Abstract
Key message
Comparative ultrastructural developmental time-course analysis has identified discrete stages at which the fruit plastids undergo structural and consequently functional transitions to facilitate subsequent development-guided understanding of the complex plastid biology.
Abstract
Plastids are the defining organelle for a plant cell and are critical for myriad metabolic functions. The role of leaf plastid, chloroplast, is extensively documented; however, fruit plastids—chromoplasts—are poorly understood, especially in the context of the diverse metabolic processes operating in these diverse plant organs. Recently, in a comparative study of the predicted plastid-targeted proteomes across seven plant species, we reported that each plant species is predicted to harbor a unique set of plastid-targeted proteins. However, the temporal and developmental context of these processes remains unknown. In this study, an ultrastructural analysis approach was used to characterize fruit plastids in the epidermal and collenchymal cell layers at 11 developmental timepoints in three genotypes of apple (Malus × domestica Borkh.): chlorophyll-predominant ‘Granny Smith’, carotenoid-predominant ‘Golden Delicious’, and anthocyanin-predominant ‘Top Red Delicious’. Plastids transitioned from a proplastid-like plastid to a chromoplast-like plastid in epidermis cells, while in the collenchyma cells, they transitioned from a chloroplast-like plastid to a chloro-chromo-amyloplast plastid. Plastids in the collenchyma cells of the three genotypes demonstrated a diverse array of structures and features. This study enabled the identification of discrete developmental stages during which specific functions are most likely being performed by the plastids as indicated by accumulation of plastoglobuli, starch granules, and other sub-organeller structures. Information regarding the metabolically active developmental stages is expected to facilitate biologically relevant omics studies to unravel the complex biochemistry of plastids in perennial non-model systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
What is the biological significance and function of plastids in fruits? This remains an intriguing question owing to the diversity of fruit types and variability in plastid-dependent pigmentation in fruit. Plastids have been shown to participate in myriad metabolic processes including carotenoid synthesis (Camara et al. 1995; Kreuz and Kleinig 1984), monoterpene and diterpene synthesis (Mettal et al. 1988), amino acid biosynthesis, and shikimate pathway, and serve as the main site of fatty acid synthesis (Fischer and Weber 2002). Some of these pathways are exclusive to the plastid, while several others utilize a network of other subcellular components.
The vast majority of fruit plastid research has been performed using tomato as a model system. Plastids in tomato fruit undergo a transition from a photosynthetic chloroplast to a non-photosynthetic chromoplast. Immature tomato fruit possess chloroplasts with thylakoid membranes and stacked grana, akin to the chloroplast of a leaf (Liu et al. 2004). During the breaker stage, where tomato fruit begin to ripen, chloroplasts undergo an unstacking of grana, degradation of thylakoid membranes, and accumulation of plastoglobuli and lycopene (Cheung et al. 1993). In mature chromoplasts, thylakoid remnants are present and can be found sporadically throughout the chromoplast. Carotenoids are more evident at this stage and are present as carotenoid-rich membrane sacs and crystalloid structures (Egea et al. 2010). Plastid division is largely absent in tomato chromoplasts and proteomic analysis has revealed that many of the proteins necessary for replication are absent (Barsan et al. 2010; Cookson et al. 2003). Mature chromoplasts also exhibit an increase in the presence and length of stroma-filled tubules, known as stromules (Egea et al. 2010; Waters et al. 2004).
Plastid developmental transition studies are limited, but it has been proposed that the transition is driven by two major mechanisms—hormonal and nutritional (Bouvier and Camara 2006). Studies on hormonal control have focused on the role of ethylene, mostly in tomato (Alexander and Grierson 2002). The nutritional hypothesis, linking plastid development to carbon/nitrogen ratio, has been limited to citrus as a model (Huff 1983, 1984; Mayfield and Huff 1986). It is possible that the transition may be controlled through different mechanisms depending upon the fruit type. In such a case, reliance upon a single model organism such as tomato limits our understanding of this ubiquitous process.
Investigations of non-model plant systems have shown a wide diversity in fruit plastid structure and developmental transition, which is suggestive of the diverse biochemistries and functions that these organelles may perform. While limited diversity has been reported in chloroplast structure, chromoplasts have been described to have crystalline, globular, tubular, membranous, tubular/fibrillar, and reticulo-globular morphologies in plants (Camara et al. 1995). In Arum italicum (Italian arum), fruit plastids transition from amyloplasts to chloroplasts and then to chromoplasts (Bonora et al. 2000). Green cultivars of Actinidia chinensis and Actinidia deliciosa (kiwi fruit) retain the traditional chloroplast structures even in ripe fruit (Montefiori et al. 2009). Chromoplasts in orange fruit of Solanum luteum maintain honeycomb-like structures (Simpson et al. 1975).
Transmission electron microscopy (TEM) analysis in apple has demonstrated that mature fruit plastids are structurally different compared to tomato with respect to thylakoid presence, amount of starch granules, as well as plastoglobule abundance (Clijsters 1969). Epidermal plastids in mature apple fruit are devoid of thylakoid membranes and starch granules and contain many plastoglobuli (Merzlyak and Solovchenko 2002). An abundance of plastoglobuli indicates enhanced secondary metabolic activities as these structures contain structural proteins as well as active enzymes, several of unknown functions (Nacir and Brehelin 2013). The collenchyma cells in mature apple were reported to possess plastids with elongated grana with interspersed starch granules, while chloroplasts within inner tissues surrounding the vasculature maintain a hypergranum structure lacking visible starch granules (Clijsters 1969; Phan 1973, 1984). Dimorphic plastids have also been reported in cherry tomato, where, interestingly, the photosynthetic efficiency of the outer region of the fruit is much higher compared to the internal regions (Laval-Martin 1974). This difference has been attributed to the light-dependent activity of phosphoenol pyruvate carboxylase (PEPC) over Rubisco. PEPC activity correlated with higher chlorophyll content and the PEPC-dependent photosynthetic activity is reminiscent of the carbon-concentrating mechanisms of C4 or CAM plants (Blanke et al. 1986). The hypergranum structures may correlate with the modified biochemistry of the fruit plastid.
