Abstract
Key message
Adapted pathogens are able to modulate cell responses of their hosts most likely due to the activity of secreted effector molecules thereby enabling colonisation by ostensible nonhost pathogens.
Abstract
It is postulated that host and nonhost pathogens of a given plant species differ in their repertoire of secreted effector molecules that are able to suppress plant resistance. We pursued the strategy of identifying novel effectors of Magnaporthe oryzae, the causal agent of blast disease, by comparing the infection process of closely related host vs. nonhost Magnaporthe species on barley (Hordeum vulgare L.). When both types of pathogen simultaneously attacked the same cell, the nonhost isolate became a successful pathogen possibly due to potent effectors secreted by the host isolate. Microarray studies led to a set of M. oryzae Hypothetical Effector Genes (MoHEGs) which were classified as Early- and LateMoHEGs according to the maximal transcript abundance during colonization of barley. Interestingly, orthologs of these MoHEGs from a nonhost pathogen were similarly regulated when investigated in a host situation, suggesting evolutionary conserved functions. Knockout mutants of MoHEG16 from the group of EarlyMoHEGs were less virulent on barley and microscopic studies revealed an attenuated transition from epidermal to mesophyll colonization. MoHEG13, a LateMoHEG, was shown to antagonize cell death induced by M. oryzae Necrosis-and ethylene-inducing-protein-1 (Nep1)-like proteins in Nicotiana benthamiana. MoHEG13 has a virulence function as a knockout mutant showed attenuated disease progression when inoculated on barley.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microorganisms have to overcome the plant immune system to be successful pathogens. This system is composed of two interconnected branches, the first of which comprises the perception of ubiquitous molecules conserved among entire classes of pathogens such as chitin and referred to as pathogen-associated molecular patterns (PAMPs) (Jones and Dangl 2006; Pritchard and Birch 2014). Recognition of PAMPs is based on pattern recognition receptors and triggers the so-called PAMP-triggered immunity (PTI) (Zipfel 2014). Pathogens can overcome PTI by delivery of specific molecules, termed effectors, which undermine or bypass this first layer of immunity, resulting in effector-triggered-susceptibility (ETS). Plants, in turn, are able to recognize effectors by immune receptors that may be either localized on the cell surface or in the cytosol and this represents the second branch of the plant immune system, named effector-triggered immunity (ETI). Effectors that are recognized by plant immune receptors are termed avirulence (AVR) proteins (Giraldo et al. 2013).
Pathogens not adapted to a plant species and not armed with appropriate effector molecules to overcome PTI fail to establish disease. This type of resistance is referred to as nonhost resistance and is believed to represent the most common form of plant resistance (Heath 2000; Mysore and Ryu 2004; Thordal-Christensen 2003). Recently it was postulated that ETI also contributes to nonhost resistance (Schulze-Lefert and Panstruga 2011) and that its contribution increases with shorter evolutionary divergence-time between host and nonhost plants. Variations in the effector repertoire occurring during evolution might lead to host shifts or host range expansions.
