeong JC, Zhu Y, Sokolchik I, Miyazaki S, Zhu J-K, Hasegawa PM, Bohnert HJ, Shi H, Yun D-J (2008) Involvement of Arabidopsis HOS15 in histone deacetylation and cold tolerance. Proc Natl Acad Sci 105:4945–4950
Acknowledgements
This research was supported by a grant from the Natural Sciences and Engineering Research Council (NSERC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no conflict of interests.
Additional information
Communicated by Wusheng Liu.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tahir, M.S., Tian, L. HD2-type histone deacetylases: unique regulators of plant development and stress responses. Plant Cell Rep 40, 1603–1615 (2021). https://doi.org/10.1007/s00299-021-02688-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-021-02688-3