Abstract
Key message
PtHSP17.8 was regulated by various abiotic stresses. Overexpression of PtHSP17.8 enhanced the tolerance to heat and salt stresses through maintain ROS homeostasis and cooperate with stress-related genes in Arabidopsis.
Abstract
Small heat shock proteins (sHSPs) play important roles in response to diverse biotic and abiotic stresses, especially in heat tolerance. However, limited information is available on the stress tolerance roles of sHSPs in woody species. To explore the function of sHSPs in poplar, we isolated and characterized PtHSP17.8 from Populus trichocarpa. Phylogenetic analysis and subcellular localization revealed that PtHSP17.8 was a cytosolic class I sHSP. The gene expression profile of PtHSP17.8 in various tissues showed that it was significantly accumulated in stem and root, which was consistent with the GUS expression pattern driven by promoter of PtHSP17.8. The expression of PtHSP17.8 could be induced by various abiotic stresses and significantly activated by heat stress. Overexpression of PtHSP17.8 enhanced the tolerance to heat and salt stresses in Arabidopsis. The seedling survival rate, root length, relative water content, antioxidative enzyme activities, proline, and soluble sugar content were increased in transgenic Arabidopsis under heat and salt stresses, but not in normal condition. The co-expression network of PtHSP17.8 were constructed and demonstrated many stress responsive genes included. The stress-related genes in the co-expression network were up-regulated in the PtHSP17.8 overexpression seedlings. These results suggest that PtHSP17.8 confers heat and salt tolerances in plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During the evolution, plants have developed multiple levels of strategies to tolerate various stresses (Zhu 2002). When plants are exposed to stress conditions, many genes are quickly induced, one class of which is heat shock proteins (HSPs) (Ahuja et al. 2010; Murakami et al. 2004; Ruibal et al. 2013; Song et al. 2009).
As molecular chaperones, HSPs are ubiquitous in plants and play key roles in variety of cellular processes, such as binding to other proteins, activation and function of target proteins, degradation of protein molecules in all organisms (Al-Whaibi 2011). Based on the molecular weights, HSPs are classifieds into five major families: small HSP (sHSP), HSP60, HSP70, HSP90, and HSP100 (Hu et al. 2009; Zhang et al. 2015). Among them, the molecular weight of sHSP is minimum and ranges 12–42 kDa (De Jong et al. 1993). sHSPs are further grouped into eight classes according to their intracellular localization—the members of classes CI, CII, CIII, and CIV are located in cytoplasm or nucleus, whereas the members in other four classes are located in plastid, endoplasmic reticulum, mitochondria, or peroxisome (Sun et al. 2002).
The expression of sHSPs can be induced by various abiotic stresses, such as extreme temperature (Sabehat et al. 1998), salinity (Jiang et al. 2009; Sun et al. 2001), and oxidative stress (Ma et al. 2006). Previous studies have shown that overexpression of certain sHSPs enhanced the stress tolerance in transgenic plants. Constitutively overexpression of AtHSP17.8 enhanced the tolerances to drought and salt stresses in both Arabidopsis and lettuce (Kim et al. 2013). In Arabidopsis, ectopic expression of a Primula sHSP ‘PfHSP21.4’ showed high survival rate and increased tolerance to heat, salt, and drought stresses through inducing the expression of other HSPs, ascorbate peroxidase (APX), and D1-pyrroline-5-carboxylate synthase (P5CS) (Zhang et al. 2014). Overexpression of ZmHSP16.9 in transgenic tobacco conferred tolerance to heat and oxidative stresses (Sun et al. 2012). Transgenic rice with overexpressed OsHsp17.0 and OsHsp23.7 showed significantly lower malondialdehyde (MDA) content but higher free proline content than control plants under both drought and salt stresses. Overexpression of RcHSP17.8 also enhanced drought and salt tolerances of transgenic plants (Jiang et al. 2009). In addition, the function of LeHSP21, OsHsp18.7, and TaHSP26 in many other plant species were also proved to involve in the abiotic stress tolerances (Chauhan et al. 2012; Wang et al. 2015; Zhang et al. 2016). These studies indicate that sHSPs play important roles in plant adaptation under environmental stresses.
Although previous studies have demonstrated that constitutive expression of sHSPs participate in stress responses in herbaceous model plants, their roles in perennial woody species undergoing environmental stresses year to year remain unclear. Poplar (Populus spp.) is an important economic and ecologic species (Bradshaw et al. 2000). Exploring the function of sHSPs in woody model plant—poplar will be helpful to understand the stress tolerance mechanism in perennial woody species.
In poplar, total of 37 sHSPs have been identified and their expression patterns under various abiotic stresses have been preliminary analyzed in our previous study. Among these poplar sHSP genes, PtHSP17.8 was significant response to various stresses (Zhang et al. 2015). In this study, we isolated PtHSP17.8 from P. trichocarpa and further characterized its function in plant stress tolerance. qRT-PCR analysis showed that the expression of PtHSP17.8 could be induced by heat, cold, salt, drought, and oxidative stresses. Overexpression of PtHSP17.8 enhanced the tolerances to heat and salinity in Arabidopsis by cooperating with other stress-related genes. Our data defines a possible mechanism of PtHSP17.8-mediated stress tolerance in plants.