Magnaporthe oryzae (anamorph Pyricularia oryzae) causes the so-called blast disease on grass species including some of our major crop plants like rice (Oryza sativa L.), wheat (Triticum sativum L.) or barley (Fisher et al. 2012). The host range of pathogens belonging to the Pyriculariaceae is typically restricted. Isolates that infect domesticated or non-domesticated grasses such as foxtail millet (Setaria italica), fountaingrass (Pennisetum setaceum) or crabgrass (Digitaria sanguinalis) are not able to colonize barley, rice or wheat (Couch and Kohn 2002; Faivre-Rampant et al. 2008; Nga et al. 2009; Zellerhoff et al. 2006). Nevertheless, also in these nonhost types of interactions a cross talk between microbe and plant, possibly involving effectors, is predicted (Delventhal et al. 2014; Zellerhoff et al. 2010). M. oryzae has a hemibiotrophic lifestyle with an initial biotrophic phase and a subsequent switch to necrotrophy (Wilson and Talbot 2009). To infect leaf tissue, a germinated conidium of M. oryzae develops a specialized dome-shaped infection structure called appressorium which generates a high turgor to break through the host leaf cuticle and cell wall. Subsequently, a penetration hypha is formed that invaginates the plant plasma membrane (Loehrer et al. 2014; Talbot 2003). Barley plants may counterattack fungal penetration attempts by formation of a cell wall apposition, called papilla, beneath appressoria (Jarosch et al. 1999). If the fungus can overcome this first defense barrier, it establishes invasive bulbous hyphae which are surrounded by an extrahaustorial membrane compartment and believed to function in nutrient uptake (Mosquera et al. 2009). When host cells are completely filled with invasive hyphae, the fungus spreads into adjacent cells possibly via plasmodesmata (Kankanala et al. 2007). However, even at the post-penetration stage, fungal invasion can be stopped in association with a hypersensitive response of the first attacked epidermal cell (Jarosch et al. 2005). For further colonization of host tissue, thin hyphae are formed and finally necrosis is initiated.
While less is known about M. oryzae effectors with virulence function, more is known about AVR proteins such as Pia, AVR-Pik, AVR-Pita and AVR1-CO39, all of which play an important role during the M. oryzae-rice interaction (Cesari et al. 2013; Jia et al. 2000; Kanzaki et al. 2012). Currently, there are only few examples of M. oryzae virulence effectors. One is Secreted LysM Protein1 (Slp1) that acts in the apoplast by binding the PAMP chitin and thereby preventing the initiation of PTI by the rice chitin-binding protein (CEBiP) (Mentlak et al. 2012). Consequently, knockout mutants show less virulence on rice plants (Mentlak et al. 2012). In 2009, a transcriptome analysis was published that identified 262 genes differentially expressed in invasive hyphae compared to vegetative mycelium (Mosquera et al. 2009). Although secretion into host cells could be shown for some of the related proteins, no virulence function could be established testing respective knockout mutants (Khang et al. 2010; Mosquera et al. 2009).
Necrosis- and ethylene-inducing protein 1 (Nep1)-like proteins (NLPs) are highly conserved among pathogenic bacteria, fungi and oomycetes and induce a specific type of necrosis in dicotyledonous but not monocotyledonous plants (Bailey 1995). It was speculated that this is due to NLP-sensitivity factors only present in the plasma membrane of dicots (Qutob et al. 2006). Genes coding for NLPs are also present in pathogens of monocotyledonous plants but their role in virulence remains unknown (Gijzen and Nürnberger 2006). The only NLP from a monocot-infecting fungus that has been studied in detail, MgNLP of the wheat pathogen Mycosphaerella graminicola, induces necrotic cell death in leaves of the dicotyledonous plant Arabidopsis thaliana and targeted gene deletion showed that it is not essential for virulence (Motteram et al. 2009). M. oryzae NLPs have not been studied apart from MoNep1 that was shown to trigger cell death in N. benthamiana (Zhang et al. 2012).
In the present study, we selected a set of small secreted proteins from M. oryzae, referred to as Hypothetical Effector Genes (MoHEGs) that are differentially expressed during the initial interaction of an adapted and non-adapted isolate from the genus Magnaporthe with barley. We grouped genes into an early and a late class, according to the infection stage at which transcripts accumulated to highest concentrations. Homologs of these MoHEGs were identified in M. grisea and most of them show similar regulation when analyzed in a host situation. MoHEG16 from the group of early expressed genes was shown to function in mesophyll colonization, while MoHEG13 from the late group was identified to suppress NLP-induced cell death. Knockout of MoHEH13 resulted in reduced virulence of the respective mutant.