Like the plastids of leaves, chloroplasts in green apple fruit are photosynthetically active. Respiration and photosynthetic activity both decrease during the phase of cell growth in developing apples (Clijsters 1969). During fruit ripening, respiration activity increases, but photosynthetic activity remains at pre-ripening levels. Throughout the fruit developmental continuum, the overall chlorophyll content of maturing apples constantly decreases, even during the plateau of photosynthesis observed during ripening. The loss of photosynthetic activity in apple fruit is accompanied by a decrease of thylakoid-associated carotenoids, and de novo synthesis and fatty acid esterification of neoxanthin and violaxanthin (Knee 1972, 1988; Merzlyak and Solovchenko 2002). Synthesis of the carotenoids marks a transition from a chloroplast to a chromoplast. This transition differs from the chloroplast transition to a gerontoplast (a senescing plastid) which is characterized by a lack of de novo carotenoid synthesis (Egea et al. 2010; Matile 2000).
In addition to photosynthesis and the synthesis and storage of carotenoids, the strongly reductive environment and abundance of precursor molecules in apple fruit plastids is ideal for the synthesis of many molecules such as fatty acid chains (Cornah et al. 2002; Rawsthorne 2002; Sivak 1998) and aromatic amino acids (Herrmann and Weaver 1999). Fatty acids are the primary precursors for straight chain aromatic compounds, which account for a large majority of total volatiles in most fruits (Sanz et al. 1996). In apple, synthesis of aromatic compounds is principally derived from fatty acids (Bartley et al. 1985). Experiments in pear, a closely related species to apple, suggest that a precursor supply of fatty acids, rather than enzymatic activity, may be the most important factor in volatile synthesis (Lara et al. 2003). Ultrastructural changes in plastids coincide with production of volatiles which are typically produced toward the end of ripening in conjunction with other ripening-related processes. Fatty acids generated during the breakdown of thylakoid membranes can serve as precursors to volatile synthesis (Echeverria et al. 2004).
Recently, we reported a comparative analysis of the predicted plastid-targeted proteomes from seven plant species and identified a large number of novel, species-specific plastid-targeted proteins along with alternatively targeted homologs across the analyzed species (Schaeffer et al. 2014). Interestingly, out of the seven species, Malus × domestica Borkh., with 10,492 proteins, possessed the largest number of predicted plastid-targeted proteins. Only 40% of these proteins were homologous to members of the Arabidopsis plastid-targeted proteome and 57% of the apple proteins shared a homolog with the Arabidopsis total proteome (Schaeffer et al. 2014). There is a strong indication that plastids in perennial, fruit bearing species such as apple have far more complex and specialized functions compared to model systems such as Arabidopsis and tomato. The perennial habit, wide diversity of plastid and vacuolar pigmentation, and longer days to maturity (DTM) index (~150 days for apple), which is almost two times that of annual plants tomato and capsicum (~70 days), enable prolonged interaction of the fruit with its cognate environment both during the autotrophic and heterotrophic phase of fruit development. Especially during the latter phase, when chloroplasts transition to chromoplasts, the epidermis of the apple fruit produces several phytochemicals and aromatic compounds which are important for seed dispersal in nature along with pathogen resistance, human health, and organoleptic experience (Bizjak et al. 2013). Several of these compounds are derived from the breakdown of the chlorophyllous thylakoid membranes which are replaced by lipoprotein structures that accumulate carotenoids and other lipophilic metabolic intermediates during ripening (Bouvier and Camara 2006; Egea et al. 2010).
In perennial crops, apple represents the model system of climacteric ripening. There is extensive information regarding the ripening physiology (Bulens et al. 2014) due to its biological, nutritional, and economical value. Except for a recent report on its predicted plastid proteome (Schaeffer et al. 2014), not much is known about the novel proteomic or metabolomic constitution of apple plastids, which carry out important biochemical processes. To further this field, a thorough characterization of apple fruit plastid development is needed. This work documents the structural modifications of plastids in epidermal and collenchyma cells during fruit development in three distinct apple genotypes. The analysis revealed a different structural progression compared to model systems highlighting the uniqueness of apple as a system. While this study describes the changes a plastid undergoes in the course of apple fruit development, it also establishes a phenotypic foundation to correlate existing metabolic information regarding plastid structures, but more importantly identifies specific developmental stages to guide subsequent transcriptomic, proteomic, and metabolomic studies.
Materials and methods
Sample collection
‘Golden Delicious’ (yellow), ‘Granny Smith’ (green), and ‘Top Red Delicious’ (red) apples were collected in the Washington State University Tukey Orchard during the 2010 growing season. Tissue was collected on 8, 22, 39, 51, 64, 78, 93, 107, 121, 134, and 148 days after anthesis (DAA) (May 17th full bloom date). These dates cover the entire development spectrum of the fruit from early stage to maturity. Photographs were recorded for three apples at each timepoint to document and track phenotypic changes throughout fruit development (Fig. 1).
Phenotypic changes in the fruit of three cultivars of Malus × domestica Borkh. ‘Golden Delicious’, ‘Granny Smith’, and ‘Top Red Delicious’. Fruit was collected throughout the 2010 growing season. Images are shown for samples collected at 8, 39, 64, 93, 121, and 148 days after anthesis (DAA) for each cultivar which represents the developmental continuum to maturity
Tissue sectioning and transmission electron microscopy of fruit peel plastids
Peel tissue from the midsection of ‘Granny Smith’, ‘Top Red’, and ‘Golden Delicious’ apples lacking surface damage or sun burn was fixed in 2% glutaraldehyde and 2% paraformaldehyde in 50 mM PIPES buffer followed by a 1% osmium tetroxide overnight post-fixation. Dehydration of samples was performed using a gradient of 30–100% ethanol. Samples were infiltrated with propylene oxide and embedded in Spurr’s resin. Thick section of 1 μm was made with a Reichert-Jung Ultracut E Ultramicrotome and stained with 1% toluidine blue in 1% sodium borate on a slide warmer for 1.5 min. Samples were rinsed with water, left to dry in the air, and imaged on an Olympus BH-2 light microscope. Thin sections of 80–100 nm were made using a Reichert-Jung Ultracut E Ultramicrotome. Sections were stained with 2 ml of 4% uranyl acetate (aq) and 5 μl of 1% potassium permanganate for 6–8 min followed by Reynold’s lead citrate staining for 6–8 min. Images were taken on an FEI TEM T20 Transmission Electron Microscope for samples collected on timepoints 1–8 and 11 and on a Philips CM-200 for samples collected on timepoints 9 and 10. Representative images are summarized in Figs. 3 and 5. A collage of three additional TEM images for each stage is available as online resource 1.