Materials and methods
Plant material and treatments
Arabidopsis thaliana (ecotype Columbia-0) and tobacco (Nicotiana benthamiana) were grown at 20–22 °C with a 16 h/8 h light/dark cycle in 1/2 MS medium or soil. P. trichocarpa and hybrid poplar (P. alba × P. glandulosa) clone 84K were grown at 23–25 °C under a 16 h/8 h light/dark cycle. For various abiotic stresses, two-month-old seedlings of P. trichocarpa were treated with 37 °C (for heat stress) or 4 °C (for cold stress), or irrigated with Hoagland solution containing 150 mM NaCl (for salinity stress), 20 % PEG (m/v, for drought stress), 10 μM methyl viologen (MV, for oxidative stress) or 100 μM ABA. During the stresses, six time points (0, 0.5, 2, 6, 12, and 24 h) under heat stress and three time points (0, 2, and 12 h) under other stresses were selected for sample collection. Leaves from each sample were immediately frozen in liquid nitrogen and stored at −80 °C until use.
RNA isolation and qRT-PCR
Total RNAs were extracted from plant samples using the RNeasy Plant Mini Kit and RNase-free DNase I set (Qiagen, Hilden, Germany). For first-strand cDNA synthesis, approximately 3 μg RNA were reverse transcribed using the SuperScript III first-strand synthesis system (Life technologies, Carlsbad, CA, USA) according to the manufacturer’s instructions.
Gene specific primers were designed using Primer3 software (http://frodo.wi.mit.edu/primer3/input.htm) with annealing temperature of 58–60 °C and amplicon 150–260 bp. qRT-PCR was performed using the SYBR Premix Ex Taq™ II Kit (TaKaRa Dalian, Dalian, China) on the Roche LightCycler 480 (Roche Applied Science, Penzberg, Upper Bavaria, Germany) according to the manufacturer’s instructions. Expression was normalized relative to the internal control (PtActin). All the experiments were repeated at least three times with similar results. The primers used in this study were listed in Table S1.
Plasmid construction, transformation, and subcellular localization
For overexpression, the full length CDS of PtHSP17.8 was constructed into the binary vector pBI121 under driven of the cauliflower mosaic virus (CaMV) 35S promoter, represented as p35S:PtHSP17.8. For promoter analysis, the promoter region of PtHSP17.8 (~2.1 kb upstream of translation initiation site) was constructed into pMDC164 containing the GUS reporter gene, represented as pPtHSP17.8::GUS. For subcellular localization, the full length CDS of PtHSP17.8 was constructed into pEarleyGate104 (ABRC stock DB3-686) to produce 35S::YFP-PtHSP17.8 construction. All the constructed vectors were subsequently transformed into Agrobacterium GV3101 by the electroporation method.
Arabidopsis transformation was carried out using the floral dip method (Clough and Bent 1998). After screened using kanamycin, more than 30 independent transgenic lines were obtained. Among the analyzed transgenic lines in our pre-experiment, at least 16 lines showed similar phenotypes. And two independent transgenic lines (L5 and L10) with high abundance of PtHSP17.8 were used for further study in here. Poplar transformation was performed according to Liu et al. (2014). After screened on selection medium with 3 mg/l hygromycin, more than 20 independent transgenic lines were obtained. At least three independently transgenic lines were used for promoter::GUS assay. All experiments were repeated at least three times with similar results. For subcellular localization analysis, the transient expression and imaging were performed according to Zhang et al. (2013).
GUS staining
Histochemical GUS staining was performed according to Liu et al. (2014).
Stress treatment of transgenic plants
For heat stress, T3 generation seeds of two independent transgenic lines (L5 and L10) and a wild type control line (WT) were germinated on 1/2 MS medium for 14 days and then treated with 45 °C for 2 or 3 h. After 4 days of recovery under normal conditions, the survival rate was calculated. For the 4-week-old seedlings, the seedlings were treated with 45 °C for 12 h and then recovered under 22 °C for 4 days.
For salt stress, T3 generation seeds were germinated on 1/2 MS medium for 3 days and then transferred into 1/2 MS medium containing 50 mM NaCl for 2 weeks. For the 4-week-old seedlings, the seedlings were irrigated with 50 ml of 300 mM NaCl for 7 days and then with pure water for 3 days (Zhang et al. 2008).
Measurement of relative water content, proline content, soluble sugar content, and enzyme assay
Relative water content (RWC) was calculated by the equation RWC (%) = [(FW − DW)/(TW − DW)] × 100 % (Smart and Bingham 1974). The proline content and soluble sugar content were measured according to Dubois et al. (1956). For enzyme assays, the activities of SOD (EC 1.5.1.1) (Beauchamp and Fridovich 1971), POD (EC 1.11.1.7) (Beers and Sizer 1952), and CAT (EC 1.11.1.6) (Kar and Mishra 1976) were analyzed as previous methods. All the experiments were performed in triplicate.
Co-expression network construction
The co-expressed biological processes database for P. trichocarpa (http://webs2.kazusa.or.jp/kagiana/cop0911/) was used to construct the co-expression network of PtHSP17.8.
