Abstract
Key message
Our results based on proteomics data and physiological alterations proposed the putative mechanism of exogenous Spd enhanced salinity tolerance in cucumber seedlings.
Abstract
Current studies showed that exogenous spermidine (Spd) could alleviate harmful effects of salinity. It is important to increase our understanding of the beneficial physiological responses of exogenous Spd treatment, and to determine the molecular responses underlying these responses. Here, we combined a physiological analysis with iTRAQ-based comparative proteomics of cucumber (Cucumis sativus L.) leaves, treated with 0.1 mM exogenous Spd, 75 mM NaCl and/or exogenous Spd. A total of 221 differentially expressed proteins were found and involved in 30 metabolic pathways, such as photosynthesis, carbohydrate metabolism, amino acid metabolism, stress response, signal transduction and antioxidant. Based on functional classification of the differentially expressed proteins and the physiological responses, we found cucumber seedlings treated with Spd under salt stress had higher photosynthesis efficiency, upregulated tetrapyrrole synthesis, stronger ROS scavenging ability and more protein biosynthesis activity than NaCl treatment, suggesting that these pathways may promote salt tolerance under high salinity. This study provided insights into how exogenous Spd protects photosynthesis and enhances salt tolerance in cucumber seedlings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Saline land that contains high levels of salt, severely limits plant growth and productivity. Salt stress is an increasingly serious problem worldwide. High salinity depresses plant growth by inducing an initial water deficit, which later leads to secondary oxidative stress and ion-specific toxicity (Zhu 2001). In leaves, water deficit from salt stress causes stomatal closure and a reduction in the photosynthetic rate (Robinson et al. 1983). Oxidative stress accelerates the generation of ROS, which disturbs the balance between formation and removal of ROS, leading to damage membrane structure and cellular apparatus (Triantaphylidès and Havaux 2009). Na+ accumulation in leaves negatively affects photosynthetic efficiency, decreases the stability of PSII and inhibits electron transport (Stepien and Johnson 2009). Furthermore, the ion imbalance aggravates hyperosmotic stress and nutrient deficiency in plants, limiting plants growth (Zhu 2001). Under salt stress, a lot of pathways are affected, including substance/energy metabolism and signal transduction. To cope with salt stress, new molecular technologies or exogenous growth regulators have been used.
Polyamines (PAs) are ubiquitous low molecular weight aliphatic nitrogen compounds that are positively charged at physiological pH and are present in animals, plants and bacteria (Groppa and Benavides 2008). In higher plants, putrescine (Put), spermidine (Spd) and spermine (Spm) are the major PAs. Many recent researchers investigated, PAs could protect negatively charged macromolecules like DNA, RNA, and some proteins (Alcázar et al. 2010). PAs participate in signal transductions. ABA can induce PAs biosynthesis genes expression. Put, one of the major PAs has a positive feedback with ABA, Put and ABA can promote each other’s biosynthesis under stress condition. PAs catabolic products include H2O2, which can associate with other signal molecule, like ABA, Ca2+ and NO. PAs are also reported to promote NO production had been reported. In addition, biosynthesis of polyamine and ethylene shared the common precursor SAM (S-Adenosyl methionine). Therefore, ABA, H2O2, NO, Ca2+, PAs, and ethylene could response to stress condition and become a complex network from cells to whole plants(Alcázar et al. 2010; Tanou et al. 2014). In many plant species, the ability to regulate PAs levels has been linked to salt stress tolerance (Kasukabe et al. 2004; Moschou et al. 2008). Transgenic plants overexpressed PAs synthesis enzymes genes, such as odc (ornithine decarboxylase) (Kumria and Rajam 2002), samdc (S-AdenosylL-methionine decarboxylase) (Wi et al. 2006) and spds (spermidine synthase) (Kasukabe et al. 2004), exhibited increased salt stress tolerance. Application of exogenous PAs also have been reported could alleviate salinity-induced damages in rice (Chattopadhayay et al. 2002; Roychoudhury et al. 2011), citrus (Tanou et al. 2014), bermudagrass (Shi et al. 2013) and cucumber.
Cucumber, an important vegetable crop, is sensitive to high salinity, and rapidly senses exogenous PAs application under salt stress (Duan et al. 2008). Recent studies in cucumber suggested that exogenous application of Spd could work at different levels to enhance plant stress tolerance, e.g., by protecting cell membrane integrity, improving ROS scavenging ability and increasing photosynthesis efficiency (Duan et al. 2008; Shu et al. 2014). Despite many studies on the putative functions of exogenous Spd in salt stress resistance, the physiological roles and molecular responses remain unclear. Therefore, in this study, we used the isobaric tags for relative and absolute quantification (iTRAQ) technology to investigate proteins change in cucumber leaves. iTRAQ is a powerful technique for identified and quantified protein, especially for hydrophobic and low-abundant proteins in environmental conditions (Wang et al. 2013). We identified specific proteins whose abundances changed under salt stress with exogenous Spd using comparative proteomic analysis. Combined with an analysis of important physiological parameters, we revealed a close link between the differentially expressed proteins and the observed physiological alterations. The molecular responses to salinity stress treated external Spd in cucumber seedling leaves was proposed.
Materials and methods
Plant materials and treatments
Seeds of cucumber (Cucumis sativus L. Jinchun No. 2) were germinated in the dark at 30°C for 20 h, sown in a quartz sand-filled plastic tray, and growth in a greenhouse at 28 ± 2°C (day)/18 ± 2°C (night), under natural light and a relative humidity of 60–70 %. After emergence of the third leaf, cucumber seedlings were transferred to 18-L containers in a hydroponic system containing full-strength Hoagland’s nutrient solution. The pH of the nutrient solution was kept between 6.3 and 6.6. The nutrient solution was renewed every 3 days and an air pump was used to maintain the dissolved oxygen at 8.0 ± 0.2 mg L−1. After 3 days of pre-culture, cucumber seedlings were received to four different nutrient solution treatments: Cont, 0 mM NaCl + 0 mM Spd; Spd, 0 mM NaCl + 0.1 mM Spd (Sigma Chemical Co); NaCl, 75 mM NaCl; and NaCl+Spd, 75 mM NaCl + 0.1 mM Spd. Total 36 plants of each treatment were measured or harvested after 1, 3, and 6 days. All plants were separated into roots, stems and leaves, and the fresh weight (FW) was recorded. For proteomic analysis, leaves from 12 plants separated into two independent biological replicates were harvested and washed with deionized water three times before immersion into liquid nitrogen after 3 days of each treatment. The samples were stored at −80°C for further analyses. Leaf determinations used the third leaf under the growing point.
Measurement of leaf gas exchange and chlorophyll fluorescence parameters
Leaf gas exchange parameters, including net photosynthetic rate (Pn), stomatal conductance (Gs) and intercellular CO2 (Ci), were measured using a portable photosynthesis system (LI-6400; Li-COR Inc., Lincoln, NE, USA), and maintained leaf temperature at 25 °C, relative humidity in the leaf chamber at 70 %, external CO2 concentration at 380 ± 10 µmol mol−1 and light intensity at 1000 µmol photons m−2 s−1. Chlorophyll fluorescence parameters were measured with a portable fluorometer (PAM-2100; Walz, Effeltrich, Germany). After dark-adaptation, the measurement of maximal photochemical efficiency of PSII (Fv/Fm), actual photochemical efficiency of PSII (ϕPSII) and non-photochemical quenching (NPQ) was measured as previously described (Zhang et al. 2009).
