Abstract
Key message
Loss-of-function of nucleoporin NUP1 in Arabidopsis causes defect in both male and female gametogenesis. Its ovules are arrested during meiosis, and its pollen grains are aborted at mitosis I.
Abstract
Nuclear pore complex (NPC) plays crucial roles in nucleocytoplasmic trafficking of proteins and RNAs. The NPC contains approximately 30 different proteins termed nucleoporins (NUPs). So far, only a few of plant NUPs have been characterized. The Arabidopsis NUP1 was identified as an ortholog of the yeast NUP1 and animal NUP153. Loss-of-function of NUP1 in Arabidopsis caused fertility defect; however, the molecular mechanism of this defect remains unknown. Here, we found that both male and female gametogenesis of the nup1 mutants were defective. nup1 ovules were arrested from the meiosis stage onward; only approximately 6.7% and 3% ovules of the nup1-1 and nup1-4 mutants developed up to the FG7 stage, respectively. Pollen development of the nup1 mutants was arrested during the first mitotic division. In addition, enlarged pollen grains with increased DNA content were observed in the nup1 mutant. RNA-sequencing showed that expression levels of genes involved in pollen development or regulation of cell size were reduced dramatically in nup1 compared with wild type. These results suggest that NUP1 plays an important role in gametogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The trafficking of RNAs and proteins between nucleus and cytoplasm is important for cellular function of eukaryotes (Xu and Meier 2008; Meier and Brkljacic 2009). The nuclear pore complex (NPC) plays crucial roles in these cellular activities. NPC is a nuclear envelope-embedded protein complex that is composed of approximately 30 nucleoporins (NUPs) (Walde and Kehlenbach 2010). The basic structure of NPC is conserved in vertebrates (Goldberg and Allen 1996), yeast (Allen and Douglas 1989) and plants (Roberts and Northcote 1970; Fiserova et al. 2009). Although NPC has been well characterized in vertebrates and yeast, the plant NPC is largely unclear.
A set of 26 NUPs were identified in Arabidopsis and rice by bioinformatics searches (Neumann et al. 2006). Then 30 Arabidopsis NUPs were identified using interactive proteomic approach (Tamura et al. 2010). So far only a few of plant NUPs have been characterized by physiological and genetic methods (Xu and Meier 2008; Meier and Brkljacic 2009; Yang et al. 2017). These studies demonstrated that plant NUPs were involved in diverse biological processes. The Arabidopsis NUP96, NUP88, NUP160, SEH1, NUP98, CPR5, NUP82, and NUP1/NUP136 were reported to play important roles in disease resistance (Zhang and Li 2005; Cheng et al. 2009; Wiermer et al. 2012; Genenncher et al. 2016; Gu et al. 2016; Tamura et al. 2017). NUP160 was required for plant response to cold stress (Dong et al. 2006; Yang et al. 2017). NUP85, NUP160, and SEH1 were essential for cell-death control (Du et al. 2016). NUP96, NUP160, and NUP62 were shown to be involved in response to the plant hormone auxin (Parry et al. 2006; Boeglin et al. 2016).
The Arabidopsis NUP1 was initially identified as a putative ortholog of yeast NUP1 and vertebrate NUP153 by bioinformatics analysis (Neumann et al. 2006). Subsequently, it was verified to be a NPC component by yeast two-hybrid screen, proteomic approach, subcellular localization analysis, and mRNA export assay (Tamura et al. 2010; Lu et al. 2010). Functional analysis demonstrated that Arabidopsis NUP1 was necessary for exportation of nuclear mRNA and maintenance of nuclear morphology (Tamura et al. 2010; Lu et al. 2010; Tamura and Hara-Nishimura 2011). nup1 mutant displayed multiple developmental defects, including early flowering, short siliques, and aborted pollen grains (Tamura et al. 2010; Lu et al. 2010). However, it is so far unclear at which stage nup1 pollen development is arrested and whether female gametogenesis in nup1 is affected. And the underlying molecular mechanisms of these defects remain to be solved.
In this study, we found that loss-of-function of NUP1 resulted in lethality in both male and female gametophyte development in Arabidopsis. nup1 ovules were arrested during meiosis; its pollen grains were arrested during mitosis I. Tetrad analysis indicated that the defective pollen phenotype was resulted from a sporophytic defect. We also observed enlarged pollen grains in nup1 mutant. DNA content was increased in nup1 pollen grains. RNA-sequencing (RNA-seq) showed that transcript levels of genes involved in reproduction and regulation of cell size were reduced in nup1 compared with the wild type (WT). Altogether our data indicate that NUP1 is required for gametogenesis in Arabidopsis.
Materials and methods
Plant materials and growth conditions
Arabidopsis thaliana ecotype Columbia (Col-0) was used in this study. Arabidopsis mutants including nup1-1 (SALK_104728), nup1-4 (GK_098G11), and qrt1 (SAIL_1159_C11) were obtained from the Nottingham Arabidopsis Stock Centre (NASC) (http://arabidopsis.info/). Arabidopsis plants were cultured under long-day condition (16 h light/8 h dark) at 22 ± 1 °C. Seeds were surface-sterilized and incubated at 4 °C for 3 days before being sown on Murashige and Skoog (MS) medium (Duchefa Biochemie, Haarlem, The Netherlands) supplemented with 1% (w/v) sucrose (Sangon Biotech, Shanghai, China) and 0.8% (w/v) plant agar (Duchefa Biochemie, Haarlem, The Netherlands).