Statistical analyses
Statistical analyses were carried out using SPSS 16.0 software (SPSS Inc, Chicago, IL, USA). Data was compared using Student’s t test. Differences were considered to be significant if P < 0.05.
Results
Isolation and characterization of PtHSP17.8
Based on the sequence of Potri.009G049800 from the Populus trichocarpa genome database, the full length CDS of PtHSP17.8 was amplified by PCR from the cDNA of P. trichocarpa. PtHSP17.8 has an ORF of 468 bp, encoding a protein of 155 amino acids with a predicted molecular mass of 17.8 kDa and predicted isoelectric point is 6.19. Multiple sequence alignment of the deduced PtHSP17.8 with its orthologous genes in other plant species showed that a typical α-crystalline domain (86 amino acids) located near the C-terminus, which contains two conserved heat shock domains (consensus I and II, Fig. 1a). PtHSP17.8 shared high sequence identities with StHSP17.8 from Solanum tuberosum (75.2 %) and SlHSP17.8 from Solanum lycopersicum (75.8 %), it was more similar with the members in dicots (~70 %) than in monocots (~30 %) (Fig. 1b).
Protein sequence multiple alignment of PtHSP17.8 with its orthologous genes in other plant species. a Alignment of PtHSP17.8 protein with its orthologous genes in other seven species. Their GenBank accession numbers are as follows: Arabidopsis thaliana (AtHSP17.8, Q9LNW0.1); Oryza sativa Japonica (OsHSP17.8, CAA53286.1); Solanum lycopersicum (SlHSP17.8, XP_011069474.1); Solanum tuberosum (StHSP17.8, XP_006350800.1); Nicotiana tomentosiformis (NtHSP17.8, XP_009625022.1); Triticum aestivum (TaHsp17.8, AAK51797); Zea mays (ZmHsp17.8, NP_001105954.1) and Setaria italica (SiHsp17.8, XP_004953663.1). The two consensus regions in sHSPs are underlined. b Phylogenetic analysis of PtHSP17.8 with its orthologous genes from different plants. The phylogenetic tree was constructed using the neighbor-joining method with 1000 bootstrap replicates in MEGA6.0. Numbers indicate the percentage of confidence
The sHSPs in Arabidopsis could be classified into at least eight subclasses based on their sequence similarity and subcellular localization. To reveal which subclass of PtHSP17.8 was, we performed a phylogenetic analysis using the amino acid sequences of PtHSP17.8 and all of Arabidopsis sHSPs (Fig. 2). The amino acid of PtHSP17.8 showed highly similar with AtHSP17.6BI (75.5 %) and AtHSP17.6AI (67.5 %) and shared 60–70 % sequence similarity with other cytosolic class I small HSPs in Arabidopsis.
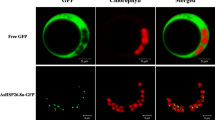
In addition, the subcellular localization prediction program CELLO suggested that PtHSP17.8 is a cytoplasmic protein. To confirm the predicted subcellular localization, PtHSP17.8 was fused to the C-terminus of YFP and then transiently expressed in tobacco leaf epidermal cells. As shown in Fig. 3, the fluorescent signal of YFP-PtHSP17.8 was detected in cytoplasm.
All the results suggested that the PtHSP17.8 was structural conserved and it belonged to cytosolic class I, which implying the PtHSP17.8 might have the similar function with the members of cytosolic class I sHSPs.
Expression pattern of PtHSP17.8 in poplar
Expression level of PtHSP17.8 across various tissues of P. trichocarpa was analyzed using qRT-PCR. PtHSP17.8 was expressed in various tissues of P. trichocarpa, including shoot apical meristem (SAM), young leaf (YL), mature leaf (ML), stem, and root. However, the highest accumulation of PtHSP17.8 transcripts was observed in stem (Fig. 4a).
qRT-PCR of PtHSP17.8 expression in poplar and GUS assays in transgenic poplar. a Expression pattern of PtHSP17.8 measured using qRT-PCR. SAM shoot apical meristem, YL young leaf, ML mature leaf. b–h GUS staining of 20-days-old pPtHSP17.8::GUS transgenic poplar. c GUS activity in the transverse sections of stem; d is the higher magnification of c; e longitudinal sections of stem, GUS is highly expressed in cambium region. GUS activity in leaf (f), adventitious root (g), and lateral root (h). Scale bars in b = 1 cm, c–e = 100 μm, f = 0.5 cm, g = 500 μm, and h = 200 μm
To further determine the expression pattern of PtHSP17.8, the promoter region of PtHSP17.8 (~2.1 kb upstream sequence of translation initiation site) was amplified and constructed into the pMDC164 vector to fuse with the reporter gene GUS, and then transformed into 84K hybrid poplar. The activity of GUS expression was detected in 20-day-old seedlings. Expression of pPtHSP17.8::GUS was closely associated with the whole plant (Fig. 4b), including stem (Fig. 4c–e), leaf (Fig. 4f), and root (Fig. 4g, h). GUS staining revealed that PtHSP17.8 was strongly expressed near the cambium region in stem (Fig. 4c–e), the column of root (Fig. 4h), the tip of adventitious root (Fig. 4g) and lateral root (Fig. 4h).