Materials and methods
Fungal isolates and inoculation
Cultivation of fungal isolates and barley as well as inoculation were performed as described previously (Delventhal et al. 2014) unless stated otherwise. The M. oryzae isolate TH6772 was kindly provided by Machida-shi (Tamagawa University, Tokyo, Japan). M. oryzae isolate US68 (70–15) and isolates derived from Pennisetum (P. penniseticola, CD180) or Digitaria (M. grisea/P. grisea, BR29) were kindly provided by Didier Tharreau [Centre de coopération internationale en recherche agronomique pour le développement (CIRAD), Montpellier, France]. M. oryzae mutant Δmoheg13 and respective wild type isolate INA72 (Saitoh et al. 2012) were both kindly provided by Hiromasa Saitoh (Iwate Biotechnology Research Center, Kitakami, Japan). Magnaporthe strains were transformed with GFP [pCAMB-HPT, (Odenbach et al. 2007)] or DsRed [pCAMDsRed, (Eckert et al. 2005)] using A. tumefaciens strain AGL1 following standard protocols (Rho et al. 2001; Tucker and Orbach 2007). AGL1 and pCAMDsRed were kindly provided by Jorrit-Jan Krijger (Martin-Luther-University Halle-Wittenberg, Germany) and pCAMB-HPT was obtained from Andrew J. Foster (Institute for Biotechnology and Drug Research, Kaiserslautern, Germany).
Magnaporthe grisea isolate BR29 was sub-cultivated on Digitaria-leaf agar [water extract of 50 g D. sanguinalis leaves per liter, 2 g L−1 Faex medicinales (brewer’s yeast extract; Biolabor, Bremen, Germany), 10 g L−1 starch (water-soluble), 15 g L−1 agar]. D. sanguinalis seeds were ordered from B & T World seeds (Aigues-Vives, France). Seeds were pre-germinated for 5 days on wet filter paper in petri dishes sealed with parafilm and placed in a growth cabinet (12 h light/12 h dark, 24 °C day/22 °C night). Seedlings were transplanted in standard soil (type ED73, Balster Einheitserdewerk GmbH, Froendenberg, Germany) and cultivated in a climate chamber (15 h light/9 h dark, 24 °C day/22 °C night, 75 % relative humidity day/80 % night). D. sanguinalis plants were inoculated 3 weeks after planting.
For inoculation, concentration of conidia was determined using a hemocytometer and adjusted to 150,000 conidia mL−1 for pathogenicity assays and 750,000 conidia mL−1 for sampling of epidermal peels, respectively. For co-inoculation experiments, 7-days-old barley primary leaves cv. Vada (kindly provided by Rients Niks, Wageningen University, Netherlands) were inoculated with the host isolate TH6772 (GFP-tagged) 6 h before the nonhost isolate CD180 (DsRed-tagged) was applied. Concentration of conidia was adjusted to 250,000 conidia mL−1 each. N. benthamiana seeds were kindly provided by Max Planck Institute for Plant Breeding Research (MPIPZ), Cologne, Germany. Plants were pre-germinated, cultivated and incubated (16 h light/8 h dark, 24 °C) in standard soil (type ED73).
Identification of candidate effector genes
For microarray analysis, 7-days-old barley plants (cv. Ingrid, kindly provided by Paul Schulze-Lefert, MPIPZ, Cologne, Germany) were inoculated with Magnaporthe isolate TH6772 or CD180 at a spore concentration of 750,000 conidia mL−1. After 24 h, epidermal peels of 200 plants were collected and RNA was extracted using the RNeasy Plant Mini Kit (Qiagen, Hilden, Germany). RNA (1600 ng) from four biological replicates of each isolate was labeled with either Cyanine 3 or Cyanine 5 according to the Two-Color Microarray-Based Gene Expression Analysis (Quick Amp Labeling) Protocol (Version 5.7, Agilent Technologies, Santa Clara, USA). Differently labelled cRNA of TH6772- and CD180-samples (1650 ng of each) was combined using dye swaps and hybridized to a Magnaporthe (V2) Gene Expression Microarray 4 × 44K slide (Design ID 15060) according to the manufacturer’s instructions. After scanning and feature extraction, microarray raw data were background-corrected, normalized by local regression (LOESS) and fitted to a linear model using R [R Development Core Team (2009), http://www.R-project.org/] with the Bioconductor (Gentleman et al. 2004) limma package (Smyth 2004). Using the linear model, 1528 sequences with a significant adjusted P value for multiple testing using a false discovery rate (Benjamini and Hochberg 1995) (P < 0.05) and at least two-fold higher transcript abundance in the TH6772 samples compared to the CD180 samples were identified. These were assigned to M. oryzae genes of assembly 6 (Broad database, http://www.broadinstitute.org/) and encoded protein sequences were analyzed for the presence of a signal peptide using SignalP [Version 4.1, (Petersen et al. 2011)] and WoLFPSORT (Horton et al. 2006, 2007). Thus, a total of 344 proteins were selected (using either SignalP or WoLFPSORT). Thereafter, 157 proteins were chosen based on a total protein length of 250 amino acids.