Vector construction and generation of transgenic plants
ProNUP1:GUS was generated by introducing the NUP1 promoter fragment (− 1229 bp upstream of ATG) into the pGEENII-0229-GUS vector between XhoI and SmaI restriction enzyme sites (Men et al. 2008). The ProNUP1:NUP1-EGFP construct was generated through a two-step reaction. First, the NUP1 promoter fragment was ligated into the pGEENII-0229-EGFP vector (Zhang et al. 2016) between XhoI and SmaI sites, then the NUP1 coding sequence without the stop codon and added with a linker (GCGGCAGCC) was introduced into the above-obtained construct between SmaI and SpeI sites (Primer sequences are available in Supplementary Table S1). These constructs were transformed into Agrobacterium tumefaciens strain C58C1 (pMP90/pJIC Sa-Rep). Arabidopsis transgenic plants were generated using floral dip method (Clough and Bent 1998).
Male and female gametophyte observation
Pollen grains were collected from opening flowers and observed using an Olympus BX63 microscope. For female gametophyte observation, floral buds at different developmental stages were fixed in ethanol:acetic acid at 3:1 (v:v) for at least 4 h, and then cleared in chloral hydrate solution (67 g chloral hydrate, 8.3 mL glycerol, and 25 mL distilled water) for 4 days. Ovules were dissected out of the pistil and observed by differential interference contrast (DIC) microscopy.
GUS staining
For GUS staining, tissues were immersed in staining solution (0.5 mg/mL 5-bromo-4-chloro-3-indolyl-β-d-glucuronide in 50 mM sodium phosphate buffer pH 7.0, 0.5 mM potassium ferricyanide, 0.5 mM potassium ferrocyanide, and 0.1% Triton X-100) and incubated at 37 °C overnight. Before observation, the stained materials were cleared in chloral hydrate solution overnight.
Pollen grain staining
For observation of pollen nuclei, pollen grains from anthers at developmental stages of 7–13 were stained with 2 µg/mL of 4′,6-diamidino-2-phenylindole (DAPI) (Sigma-Aldrich, Steinheim, Germany) for 20 min at room temperature. And then observe by fluorescence microscopy. For pollen viability assay, anthers at developmental stage 12 were dipped in the Alexander’s solution as described (Alexander 1969; Johnson-Brousseau and McCormick 2004).
Callose deposition observation
Inflorescences of WT and nup1 mutant were fixed in FAA solution for 24 h and stained in 0.1% aniline blue in 100 mM Tris (pH 8.5) for 10 h. Female gametophytes with staining were separated from pistil using dissecting needle, and then callose deposition during megasporogenesis was observed using an Olympus BX63 microscope.
Paraffin section
Arabidopsis floral buds at different developmental stages were fixed in formalin-acetic acid-alcohol (FAA) fixative (50% ethanol, 10% acetic acid, 5% formaldehyde) for 24 h at 4 °C. The samples were dehydrated through a series of ethanol (70, 80, 90, and 100%, 1 h each), cleared with the 1:1 mixture of xylene and ethanol for 30 min, and then cleared with xylene for 1 h. Then the samples were embedded in paraffin and sectioned at 8–10 µm using a microtome (Leica RM2125RTS, Leica, Germany) and stained with safranin and fast green. The sections were observed under an Olympus BX63 microscope.
Flow cytometry
For nuclei preparation, mature pollen grains from 13-stage flowers were extracted in 1 mL nuclei extraction buffer (Galbraith et al. 1983). The nuclei suspension was then filtered, and propidium iodide was added to the nuclei solution at the final concentration of 50 g/mL and stained at 4 °C for 20 min before flow cytometry testing. Approximately 20,000 stained nuclei were detected using flow cytometry equipment (FACS Calibur, BD).
Quantitative RT-PCR
For quantitative RT-PCR (qRT-PCR) analysis of NUP1 expression pattern, total RNA was extracted from 7-day-old seedling, root, rosette leaf, cauline leaf, stem, inflorescence, and silique using Trizol reagent. For qRT-PCR verification of the expression levels of pollen development-related genes, total RNA was extracted from inflorescences containing stages 1–13 floral buds. The first-strand cDNA was prepared from 2 µg total RNA using EasyScript First-Strand cDNA Synthesis SuperMix kit (TransGen Biotech, Beijing, China). qRT-PCR was performed using Eppendorf Realplex2 Detection System according to the manufacturer’s protocol with qPCR SYBR Green Mixes (Takara, Dalian, China) in a final volume of 20 µL. Three technical repeats and three biological repeats were done for each reaction. Primer sequences are available in Supplementary Table S1.
RNA-seq and data analysis
Total RNA was extracted from inflorescences of WT and nup1-4 plants using Trizol, and mRNA was enriched by oligo (dT) magnetic beads. The mRNA was fragmented into short fragments using fragmentation buffer and the first strand of cDNA was synthesized using random hexamer primers. Second-strand cDNA was synthesized by DNA polymerase I and RNase H. Then the cDNA libraries were constructed and sequenced using Illumina HiSeq2500 by Biomarker (Beijing, China). Clean reads were aligned to the Arabidopsis genome (TAIR10) using TopHat2 software (Kim et al. 2013). The Cuffdiff software was used to screen differentially expressed genes (Trapnell et al. 2012). Gene ontology (GO) analysis was performed using AgriGO (Du et al. 2010).