Expression of PtHSP17.8 in response to various stresses
To analyze the stress responses of PtHSP17.8, the cis-elements of the PtHSP17.8 were firstly analyzed. The 1.5 kb upstream to 0.5 kb downstream sequences of the transcription starting site of the PtHSP17.8 gene were used to search the PlantCARE database. A series of cis-acting elements involved in abiotic stress responses (five), phytohormone responses (seven), or developmental processes (eight) were identified (Fig. S1). Noticeably, three heat responsive elements (HSEs) presented in the promoter of PtHSP17.8 and located near the transcription starting site, which was the most abundant element in our analyzed sequence (Fig. S1). It indicated that PtHSP17.8 could be activated by heat shock transcription factors (HSFs) to response various stresses.
The expression patterns of genes can provide useful clues for the functions of these genes. To investigate the stress responses of PtHSP17.8, the qRT-PCR analysis of its expression under various abiotic stresses (including heat, cold, drought, salt, oxidative stress, and ABA treatments) was performed. To response heat stress, the PtHSP17.8 mRNA accumulated quickly and reached to ~3000-folds at 0.5 h, while its expression was tend to stable level in 220–230-folds at 6–12 h (Fig. 5a). After treated with 4 °C, the transcription level of PtHSP17.8 peaked at 2 h and then began to decline. While in other treatments (drought, salinity, oxidative stress, and ABA treatment), the transcription level of the PtHSP17.8 increased overall especially at 12 h. Noticeably, the expression of PtHSP17.8 was significantly induced (~22-folds) at 12 h under ABA treatment (Fig. 5b).
The expression of PtHSP17.8 in response to various stresses. a, b Expression pattern of PtHSP17.8 in P. trichocarpa seedlings treated with heat stress (37 °C), low temperature (4 °C), 20 % PEG (drought stress), 150 mM NaCl (salinity stress), 100 μM ABA, or 10 μM methyl viologen (oxidative stress). Data are mean ± SD from three independent experiments. The asterisks on the top of the columns indicate significantly differences at P < 0.05. c The GUS staining of leave of pPtHSP17.8::GUS transgenic poplar seedlings treated wit 37 °C for 6 h, CK represents without any treatment
To further confirm the expression pattern of PtHSP17.8 in heat response, the transgenic poplar seedlings containing pPtHSP17.8::GUS were treated with 37 °C for 6 h, in which time point the expression of PtHSP17.8 was induced to a relatively stable level (Fig. 5a). Consistently, the strong GUS staining was detected in the leaves of transgenic seedlings at 6 h after heat treatment (Fig. 5c).
Ectopic expression of PtHSP17.8 in Arabidopsis confers thermotolerance
In order to gain more insight into the function of PtHSP17.8 in vivo, Arabidopsis overexpression lines were generated. More than 30 independent transgenic lines were obtained, and at least 16 lines showed similar phenotypes. In this study, two independent T3 transgenic Arabidopsis lines (L5 and L10) with high abundance of PtHSP17.8 were used for further functional analysis. The expression of exogenous PtHSP17.8 was detected using RT-PCR and the result was shown in Fig. 6a.
Overexpression of PtHSP17.8 enhanced thermotolerance in Arabidopsis. a Semi-quantitative RT-PCR analysis of PtHSP17.8 expression in WT and transgenic plants. b Photograph of WT and transformants in four conditions: before treatment, no treat control, 45 °C treatment for 2 h and then recovery for 4 days, and 45 °C treatment for 3 h and then recovery for 4 days, respectively (from top to bottom). c Survival rate of 22 °C (CK) and 45 °C (heat stress, 2 or 3 h). d Growth of PtHSP17.8-overexpression transgenic plants under heat stress conditions. Photograph 4-week-old WT and transgenic plants exposed to 22 °C (control) and 45 °C (heat stress) for 12 h. e–j Physiological characterization of PtHSP17.8-transformed and WT plants. Quantification of the leaf water content (e), proline content (f), SOD (g), POD (h), CAT (i) and solution sugar content (j). Data are mean ± SD from three independent experiments. The asterisks on the top of the columns indicate significantly differences at P < 0.05
Here, the transgenic and control seedlings in two developmental stages (2- and 4-week-old) were chosen to assess their thermotolerance. For 2-week-old plants, the T3 generation seeds of transgenic Arabidopsis and wild-type control were germinated on 1/2 MS medium for 2 weeks and then treated with 45 °C for 2 or 3 h (Fig. 6b). After 4 days of recovery, the survival rate was calculated. With 2 h 45 °C treatment, the survival rates of L5 and L10 transgenic seedlings were over 1.5-folds higher than that of the WT seedlings. With 3 h 45 °C treatment, approximate 70 % of L5 and L10 seedlings survived, while only 35 % of WT control seedlings did (Fig. 6c).