Determination of transcript abundances
Epidermal peels were harvested from infected barley plants (cv. Ingrid or Nigrate, the latter kindly provided by Yukio Tosa, Kobe University, Japan) for quantifying transcript abundances of fungal genes under investigation. For that purpose, the abaxial epidermis of at least 20 primary leaves was stripped at different time points after inoculation and immediately frozen in liquid nitrogen. RNA was extracted using a standard extraction protocol with hot phenol (Dudler and Hertig 1992).
For in vitro gene expression studies, conidia were germinated on glass slides in a plastic box and scraped-off with a razor blade at different time points. In this case, RNA was extracted from the fresh material using a protocol described in Voegele and Schmid (2011). Thus, the material was suspended in 600 μl cell lysis buffer (20 g L−1 sodium dodecyl sulfate ultrapure, 68 mM sodium citrate, 132 mM citric acid, 10 mM Ethylenediaminetetraacetic acid, pH 3.5). Subsequently, proteins and DNA were precipitated with 200 μl precipitation buffer (4 M sodium chloride, 17 mM sodium citrate, 33 mM citric acid, pH 3.5). RNA was precipitated from the supernatant with isopropanol, washed in 70 % ethanol and solved in double-distilled water. After removal of genomic DNA by treatment with DNase I (Fisher Scientific GmbH, Schwerte, Germany), cDNA synthesis was performed using HindAnchorT-primer [AAGCTTTTTTTTTTTTTTT(AGC)] and RevertAid Reverse Transcriptase (Thermo Fisher Scientific Inc., Germany). Gene-specific primers were designed using Primer3Plus software (Untergasser et al. 2007). RT-qPCR was performed using SYBR Green qPCR SuperMix-UDG with ROX (Invitrogen, Carlsbad, USA) with ABI Prism 7300 sequence detection system (Applied Biosystems, Life Technologies, Darmstadt, Germany). After activation cycles for 2′ at 50 °C and for 10′ at 95 °C, samples were exposed to 40 cycles of 15″ at 95 °C and 1′ at 60 °C. Afterwards, a melting-curve analysis was performed for each sample. For analysis of relative transcript abundances, only cycle threshold (ct)-values with specific melting points were evaluated. Transcript accumulation was evaluated relative to a constitutive reference gene (2(Ct(reference)−Ct(target))) (Livak and Schmittgen 2001). For normalization, fungal αTubulin was used as reference gene. For comparison and as an internal control, results were also calculated relative to the fungal gene Elongation factor 1α (EF1α) and this obtained similar results (data not shown). For primer sequence information see Online Resource 1.
Fungal transformation and microscopic studies
Knockout constructs were generated via Double Joint-PCR using a protocol kindly provided by Ralf Horbach (Martin-Luther-University Halle-Wittenberg, Germany) based on Yu et al. (2004). Knockout constructs were transformed into M. oryzae using split-marker method (Catlett et al. 2003). For generating split knockout constructs, the primer combinations 5′UTR_for and HY or 3′UTR_rev and YG were used, respectively. PCRs were performed using proof-reading Phusion Polymerase (Thermo Fisher Scientific Inc., Germany). Fungal gene knockout was done using a polyethylene glycol-mediated method kindly provided by Lauren Ryder (University of Exeter, UK) as described in Kershaw and Talbot (2009). Deletion of the target gene was verified by PCR using a gene-specific UTR-primer together with the respective qRT-PCR-primer. For primer sequence information see Online Resources 1 and 2.