The internal structures were annotated by visually comparing the sub-organeller structure with previously published studies. Phytoferritins were annotated based on plastid ultrastructure studies performed in kiwi fruit and S. luteum (Montefiori et al. 2009; Simpson et al. 1975). Studies performed on apple fruit plastids were used as a reference to annotate starch and plastoglobuli (Clijsters 1969; Kovacs and Eads 1999; Merzlyak and Chivkunova 2000). Osmiophilic bodies were identified based on prior studies in Theobroma and S. luteum (Martini et al. 2008; Simpson et al. 1975).
Statistics
Plastid size and dimensions of the internal structures were quantified by analyzing transmission electron micrographs using image analysis program ImageJ (Abramoff et al. 2004). To perform the statistical analysis of stereological measurements, first, it was determined how many plastids would represent a given cell type and developmental stage. This was accomplished by imaging a large number (20–108) of plastids from 4 out of the 11 sampling timepoints representing epidermal and collenchymal cells across the three genotypes. Plastid size and dimensions of the internal structures were quantified and means, standard deviation, and population variance were generated for the six random sets of plastid images for different sample sizes. An n of 6 was found to be optimal in even a completely random distribution. Furthermore, when plastid images with nearly equivalent dimensions representing the approximate median of the three-dimensional structure were selected for stereology, none of the sample means fell outside the standard deviation of the true mean, and the total population mean fell within one standard deviation of the selected sample set mean (please refer to online resource 2). Therefore, for each developmental stage and sampling time, six arbitrarily selected plastids were used for quantifying total plastid area, length, width, and internal structures. All the measurements were also represented as a ratio of the total plastid area to account for plastid size differences across different sampling times and different tissues. Data were represented as mean ± SE. All the measurements and analysis are summarized in online resource 3.
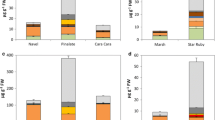
A semi-quantitative and comparative representation of the approximate fold-change in the area of internal structures during fruit development is summarized in Fig. 9. The ratios of average plastid area, average starch area per plastid, and average plastoglobule area per plastid were calculated across subsequent sampling timepoints. Stages showing greater than twofold increase were selected for representation. The ratio for a given feature was normalized to the largest value across the three genotypes and depicted, as shown in Fig. 9.
Results
Changes in fruit peel cell morphology
‘Golden Delicious’, ‘Granny Smith’, and ‘Top Red Delicious’ apples were chosen for this study due to the differences in pigmentation with the latter variety being the only one that predominantly accumulates anthocyanins in the cellular vacuoles, while the other two accumulate carotenoid and xanthophylls and chlorophyll in their chromoplasts. Three apples per genotype were sampled at each developmental timepoint to study the intracellular changes during fruit development (Fig. 1). Sections were taken from apple fruit peel consisting of epidermal and collenchyma cell layers. The development of apple fruit peel cells has been previously investigated in ‘Cox’s Orange Pippin’ (Jackson 2003; Skene 1962). In this cultivar, fruit peel development was typified by the accumulation of cuticular wax, cellular expansion in both epidermal and collenchyma tissues, and cell layer transition from easily distinguishable layers of cells to indistinguishable cell layers. Apple fruit cell development from ‘Golden Delicious’, ‘Granny Smith’, and ‘Top Red Delicious’ largely conformed to a similar progression of cellular development (Fig. 2). The epidermal layers were largely comprised of uniformly sized cells all of which were elongated in young fruit. As the fruit matured these cells lost uniformity in size and also flattened out. During earlier developmental stages, epidermal cell layers were easily distinguishable from collenchyma cells; however, during later stages of fruit development, these layers were less uniformly distributed.
Changes in the epidermal and outer collenchyma cellular structures of three cultivars of Malus × domestica Borkh. Thick sections were made from tissue derived from fruit of ‘Golden Delicious’, ‘Granny Smith’, and ‘Top Red Delicious’ at 8, 39, 64, 93, 121, and 148 days after anthesis (DAA). The outermost layer with elongated cells is the epidermal layer and the rounded cells visible 2–3 layers deep represent the collenchymal layer. Images were recorded at ×400 magnification
Ultrastructural changes in plastids of Malus × domestica Borkh. fruit during development
Fruit peel plastids were imaged using transmission electron microscopy (TEM) in epidermal cells and the outermost collenchyma cell layers. Plastids in the youngest apple epidermal cell layers resembled proplastids with few internal structures aside from minimal amounts of plastoglobuli (Pg), osmiophilic stacked membranes (SM), and dark-staining globulous structures (Fig. 3a, d, g). Some plastids in intermediate stages of developing apples possessed dark-staining stroma revealing vesiculate lamellae (VL—Fig. 3e). Interestingly, dark-staining aggregations, likely phytoferritin (Pf), were visible only in the intermediate and mature plastids of ‘Golden Delicious’ along with large osmiophilic bodies (Fig. 3b, c). A large number of mitochondria appear to surround epidermal plastids in later developmental timepoints, but are not present in high abundance around plastids in early stages of development (Fig. 3c, f, g). Mature fruit epidermal plastids appear to possess an enhanced number of plastoglobuli (Pg) and dark-staining membranous structures in all genotypes (Fig. 3c, i). Plastoglobuli and dark-staining membranous structures appear to coalesce, contributing to the formation of the large osmiophilic body often found to be centrally located in a plastid (Fig. 4).