Results
NUP1 is highly expressed in male and female gametophytes
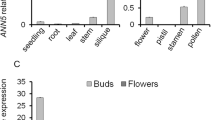
To investigate expression pattern of the Arabidopsis NUP1 gene, qRT-PCR was performed. The results showed that the NUP1 gene is expressed in most tissues examined, with the strongest expression level identified in inflorescence (Fig. 1a). To examine the NUP1 expression in detail, the native NUP1 promoter sequences (− 1229 bp upstream of ATG) were fused with the GUS reporter gene, and transformed into WT Arabidopsis. In 2-week-old ProNUP1:GUS transgenic plants, GUS staining was detected in hypocotyl, root tip and root vascular, and cotyledons (Fig. 1b; Supplementary Fig. S1). In cotyledons and hypocotyl, the GUS signal was not evenly distributed, some cells showed stronger GUS signal than other cells (Supplementary Fig. S1a-d). In root tip, the root cap cells showed stronger GUS signal than cells in the meristem and elongation zone (Supplementary Fig. S1f). In the inflorescence, the strongest GUS signal was detected in the anthers of opening flower (Fig. 1c). Detailed examination showed that GUS signal was mainly detected in pollen grains and ovules (Fig. 1d–h). ProNUP1:GUS was expressed throughout pollen and ovule development (Supplementary Fig. S2a–d), and its expression was highest in mature pollen grains (Supplementary Fig. S2c).
Expression patterns of the Arabidopsis NUP1 gene. a Analysis of NUP1 expression levels by qRT-PCR. Expression levels relative to ACTIN2 are displayed. The data represent the mean values ± SD of three experiments. b–h Expression of ProNUP1:GUS in various tissues, including 2-week-old plant (b), inflorescence (c), flower (d), stamen (e), pollen grains (f), pistil (g), and ovule (h)
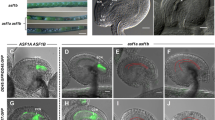
To investigate subcellular localization of NUP1, we analyzed expression of a C-terminal EGFP-tagged NUP1 fusion protein in root, which was under control of the native NUP1 promoter (ProNUP1:NUP1-EGFP). The results showed that NUP1 is localized to nuclear envelope (Supplementary Fig. S3). We then analyzed ProNUP1:NUP1-EGFP expression during ovule and pollen development. NUP1-EGFP was expressed in all ovule cells throughout the ovule development (Fig. 2a–e). And GFP signal was also detected throughout the pollen development (Fig. 2f–i). In bicellular pollen, NUP1-EGFP was equally distributed in vegetative and generative nuclei (Fig. 2h), whereas in mature pollen grains, NUP1-EGFP signal was stronger in the vegetative nuclear envelope than in the sperm nuclei envelope (Fig. 2i).
Together, these results suggest that NUP1 plays a role in male and female gametophyte development.
Deficiency of NUP1 affects both male and female gametophyte development
To study the potential role of Arabidopsis NUP1 in gametogenesis, we isolated two T-DNA insertion mutant alleles, nup1-1 (SALK_104728) and nup1-4 (GK_098G11) (Fig. 3a). nup1-1 has been reported previously (Tamura et al. 2010; Lu et al. 2010), whereas nup1-4 is a new mutant allele of the NUP1 gene. RT-PCR analysis revealed that residual NUP1 transcripts were present in the nup1-1 mutant, whereas no NUP1 transcripts were detected in the nup1-4 mutant (Fig. 3b). Therefore, the nup1-1 allele is a knock down mutant, whereas the nup1-4 allele is a knock out mutant. Both nup1-1 and nup1-4 exhibit early flowering and short siliques phenotypes, and the siliques of nup1-4 were shorter than that of nup1-1 (Supplementary Fig. S4). Short undeveloped siliques suggest fertility defect. Therefore, we examined seed set in the nup1 siliques. There were approximately 60 seeds per WT silique (Fig. 3c, f). However, in nup1-1 and nup1-4 siliques there were only approximately 10 and 1 seeds, respectively (Fig. 3d–f). In addition, siliques from nup1-1 and nup1-4 were found to have approximately 82% and 97% unfertilized ovules, respectively, compared with < 2% in WT (Fig. 3c–e, g). These results suggest that gametogenesis is affected in the nup1 mutant. To verify this, we performed reciprocal crosses between nup1 mutants and WT. When nup1-1 or nup1-4 pollen grains were used to fertilize WT ovaries, more than 60% of ovaries were not fertilized (60.4 ± 18.7 for nup1-1, 61.6 ± 25.5 for nup1-4) (Table 1). Similarly, when WT pollen grains were used to pollinate nup1-1 or nup1-4 ovaries, more than 80% of ovaries were not fertilized (81.5 ± 7.2 for nup1-1, 97.6 ± 2.0 for nup1-4) (Table 1). Together, these data demonstrate that both male and female gametes of nup1 plants are defective.
Defective seed set in nup1 mutants. a Schematic of T-DNA insertion sites in the NUP1 gene. Black boxes indicate exons; white boxes indicate 5′ and 3′ untranslated regions; black lines indicate introns; triangles indicate T-DNA insertion sites; Arrows indicate the positions of primers. b RT-PCR analysis of the expression levels of the NUP1 gene. The ACTIN2 (ACT2) gene was used as an internal control. Total RNA was extracted from inflorescence. c–e Siliques 7–10 days post pollination from WT (c), nup1-1 (d) and nup1-4 (e) plants. Figures (c-e) share the same scale bar as shown in (e). f, g Quantification of total seeds and unfertilized ovules in siliques from WT, nup1-1 and nup1-4 plants. The data represent mean values ± SD (n = 36 siliques). **P < 0.01 by Student’s t test
To confirm that these fertility defects were caused by NUP1 loss-of-function, we introduced the ProNUP1:NUP1-EGFP into the nup1-4 mutant. The transgene rescued all of the defects of the nup1-4 mutant (Supplementary Fig. S5).