For 4-week-old plants, the seedlings grown in soil were exposed to 45 °C for 12 h and then recovered in normal temperature for 4 days. Then the phenotypes and physiological characteristics of transgenic and control plants were measured (Fig. 6d–j). The relative water content in WT leaves was significantly decreased than in transgenic plants after heat stress (Fig. 6d, e). Under normal condition, there were no significant differences in proline and total soluble sugars contents between transgenic lines and the WT plants (Fig. 6f, j). Heat stress increased the levels of proline and total soluble sugars in both transgenic lines and WT plants, but the increases were greater in transgenic lines than in WT (Fig. 6f, j). Antioxidant enzymes, such as POD, SOD, and CAT, have been shown to remove reactive oxygen species (ROS) and maintain ROS balance effectively. Although there were no significant differences in the levels of SOD, POD, and CAT between transgenic lines and WT plants under normal condition (Fig. 6g–i), the increased activities of POD, SOD, and CAT were observed in PtHSP17.8 transgenic Arabidopsis compared with that in WT plants under heat stress, especially CAT. These findings suggested that overexpression of PtHSP17.8 enhanced the thermotolerance in transgenic Arabidopsis.
Ectopic expression of PtHSP17.8 in Arabidopsis confers salt stress tolerance
Under salt stress, the expression of PtHSP17.8 was obviously up-regulated (Fig. 5b). To demonstrate the function of PtHSP17.8 in defense to salt stress. The seeds of WT and transgenic lines were germinated on 1/2 MS medium germinated for 3 days and then transferred onto the 1/2 MS medium containing 50 mM NaCl for 2 weeks. As shown in Fig. 7a, the root growth was inhibited by salt stress in WT plants, while the inhibition was alleviated by overexpression of PtHSP17.8. After salt stress, the average root lengths of transgenic lines L5 and L10 were 2.01 and 2.12 cm, while only 0.9 cm for WT seedlings (Fig. 7b).
Overexpression of PtHSP17.8 enhanced salt tolerance in Arabidopsis. a Two transgenic lines and a wild-type control were germinated on 1/2 MS medium for 3 days and then transferred onto 1/2 MS containing 50 mM NaCl for 2 weeks. b Measurement of the root lengths of PtHSP17.8-overexpression and WT plants. c 4-week-old T3 plants were irrigated with 50 ml of a 300 mM NaCl for 7 days and then with pure water for recovery. Photographs were taken after recovery for 5 days. d–i Physiological characteristics of PtHSP17.8-transformed and WT plants. Quantification of the leaf water content (d), proline content (e), SOD (f), POD (g), CAT (h) and solution sugar (i). Data are mean ± SD from three independent experiments. The asterisks on the top of the columns indicate significantly differences at P < 0.05
In addition, 4-week-old seedlings were also used to test the tolerance to salt stress (Fig. 7c). After 7 days treatment with 300 mM NaCl, physiological characteristics of transgenic lines and WT were measured (Fig. 7c–i). Under salt stress, the leaf relative water content (Fig. 7d), proline content (Fig. 7e), antioxidative enzymes activities (Fig. 7f–h), and soluble sugar content (Fig. 7i) maintained in higher levels in transgenic lines than WT. These results demonstrated that overexpression of PtHSP17.8 improved the salt tolerance in Arabidopsis.
Constitutive expression of PtHSP17.8 activates the expression of stress-related genes in transgenic Arabidopsis
Based on the co-expressed biological processes database for P. trichocarpa, we constructed a co-expression network of PtHSP17.8. As shown in Fig. 8a, many stress-related genes co-expressed with PtHSP17.8, implying that these genes might be cooperated with PtHSP17.8. We then selected eight genes from the network to validate the co-expression relationships using qRT-PCR. The eight genes including JAZ1 (At1G19180), JAZ10 (At5G13220), WRKY40 (At1G80840), CYP70A1 (At4G19230), TPPJ (At5G65140), BAP2.1 (At2G45760), PIP3 (At4G35100), and wound-response gene (At4G10265). These genes were significantly up-regulated by a fold change >2.0 in transgenic lines even under normal conditions (Fig. 8b). These results indicated that PtHSP17.8 involved in abiotic stresses through cooperate with a series of stress-related genes.
Discussion
In plants, sHSPs as molecular chaperons bind to unfolded proteins, prevent aggregation, induce correct refolding, and facilitate correct cell function under stress conditions (Al-Whaibi 2011). Although there are many studies focusing on the function of sHSPs in several plant species, their roles have not been established in woody species, which frequently undergoing secondary growth, dormancy, and various abiotic and biotic stresses year by year.
In this study, we cloned the full length CDS of PtHSP17.8. Based on the phylogenetic analysis and subcellular localization, PtHSP17.8 was determined as a cytosolic class I sHSP and contained two conserved domains, which indicated it may have the similar function with the members in cytosolic class I sHSP. The results of qRT-PCR and promoter driven GUS assay showed PtHSP17.8 was ubiquitously expressed in different tissues, but strongly expressed in the region near cambium of stem and in the root tip, where are the sensitive tissues in prompt response to abiotic stresses. It is worth noting that the expression of PtHSP17.8 was significantly higher in stem than in other tissues, indicating that it may have potential roles in adjustment stem development under diverse stresses. For instance, the stem was exposed in the high temperature frequently during the summer, the high expression of PtHSP17.8 might protect the proteins in function under the heat stress and then maintain the high activity of cambium.