Ultrastructural changes in the epidermal cell plastids of ‘Golden Delicious’, ‘Granny Smith’, and ‘Top Red Delicious’ apple fruit during development. Transmission electron microscopy of plastids of the three investigated cultivars was performed throughout fruit development. Micrographs display plastid ultrastructure from early (8 DAA—a, d, g), intermediate (64 DAA—b, e, h) and mature (148 DAA—c, f, i) developmental stages. Structures including plastoglobuli (Pg), osmiophilic bodies (OB), phytoferritin (Pf), starch granules (SG), osmiophilic stacked membranes (SM), vesiculate lamellae (VL), and neighboring mitochondria (Mt) are labeled. Phytoferritins are visible in b and c during the intermediate and mature stage in ‘Golden Delicious’ cultivar only
Representative plastid from epidermal tissue of ‘Golden Delicious’ apple fruit at 78 DAA highlighting the area of membrane coalescence (AOC). Early plastids contain small globulous structures and osmiophilic stacking membranes, while later plastids largely contain a single large osmiophilic body (OB). It appears that these two structures are related and perhaps the membranes coalesce and contribute to the formation of the large osmiophilic body. Other structures visible in the micrograph include plastoglobuli (Pg) and osmiophilic stacked membranes (SM)
Plastids in the collenchyma cells followed a different and novel developmental program compared to the proplastid to chromoplast transition observed in the plastids of the epidermal layer (Fig. 5). Plastids in the collenchyma layer of immature fruit possessed well-formed thylakoid membranes and granal stacks, typical of mature chloroplasts (Fig. 5a, d). The plastids at eight DAA in ‘Top Red Delicious’ collenchyma layer were similar to the ones in the epidermis (Figs. 3g, 5g). As the fruit matured, the plastids transitioned to a chloro-amyloplast which retained the photosynthetic membranes, but also accumulated starch granules. The plastids in ‘Top Red Delicious’, an anthocyanin accumulating cultivar, exhibited accumulation of additional structures such as vesiculate lamellae (VL) and osmiophilic stacked membranes (SM) (Fig. 5h). Mature fruit largely retained starch, thylakoid membranes, and accumulated higher levels of plastoglobuli suggesting that these plastids may, in fact, be properly classified as chloro-chromo-amyloplasts. Thylakoid membranes in the 148 DAA samples differed between cultivars with respect to the thylakoid membrane and grana properties. ‘Granny Smith’, which remains green at maturity, retained the traditional thylakoid membrane structures which encompassed starch granules and did not assemble into grana stacks (Fig. 5f). In ‘Golden Delicious’, a carotenoid and xanthophyll predominant fruit, plastids were typified by stacked interconnecting dark-staining membranes throughout the plastid. Finally, in ‘Top Red Delicious’, collenchyma plastids at 148 DAA retained structures similar to early stage epidermal plastids with osmiophilic stacked membranes (SM) (Fig. 5i).
Ultrastructural changes in plastids of the outer collenchyma cell layer of ‘Golden Delicious’, ‘Granny Smith’, and ‘Top Red Delicious’ fruit during development. Transmission electron microscopy of plastids of the three investigated cultivars was performed throughout fruit development. Representative micrographs display early (8 DAA—a, d, g), intermediate (64 DAA—b, e, h) and mature (148 DAA—c, f, i) plastid morphologies. ‘Golden Delicious’ distinctively demonstrates the presence of osmiophilic stacked membranes (SM), while ‘Granny Smith’ retains thylakoid membranes (Th) compared to the other two cultivars. Other structures visible in the micrograph include plastoglobuli (Pg), starch granules (SG), vesiculate lamellae (VL), and neighboring mitochondria (Mt)
Changes in plastid area and shape
The ultrastructural transitions in plastids were quantified by analyzing whole plastid dimensions and those of the internal structures with ImageJ (Abramoff et al. 2004). Plastid sizes were similar in both epidermal and collenchyma cells averaging 2 μm2 in apples at 8 DAA and increasing to around 4 μm2 in mature apple fruit. Epidermal plastid area increased throughout the development of the three apple genotypes; however, ‘Golden Delicious’ exhibited a significant enlargement between 107 and 121 DAA (Fig. 6a). Noticeably, collenchyma plastids from ‘Granny Smith’ increased to an average area of 12 μm2 at 121 DAA but decreased in average area comparable to the other two cultivars at 148 DAA (Fig. 6b). As in collenchyma cell plastids, the overall plastid size in epidermal cell layers increased in size throughout apple fruit growth in each of the investigated genotypes.
Changes in area of plastids in epidermal (a) and collenchymal (b) cells of ‘Golden Delicious’, ‘Granny Smith’, and ‘Top Red Delicious’ fruit during development. Plastid area was measured at each timepoint for six plastids in each cultivar using ImageJ. Average plastid area with standard error was plotted against sampling date. Standard error bars (n = 6) are provided for each timepoint
Changes in plastoglobule abundance and area
While an increase in the number of plastoglobuli for the late stage plastids of ‘Top Red Delicious’ collenchyma was not observed (Fig. 7a, b), the average plastoglobule area increased in both the epidermal and collenchyma plastids as the fruit developed (Fig. 7c, d). The increase in area occured in two phases in epidermal plastids. The first of these was observed from 8 DAA through 78 DAA and the second, more significant increase, occured between 107 DAA and 121 DAA in ‘Golden Delicious’ and ‘Granny Smith’ (Fig. 7c). ‘Top Red Delicious’ exhibited the largest increase in plastoglobule area at 134 DAA stage increasing 2.5 fold over ‘Golden Delicious’ (Fig. 7c). Collenchyma plastoglobuli demonstrated a later and a single phase of enlargement beginning at 107 DAA in ‘Granny Smith’ and ‘Top Red Delicious’ and at 134 DAA in ‘Golden Delicious’ (Fig. 7d). The increase in plastoglobule average area was accompanied by an increase in the number of plastoglobuli per plastid. Proliferation of plastoglobuli peaked at 78 DAA in epidermal plastids and at 148 DAA for ‘Golden Delicious’ and ‘Granny Smith’ in collenchyma plastids. Average plastoglobule area per plastid was also calculated which seemed to peak first around 78 DAA and then after 121 DAA in all the cultivars in the epidermal plastids (Fig. 7e). Average plastoglobule area in the collenchyma plastid increased only after 120 DAA (Fig. 7f).