nup1 female gametophyte is arrested during meiosis
Because reciprocal crosses indicated that nup1 mutants had defects in both male and female gametogenesis, we first analyzed the final phenotype of its female gametophyte (FG) by whole-mount clearing of ovules at mature stage. In Arabidopsis, megaspore mother cell (MMC) undergoes meiosis, resulting in four megaspores. Subsequently, three megaspores degenerate, leaving one functional megaspore (FM). Then the FM undergoes three rounds of mitosis without cytokinesis. After cellularization and polar nuclei fusion, the FM becomes a seven-celled female gametophyte, containing an egg cell, two synergids, a central cell, and three antipodals, which undergo cell death before fertilization (Fig. 4a). At maturity, approximately 97.7% of WT gametophytes reached FG7 stage (n = 305) (Fig. 4b; Table 2). By contrast, at maturity, 52.5% of the nup1-1 ovules and 80% of the nup1-4 ovules were collapsed and degenerated (Fig. 4c; Table 2); 10.2% of nup1-1 ovules and 3.9% of nup1-4 ovules were arrested either before or during the meiosis stage, as some ovules contained a MMC (Fig. 4d, e), some ovules contained a meiocyte in a dyad (Fig. 4f) and some ovules contained a tetrad (Fig. 4g, h); 15.9% of nup1-1 ovules and 8.2% of nup1-4 ovules were arrested at FG1 stage (Fig. 4i); 7.5% of nup1-1 ovules and 2.4% of nup1-4 ovules were arrested at FG2-3 stage (Fig. 4j); 3.6% of nup1-1 ovules and 0.9% of nup1-4 ovules were developed to FG4 stage (Fig. 4k); 3.6% of nup1-1 ovules and 1.5% of nup1-4 ovules reached FG5-6 stage (Fig. 4l); only 6.7% of nup1-1 ovules and 3.0% of nup1-4 ovules reached FG7 stage (n = 668 for nup1-1, 330 for nup1-4) (Fig. 4m; Table 2).
nup1 embryo sac phenotypes at maturity. a Diagram of female gametophyte development. Black dots indicate nuclei, grey ovals indicate vacuole. MMC megaspore mother cell, Nu nucellus, FM functional megaspore, DM degenerated megaspore, V vacuole, AC antipodal cell, PN polar nuclei, EC egg cell, SC synergid cell. b–m Differential interference contrast (DIC) images of embryo sacs dissected from WT (b) and nup1-1 (c–m) flowers at developmental stage of 13. b WT female gametophyte at stage FG7. c Degenerated nup1-1 female gametophyte. d–l Arrested nup1-1 female gametophytes at stages of MMC (d, e), meiosis I (f), tetrad (g and h; h is a high-magnification image of g), FG1 (i) and FG2 (j). k, l delayed nup1-1 female gametophytes at stages of FG4 (k) and FG6 (l). m nup1-1 female gametophyte at stage FG7. CN central cell nucleus, EN egg cell nucleus, SN synergid cell nuclei, N nucleus, DN degenerating nucleus, CW cell wall, DM degenerating megaspore, PN polar nuclei, AN antipodal cell nuclei, DES degenerated embryo sac. Figures (b–g, i–m) share the same scale bar as shown in m
To determine when the collapsed and degenerated nup1 ovules were arrested, we analyzed WT and nup1 ovules at various stages of development. In WT, female gametogenesis proceeds normally from MMC to FG7 (Supplementary Fig. S6), and is fairly synchronous within a pistil, spanning at most three neighboring developmental stages (Table 3). However, the developmental synchronicity was already impaired in the nup1 pistils at the transition from MMC to FG1 stage, approximately 45% of nup1-1 ovules (n = 171) and 78.2% of nup1-4 ovules (n = 202) were degenerated before FG1 stage (Supplementary Fig. S6; Table 3). Taken together, these results indicate that most of nup1 ovules were arrested either before or during the process of meiosis.
To further determine whether meiosis of ovules was affected in nup1 mutant, we investigated the callose deposition which is a cytological marker for positioning of newly formed cell plate during first and second division of meiosis. In WT ovules, callose signal showed a bright band at the newly formed cell plates after the first meiotic division, called dyad (Fig. 5a, e). Then two bright bands of callose signal were observed at the early stage of second meiotic divison, called triad (Fig. 5b, f). After that, two bright bands and partial accumulation at the micropylar end of callose signal were observed after the second meiotic division, called tetrad (Fig. 5c, g). The callose signal almost disappeared after functional megaspore formation (Fig. 5d, h). In nup1 ovules, the callose deposition showed dramatically abnormal distribution compared with WT, including the callose signal mainly focused at the micropylar end, named abnormal 1 (22.8%, n = 114) (Fig. 5i, m, q); all of the callose signal focused at the micropylar end, named abnormal 2 (18.4%, n = 114) (Fig. 5j, n, q); callose signal distributed pell-mell in embryo sac and no signal distributed at the micropylar end, named abnormal 3 (25.4%, n = 114) (Fig. 5k, o, q); and callose signal distributed on one side of embryo sac, named abnormal 4 (5.3%, n = 114) (Fig. 5l, p, q). The percentages of ovules at the dyad, triad, and tetrad stage of nup1-4 were obviously reduced compared with WT (Fig. 5q). These results suggest that formed position of cell plate was affected during first and second meiotic division of nup1 ovules.
Callose depositions during megasporogenesis are defective in nup1 mutant. a–h Callose depositions during megasporogenesis in WT. i–p Abnormal callose depositions during megasporogenesis in nup1-4 mutant. q Quantification of callose deposition at various stages of meiosis in 2-IV stage ovules in WT (n = 46) and nup1-4 (n = 114). Figures (a–p) share the same scale bar as shown in (p)
Sporophytic mutation results in abortive pollen grains in nup1 mutant
We next examined pollen development in nup1 mutant. Alexander staining showed that the number of viable pollen grains in nup1 anthers was significantly reduced compared with that of the WT (Supplementary Fig. S7). Approximately 53.5% of nup1-1 pollen grains (n = 1206) and 59.3% of nup1-4 pollen grains (n = 1163) were shriveled (Supplementary Fig. S7e, f). By contrast, less than 0.5% of the pollen was shriveled in WT anthers (n = 1118). We further examined the pollen morphology by scanning electron microscopy. Pollen grains from WT were morphologically normal (Supplementary Fig. S8a), whereas many nup1 pollen grains were collapsed (Supplementary Fig. S8b, c). In addition, viable nup1 pollen grains exhibited a thinner exine surface compared with WT (Supplementary Fig. S8d-k).