Previous studies have demonstrated that the induction of sHSP play important roles in plant adaptation to heat stress (Wang et al. 2012). During heat shock response, the HSFs bind to the HSE, which generally enriched in the promoters of numerous HSPs, eventually resulting in abundant expression of downstream HSPs (Pirkkala et al. 2001). Three HSEs were identified in the promoter region of PtHSP17.8 (Fig. S1), this was consistent with its rapid induction under heat stress. The finding supports the view that the expression of PtHSP17.8 like other sHSPs, which is one of the mechanisms for the rapid adaptation of plants to heat stress (Sun et al. 2002). As an endogenous phytohormone, ABA plays an important role in controlling various stress responses (Fujita et al. 2011). In here, the transcription of PtHSP17.8 was significantly increased after ABA treated for 12 h (Fig. 5b), indicating that PtHSP17.8 might be participated in the ABA-mediated pathway in stress tolerance (Zhu et al. 2014), also in poplar.
In this study, overexpression of PtHSP17.8 enhanced tolerance to heat and salt stresses in Arabidopsis (Figs. 6, 7). The similar results were also observed in transgenic plants overexpression sHSPs from other species. For instance, OsHSP18.6 from rice, PfHSP21.4 from Primula, ZmHSP16.9 from maize, and sHSP26.8 from wheat, all these sHSPs enhanced various stress tolerances in transgenic plants (Chauhan et al. 2012; Sun et al. 2012; Wang et al. 2015; Zhang et al. 2014). As important physiology indexes, relative water content, proline and soluble sugar contents, and antioxidative enzymes (SOD, POD, and CAT) activities could be as indicators to evaluate the stress tolerance in plants (Kar and Mishra 1976). Our results showed that overexpression of PtHSP17.8 increased the antioxidative enzyme activities, proline and soluble sugar contents, and leaf relative water content under both heat and salt stresses, but not in normal growth condition (Figs. 6, 7). As a molecular chaperon, PtHSP17.8 might not be involved in the biosynthesis of antioxidative enzymes, proline, or soluble sugar directly, so the enzymes activities and contents of proline and soluble sugar were not significantly changed in transgenic lines under normal condition despite the PtHSP17.8 was driven by constitutive 35S promoter. However, the over-accumulated PtHSP17.8 could protect the enzymes and proteins such as proline to prevent they were destroyed or degraded under stress conditions in transgenic Arabidopsis.
Plants produce various defense related proteins that work cooperatively to maintain the stability of the plant cell (Golldack et al. 2011). The co-expression network of PtHSP17.8 showed that several stress-related genes were co-expressed with PtHSP17.8, as well as the expression of these genes (JAZ1, JAZ10, WRKY40, PIP3, TPPJ, BAP2.1) have been up-regulated, which implying that these co-expressed genes might be work in collaboration with PtHSP17.8 under stress conditions. JAZ protein act as repressor of jasmonate signaling via their physical interactions with a series of transcription factors. Overexpression of a modified form of JAZ1/TIFY10 (JAZ1Δ3A) that is stable in the presence of JA compromises host resistance to feeding by Spodoptera exigua larvae (Chung et al. 2008). While the knockout mutant jaz1 showed decreased alkaline tolerance in Arabidopsis, and the function was also confirmed in its homologous gene GsTIFY10a (Zhu et al. 2014). Overexpression of AtJAZ10/AtJAS1 in Arabidopsis resulted in a decreased sensitivity to methyl jasmonate (MeJA) and alleviated wound-induced growth inhibition, RNAi construct of targeting AtJAZ10 showed increased MeJA sensitivity (Yan et al. 2007). MeJA and JA are referred to as jasmonates and activate plant defense mechanisms in response to various biotic or abiotic stresses. In wheat, exogenous JA effectively protect seedlings from salinity damage by activating antioxidative enzymes (Qiu et al. 2014). Which was consistent with the induced JA-related genes and enhanced activities of antioxidative enzymes in Arabidopsis by overexpression of PtHSP17.8 in this study. WRKY transcription factors act as important components in the complex signaling processes that occur during plant stress responses. To response ABA and abiotic stresses, WRKY40 could be interact with WRKY18 and WRKY60 both in physically and functionally in Arabidopsis (Chen et al. 2010). In addition, WRKY40 from pepper plays an important role in tolerance to heat stress and resistance to Ralstonia solanacearum infection (Dang et al. 2013). In addition, many other stress related genes, such as PIP3 (del Carmen and Carvajal 2014), TPPJ (Hubberten et al. 2015), and BAP2.1 (Topp 2008; Yang et al. 2007) were also co-expressed with PtHSP17.8. The function of these genes in stress tolerance indicate these genes might play the cooperator roles with PtHSP17.8 under stress conditions, however detailed work will be performed in the future to confirm the regulatory network of PtHSP17.8 in abiotic stress signaling.
In conclusion, this study isolated and characterized the PtHSP17.8 and also provides evidence that the constitutive expression of PtHSP17.8 confers tolerance to heat and salt stresses. In addition, the mechanism of PtHSP17.8 involved in the stress tolerance is a complex network which cooperated with many stress-related genes and regulation pathways. However, further studies are needed to clarify the detailed function and regulatory mechanism of PtHSP17.8 in the woody plant poplar stress response.