Measurements of average number of plastoglobuli per plastid, average area, average area per plastid and plastoglobule area as a percent of total plastid area in epidermal (a, c, e, g) and collenchyma (b, d, f, h) cells. Plastoglobuli measurements were made using ImageJ in six plastids representing each sample. Average plastoglobule size, area, and percent area of total plastid area were plotted against stage of growth. Standard error bars (n = 6) are provided for each timepoint
The percent of plastid area occupied by the plastoglobuli was measured by dividing plastoglobule area by total plastid area to account for differences in the plastid area. Plastoglobuli comprised a minimal amount (~10%) of the plastid area in immature epidermal cell plastids and increased to about 60% of the total plastid area at 78 DAA (Fig. 7g). Collenchyma plastids had less than 5% of their total area comprised of plastoglobuli at 8 DAA which peaked to ~20% in ‘Golden Delicious’ and ~35% in ‘Granny Smith’ at 148 DAA (Fig. 7h).
Changes in starch granule abundance and area
Starch granule area was lower in the epidermal cell plastids compared to the collenchyma cell plastids (Fig. 8a, b). Starch abundance appeared to be nominal from 64 to 93 DAA in both ‘Golden Delicious’ and ‘Top Red Delicious’ epidermal plastids, while small amounts of starch were present in ‘Granny Smith’ (Fig. 8c). Starch accumulation in epidermal cells commenced at 107 DAA, later than those in collenchyma cells. The total percentage of starch accumulation reached about 15–25% of the total plastid area in the epidermal cells during later stages (Fig. 8e).
Measurements of starch granule average area, total area, and as a percent of total plastid area in epidermal (a, c, e) and collenchyma (b, d, f) cells. Starch granules were measured using ImageJ in six plastids representing each sample. Average starch granule size, area, and percent area of total plastid area were plotted against stage of growth. Standard error bars (n = 6) are provided for each timepoint
The outer collenchyma cell plastids accumulated a much higher percentage of starch as evident by the overall area of starch granules and area of starch granules per plastid (Fig. 8b, d). In the plastids of fruit at 8 DAA, starch comprised less than 10% of the total plastid area. However, it increased to 25–60% at 107 DAA (Fig. 8f).
When the increases in the average areas of plastids, average starch area/plastid and average plastoglobule area/plastid for all three genotypes and two cell types were compared in the context of the fruit developmental timeline, shared and interesting patterns emerged (Fig. 9). Average starch area per plastid increased mostly as the fruit entered the ripening stage at 93 DAA. While ‘Granny Smith’ and ‘Golden Delicious’ exhibited an increase in average starch area per plastid only in their epidermal cells, the collenchymal cells in ‘Red Delicious showed a major increase with the epidermal cells only showing a marginal increase. Interestingly, the average plastid area showed a general increase in all genotypes and both plastid types at or after 93 DAA. Average plastoglobule area/plastid showed a marginal increase only in the epidermal plastids of all three genotypes prior to 78 DAA. Thereafter, a major increase was observed in the collenchymal plastids of ‘Granny Smith’, in both epidermal and collenchymal plastids of ‘Golden Delicious’, and marginal increase in the collenchymal plastids of ‘Red Delicious’. The magnitude of increase for all sub-organeller structures was more pronounced in ‘Granny Smith’ and ‘Golden Delicious’.
Discussion
Across the three genotypes of Malus × domestica Borkh. investigated in this study, fruit epidermal cell plastids transitioned from canonical proplastids, possessing minimal amounts of plastoglobuli and osmiophilic inclusion bodies, lacking internal structure, into globular chromoplasts containing an abundance of plastoglobuli and a large central polyphenolic or inclusion body. There was a marked increase in average area (Fig. 6) with plastids becoming more rotund, and plastoglobuli increasingly occupying a larger area as the fruit matured. In contrast, plastids within the outer collenchyma cells exhibited increased structural complexity and contained characteristic features of chloroplasts, chromoplasts, and amyloplasts all at once. In fact, a hybrid chloro-chromo-amyloplast-like plastid has been previously described in Zamia floridana root tissue exposed to light (Webb 1982). Plastid transition in collenchyma cells was exemplified by the increase in size, enlargement of starch granules, and increase in plastoglobule abundance and size while mostly maintaining the traditional “jellybean” chloroplast shape except in plastids with large starch deposits. Plastoglobuli in the chromoplasts were considered to be lipid droplets that stored carotenoids and lipids derived from thylakoid turn-over (Nacir and Brehelin 2013). Proteomic studies have shown that plastoglobuli contain structural proteins and several active enzymes involved in tocopherol biosynthesis and several enzymes of unknown function in Arabidopsis (Vidi et al. 2006; Ytterberg et al. 2006). The increase of plastoglobuli during later stages of apple fruit development is strongly indicative of novel, yet uncharacterized, biochemical processes which may include tocopherol biosynthesis.
Interestingly, the observed increase in starch abundance contrasts from the starch accumulation trend observed in whole fruit (Janssen et al. 2008). ‘Royal Gala’ apple fruits were previously described to accumulate starch from 35 to 87 DAA and exhibit a decline in starch from 87 to 132 DAA (Janssen et al. 2008). In this study, plastids in outer collenchyma cells accumulated starch at 93–134 DAA and the small accumulation of epidermal plastid-localized starch began at 107 DAA. In ‘Granny Smith’, plastids underwent a comparatively higher spike in starch abundance at 121 and 134 DAA (Fig. 8). These differences could be attributed to the differences in cultivars examined; however, it is also likely that different cell layers accumulate and catabolize starch differently during fruit development. Apples have been shown to have different rates of starch degradation between the core and outer cortex tissues as well as between cultivars (Brookfield et al. 1997). Starch granules have previously been shown to be present in peel tissue and decrease in size from harvest to 1 week after harvest (Kovacs and Eads 1999). As ripening begins within the inside of fruit and spreads outwards, perhaps, the starch metabolism of peel tissue differs from internal tissues.