Chlorophyll content
After 3 and 6 days of treatment, for chlorophyll measurement, the FW of leaves from different treated plants was measured after washing with distilled water; the tissues were then extracted in 80 % v/v acetone. Chlorophyll content was determined by the method previously described (Porra et al. 1989). Six replicates were measured for each treatment.
Protein extraction, digestion and iTRAQ labeling
Total protein was extracted using the cold acetone method (Liu et al. 2014). Two biological replicates were carried out for each sample. The sample was grinded to powder with liquid nitrogen, then added 10 mL cooled acetone contained 10 % trichloroacetic acid (TCA) to 1 g sample powder at −20 °C for 1 h. The samples were then centrifuged at 15,000 g for 15 min at 4 °C, collected the deposit and added cooled acetone at −20 °C for 1 h, then repeated. Suspended the deposit in cold phenol extraction buffer and added equal volume of phenol saturated with Tris–HCl (pH 7.5), then waggled the mixture for 30 min at 4 °C. After centrifuged (5000 g, 30 min at 4 °C), transferred the upper phenol phase to a new centrifuge tube and repeated two to three times. Added five volumes of cold ammonium sulfate-saturated methanol to precipitated the proteins, and incubated at −20 °C for 3 h. After centrifuged (5000 g, 30 min at 4 °C), the protein were suspended and rinsed t with ice-cold methanol. Washed the pellet with ice-cold acetone and centrifuged at 5000 g for 30 min at 4 °C more than three times. After final centrifuged, the supernatant was decanted carefully and pellets were air-dried for use. The concentrations of the protein extracts were determined according to the Bradford method (Bradford 1976). 100 μg protein samples were digested with 50 μL of trypsin (50 ng μL−1) (Promega, Madison, WI, USA) at 37°C for 12 h. The samples were then labeled using iTRAQ 8plex kits (AB Sciex Inc., Framingham, MA, USA), according to the manufacturer’s protocol. The control samples (Cont) were labeled with iTRAQ tags 113 and 114; Spd-treated samples were labeled with iTRAQ tags 115 and 116; NaCl-treated samples were labeled with 117 and 118; and NaCl+Spd-treated samples were labeled with 119 and 121.
Strong cation exchange and LC–MS/MS analysis
After labeling, all samples were pooled and purified using a strong cation exchange chromatography (SCX) column (Agilent 1200 HPLC). The parameter of SCX column was: Poly-SEA 5 μm 300 Å2.0 × 150 mm with 215 and 280 nm UV detection. Separation was performed at 0.3 mL/min using a nonlinear binary gradient starting with buffer A (10 mM KH2PO4 in an aqueous solution of 25 % acetonitrile and acidified to a pH of 3.0 with H3PO4) and transitioning to buffer B (add 2 M KCl to butter A). The gradient was set as 0–50 % buffer B for 30 min, 50–80 % buffer B for 5 min, and 80–100 % buffer B for 15 min. The fractions were collected every 4 min, and collected total 12 fractions. Eluted fractions were dried for LC-MSMS analysis. The supernatant separated by liquid chromatography (LC) using an Eksigent nanoLC-Ultra 2D system (AB SCIEX). The LC fractions were analyzed using a Triple TOF 5600 mass spectrometer (AB SCIEX). Mass spectrometer data acquisition was performed with the Triple TOF 5600 System (AB SCIEX, USA) fitted with a Nanospray III source (AB SCIEX, USA) and a pulled quartz tip as the emitter (New Objectives, USA). Data were acquired using an ion spray voltage of 2.5 kV, curtain gas of 30 PSI, nebulizer gas of 5 PSI, and an interface heater temperature of 150°C. For information-dependent acquisition (IDA), survey scans were acquired in 250 ms and as many as 35 product ion scans were collected if they exceeded a threshold of 150 counts per second (counts/s) with a 2+ to 5+ charge-state. The total cycle time was fixed at 2.5 s. A rolling collision energy setting was applied to all precursor ions for collision-induced dissociation (CID). Dynamic exclusion was set for the K value of the peak width (18 s), and the precursor was then refreshed off the exclusion list, as previously described (Zhang et al. 2014).
Database searching and protein quantification
The iTRAQ data were processed with Protein Pilot Software v4.0 against the Cucumis sativus L. database, using the Paragon algorithm (Shilov et al. 2007). Protein identification was performed with the search option that emphasized biological modifications. The database search parameters were as follows: the instrument was TripleTOF 5600, iTRAQ quantification, cysteine modified with iodoacetamide; biological modifications were selected as ID focus, trypsin digestion. For the false discovery rate (FDR) calculation, an automatic decoy database search strategy was employed to estimate FDR using PSPEP (Proteomics System Performance Evaluation Pipeline Software, integrated in the Protein Pilot Software). The FDR was calculated as the number of false positive matches divided by the number of total matches. Then, iTRAQ was chosen for protein quantification with unique peptides during the search, and peptides with global FDR values less than 1 % were considered for further analysis. Within each iTRAQ run, the p values were generated by Protein Pilot using the peptides used to quantitate the respective protein (Zhang et al. 2014). Finally, for differential expression analysis, fold change was calculated as the average ratio of two independent biological replicates, respectively. Proteins with a fold change of 1.5 (increased abundance) or 0.67 (decreased abundance) and p value less than 0.05 were considered to be differentially expressed.
Protein functional classification analysis and hierarchical cluster analysis
The annotations of the identified proteins, combined with the result of BLAST alignments, provided the biological function of the proteins. The proteins were classified functionally according to the MapMan ontology (http://mapman.gabipd.org/). Hierarchical cluster analysis were used the HemI 1.0 software.
Statistical analysis
The statistical data are presented as the mean ± standard deviation (SD). Statistical analysis was performed by one-way ANOVA and Duncan’s multiple range tests, with a 5 % level of significance, using the SPSS software (version 12.0).
Results and discussion
Plant development
Depending on analysis of cucumber seedlings organ (shoots or roots), we found the effects of different treatments applied were different (Fig. 1). During 6 days of treatment, the FW values of shoots and roots of plants grown under exogenous Spd were very similar to those obtained under control conditions (Fig. 1). However, salt treatment (75 mM NaCl) affected the growth of cucumber seedlings’ shoots and roots after 3 days of treatment, with a reduction of 37 % in the shoots (Fig. 1a) and 36 % in the roots (Fig. 1b) compared with control plants (P < 0.01). In contrast with salt treatment, applied exogenous Spd alleviated the inhibition of cucumber seedling growth, and the FW values of the shoots and roots were significantly increased after 3 days of treatment. Therefore, we chose 3 days as the key time point for further experiments.