Author contribution statement
J.L. performed most of the experiments and drafted the manuscript. J.Z. and M.L. coordinated the project, conceived and designed the experiments and edited the manuscript. H.J., Y.L., and X.X. helped in data collection, sample preparation, and RNA extraction. L.W. and M.L. contributed with valuable discussions. All authors read and approved the final manuscript.
References
Ahuja I, de Vos RC, Bones AM, Hall RD (2010) Plant molecular stress responses face climate change. Trends Plant Sci 15:664–674
Al-Whaibi MH (2011) Plant heat-shock proteins: a mini review. J King Saud Univ Sci 23:139–150
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Beers RF, Sizer IW (1952) A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 195:133–140
Bradshaw H, Ceulemans R, Davis J, Stettler R (2000) Emerging model systems in plant biology: poplar (Populus) as a model forest tree. J Plant Growth Regul 19:306–313
Chauhan H, Khurana N, Nijhavan A, Khurana JP, Khurana P (2012) The wheat chloroplastic small heat shock protein (sHSP26) is involved in seed maturation and germination and imparts tolerance to heat stress. Plant Cell Environ 35:1912–1931
Chen H, Lai Z, Shi J, Xiao Y, Chen Z, Xu X (2010) Roles of Arabidopsis WRKY18, WRKY40 and WRKY60 transcription factors in plant responses to abscisic acid and abiotic stress. BMC Plant Biol 10:281
Chung HS, Koo AJ, Gao X, Jayanty S, Thines B, Jones AD, Howe GA (2008) Regulation and function of Arabidopsis JASMONATE ZIM-domain genes in response to wounding and herbivory. Plant Physiol 146:952–964
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Dang FF, Wang YN, Yu L, Eulgem T, Lai Y, Liu ZQ, Wang X, Qiu AL, Zhang TX, Lin J (2013) CaWRKY40, a WRKY protein of pepper, plays an important role in the regulation of tolerance to heat stress and resistance to Ralstonia solanacearum infection. Plant Cell Environ 36:757–774
De Jong W, Leunissen J, Voorter C (1993) Evolution of the alpha-crystallin/small heat-shock protein family. Mol Biol Evol 10:103–126
del Carmen Martinez-Ballesta M, Carvajal M (2014) New challenges in plant aquaporin biotechnology. Plant Sci 217:71–77
Dubois M, Gilles KA, Hamilton JK, Rebers P, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Fujita Y, Fujita M, Shinozaki K, Yamaguchi-Shinozaki K (2011) ABA-mediated transcriptional regulation in response to osmotic stress in plants. J Plant Res 124:509–525
Golldack D, Lüking I, Yang O (2011) Plant tolerance to drought and salinity: stress regulating transcription factors and their functional significance in the cellular transcriptional network. Plant Cell Rep 30:1383–1391
Hu W, Hu G, Han B (2009) Genome-wide survey and expression profiling of heat shock proteins and heat shock factors revealed overlapped and stress specific response under abiotic stresses in rice. Plant Sci 176:583–590
Hubberten H-M, Watanabe M, Bielecka M, Heyneke E, Aarabi F, Hoefgen R (2015) More than a substrate: the O-acetylserine responsive transcriptome. In: Molecular physiology and ecophysiology of sulfur. Springer, Berlin, pp 133–143
Jiang C, Xu J, Zhang H, Zhang X, Shi J, Li M, Ming F (2009) A cytosolic class I small heat shock protein, RcHSP17. 8, of Rosa chinensis confers resistance to a variety of stresses to Escherichia coli, yeast and Arabidopsis thaliana. Plant Cell Environ 32:1046–1059
Kar M, Mishra D (1976) Catalase, peroxidase, and polyphenol oxidase activities during rice leaf senescence. Plant Physiol 57:315–319
Kim DH, Xu Z-Y, Hwang I (2013) AtHSP17.8 overexpression in transgenic lettuce gives rise to dehydration and salt stress resistance phenotypes through modulation of ABA-mediated signaling. Plant Cell Rep 32:1953–1963
Liu B, Wang L, Zhang J, Li J, Zheng H, Chen J, Lu M (2014) WUSCHEL-related Homeobox genes in Populus tomentosa: diversified expression patterns and a functional similarity in adventitious root formation. BMC Genom 15:296
Ma C, Haslbeck M, Babujee L, Jahn O, Reumann S (2006) Identification and characterization of a stress-inducible and a constitutive small heat-shock protein targeted to the matrix of plant peroxisomes. Plant Physiol 141:47–60
Murakami T, Matsuba S, Funatsuki H, Kawaguchi K, Saruyama H, Tanida M, Sato Y (2004) Over-expression of a small heat shock protein, sHSP17.7, confers both heat tolerance and UV-B resistance to rice plants. Mol Breed 13:165–175
Pirkkala L, Nykänen P, Sistonen L (2001) Roles of the heat shock transcription factors in regulation of the heat shock response and beyond. FASEB J 15:1118–1131