While the obvious differences between ‘Golden Delicious’, ‘Granny Smith’, and ‘Top Red Delicious’ relate to differences in peel pigmentation, these genotypes differ in several other traits. The best studies of these differences are mostly related to the aroma and flavor profiles of apples. ‘Red Delicious’ and ‘Granny Smith’ differ greatly in the content and repertoire of 2-methylbutanoate esters, major contributors to apple aroma (Rowan et al. 1996). Isoleucine is the biosynthetic precursor for these compounds, and as plastids play a major role in its synthesis, it may directly contribute to production and differences in aromatic compounds. In fact, of six branched chain amino acid transferases found in tomato, two were found to be plastid-localized (Maloney et al. 2010).
One significant morphological difference that was observed between the three cultivars was the thylakoid membrane state in collenchyma plastids at 148 DAA. ‘Granny Smith’ maintained traditional thylakoid membrane structures which encircled central starch granules, ‘Golden Delicious’ contained interconnecting dark-staining membranes with grana-like stacks throughout the plastid, while ‘Top Red Delicious’ retained globulous and stacking osmiophilic bodies (Fig. 5). These differences could potentially be related to photoprotective mechanisms which differ between the genotypes and may account for differential susceptibility to sunburn. ‘Granny Smith’ retains chlorophyll which is likely still present within these plastids, potentially allowing for reactive oxygen species (ROS)-protective mechanisms associated with photosynthesis such as non-photochemical quenching (NPQ). ‘Top Red Delicious’ on the other hand maintains significant amounts of anthocyanins which, themselves, are considered to be photoprotective (Merzlyak and Chivkunova 2000). Perhaps, the ‘Golden Delicious’ dark-staining membranes, with grana-like stacks, maintain a photoprotective function. Carotenoid photostability has been shown to increase as apples ripen and it is potentially involved in providing protection to light sensitive components in apples. This role could be increasingly important in apple fruit as the photosynthetic machinery and activity subsides in developing apple fruit and solar radiation may cause generation of ROS and fruit burning.
In this study, DAA was used as an indicator of apple fruit maturity as common in other studies in apple (Echeverria et al. 2004; Janssen et al. 2008). However, fruit maturity may in fact differ between the cultivars investigated or even be different within apples on the same tree. This variation in fruit maturity has necessitated the development of fruit maturity tests such as measurements of starch pattern, soluble sugars, and fruit firmness (Reid et al. 1982). Variation in maturity could have an effect on the changes in plastid size and morphology of internal structures. Perhaps, plastid morphology could also be used as an indicator of fruit maturity in addition to these tests.
Several novel structures were identified in the developing plastids of Malus × domestica Borkh. genotypes. The presence of dark-staining aggregations observed in later stages of fruit epidermal cell plastids is likely phytoferritin (Fig. 3e, f). These complexes, derived from the aggregation of iron and proteins from disassembled thylakoid membranes, have been previously observed in developing chromoplasts of S. luteum epidermal fruit cells as well as in kiwifruit collenchyma plastids (Montefiori et al. 2009; Simpson et al. 1975). Interestingly, in this study, these bodies were observed in epidermal cells which appeared to lack thylakoids throughout their development. This suggests that phytoferritins may also be derived from catabolism of complexes and structures other than thylakoid membranes.
A second uncommon structure seen in the epidermal plastids of the fruit in later stages was the large osmiophilic circular body (Fig. 3c, e, h, i). Epidermal cells from ripe yellow S. luteum fruit were described previously to have a similar structure referred to as inclusion bodies (Simpson et al. 1975). The authors hypothesized that these bodies are rich in protein and nucleic acids. A study in loquat (Eriobotrya japonica) fruit at the breaker stage referred to similar structures within the plastids as “prolamellar-like bodies” (Fu et al. 2012). A third study identified yet another comparable structure in plastids of Theobroma cacao cotyledon mesophyll plastids and hypothesized that they were polyphenol-rich bodies (Martini et al. 2008). In fact, young apple fruits contain plastids with high levels of polyphenol oxidase (PPO) which has been shown to then accumulate in the vacuole in more mature apples (Murata et al. 1997). Interestingly, it appears that these structures may originate early in young epidermal plastids as smaller inclusion bodies and thick osmiophilic membranous stacks (Fig. 3e, h). As the plastids mature these stacks, small inclusion bodies appear to coalesce and form a single large structure in the plastid (Fig. 4). The presence of such bodies may have multiple functions serving both as a pool of antioxidant-rich compounds that could aid in prevention of ROS-induced damage and serving as a pool to be utilized in the synthesis of phenolic and volatile compounds as the fruit matures.
The comparative representation of increase in the average area of plastids and sub-organeller structures in the context of the developmental continuum of the fruit reveals metabolic transition phases (Fig. 9). One would expect the autotrophic plastids to transition from source to sink status as ripening commences. It would be very enriching to perform proteomics studies during the entire continuum but in particular at the transition stages to understand the proteomic and metabolic composition of fruit plastids as identified in this study.
Conclusions
It is an accepted fact that plastid structure and function change both developmentally and temporally in fruits and other organs. This study has demonstrated just how dynamic and unique, the structural, and by inference, functional transitions in plastids of developing apple fruits are. Significant differences were observed even between the plastids of epidermal cells compared to those of the outer layer of collenchyma and, additionally, across the three investigated cultivars. Apple plastids in the epidermal cell layer follow a proplastid to chromoplast transition, while those in the outer collenchyma transition from chloroplast to chloro-amyloplast to chloro-chromo-amyloplast. The observations from this ultrastructural study have enabled the identification of discrete developmental stages during which specific functions are most likely being performed by the plastid as indicated by accumulation of plastoglobuli, starch granules, and other sub-organeller structures. Prior information regarding the metabolically active developmental stages is expected to facilitate biologically relevant transcriptomic, proteomic, and metabolomic studies to unravel the complex biochemistry of plastids in perennial non-model systems.