To distinguish lethal pollen in nup1 mutant was due to gametophytic or sporophytic defect, we analyzed self progenies of NUP1 heterozygous plant (A/a). Among 229 descendants, 56 plants (about 24.5%) displayed sterile phenotype (aa). In addition, we crossed nup1-4 with quartet1 (qrt1) mutant (Preuss et al. 1994). In qrt1 plants, the four products of a single meiosis are attached together throughout pollen development (Supplementary Fig. S9a). The nup1/NUP1 qrt1 plant produced four normal attached mature pollen grains (Supplementary Fig. S9b). However, zero to four shriveled pollen grains were detected in tetrads from nup1qrt1 mutant (Supplementary Fig. S9c-g). These results indicate that sporophytic defect give rise to aborted pollen grains in nup1 mutant.
nup1 mutants produce large pollen grains
We also observed that nup1 mutants produced large pollen grains (Fig. 6a, c; Supplementary Fig. S10a, c). The average diameter of WT pollen grains was approximately 21 µm, whereas the average diameter of nup1 pollen grains was approximately 25 µm (Fig. 6b, d; Supplementary Fig. S10b, d). Since cell size is usually correlated with endoreduplication (Barow 2006; Lee et al. 2009), we performed flow cytometry analysis to see if the large pollen grain phenotype of nup1 was accompanied by increased endoreduplication. Indeed, nup1 pollen had a higher ploidy level compared with that of the WT (Fig. 6e; Supplementary Fig. S10e).
nup1 mutants produce large pollen grains with increased DNA content. a, c Mature pollen grains from WT (a) and nup1-1 plants (c). Arrowheads indicate large pollen grains; arrows indicate aborted pollen grains. b, d Pollen grain size distribution of WT (b) and nup1-1 (d). n = 5203 for WT and 5222 for nup1-1. e DNA content in pollen grains of WT and nup1 mutant. Mature pollen grains from 13-stage flowers of WT and nup1-1 mutant were collected, and DNA content was determined by flow cytometry. Approximately 20,000 pollen grains were analyzed for each genotype
Pollen development in nup1 is arrested during mitosis I
To determine at which developmental stage nup1 pollen was abnormal, we performed transverse sections of nup1 anthers at various developmental stages. Pollen from nup1 anthers appeared normal during the tetrad and microspore stages (Fig. 7a). However, by the early bicellular stage, some nup1 pollen grains were obviously smaller and exhibited collapsed cytosol (Fig. 7a). At later stages, shriveled pollen grains were observed (Fig. 7a).
nup1 pollen grains were arrested at mitosis I. a Parafin sections of WT and nup1 anthers at different developmental stages. Aborted pollen grains (indicated by black arrows) were observed in nup1anthers at the early bicellular stage. Arrowheads indicate the tapetum. b DAPI staining of WT and nup1 pollens at different developmental stages. Pollen grains from nup1-1 and WT anthers were stained with DAPI to label nuclei and visualized with fluorescent microscope. Nonstaining pollen grains (indicated by arrows) were first observed at the bicellular stage
We further stained nup1 and WT pollen grains with DAPI to visualize the number and position of nuclei. At the microspore stage, there was no difference between nup1 and WT pollen grains (Fig. 7b). However, at the bicellular stage, some nup1 mutant pollen grains exhibited no DAPI staining (Fig. 7b). The number of unstained nup1 pollen grains increased at tricellular stage (Fig. 7b).
Together, these results indicate that nup1 pollen grains were arrested during the first mitosis process.
Reproduction-related genes are downregulated in nup1 mutants
To gain insight into the molecular mechanism by which NUP1 affects male and female gametogenesis, we performed transcriptome analysis using RNA-sEq. We found that 134 genes were upregulated and 689 genes were downregulated in nup1, respectively [fold change > 2 and false discovery rate (FDR) < 0.01] (Fig. 8a; Supplementary Dataset 1). Consistent with the observed phenotypes, GO analysis of the downregulated genes showed a significant enrichment for genes involved in pollination, reproductive developmental process, cell differentiation, and regulation of cell size (ranked by FDR < 0.01) (Fig. 8b; Supplementary Dataset 2; Supplementary Fig. S11). Whereas top recurring GO terms among the upregulated genes were related to response to stimulus and cell death (Supplementary Fig. S12). Given that mitosis of male and female gametophyte was affected in nup1 mutant, we identified many genes involved in mitotic process in our RNA-seq data (Supplementary Dataset 3). For validation, the transcript levels of 19 reproduction-related genes were analyzed by qRT-PCR. The qRT-PCR results were consistent with the RNA-seq results (Fig. 8c). Altogether, these data suggest that the gametogenesis defects observed in nup1 mutants are caused by downregulation of reproduction-related genes.