Qiu Z, Guo J, Zhu A, Zhang L, Zhang M (2014) Exogenous jasmonic acid can enhance tolerance of wheat seedlings to salt stress. Ecotox Environ Safe 104:202–208
Ruibal C, Castro A, Carballo V, Szabados L, Vidal S (2013) Recovery from heat, salt and osmotic stress in Physcomitrella patens requires a functional small heat shock protein PpHsp16.4. BMC Plant Biol 13:174
Sabehat A, Lurie S, Weiss D (1998) Expression of small heat-shock proteins at low temperatures a possible role in protecting against chilling injuries. Plant Physiol 117:651–658
Smart RE, Bingham GE (1974) Rapid estimates of relative water content. Plant Physiol 53:258–260
Song H, Fan P, Li Y (2009) Overexpression of organellar and cytosolic AtHSP90 in Arabidopsis thaliana impairs plant tolerance to oxidative stress. Plant Mol Biol Rep 27:342–349
Sun W, Bernard C, Van De Cotte B, Van Montagu M, Verbruggen N (2001) At-HSP17.6A, encoding a small heat shock protein in Arabidopsis, can enhance osmotolerance upon overexpression. Plant J 27:407–415
Sun W, Van Montagu M, Verbruggen N (2002) Small heat shock proteins and stress tolerance in plants. BBA Gene Struct Expr 1577:1–9
Sun L, Liu Y, Kong X, Zhang D, Pan J, Zhou Y, Wang L, Li D, Yang X (2012) ZmHSP16. 9, a cytosolic class I small heat shock protein in maize (Zea mays), confers heat tolerance in transgenic tobacco. Plant Cell Rep 31:1473–1484
Topp SD (2008) Regulation of defense responses mediated by Bon1 and Bap2 in Arabidopsis thaliana. Cornell University, Ithaca
Wang F, Dong Q, Jiang H, Zhu S, Chen B, Xiang Y (2012) Genome-wide analysis of the heat shock transcription factors in Populus trichocarpa and Medicago truncatula. Mol Biol Rep 39:1877–1886
Wang A, Yu X, Mao Y, Liu Y, Liu G, Liu Y, Niu X (2015) Overexpression of a small heat-shock-protein gene enhances tolerance to abiotic stresses in rice. Plant Breed 134:384–393
Yan Y, Stolz S, Chételat A, Reymond P, Pagni M, Dubugnon L, Farmer EE (2007) A downstream mediator in the growth repression limb of the jasmonate pathway. Plant Cell 19:2470–2483
Yang H, Yang S, Li Y, Hua J (2007) The Arabidopsis BAP1 and BAP2 genes are general inhibitors of programmed cell death. Plant Physiol 145:135–146
Zhang X, Liu S, Takano T (2008) Two cysteine proteinase inhibitors from Arabidopsis thaliana, AtCYSa and AtCYSb, increasing the salt, drought, oxidation and cold tolerance. Plant Mol Biol 68:131–143
Zhang J, Li J, Liu B, Zhang L, Chen J, Lu M (2013) Genome-wide analysis of the Populus Hsp90 gene family reveals differential expression patterns, localization, and heat stress responses. BMC Genom 14(1):532
Zhang L, Zhang Q, Gao Y, Pan H, Shi S, Wang Y (2014) Overexpression of heat shock protein gene PfHSP21.4 in Arabidopsis thaliana enhances heat tolerance. Acta Physiol Plant 36:1555–1564
Zhang J, Liu B, Li J, Zhang L, Wang Y, Zheng H, Lu M, Chen J (2015) Hsf and Hsp gene families in Populus: genome-wide identification, organization and correlated expression during development and in stress responses. BMC Genom 16(1):1
Zhang J, Chen H, Wang H, Li B, Yi Y, Kong F, Liu J, Zhang H (2016) Constitutive expression of a tomato small heat shock protein gene LeHSP21 improves tolerance to high-temperature stress by enhancing antioxidation capacity in tobacco. Plant Mol Biol Rep 34:399–409
Zhu J-K (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53:247
Zhu D, Li R, Liu X, Sun M, Wu J, Zhang N, Zhu Y (2014) The positive regulatory roles of the TIFY10 proteins in plant responses to alkaline stress. PLoS One 9:e111984
Acknowledgments
This work was supported by the China Postdoctoral Science Foundation [2014 M550104] to J.Z. and the National Key Basic Research Program of China [2012CB114500] and a Collaborative Innovation Plan of Jiangsu Higher Education to M.L.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Communicated by K Chong.
J. Li and J. Zhang contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
299_2016_1973_MOESM1_ESM.tif
Fig. S1 The cis- acting elements in promoter of PtHSP17.8 gene were searched in PlantCARE database. The promoter region (1.5 kb upstream to 0.5 kb downstream of the transcription starting site) of PtHSP17.8 was analyzed in PlantCARE database (a). The description and statistics of cis-acting elements were shown in (b). (TIFF 1107 kb)
Rights and permissions
About this article
Cite this article
Li, J., Zhang, J., Jia, H. et al. The Populus trichocarpa PtHSP17.8 involved in heat and salt stress tolerances. Plant Cell Rep 35, 1587–1599 (2016). https://doi.org/10.1007/s00299-016-1973-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-016-1973-3