Shoots (a) and roots (b) fresh weight (FW) obtained from cucumber seedlings grown under Cont, Spd, Salt or Salt+Spd treatments. The Fig is representative of two independent experiments. Values represent mean ± SD (n = 6). The asterisks indicate the significance of differences between treatments and their corresponding controls (*P < 0.05)
Photosynthesis performances
The changes in gas exchange parameters were both measured under saturating light and atmospheric CO2. Pn, Gs and Ci showed no significant differences between applied exogenous Spd and control plants, while both groups of plants showed drastic reductions in these three parameters under 75 mM NaCl treatment. After 3 days of treatment, plants treated with exogenous Spd and NaCl showed a higher photosynthetic rate than the plants treated with NaCl alone; however, Pn increased without a rise in Ci, which implied that exogenous Spd may regulate non-stomatal factors to enhance photosynthetic efficiency under salt stress (Fig. 2a–c). Salt-treated leaves and non-saline-treated leaves had no significantly different of chlorophyll a and b after 3 days of treatment; however, the salt-treated leaves had lower chlorophyll a and b contents after 6 days. This probably resulted from an imbalance of chlorophyll synthesis and degradation, along with exacerbated salt damage (Fig. 2d, e). The chlorophyll a and b levels in the NaCl+Spd-treated plants were lower than in the controls, but higher than in the NaCl alone-treated plants. Chlorophyll fluorescence parameters related to PSII were then determined (Fig. 2f–h). Fv/Fm showed no significant difference in all treatments after 3 days. However, ϕPSII showed slight decreases, and NPQ was increased after 3 days of treatment with NaCl alone. After 6 days of salt treatment, chlorophyll fluorescence parameters including Fv/Fm, ϕPSII and NPQ had all decreased. By contrast, in the leaves of plants treated with exogenous Spd and NaCl, the chlorophyll fluorescence parameters were considerably higher than those under salt treatment alone, suggesting that the exogenous polyamine protected PSII. Our results are similar to previous studies in cucumber (Shu et al. 2014).
Effect of exogenous Spd on photosynthesis parameters and chlorophyll content in cucumber seedlings exposed to salinity. Gas exchange parameters of Pn (a), Gs (b), Ci (c) and chlorophyll content of Chla (d), Chlb (e) in cucumber seedlings treated with Spd and/or NaCl for 3 and 6 days were measured. Changes in chlorophyll fluorescence parameters, Fv/Fm (maximum quantum efficiency of PSII) (f), ϕPSII (actual photochemical efficiency of PSII) (g) and NPQ (non-photochemical quenching) (h) were measured. The Fig is representative of two independent experiments. Values represent mean ± SD (n = 6). Different letters indicate that they are significantly different from each other (P < 0.05)
Protein identification and functional classification
After 3 days of treatment, a total of 17,618 identified peptides and 2970 proteins were identified. Among those proteins, 221 proteins were characterized as differentially expressed proteins, with at least two significant peptide sequences and an expression ratio >1.50 or <0.67 (Liu et al. 2014; Wang et al. 2013) under exogenous Spd or NaCl compared with the control and in Spd+NaCl-treated plants compared with NaCl alone (Supporting Information Table 1 and 2). Compared with the control, exogenous Spd treatment changed the abundances of 45 proteins: 40 proteins were increased and five were decreased. During salt stress, a total 189 proteins were differently expressed compared with control, the proteins levels of 95 proteins were increased and 94 were decreased. Compared with salt-treated leaves, exogenous Spd treatment changed the expression levels of 74 proteins: 52 were increased levels and 22 were decreased in proteins levels. Among the proteins affected by application of Spd and NaCl treatment, 35 proteins were increased that had been decreased abundance by salt stress and 18 were decreased that had been increased proteins levels by NaCl alone (Fig. 3a). In addition, there were 17 proteins which were only increased protein abundance in NaCl+Spd treatment. There results suggested that different strategies might be activated in response to salt stress in cucumber seedlings treated with exogenous Spd and NaCl.
Hierarchical clustering analysis of differentially expressed proteins (a) and functional classification (b). a Hierarchical cluster analysis of the differentially expressed proteins respond to exogenous Spd applied with NaCl treatment in cucumber leaves under 3 days treated, compared with other treated plants. The ranks represent individual proteins. b Functional characterization of protein abundances changed in response to 3 days of treatment with exogenous Spd with/without salt stress in cucumber leaves. The classification is based on Mercator (MapMan) ontology (http://mapman.gabipd.org/)
To further analyze the differentially expressed proteins in leaves caused by salt and/or Spd treatment, the identified proteins were grouped into 30 functional categories using the Mercator (MapMan) ontology (Fan et al. 2011; Liu et al. 2014; Lohse et al. 2014), as shown in Fig. 3b. Among the functional groups, Spd treatment affected two major categories: photosynthesis (33 %) and protein metabolism (13 %), in which most proteins were upregulated, which indicated that exogenous Spd might have a positive effect on the photosystem and protein metabolism. Compared with the control, complicated changes occurred under high salt stress. The differentially expressed proteins were classified into all 30 functional categories, and proteins in photosynthesis, major and minor CHO metabolism, and respiratory metabolism (glycolysis, OPP, TCA and mitochondrial electron transport) were upregulated; however, proteins in other metabolisms were downregulated. In addition, compared with salt stress alone, exogenous Spd+NaCl changed the levels of proteins enriched into photosynthesis, tetrapyrrole synthesis, redox pathway, and protein metabolism. These differentially expressed proteins might be helpful to understand the mechanism by which exogenous Spd enhances salinity tolerance in cucumber seedlings.
Proteins involved in photosynthesis
Photosynthesis is sensitive to salt stress (Sudhir and Murthy 2004). In the present study we identified 27 differentially expressed proteins in the light reactions, Calvin cycle and photorespiration, in response to exogenous Spd with/without NaCl.
The light reaction is the first stage of photosynthesis whereby light energy is converted into chemical energy, including ATP and NADPH. In this study, the levels of the PSII47KD protein (CP47, gi|74027126), 44-kDa protein (CP43, gi|74027097) and reaction center D2 protein (gi|75317322) increased under salt stress (Fig. 4a). These proteins are responsible for receiving and transferring photon in PSII, and damage to them under stress could lead to photoinhibition or photodamage (Takahashi and Murata 2008). We hypothesized that the increased abundance of these three proteins was a manifestation of repair to PSII under salt stress. In the middle stage of salt stress, the levels of ϕPSII slightly decreased, but Fv/Fm showed no significant difference (Fig. 2f, g). On the contrary, application of exogenous Spd under NaCl treatment decreased proteins levels of the three proteins compared with salt treatment alone, but their levels were still higher than the control (Fig. 4b). This indicated exogenous PAs might reduce damage to PSII under salinity. Chlorophyll a–b binding proteins CP26 (gi|449510691), CP24 (gi|449497079) and CP29 (gi|449516143) are small subunits of LHCII, which bind with zeaxanthin and function in heat dissipation (Amarie et al. 2009). The levels of these proteins increased under salt treatment, indicating that NPQ was activated, as shown by the measured chlorophyll fluorescence parameter (Fig. 2h). Cytochrome f (gi|74027114), part of the cytochrome b6f complex (Kurisu et al. 2003), two PSI P700 chlorophyll a apoproteins (A1, gi|68164803 and A2, gi|75317318), and PSI reaction center subunit III (gi|449487680) all showed increased abundance under salt stress. These results implied that the levels of light reaction proteins were maintained to permit sufficient transfer of excitation energy in the middle phase of salt stress. To cope with high salinity, more energy is required, which correlated with the increased levels of the ATPase alpha subunit (gi|74027087) and gamma chain (gi|449488358), and ferredoxin-NADP reductase (gi|449456811) under NaCl treatment. Similar results were observed in wheat (Kamal et al. 2012). The protein level of ATP synthase subunit b was increased under NaCl+Spd treatment.