NUP1 mutation causes downregulation of genes involved in reproduction. a RNA-seq scatter plot showing differentially transcribed genes in WT and nup1-4. FDR, false discovery rate; FPKM, fragments per kilobase of transcript per million fragments mapped. b GO analysis of the downregulated genes in nup1-4. GO functional enrichment was analyzed using the online tool AgriGO (http://bioinfo.cau.edu.cn/agriGO/index.php). c qRT-PCR analysis of downregulated genes involved in pollen development. Expression levels relative to TIP41 are displayed. Similar results were obtained from three independent experiments. Shown are results from one experiment. Error bars indicate the standard deviation (SD). **P < 0.01 by Student’s t test
Discussion
In this study, we analyzed the fertility defects of nup1 mutant. We found that both male and female gametogenesis were aberrant in nup1 mutant. nup1 female gametophytes were arrested during meiosis; its male gametophytes were arrested during mitosis I. The developmental defect in nup1 gametophytes was accompanied by reduced expression of reproduction-related genes. These results suggest that NUP1 plays a role in gametogenesis.
NUP1 affects meiosis of MMC
NUPs are components of NPC, which mediates transport of RNAs and proteins between the nucleus and cytoplasm (Xu and Meier 2008; Meier and Brkljacic 2009). Mutants of NUP genes share some common phenotypes such as retarded growth, early flowering, blocked mRNA export, and altered nuclear morphology (Tamura et al. 2010; Lu et al. 2010; Tamura and Hara-Nishimura 2011; Parry 2014; Du et al. 2016). However, more evidences indicate that individual NUPs play specific cellular roles and influence plant growth and development by different molecular mechanisms. For example, NUP98 regulates the shade avoidance response in Arabidopsis by activating shade-induced gene expression (Gallemí et al. 2016). NUP88/MOS7 was demonstrated to be required for spindle assembly and cell plate formation during gametophyte mitosis (Park et al. 2014). Here we show for the first time that NUP1 is required for the meiosis of MMC. More than half of nup1 female gametophytes could not develop to stage FG1 (Tables 2, 3; Fig. 4; Supplementary Fig. S6), with some ovules arrested prior to meiosis (Fig. 4d, e), some ovules arrested after meiosis I (Fig. 4f; Supplementary Fig. S6), and some aborted just after meiosis II (Fig. 4g, h). In addition, our results indicated that formed position of cell plate was affected during meiosis of nup1 ovules (Fig. 5). These results indicate that NUP1 influences the initiation, execution, exit, and cell plate formation during the meiosis of MMC. NUPs have been demonstrated to play important roles during meiosis in yeast and animals. In Saccharomyces cerevisiae, NUP2, NUP60 and NUP84 were found to have a meiotic function (Chu et al. 2017). A 125 aa region of NUP2 was necessary and sufficient for its meiotic role, therefore this region was called meiotic autonomous region (MAR) (Chu et al. 2017). NUP2-MAR was required for meiotic chromosome organization (Chu et al. 2017). In Schizosaccharomyces pombe, Nup132 regulates kinetochore assembly during meiotic prophase (Yang et al. 2015). The C. elegans nucleoporin MEL-28/ELYS interacts with PP1 (protein phosphatase 1) to mediate meiotic chromosome segregation (Hattersley et al. 2016). Nup107 is required for cytokinesis in Drosophila male meiosis (Hayashi et al. 2016). Whether the Arabidopsis NUP1 affects MMC meiosis through regulating chromosome organization or segregation requires further analysis.
NUP1 is required for mitosis of both male and female gametophytes
Mitosis of both male and female gametophytes of nup1 mutants was defective. Male gametophytes of nup1 were arrested at mitosis I (Fig. 7). Less than 17% of nup1 female gametophytes could finish the three rounds of mitosis to form mature female gametes (Tables 2, 3). Therefore, ovules stayed at FG1, FG2-3, and FG4 were observed in mature pistils of nup1 mutants (Table 2; Fig. 4i–k). These results indicate a mitotic function of the Arabidopsis NUP1. Mitotic functions have been reported for several NUPs including the Arabidopsis MOS7/NUP88 (Park et al. 2014), the vertebrate Nup98, Nup133, Nup188, and Nup153 (Lussi et al. 2010; Cross and Powers 2011; Bolhy et al. 2011; Itoh et al. 2013), the C. elegans NPP-10, NPP-13, and NPP-20 (Ferreira et al. 2017), and the yeast Nup1 (Harper et al. 2008). These NUPs participate in mitosis by affecting spindle assembly, kinetochore organization, and spindle checkpoint activity (Harper et al. 2008; Lussi et al. 2010; Cross and Powers 2011; Bolhy et al. 2011; Itoh et al. 2013; Ferreira et al. 2017). Nup153 interacts with the spindle assembly checkpoint protein Mad1 and affects its phosphorylation status (Lussi et al. 2010). The Arabidopsis NUP1 is an ortholog of the yeast NUP1 and animal NUP153 (Neumann et al. 2006; Lu et al. 2010). The Arabidopsis NUP1 is highly dynamic on the nuclear envelope, suggesting a dynamic interaction with other proteins (Tamura et al. 2010). Future work is required to determine whether the Arabidopsis NUP1 interact with the microtubule or the spindle assembly checkpoint proteins.
NUP1 deficiency reduces expression level of reproduction-related genes
Increasing evidence has pointed to a transport-independent role for the NUPs in regulating gene expression. NPC provides a platform for regulating gene transcription by anchoring genes, recruiting transcription factors, or post-transcriptional modification (Dieppois and Stutz 2010). A recent research found that Nup62, Nup93 and Nup155 form a negative regulatory loop to control the chromatin tethering state in Drosophila male germline and somatic cells (Breuer and Ohkura 2015). In plants, NUPs have also been reported to regulate gene expression through tethering of certain genes (Smith et al. 2015; Gallemí et al. 2016). In mouse embryonic stem cells, Nup153 recruits the polycomb-repressive complex 1 (PRC1) to a subset of developmental genes to inhibit their expression to maintain the pluripotency of the stem cells (Jacinto et al. 2015). Nup98 interacts with the 3′-UTR of p21 (a target gene of the p53 tumor suppressor) mRNA to protect it from degradation by the exosome (Singer et al. 2012). Nup106, Nup120, and Rae1 selectively destabilize meiotic mRNAs in vegetative fission yeast (Sugiyama et al. 2013). Our transcriptome analysis results showed that expression levels of reproduction-related genes were reduced dramatically in the Arabidopsis nup1 mutant (Fig. 8; Supplementary Fig. S11, S12 and supplementary dataset). This finding implicates that NUP1 may regulate expression level of reproduction-related genes. Similar to those NUPs that can regulate gene expression, NUP1 is a peripheral NUP of the NPC and is highly mobile (Tamura et al. 2010; Lu et al. 2010). Further experiments are required to determine whether NUP1 can directly regulate gene transcription.