Author contribution statement
SMS and AD designed the study. AD supervised the study. SMS collected and fixed samples. VL, RC, and SS performed transmission electron microscopy imaging. SMS, RC, NV, and BH analyzed and measured images and contributed to statistical analysis. SMS and AD conceived the project and prepared the manuscript with contributions from all authors.
References
Abramoff M, Magalhaes P, Ram S (2004) Image processing with ImageJ. Biophoton Int 11:36–42
Alexander L, Grierson D (2002) Ethylene biosynthesis and action in tomato: a model for climacteric fruit ripening. J Exp Bot 53:2039–2055
Barsan C, Sanchez-Bel P, Rombaldi C, Egea I, Rossignol M, Kuntz M, Zouine M, Latche A, Bouzayen M, Pech JC (2010) Characteristics of the tomato chromoplast revealed by proteomic analysis. J Exp Bot 61:2413–2431
Bartley IM, Stoker PG, Martin ADE, Hatfield SGS, Knee M (1985) Synthesis of aroma compounds by apples supplied with alcohols and methyl-esters of fatty-acids. J Sci Food Agric 36:567–574
Bizjak J, Mikulic-Petkovsek M, Stampar F, Veberic R (2013) Changes in primary metabolites and polyphenols in the peel of “Braeburn” apples (Malus domestica Borkh.) during advanced maturation. J Agric Food Chem 61:10283–10292
Blanke MM, Notton BA, Hucklesby DP (1986) Physical and kinetic-properties of photosynthetic phosphoenolpyruvate carboxylase in developing apple fruit. Phytochemistry 25:601–606
Bonora A, Pancaldi S, Gualandri R, Fasulo MP (2000) Carotenoid and ultrastructure variations in plastids of Arum italicum Miller fruit during maturation and ripening. J Exp Bot 51:873–884
Bouvier F, Camara B (2006) The role of plastids in ripening fruits. In: Wise RR, Hoober JK (eds) Advances in photosynthesis and respiration. Springer, Dordrecht, pp 419–432
Brookfield P, Murphy P, Harker R, MacRae E (1997) Starch degradation and starch pattern indices; Interpretation and relationship to maturity. Postharvest Biol Technol 11:23–30
Bulens I, Van de Poel B, Hertog M, Cristescu SM, Harren FJM, De Proft MP, Geeraerd AH, Nicolai BM (2014) Dynamic changes of the ethylene biosynthesis in ‘Jonagold’ apple. Physiol Plant 150:161–173
Camara B, Hugueney P, Bouvier F, Kuntz M, Moneger R (1995) Biochemistry and molecular biology of chromoplast development. Int Rev Cytol 163(163):175–247
Cheung AY, McNellis T, Piekos B (1993) Maintenance of chloroplast components during chromoplast differentiation in the tomato mutant green flesh. Plant Physiol 101:1223–1229
Clijsters H (1969) On the photosynthetic activity of developing apple fruits. Qualitas Plantarum et Materiae Vegetabiles 19:129–140
Cookson PJ, Kiano JW, Shipton CA, Fraser PD, Romer S, Schuch W, Bramley PM, Pyke KA (2003) Increases in cell elongation, plastid compartment size and phytoene synthase activity underlie the phenotype of the high pigment-1 mutant of tomato. Planta 217:896–903
Cornah JE, Roper JM, Singh DP, Smith AG (2002) Measurement of ferrochelatase activity using a novel assay suggests that plastids are the major site of haem biosynthesis in both photosynthetic and non-photosynthetic cells of pea (Pisum sativum L.). Biochem J 362:423–432
Echeverria G, Graell J, Lopez ML, Lara I (2004) Volatile production, quality and aroma-related enzyme activities during maturation of ‘Fuji’ apples. Postharvest Biol Technol 31:217–227
Egea I, Barsan C, Bian W, Purgatto E, Latche A, Chervin C, Bouzayen M, Pech J-C (2010) Chromoplast differentiation: current status and perspectives. Plant Cell Physiol 51:1601–1611
Fischer K, Weber A (2002) Transport of carbon in non-green plastids. Trends Plant Sci 7:345–351
Fu XM, Kong WB, Peng G, Zhou JY, Azam M, Xu CJ, Grierson D, Chen KS (2012) Plastid structure and carotenogenic gene expression in red- and white-fleshed loquat (Eriobotrya japonica) fruits. J Exp Bot 63:341–354
Herrmann KM, Weaver LM (1999) The shikimate pathway. Annu Rev Plant Physiol Plant Mol Biol 50:473–503
Huff A (1983) Nutritional control of regreening and degreening in citrus peel segments. Plant Physiol 73:243–249
Huff A (1984) Sugar regulation of plastid interconversions in epicarp of citrus-fruit. Plant Physiol 76:307–312
Jackson JE (2003) Fruit skin color, russet and cracking. Biology of apples and pears. Cambridge University Press, Cambridge, pp 317–324
Janssen BJ, Thodey K, Schaffer RJ, Alba R, Balakrishnan L, Bishop R, Bowen JH, Crowhurst RN, Gleave AP, Ledger S, McArtney S, Pichler FB, Snowden KC, Ward S (2008) Global gene expression analysis of apple fruit development from the floral bud to ripe fruit. BMC Plant Biol 8:16
Knee M (1972) Anthocyanin, carotenoid, and chlorophyll changes in the peel of Cox’s Orange Pippin apples during ripening on and off the tree. J Exp Bot 23:184–196
Knee M (1988) Carotenol esters in developing apple fruits. Phytochemistry 27:1005–1009
Kovacs E, Eads TM (1999) Morphologic changes of starch granules in the apple cv. Mutsu during ripening and storage. Scanning 21:326–333
Kreuz K, Kleinig H (1984) Synthesis of prenyllipids in cells of spinach leaf. Compartmentation of enzymes for formation of isopentenyl diphosphate. Eur J Biochem 141:531–535
Lara I, Mio RM, Fuentes T, Sayez G, Graell J, Lopez ML (2003) Biosynthesis of volatile aroma compounds in pear fruit stored under long-term controlled-atmosphere conditions. Postharvest Biol Technol 29:29–39
Laval-Martin D (1974) Maturation of the cherry tomato fruit: evidence, by freeze-etched studies, of the evolution of chloroplasts in two classes of chromoplasts (author’s transl). Protoplasma 82:33–59