The Calvin cycle consumes the energy produced by the light reaction to fix CO2 into carbon skeletons, which are used for synthesis starch and sucrose (Raines 2003). Salt stress causes water deficit, leading CO2 diffusion restriction via limitation on stomatal opening (Chaves et al. 2009). Under CO2 limited conditions, ribulose-1,5-bisphospate carboxylase/oxygenase (Rubisco) tends to catalyze the oxygenation of RuBP to produce glycolate-2-P, rather than the carboxylation of RuBp to produce 3-phosphoglycerate (3-PGA). Glycolate-2-P is subsequently metabolized in the photorespiration carbon cycle to generate the 3-PGA and CO2, which can supply the Calvin cycle, thus avoiding photodamage by maintaining energy utilization via the Calvin cycle (Takahashi and Badger 2011). However, the photorespiration carbon cycle only recovers 75 % of the carbon from RuBp, to generate 3-PGA and CO2, and generates toxic intermediates such as glyoxylic acid and H2O2 (Bauwe et al. 2010), which, if not scavenged quickly, might aggravate oxidative stress. In this study, proteins belonging to CO2 carboxylation and the reduction stage of Calvin cycle were decreased abundances after 3 days of salt stress (Fig. 4a), including ribulose bisphosphate carboxylase/oxygenase activase (RA, gi|449459892), Rubisco large subunit-binding protein subunit alpha (gi|449496181) and phosphoglycerate kinase (PGK, gi|449475841). Proteins belonging to photorespiration were upregulated, including glycerate dehydrogenase (HPR, hydroxypyruvate reductase, gi|525507045), peroxisomal (S)-2-hydroxy-acid oxidase (GOX, glycolate oxidase, gi|449526029), serine-glyoxylate aminotransferase (SGAT, gi|449480967), glycine dehydrogenase (GDC, gi|449450349), phosphoglycolate phosphatase (PGP, gi|449485338) and ferredoxin-dependent glutamate synthase (FxGS, gi|449438000). Proteins involved in the Calvin cycle’s regeneration of RuBp were upregulated, including fructose-bisphosphate aldolase (ADL, gi|449464838), transketolases (TK, gi|525507124) and fructose-1,6-bisphosphatase (FBP, gi|449506460). These results suggested that salt stress inhibits CO2 carboxylation and triggers photorespiration. Research using salt-treated rice showed that increases of these enzymes in the photorespiratory pathway are indicative of oxidative stress (Abbasi and Komatsu 2004). Compared with NaCl treatment, applied exogenous Spd under salinity stress were induced RA and PGK, and were reduced the photorespiration proteins (GOX, SGAT, GDC and FxGS) (Fig. 4a). In addition, NaCl+Spd treatment increased the abundance of thioredoxin M (Trx, gi|449516397) and CBS domain-containing proteins 1 (CBSX1, gi|449525190) (Fig. 6), which are thought to regulate the Calvin cycle and CO2 assimilation via the ferredoxin-Trx system (FTS) (Cheng et al. 2014; Yoo et al. 2011). In this context, it is suggested that exogenous Spd upregulated CO2 carboxylation under salt stress by increasing the protein levels of Rubisco activase and PGK, reduced the competition for Rubisco by photorespiration, and regulated the Calvin cycle flux via FTS, thereby enabling coordinated enhancement of photosynthetic efficiency.
Proteins involved in metabolism
Metabolism represents the basic physiological processes that maintain cell living. Metabolic proteins belonging to carbohydrate metabolism, respiratory metabolism and other metabolisms were changed their abundances after different treatment in this study (Figs. 3, 5). Proteins related to carbohydrate metabolism, especially in small molecule osmolytes synthesis, were increased their abundances under 3 days salt stress. Also, proteins involved in energy production processes, including glycolysis, TCA and OPP, were upregulated. By contrast, proteins related to lipid metabolism, amino acid metabolism, tetrapyrrole synthesis, S assimilation, secondary metabolism, and cofactors and vitamin metabolism showed reduced abundance in highly salinity. These results indicated that cucumber seedlings in the middle stage of salt stress shifted their metabolic focus away from low-priority activities toward high-priority activities including essential carbohydrates and energy production; however, some other metabolisms are also important in coping with stress.
Overview of metabolic pathways of differentially expressed proteins used MapMan tool. The red–green scale represents proteins changed in the NaCl treatment contrast control. The yellow–blue scale represents proteins changed in NaCl+Spd treatment compared with treatment by NaCl alone. The boxes in yellow/blue near the boxes in red/green and arranged with the same shape mean that they represent the same proteins (color figure online)
Many metabolic processes require the assistance of cofactors. Coenzyme A (CoA) is a key co-factor of certain important biosynthetic, degradative and energy production pathways (Begley et al. 2001). Phosphopantetheine adenylyltransferase (PPAT, gi|449529337), an important biosynthetic enzyme of CoA, was increased protein level in plants treated with NaCl+Spd. Overexpression of PPAT in Arabidopsis thaliana increased resistance to salt stress and osmotic stress (Rubio et al. 2008). Salt stress reduced the protein levels of ATP sulfurylase 1 (gi|449514837), ATP sulfurylase 2 (gi|449505729) and 5′-adenylylsulfate (APS) reductase 3 (gi|449433968), which participate in the initial steps of S assimilation, and catalyze the transformation of SO4 2− to cysteine (Tsakraklides et al. 2002). Applying exogenous Spd under NaCl raised the protein expression of APS reductase 3, which could help to increase cysteine level. Cysteine play important role in ROS scavenging via form to glutathione (Youssefian et al. 2001). In addition, a chloroplastic soluble inorganic pyrophosphatase (PPasee, gi|449527824), which catalyzes the conversion of pyrophosphate (PPi) to orthophosphoric acid (Pi), was decreased protein level under salt stress. PPi is generated by multiple metabolic pathways, and an accumulated excess would lead to feedback inhibition on these reactions, so removing it quickly could be very important (Geigenberger et al. 1998). In the chloroplast, PPi could be generated during the metabolism of chlorophyll, starch, nucleic acids, fatty acids and amino acids; therefore, deficiencies in PPi removal might inhibit the activity of these pathways. Silencing the PPase gene in tobacco led to growth restriction and sensitivity to stress (George et al. 2010). The amount of the chloroplast PPase protein was reduced under salt stress, which might have exacerbated the accumulation of PPi, resulting in feedback inhibition of the above-mentioned metabolisms. However, the application of exogenous Spd under salt stress increased the abundance of PPase. This result suggested that polyamines might help to maintain the operation of chlorophyll synthesis, amino acid and fatty acid synthesis under salt stress.