Author contribution statement
SM conceived the project and designed experiments. SB performed most of the experiments; GS and ZL helped with parafin sections; GL, MA, and QW helped with cloning and genotyping. SM analyzed the data and wrote the article.
Abbreviations
- NPC:
-
Nuclear pore complex
- NUPs:
-
Nucleoporins
- NUP1:
-
Nucleoporin 1
- MMC:
-
Megaspore mother cell
- FG:
-
Female gametophyte
- FM:
-
Functional megaspore
- RNA-seq:
-
RNA-sequencing
References
Alexander MP (1969) Differential staining of aborted and nonaborted pollen. Stain Technol 44:117–122
Allen JL, Douglas MG (1989) Organization of the nuclear pore complex in Saccharomyces cerevisiae. J Ultra Mol Struct Res 102:95–108
Barow M (2006) Endopolyploidy in seed plants. Bioessays 28:271–281
Boeglin M, Fuglsang AT, Luu DT et al (2016) Reduced expression of AtNUP62 nucleoporin gene affects auxin response in Arabidopsis. BMC Plant Biol 16:2
Bolhy S, Bouhlel I, Dultz E et al (2011) A Nup133-dependent NPC-anchored network tethers centrosomes to the nuclear envelope in prophase. J Cell Biol 192:855–871
Breuer M, Ohkura H (2015) A negative loop within the nuclear pore complex controls global chromatin organization. Genes Dev 29:1789–1794
Cheng YT, Germain H, wiermer M et al (2009) Nuclear pore complex component MOS7/Nup88 is required for innate immunity and nuclear accumulation of defense regulators in Arabidopsis. Plant Cell 21:2503–2516
Chu DB, Gromova T, Newman TAC, Burgess SM (2017) The nucleoporin Nup2 contains a meiotic-autonomous region that promotes the dynamic chromosome events of meiosis. Genetics 206:1319–1337
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Cross MK, Powers MA (2011) Nup98 regulates bipolar spindle assembly through association with microtubules and opposition of MCAK. Mol Biol Cell 22:661–672
Dieppois G, Stutz F (2010) Connecting the transcription site to the nuclear pore: a multi-tether process that regulates gene expression. J Cell Sci 123:1989–1999
Dong CH, Hu X, Tang W et al (2006) A putative Arabidopsis nucleoporin, AtNUP160, is critical for RNA export and required for plant tolerance to cold stress. Mol Cell Biol 26:9533–9543
Du Z, Zhou X, Ling Y et al (2010) agriGO: a GO analysis toolkit for the agricultural community. Nucleic Acids Res 38:W64–W70
Du J, Gao Y, Zhan Y et al (2016) Nucleocytoplasmic trafficking is essential for BAK1- and BKK1-mediated cell-death control. Plant J 85:520–531
Ferreira J, Stear JH, Saumweber H (2017) Nucleoporin NPP-10, NPP-13 and NPP-20 are required for HCP-4 nuclear import to establish correct centromere assembly. J Cell Sci 130:963–974
Fiserova J, Kiseleva E, Goldberg MW (2009) Nuclear envelope and nuclear pore complex structure and organization in tobacco BY-2 cells. Plant J 59:243–255
Galbraith DW, Harkins KR, Maddox JM et al (1983) Rapid flow cytometric analysis of the cell cycle in intact plant tissues. Science 220:512–527
Gallemí M, Galstyan A, Paulišić S et al (2016) DRACULA2 is a dynamic nucleoporin with a role in regulating the shade avoidance syndrome in Arabidopsis. Development 143:1623–1631
Genenncher B, Wirthmueller L, Roth C et al (2016) Nucleoporin-regulated MAP kinase signaling in immunity to a necrotrophic fungal pathogen. Plant Physiol 172:1293–1305
Goldberg MW, Allen TD (1996) The nuclear pore complex and lamina: Three-dimensional structures and interactions determined by field emission in-lens scanning electron microscopy. J Mol Biol 257:848–865
Gu Y, Zebell SG, Liang Z et al (2016) Nuclear pore permeabilization is convergent signaling event in effector-triggered immunity. Cell 166:1526–1538
Harper NC, Al-Greene NT, Basrai MA, Belanger KD (2008) Mutations affecting spindle pole body and mitotic exit network function are synthetically lethal with a deletion of the nucleoporin NUP1 in S. cerevisiae. Curr Genet 53:95–105
Hattersley N, Cheerambathur D, Moyle M et al (2016) A nucleoporin docks protein phosphatase 1 to direct meiotic chromosome segregation and nuclear assembly. Dev Cell 38:463–477
Hayashi D, Tanabe K, Katsube H, Inoue YH (2016) B-type nuclear lamin and the nuclear pore complex Nup107-160 influences maintenance of the spindle envelope required for cytokinesis in Drosophila male meiosis. Biol Open 5:1011–1021
Itoh G, Sugino S, Ikeda M et al (2013) Nucleoporin Nup188 is required for chromosome alignment in mitosis. Cancer Sci 104:871–879
Jacinto FV, Benner C, Hetzer MW (2015) The nucleoporin Nup153 regulates embryonic stem cell pluripotency through gene silencing. Genes Dev 29:1224–1238
Johnson-Brousseau SA, McCormick S (2004) A compendium of methods useful for characterizing Arabidopsis pollen mutants and gametophytically-expressed genes. Plant J 39:761–775
Kim D, Pertea G, Trapnell C et al (2013) TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol 14:R36