Liu Y, Roof S, Ye Z, Barry C, van Tuinen A, Vrebalov J, Bowler C, Giovannoni J (2004) Manipulation of light signal transduction as a means of modifying fruit nutritional quality in tomato. Proc Natl Acad Sci USA 101:9897–9902
Maloney GS, Kochevenko A, Tieman DM, Tohge T, Krieger U, Zamir D, Taylor MG, Fernie AR, Klee HJ (2010) Characterization of the branched-chain amino acid aminotransferase enzyme family in tomato. Plant Physiol 153:925–936
Martini M, Figueira A, Lenci C, Tavares D (2008) Polyphenolic cells and their interrelation with cotyledon cells in seven species of Theobroma (Sterculiaceae). Braz J Bot 31:425–431
Matile P (2000) Biochemistry of Indian summer: physiology of autumnal leaf coloration. Exp Gerontol 35:145–158
Mayfield SP, Huff A (1986) Accumulation of chlorophyll, chloroplastic proteins, and thylakoid membranes during reversion of chromoplasts to chloroplasts in citrus-sinensis epicarp. Plant Physiol 81:30–35
Merzlyak MN, Chivkunova OB (2000) Light-stress-induced pigment changes and evidence for anthocyanin photoprotection in apples. J Photochem Photobiol B 55:155–163
Merzlyak MN, Solovchenko AE (2002) Photostability of pigments in ripening apple fruit: a possible photoprotective role of carotenoids during plant senescence. Plant Sci 163:881–888
Mettal U, Boland W, Beyer P, Kleinig H (1988) Biosynthesis of monoterpene hydrocarbons by isolated chromoplasts from daffodil flowers. Eur J Biochem 170:881–888
Montefiori M, McGhie TK, Hallett IC, Costa G (2009) Changes in pigments and plastid ultrastructure during ripening of green-fleshed and yellow-fleshed kiwifruit. Sci Hortic 119:377–387
Murata M, Tsurutani M, Hagiwara S, Homma S (1997) Subcellular location of polyphenol oxidase in apples. Biosci Biotechnol Biochem 61:1495–1499
Nacir H, Brehelin C (2013) When proteomics reveals unsuspected roles: the plastoglobule example. Front Plant Sci 4:114
Phan CT (1973) Chloroplasts of the peel and the internal tissues of apple-fruits. Experientia 29:1555–1557
Phan CT (1984) All-granal chloroplasts of apple fruit. In: Sybesma C (ed) Advances in photosynthesis research research. Martinus Nijhoff/Dr. W. Junk Publishers, The Hague, pp 63–66
Rawsthorne S (2002) Carbon flux and fatty acid synthesis in plants. Prog Lipid Res 41:182–196
Reid M, Padfiels C, Watkins P, Harman J (1982) Starch iodine pattern as a maturity index for Granny Smith apples. N Z J Agric Res 25:229–273
Rowan DD, Lane HP, Allen JM, Fielder S, Hunt MB (1996) Biosynthesis of 2-methylbutyl, 2-methyl-2-butenyl and 2-methylbutanoate esters in Red Delicious and Granny Smith apples using deuterium-labeled substrates. J Agric Food Chem 44:3276–3285
Sanz C, Olias JM, Perez AG (1996) Aroma biochemistry of fruits and vegetables. In: Proceedings of the Phytochemical Society of Europe. Oxford University Press Inc., Oxford, pp 125–156
Schaeffer S, Harper A, Raja R, Jaiswal P, Dhingra A (2014) Comparative analysis of predicted plastid-targeted proteomes of sequenced higher plant genomes. PLoS One 9:e112870
Simpson DJ, Baqar M, Lee T (1975) Unusual ultrastructural features of the chloroplast-chromoplast transformation in Solanum luteum fruit. Aust J Plant Physiol 2:235–245
Sivak M (1998) Biochemistry, molecular biology and regulation of starch synthesis. Genet Eng Princ Methods 20:177–223
Skene DS (1962) Fruit skin structure in some tree fruits with special reference to russeting of apples, Ph.D. thesis. University of London, London
Vidi PA, Kanwischer M, Baginsky S, Austin JR, Csucs G, Dormann P, Kessler F, Brehelin C (2006) Tocopherol cyclase (VTE1) localization and vitamin E accumulation in chloroplast plastoglobule lipoprotein particles. J Biol Chem 281:11225–11234
Waters MT, Fray RG, Pyke KA (2004) Stromule formation is dependent upon plastid size, plastid differentiation status and the density of plastids within the cell. Plant J 39:655–667
Webb DT (1982) Structure and ultrastructure of plastids in Light-Grown and Dark-Grown Zamia-Floridana Dc—seedling roots invitro. New Phytol 91:721–725
Ytterberg AJ, Peltier JB, van Wijk KJ (2006) Protein profiling of plastoglobules in chloroplasts and chromoplasts. A surprising site for differential accumulation of metabolic enzymes. Plant Physiol 140:984–997
Acknowledgements
The authors thank Deb Pehrson for assistance with sample procurement at Washington State University Tukey Orchard and Chris Davitt for assistance with sample fixation. This work was supported in part by WSU Startup and ARC Hatch Funds to AD. NCV acknowledges the McNair Fellowship program and RC acknowledges the WSU CAHNRS undergraduate research fellowship and Auvil Fellowship for undergraduate research experience. S.M.S. and R.C. acknowledge the support received from National Institutes of Health/National Institute of General Medical Sciences through an institutional training grant award T32-GM008336. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIGMS or NIH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Prakash P. Kumar.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Schaeffer, S.M., Christian, R., Castro-Velasquez, N. et al. Comparative ultrastructure of fruit plastids in three genetically diverse genotypes of apple (Malus × domestica Borkh.) during development. Plant Cell Rep 36, 1627–1640 (2017). https://doi.org/10.1007/s00299-017-2179-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-017-2179-z