Tetrapyrroles, such as chlorophyll and hemoglobin, play important roles in plant. Their synthesis starts from glutamyl-tRNA, generated from 5-Aminolevulinic acid (ALA) by glutamate-1-semialdehyde 2,1-aminomutase (GSAAT, gi|449527822), and follows several steps to generate protoporphyrin IX. Then protoporphyrin IX binds to magnesium-chelating enzyme or iron-chelating enzyme to generate chlorophyll or hemoglobins, respectively. Recent research has suggested that enzymes related to tetrapyrrole synthesis participate in the signal transduction of the plastid to the nucleus and are involved in environmental stress resistance; however, the specific regulation pathways remain unknown (Nagahatenna et al. 2015; Zhang et al. 2015). Meanwhile, the intermediate product ALA is considered a plant regulator that has been reported to play an important role in salt stress tolerance (Akram and Ashraf 2013; Akram et al. 2012). In this experiment, proteins involved in the generation from glutamyl-tRNA to chlorophyll, including GSAAT, coproporphyrin oxidase III (COO, gi|449527089), tetrapyrrole-binding protein and uroporphyrinogen decarboxylase (UPD, gi|449457423), magnesium-chelatase subunit ChlI (Mg-C, gi|449433772) and protochlorophyllide reductase (PPR, gi|525507350) (Fig. 5), showed reduced abundance under salt stress. In addition, salt stress also caused a reduction in the amount of a geranylgeranyl diphosphate reductase (GGDR, gi|449477252), which is involved in generating chlorophyll via isoprene (Laule et al. 2003). These results indicated salt stress could strongly inhibit the synthesis of chlorophyll and other tetrapyrrole. However, the application of exogenous Spd under NaCl treatment resulted in increased abundances of COO, UPD, Mg-C, PPR and GGDR (Figs. 5, 6). This finding suggested that exogenous polyamines could alleviate the inhibition of tetrapyrrole synthesis under salt stress, and might influence the ALA or tetrapyrrole signal pathway to enhance salt tolerance. To a certain extent, these results confirmed our chlorophyll content determinations (Fig. 2d, e). Collective observations of physiological and proteomic analyses showed that exogenous Spd had protective effect of various metabolic pathways under salt stress.
The putative mechanism of exogenous Spd enhanced salinity tolerance in cucumber seedlings. Most differentially expressed proteins which changed abundance in NaCl+Spd treatment compared with treatment by NaCl, were integrated and are indicated in the blue circle (upregulated under NaCl+Spd treatments contrast NaCl treatment) or yellow (down-regulated), respectively. ALA 5-Aminolevulinic acid, CAT catalases, CBSX1 CBS domain-containing proteins 1, CCS copper chaperone for Cu/ZnSOD; COO coproporphyrin oxidase III, DHAR dehydroascorbate reductase, EF-Tu elongation factor Tu, FNR ferredoxin-NADP(H) oxidoreductase, FxGS ferredoxin-dependent glutamate synthase, GDC glycine dehydrogenase, GGDR geranylgeranyl diphosphate reductase, GOX glycolate oxidase (peroxisomal (S)-2-hydroxy-acid oxidase), Mg-C magnesium-chelatase subunit ChlI, PGK phosphoglycerate kinase, PGP phosphoglycolate phosphatase, PPR protochlorophyllide reductase, Proto protoporphyrin IX, PrxR peroxiredoxin, RA ribulose bisphosphate carboxylase/oxygenase activase, SGAT serine-glyoxylate aminotransferase, Trx thioredoxin M. UCF ubiquitin combination factor E4 protein, UPD uroporphyrinogen decarboxylase (color figure online)
Proteins involved in the ROS scavenging system
Salt stress often causes the rapid production of ROS, which involves processes like electron transport chain in chloroplasts and mitochondria, photorespiration, fatty acid oxidation and amine oxidases in the apoplast (Miller et al. 2010). Under stress conditions, the excess photon intensity from reduced CO2 assimilation caused by stomata closure would be transferred toward molecular oxygen to generate O2 – at PSI (Asada 2006). In addition, a copper/zinc superoxide dismutase (Cu/ZnSOD) in the vicinity of PSI converts O2 – to H2O2, and a glutathione-ascorbate cycle in the chloroplast converts H2O2 to water, this process being referred to as the water–water cycle. In this study, a copper chaperone for Cu/ZnSOD (CCS, gi|449524344) was induced under exogenous Spd plus NaCl treatment, which is a key factor integrating copper into Cu/ZnSOD, and regulates posttranslational activation of copper/zinc superoxide (Brown et al. 2004; Chu et al. 2005). Moreover, a MnSOD increased protein level in Spd with NaCl treatment, which did not change abundance under salt stress. After 3 days of salt stress, in the water–water cycle, proteins level of monodehydroascorbate reductase (MDAR, gi|525507286), glutathione reductase (GR, gi|449517205) and L-ascorbate peroxidase 3 (gi|449507004) were increased. But glutathione S-transferase DHAR2 (gi|449513501) was decreased by salt, but increased abundance by exogenous Spd treatment. In addition, the PrxR (peroxiredoxin)/Trx pathway is a central antioxidant defense system in chloroplasts (Zhang et al. 2011). CBSX1 could directly regulate PrxR/Trx via interaction, thereby controlling H2O2 levels. Overexpression of CBSX1 decreases the level of ROS in Arabidopsis thaliana (Yoo et al. 2011).The proteins level of CBSX1 and Trx M were increased under exogenous Spd treated with NaCl, suggesting that exogenous Spd could enhance detoxification of photochemically produced H2O2 in chloroplasts by positively regulating the CBSX-Trx/PrxR pathway.
Salt stress causes reduced water availability, induces stomatal closure, decreases the CO2 to O2 ratio in leaves and increases photorespiration, which produces glycolate in chloroplasts. Oxidation of glycolate generates the majority of H2O2 in peroxisomes (Bauwe et al. 2010). Catalases (CAT) are major antioxidative enzymes in peroxisomes that detoxify the H2O2 produced by photorespiration. CAT-deficient plants showed H2O2 accumulation and cell death triggered by high light (Vandenabeele et al. 2004). In our data, the abundance of CAT (gi|449485121) protein was decreased under salt stress, but was increased under Spd+NaCl, indicating that exogenous Spd helped to remove H2O2 via increased CAT.
Vitamin B6 (pyridoxine) is an essential coenzyme for various metabolism enzymes (John 1995; Mittenhuber 2001). Recently, vitamin B6 was reported to play a role in antioxidation. Pyridoxal biosynthesis protein 1 (PDX1) is an essential vitamin B6 biosynthetic protein that participates in stress tolerance and photoprotection, and its deficiency increased salt sensitivity and photooxidative stress (Titiz et al. 2006). In this study, the PDX1 protein (gi|449523121) level decreased under salt stress, but increased under Spd with NaCl. Additionally, polyamines could worked as efficient non-enzymatic scavengers of ROS (Alcázar et al. 2010), which suggested that application of exogenous Spd may upregulate non-enzymatic ROS scavenger, such as vitamin B6 and polyamine, to cope with high salt stress. Based on our results, exogenous Spd might activating multiple antioxidant pathways, including SOD, CAT, Trx/PrxR, the water–water cycle, and non-enzymatic ROS scavenger, to increase antioxidant capacity under stress condition. Exogenous Spd enhanced antioxidant capacity and reduced accumulation of H2O2 and O2 −, alleviated oxidative damage (Supplementary Fig. 1).