Lee HO, Davidson JM, Duronio RJ (2009) Endoreplication: polyploidy with purpose. Genes Dev 23:2461–2477
Lu Q, Tang X, Tian G et al (2010) Arabidopsis homolog of the yeast TREX-2 mRNA export complex: components and anchoring nucleoporin. Plant J 61:259–270
Lussi YC, Shumaker DK, Shimi T, Fahrenkrog B (2010) The nucloporin Nup153 affects spindle checkpoint activity due to an association with MadI. Nucleus 1:71–84
Meier I, Brkljacic J (2009) The nuclear pore and plant development. Curr Opin Plant Biol 12:87–95
Men S, Boutté Y, Ikeda Y et al (2008) Sterol-dependent endocytosis mediates post-cytokinetic acquisition of PIN2 auxin efflux carrier polarity. Nat Cell Biol 10:237–244
Neumann N, Jeffares DC, Poole AM (2006) Outsourcing the nucleus: nuclear pore complex genes are no longer encoded in nucleomorph genomes. Evol Bioinform 2:23–34
Park GT, Frost JM, Park JS et al (2014) Nucleoporin MOS7/Nup88 is required for mitosis in gametogenesis and seed development in Arabidopsis. Proc Natl Acad Sci USA 111:18393–18398
Parry G (2014) Components of the Arabidopsis nuclear pore complex play multiple diverse roles in control of plant growth. J Exp Bot 65:6057–6067
Parry G, Ward S, Cernac A et al (2006) The Arabidopsis SUPPRESSOR OF AUXIN RESISTANCE proteins are nucleoporins with an important role in hormone signaling and development. Plant Cell 18:1590–1603
Preuss D, Rhee SY, Davis RW (1994) Tetrad analysis possible in Arabidopsis with mutation of the QUARTET (QRT) genes. Science 264:1458–1460
Roberts K, Northcote DH (1970) Structure of the nuclear pore in higher plants. Nature 228:385–386
Singer S, Zhao R, Barsotti AM et al (2012) Nuclear pore component Nup98 is a potential tumor suppressor and regulates post-transcriptional expression of select p53 target genes. Mol Cell 48:799–810
Smith S, Galinha C, Desset S et al (2015) Marker gene tethering by nucleoporins affects gene expression in plants. Nucleus 6:471–478
Sugiyama T, Wanatabe N, Kitahata E et al (2013) Red5 and three nuclear pore components are essential for efficient suppression of specific mRNAs during vegetative growth of fission yeast. Nucleic Acids Res 41:6674–6686
Tamura K, Hara-Nishimura I (2011) Involvement of the nuclear pore complex in morphology of the plant nucleus. Nucleus 2:168–172
Tamura K, Fukao Y, Iwamoto M et al (2010) Identification and characterization of nuclear pore complex components in Arabidopsis thaliana. Plant Cell 22:4084–4097
Tamura K, Fukao Y, Hatsugai N et al (2017) Nup82 functions redundantly with Nup136 in a salicylic acid-dependent defense response of Arabidopsis thaliana. Nucleus 8:301–311
Trapnell C, Roberts A, Goff L et al (2012) Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc 7:562–578
Walde S, Kehlenbach RH (2010) The Part and the Whole: functions of nucleoporins in nucleocytoplasmic transport. Trends Cell Biol 20:461–469
Wiermer M, Cheng YT, Imkampe J et al (2012) Putative members of the Arabidopsis Nup107-160 nuclear pore sub-complex contribute to pathogen defense. Plant J 70:796–808
Xu XM, Meier I (2008) The nuclear pore comes to the fore. Trends Plant Sci 13:20–27
Yang HJ, Asakawa H, Haraguchi T, Hiraoka Y (2015) Nup132 modulates meiotic spindle attachment in fission yeast by regulating kinetochore assemble. J Cell Biol 211:295–308
Yang Y, Wang W, Chu Z et al (2017) Roles of nuclear pores and nucleo-cytoplasmic trafficking in plant stress responses. Front Plant Sci 8:574
Zhang Y, Li X (2005) A putative nucleoporin 96 is required for both basal defense and constitutive resistance responses mediated by suppressor of npr1-1, constitutive 1. Plant Cell 17:1306–1316
Zhang X, Sun S, Nie X et al (2016) Sterol methyl oxidases affect embryo development via auxin-associated mechanisms. Plant Physiol 171:468–482
Acknowledgements
We thank The Nottingham Arabidopsis Stock Center (NASC) for the T-DNA insertions, John Innes Centre for the pGREENII0229 vector. We thank Ruming Liu and Yajuan Wan for technical assistance in the use of confocal and cell cytometry equipment, respectively. This work was supported by grants from the National Science Foundation of China (31570247, 91417308, and 31460453); the Natural Science Foundation of Tianjin (no. 14JCYBJC41200).
Funding
This work was supported by grants from the National Natural Science Foundation of China (31570247, 91417308, and 31460453).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Xian Sheng Zhan.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bao, S., Shen, G., Li, G. et al. The Arabidopsis nucleoporin NUP1 is essential for megasporogenesis and early stages of pollen development. Plant Cell Rep 38, 59–74 (2019). https://doi.org/10.1007/s00299-018-2349-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-018-2349-7