Proteins involved in signal transduction
Salt stress can induce a variety of stress signal transductions, and their interactions form a network regulation leading to a variety of physiological responses. In this experiment, salt stress induced a lipoxygenase (LOX, gi|449523035) protein that participates in jasmonic acid signaling, and induced a 1-aminocyclopropane-1-carboxylate (ACC) oxidase (gi|449492729) protein associated with ethylene synthesis. Previous reports have documented that increased ethylene synthesis might cause leaf senescence and inhibit root elongation. ACC oxidase is a key enzyme in ethylene synthesis and overexpression of ACC oxidase gene in Arabidopsis thaliana increased salt sensitivity, possibly as the result of inhibition of ABA-dependent stress response way (Chen et al. 2014). In this study, exogenous Spd reduced ACC oxidase protein expression, possibly because the exogenous polyamine promoted the synthesis of polyamines, which consumed the common precursor of ethylene production, S-adenosylmethionine, thereby decreasing ACC synthesis (Slocum et al. 1984).
Proteins involved in protein synthesis, folding, transportation and degradation
Protein synthesis plays an important role in plants during abiotic stress. Many proteins belonging to protein synthesis pathways were downregulated under salt stress, such as 29 kDa (gi|449483348), 31 kDa (gi|449501439) chloroplast ribonucleoproteins, and a number of chloroplast ribosome proteins and cytoplasmic ribosome proteins. This suggested that inhibition of protein synthesis occurred under salt stress, which was similar to the results of a previous study (Tuteja 2007). The proteins levels of multiple ribonucleoproteins (28, 29, 31 kDa) and ribosomal proteins (30 S and 50 S) were increased in chloroplasts under exogenous Spd with NaCl, which might be related to polyamines being able to protect biological macromolecules by a positive charge. The chloroplastic elongation factor Tu (EF-Tu) (gi|449529128) and the mitochondrial elongation factor Tu (EF-Tu) (gi|449515049), which function in elongation of the growing peptide chains (Momcilovic and Ristic 2007; Suzuki et al. 2007), were down-regulated under salt stress, but were induced by NaCl applied with Spd. Exogenous Spd also induced three proteins that decreased abundances of proteins under salt stress, a 10 kDa chaperonin (gi|449470662), a 20 kDa chaperonin (gi|449521557) and a trigger factor-like protein TIG (gi|449463242), which function in chloroplast protein folding (Geisler and Bailly 2007; Sharkia et al. 2003). These results suggested that high level of polyamines might assist their molecular partners and play roles in protein synthesis, processing and assembly. In addition, application of exogenous Spd under salt stress induced a TIC40 protein (gi|449462371), which participated in protein transport from the cytoplasm to the chloroplast (Kalanon and McFadden 2008). Application of exogenous Spd under salt stress decreased the protein level of a ubiquitin combination factor E4 protein (UCF, gi|449494681), which is involved in the ubiquitin degradation pathway (UP) (Azevedo et al. 2001). These results suggested that exogenous Spd enhanced protein biosynthesis, folding and transport, and depressed protein degradation, thereby protecting protein metabolism under salt stress.
The putative mechanism of exogenous Spd enhanced salinity tolerance in cucumber seedlings
In the present study, based on the proteomics data and the observed physiological alterations, the possible mechanisms of exogenous Spd enhancing salinity tolerance in cucumber seedling leaves were proposed (Fig. 6). Application of exogenous Spd could alleviate the damage caused by salt stress in the following ways: (1) Spd activated Rubisco’s carboxylation ability and increased Calvin cycle flux, inhibition of photorespiration, increasing CO2 fixation by decreasing photoinhibition and the oxidative damage to photorespiration, all of which resulted in enhanced photosynthetic efficiency. (2) Spd regulated the flux of some important metabolic pathways, including chlorophyll biosynthesis and other important metabolism pathways. (3) Spd enhanced the antioxidant capacity by increasing ROS scavenging in multiple antioxidant pathways, such as SOD, CAT, Trx/PrxR, non-enzymatic scavengers and the water–water cycle. (4) Spd protected protein biosynthesis, folding and transport and inhibited protein degradation.
Conclusions
On the basis of our findings from the proteomics data and the observed physiological alterations, we found that compared with salt treatment alone, cucumber seedlings treated with exogenous Spd and NaCl had higher photosynthesis efficiency via upregulated carboxylation ability. Moreover, upregulated some important metabolisms, increased ROS scavenging ability and promoted more protein biosynthesis, might be contributed to protect metabolism homeostasis and maintain cell survival in plants treated with NaCl+Spd. Through synergistic effects of these pathways, exogenous Spd significantly increased biomass, enhanced photosynthetic performance, and maintained the growth of cucumber seedlings under salt stress.
Author contribution statement
TS carried out the experimental design, data analysis and drafted the manuscript. XS contributed to the data analysis. BL participated in the preparation of the Figs. JS and SS revised the language of the manuscript. SRG managed and designed the research and experiments.
Abbreviations
- ALA:
-
5-Aminolevulinic acid
- Ci:
-
Intercellular CO2
- FW:
-
Fresh weight
- Gs:
-
Stomatal conductance
- iTRAQ:
-
Isobaric tags for relative and absolute quantification
- NPQ:
-
Non-photochemical quenching
- PAs:
-
Polyamines
- Pn:
-
Net photosynthetic rate
- ROS:
-
Reactive oxygen species
- Spd:
-
Spermidine
- TCA:
-
Tricarboxylic acid cycle
- OPP:
-
Oxidative pentose phosphate pathway
References
Abbasi FM, Komatsu S (2004) A proteomic approach to analyze salt-responsive proteins in rice leaf sheath. Proteomics 4:2072–2081
Akram NA, Ashraf M (2013) Regulation in plant stress tolerance by a potential plant growth regulator, 5-aminolevulinic acid. J Plant Growth Regul 32:663–679
Akram NA, Ashraf M, Al-Qurainy F (2012) Aminolevulinic acid-induced changes in some key physiological attributes and activities of antioxidant enzymes in sunflower (Helianthus annuus L.) plants under saline regimes. Sci Hortic-amsterdam 142:143–148
Alcázar R, Altabella T, Marco F, Bortolotti C, Reymond M, Koncz C, Carrasco P, Tiburcio AF (2010) Polyamines: molecules with regulatory functions in plant abiotic stress tolerance. Planta 231:1237–1249
Amarie S, Wilk L, Barros T, Kühlbrandt W, Dreuw A, Wachtveitl J (2009) Properties of zeaxanthin and its radical cation bound to the minor light-harvesting complexes CP24, CP26 and CP29. BBA-Bioenergetics 1787:747–752
Asada K (2006) Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol 141:391–396
Azevedo C, Santos-Rosa MJ, Shirasu K (2001) The U-box protein family in plants. Trends Plant Sci 6:354–358
Bauwe H, Hagemann M, Fernie AR (2010) Photorespiration: players, partners and origin. Trends Plant Sci 15:330–336
Begley TP, Kinsland C, Strauss E (2001) The biosynthesis of coenzyme A in bacteria. Vitam Horm 61:157–171
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brian D, Ian B (1984) Estimation of hydrogen peroxide in plant extracts using titanium. Anal Chem 139:487–492
Brown NM, Torres AS, Doan PE, O’Halloran TV (2004) Oxygen and the copper chaperone CCS regulate posttranslational activation of Cu, Zn superoxide dismutase. Proc Natl Acad Sci 101:5518–5523
Chattopadhayay MK, Tiwari BS, Chattopadhyay G, Bose A, Sengupta DN, Ghosh B (2002) Protective role of exogenous polyamines on salinity-stressed rice (Oryza sativa) plants. Physiol Plant 116:192–199
Chaves M, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot 103:551–560
Chen D, Ma X, Li C, Zhang W, Xia G, Wang M (2014) A wheat aminocyclopropane-1-carboxylate oxidase gene, TaACO1, negatively regulates salinity stress in Arabidopsis thaliana. Plant Cell Rep 33:1815–1827
Cheng F, Zhou Y-H, Xia X-J, Shi K, Zhou J, Yu J-Q (2014) Chloroplastic thioredoxin-f and thioredoxin-m1/4 play important roles in brassinosteroids-induced changes in CO2 assimilation and cellular redox homeostasis in tomato. J Exp Bot 65:4335–4347
Chu C-C, Lee W-C, Guo W-Y, Pan S-M, Chen L-J, H-m Li, Jinn T-L (2005) A copper chaperone for superoxide dismutase that confers three types of copper/zinc superoxide dismutase activity in Arabidopsis. Plant Physiol 139:425–436
Duan J, Li J, Guo S, Kang Y (2008) Exogenous spermidine affects polyamine metabolism in salinity-stressed Cucumis sativus roots and enhances short-term salinity tolerance. J Plant Physiol 165:1620–1635
Elstner EF, Heupel A (1976) Inhibition of nitrite formation from hydroxylammoniumchloride: a simple assay for superoxide dismutase. Anal Biochem 70:616–620
Fan P, Feng J, Jiang P, Chen X, Bao H, Nie L, Jiang D, Lv S, Kuang T, Li Y (2011) Coordination of carbon fixation and nitrogen metabolism in Salicornia europaea under salinity: comparative proteomic analysis on chloroplast proteins. Proteomics 11:4346–4367
Geigenberger P, Hajirezaei M, Geiger M, Deiting U, Sonnewald U, Stitt M (1998) Overexpression of pyrophosphatase leads to increased sucrose degradation and starch synthesis, increased activities of enzymes for sucrose-starch interconversions, and increased levels of nucleotides in growing potato tubers. Planta 205:428–437
Geisler M, Bailly A (2007) Tete-a-tete: the function of FKBPs in plant development. Trends Plant Sci 12:465–473
George GM, Van Der Merwe MJ, Nunes-Nesi A, Bauer R, Fernie AR, Kossmann J, Lloyd JR (2010) Virus-induced gene silencing of plastidial soluble inorganic pyrophosphatase impairs essential leaf anabolic pathways and reduces drought stress tolerance in Nicotiana benthamiana. Plant Physiol 154:55–66
Groppa M, Benavides M (2008) Polyamines and abiotic stress: recent advances. Amino Acids 34:35–45
John RA (1995) Pyridoxal phosphate-dependent enzymes. BBA-Protein. Struct M 1248:81–96
Kalanon M, McFadden GI (2008) The chloroplast protein translocation complexes of Chlamydomonas reinhardtii: a bioinformatic comparison of Toc and Tic components in plants, green algae and red algae. Genetics 179:95–112
Kamal AHM, Cho K, Kim D-E, Uozumi N, Chung K-Y, Lee SY, Choi J-S, Cho S-W, Shin C-S, Woo SH (2012) Changes in physiology and protein abundance in salt-stressed wheat chloroplasts. Mol Biol Rep 39:9059–9074
Kasukabe Y, He L, Nada K, Misawa S, Ihara I, Tachibana S (2004) Overexpression of spermidine synthase enhances tolerance to multiple environmental stresses and up-regulates the expression of various stress-regulated genes in transgenic Arabidopsis thaliana. Plant Cell Physiol 45:712–722
Kumria R, Rajam MV (2002) Ornithine decarboxylase transgene in tobacco affects polyamines, in vitro-morphogenesis and response to salt stress. J Plant Physiol 159:983–990
Kurisu G, Zhang H, Smith JL, Cramer WA (2003) Structure of the cytochrome b6f complex of oxygenic photosynthesis: tuning the cavity. Science 302:1009–1014
Laule O, Fürholz A, Chang H-S, Zhu T, Wang X, Heifetz PB, Gruissem W, Lange M (2003) Crosstalk between cytosolic and plastidial pathways of isoprenoid biosynthesis in Arabidopsis thaliana. Proc Natl Acad Sci 100:6866–6871
Liu G-T, Ma L, Duan W, Wang B-C, Li J-H, Xu H-G, Yan X-Q, Yan B-F, Li S-H, Wang L-J (2014) Differential proteomic analysis of grapevine leaves by iTRAQ reveals responses to heat stress and subsequent recovery. BMC Plant Biol 14:110
Lohse M, Nagel A, Herter T, May P, Schroda M, Zrenner R, Tohge T, Fernie AR, Stitt M, Usadel B (2014) Mercator: a fast and simple web server for genome scale functional annotation of plant sequence data. Plant, Cell Environ 37:1250–1258
Miller G, Suzuki N, CIFTCI-YILMAZ S, Mittler R (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant, Cell Environ 33:453–467
Mittenhuber G (2001) Phylogenetic analyses and comparative genomics of vitamin B6 (pyridoxine) and pyridoxal phosphate biosynthesis pathways. J Mol Microbiol Biotechnol 3:1–20
Momcilovic I, Ristic Z (2007) Expression of chloroplast protein synthesis elongation factor, EF-Tu, in two lines of maize with contrasting tolerance to heat stress during early stages of plant development. J Plant Physiol 164:90–99
Moschou PN, Paschalidis KA, Delis ID, Andriopoulou AH, Lagiotis GD, Yakoumakis DI, Roubelakis-Angelakis KA (2008) Spermidine exodus and oxidation in the apoplast induced by abiotic stress is responsible for H2O2 signatures that direct tolerance responses in tobacco. Plnat Cell 20:1708–1724
Nagahatenna DS, Langridge P, Whitford R (2015) Tetrapyrrole-based drought stress signalling. Plant Biotechnol J 13:447–459
Porra R, Thompson W, Kriedemann P (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. BBA-Bioenergetics 975:384–394
Raines CA (2003) The Calvin cycle revisited. Photosynth Res 75:1–10
Robinson SP, Downton WJS, Millhouse JA (1983) Photosynthesis and ion content of leaves and isolated chloroplasts of salt-stressed spinach. Plant Physiol 73:238–242
Roychoudhury A, Basu S, Sengupta DN (2011) Amelioration of salinity stress by exogenously applied spermidine or spermine in three varieties of indica rice differing in their level of salt tolerance. J Plant Physiol 168:317–328
Rubio S, Whitehead L, Larson TR, Graham IA, Rodriguez PL (2008) The coenzyme a biosynthetic enzyme phosphopantetheine adenylyltransferase plays a crucial role in plant growth, salt/osmotic stress resistance, and seed lipid storage. Plant Physiol 148:546–556
Sharkia R, Bonshtien AL, Mizrahi I, Weiss C, Niv A, Lustig A, Viitanen PV, Azem A (2003) On the oligomeric state of chloroplast chaperonin 10 and chaperonin 20. BBA-Proteins Proteom 1651:76–84
Shi H, Ye T, Chan Z (2013) Comparative proteomic and physiological analyses reveal the protective effect of exogenous polyamines in the bermudagrass (Cynodon dactylon) response to salt and drought stresses. J Proteome Res 12:4951–4964
Shilov IV, Seymour SL, Patel AA, Loboda A, Tang WH, Keating SP, Hunter CL, Nuwaysir LM, Schaeffer DA (2007) The Paragon Algorithm, a next generation search engine that uses sequence temperature values and feature probabilities to identify peptides from tandem mass spectra. Mol Cell Proteomics 6:1638–1655
Shu S, Chen L, Lu W, Sun J, Guo S, Yuan Y, Li J (2014) Effects of exogenous spermidine on photosynthetic capacity and expression of Calvin cycle genes in salt-stressed cucumber seedlings. J Plant Res 127:763–773
Slocum RD, Kaur-Sawhney R, Galston AW (1984) The physiology and biochemistry of polyamines in plants. Arch Biochem Biophys 235:283–303
Stepien P, Johnson GN (2009) Contrasting responses of photosynthesis to salt stress in the glycophyte Arabidopsis and the halophyte Thellungiella: role of the plastid terminal oxidase as an alternative electron sink. Plant Physiol 149:1154–1165
Sudhir P, Murthy S (2004) Effects of salt stress on basic processes of photosynthesis. Photosynthetica 42:481–486
Suzuki H, Ueda T, Taguchi H, Takeuchi N (2007) Chaperone properties of mammalian mitochondrial translation elongation factor Tu. J Biol Chem 282:4076–4084
Takahashi S, Badger MR (2011) Photoprotection in plants: a new light on photosystem II damage. Trends Plant Sci 16:53–60
Takahashi S, Murata N (2008) How do environmental stresses accelerate photoinhibition? Trends Plant Sci 13:178–182
Tanou G, Ziogas V, Belghazi M, Christou A, Filippou P, Job D, Fotopoulos V, Molassiotis A (2014) Polyamines reprogram oxidative and nitrosative status and the proteome of citrus plants exposed to salinity stress. Plant, Cell Environ 37:864–885
Titiz O, Tambasco-Studart M, Warzych E, Apel K, Amrhein N, Laloi C, Fitzpatrick TB (2006) PDX1 is essential for vitamin B6 biosynthesis, development and stress tolerance in Arabidopsis. Plant J 48:933–946
Triantaphylidès C, Havaux M (2009) Singlet oxygen in plants: production, detoxification and signaling. Trends Plant Sci 14:219–228
Tsakraklides G, Martin M, Chalam R, Tarczynski MC, Schmidt A, Leustek T (2002) Sulfate reduction is increased in transgenic Arabidopsis thaliana expressing 5′-adenylylsulfate reductase from Pseudomonas aeruginosa. Plant J 32:879–889
Tuteja N (2007) Chapter twenty-four-mechanisms of high salinity tolerance in plants. Methods Enzymol 428:419–438
Vandenabeele S, Vanderauwera S, Vuylsteke M, Rombauts S, Langebartels C, Seidlitz HK, Zabeau M, Van Montagu M, Inzé D, Van Breusegem F (2004) Catalase deficiency drastically affects gene expression induced by high light in Arabidopsis thaliana. Plant J 39:45–58
Wang L, Liang W, Xing J, Tan F, Chen Y, Huang L, Cheng C-L, Chen W (2013) Dynamics of chloroplast proteome in salt-stressed mangrove Kandelia candel (L.) Druce. J Proteome Res 12:5124–5136
Wi SJ, Kim WT, Park KY (2006) Overexpression of carnation S-adenosylmethionine decarboxylase gene generates a broad-spectrum tolerance to abiotic stresses in transgenic tobacco plants. Plant Cell Rep 25:1111–1121
Yoo KS, Ok SH, Jeong B-C, Jung KW, Cui MH, Hyoung S, Lee M-R, Song HK, Shin JS (2011) Single cystathionine β-synthase domain–containing proteins modulate development by regulating the thioredoxin system in Arabidopsis. Plnat Cell 23:3577–3594
Youssefian S, Nakamura M, Orudgev E, Kondo N (2001) Increased cysteine biosynthesis capacity of transgenic tobacco overexpressing an O-acetylserine (thiol) lyase modifies plant responses to oxidative stress. Plant Physiol 126:1001–1011
Zhang RH, Li J, Guo SR, Tezuka T (2009) Effects of exogenous putrescine on gas-exchange characteristics and chlorophyll fluorescence of NaCl-stressed cucumber seedlings. Photosynth Res 100:155–162
Zhang H, Han B, Wang T, Chen S, Li H, Zhang Y, Dai S (2011) Mechanisms of plant salt response: insights from proteomics. J Proteome Res 11:49–67
Zhang P, Li C, Zhang P, Jin C, Pan D, Bao Y (2014) iTRAQ-based proteomics reveals novel members involved in pathogen challenge in sea cucumber Apostichopus japonicus. PLoS One 9:e100492
Zhang Z-W, Zhang G-C, Zhu F, Zhang D-W, Yuan S (2015) The roles of tetrapyrroles in plastid retrograde signaling and tolerance to environmental stresses. Planta 242:1263–1276
Zhu J-K (2001) Plant salt tolerance. Trends Plant Sci 6:66–71
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Nos. 31401919, 31471869 and 31272209), the Central Research Institutes of Basic Research Fund (6J0745), the China Earmarked Fund for Modern Agro-industry Technology Research System (CARS-25-C-03), the Jiangsu Province Scientific and Technological Achievements into Special Fund (BA2014147), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Communicated by Qiao Zhao.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
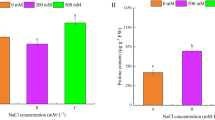
Effects of exogenous spermidine application on superoxide production rate and hydrogen peroxide content in leaves of cucumber under salt stress for 3 d. The Fig is representative of two independent experiments. Values represent mean ± SD (n = 6). Different letters indicate that they are significantly different from each other (P < 0.05). Determination method as previously described (Brian and Ian 1984; Elstner and Heupel 1976) (JPEG 923 kb)
Table S1
Differentially expressed cucumber proteins under exogenous Spd with/without NaCl treatment. (XLS 70 kb)
Table S2
Details of peptides in proteins whose response to NaCl or exogenous Spd with/without NaCl treatment. (XLS 4782 kb)
Rights and permissions
About this article
Cite this article
Sang, T., Shan, X., Li, B. et al. Comparative proteomic analysis reveals the positive effect of exogenous spermidine on photosynthesis and salinity tolerance in cucumber seedlings. Plant Cell Rep 35, 1769–1782 (2016). https://doi.org/10.1007/s00299-016-1995-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-016-1995-x