Clearing of infected barley leaves and cytological analysis were performed as described elsewhere (Delventhal et al. 2014). Confocal images were obtained with a Leica TCS SP8 (Leica Microsystems GmbH, Wetzlar, Germany) microscope (GFP: 488 nm excitation/498–520 nm emission; DsRed: 561 nm excitation/580–650 nm emission) using samples of freshly harvested leaves in 10 % (v/v) glycerol.
Transient cell death assay in N. benthamiana
Recombinant A. tumefaciens C58C1 carrying constructs in pB7WG2 were cultivated in Yeast Extract Phosphate-medium with appropriate antibiotics (rifampicin, carbenicillin, spectinomycin and streptomycin, all at 50 µg mL−1) at 28 °C and 210 rpm for 36 h. Subsequently, bacteria were pelleted and re-suspended in infiltration buffer [10 mM MgCl2, 5 mM 2-(N-morpholino)ethanesulfonic acid pH 5.6, supplemented with 200 µM acetosyringone] to OD = 1. After further incubation for 2 h at room temperature at 100 rpm, the bacterial suspension was infiltrated into the abaxial leaf side of 5–6 week old N. benthamiana plants using Omnifix®-F Tuberculin syringes without needle (0.01–1 mL, B. Braun, Melsungen, Germany). N. benthamiana plants with treated leaves were cultivated as described above and infiltration sites were evaluated 6 days after infiltration. A. tumefaciens strains C58C1 pGV2260 and constructs of ChNLP1, ChEC3 and YFP were kindly provided by Jochen Kleemann (MPIPZ, Cologne, Germany).
Results
Co-inoculation of barley with Magnaporthe host and nonhost isolates breaks nonhost resistance
To investigate whether non-adaptation of Magnaporthe isolates on certain hosts may be due to insufficient suppression of host immunity, we performed co-inoculation of barley leaves with an adapted isolate that causes disease (M. oryzae, TH6772) and a non-adapted isolate that triggers non-host resistance on this plant (Pyricularia penniseticola, CD180) (Fig. 1a, b). Since the two isolates cannot be discriminated visually, we created a transgenic CD180 isolate tagged with the red fluorescent protein DsRed (CD180-DsRed) and a TH6772 isolate tagged with the green fluorescent protein (TH6772–GFP). In accordance with previously published results on the non-host isolate CD180, penetration of CD180-DsRed was stopped at sites where an effective papilla was formed beneath the appressorium (Fig. 1c) (Zellerhoff et al. 2006). The GFP-tagged host isolate TH6772-GFP, by contrast, was able to overcome penetration resistance and formed invasive hyphae in attacked epidermal cells (Fig. 1d). When nonhost and host isolates attacked the same epidermal cell, invasive hyphae of both isolates were found (Fig. 1e), indicating that the pathogenic Magnaporthe isolate repressed penetration resistance against the nonhost pathogen. The bulbous shape of the infection hyphae is typical for the biotrophic growth phase of the fungus and shows that the infection by the nonhost-isolate CD180 happened on living tissue (Zhang and Xu 2014). The occurrence of spindle-shaped blast-disease symptoms filled with mycelium of host and nonhost isolates at later infection stages (Fig. 1f), revealed that also post-penetration resistance against the nonhost isolate was repressed at co-penetrated infection sites. Individual infection sites were analyzed 3 days after inoculation to examine quantitatively infection by the nonhost isolate at the cellular level. Leaves inoculated solely with the nonhost CD180-DsRed isolate served as a control in this experiment. On these single inoculated leaves, 90 % of all penetration attempts were stopped in association with formation of an autofluorescent papilla (Fig. 1g, CD180 single inoculation, left). On co-inoculated leaves, the same result was obtained for epidermal cells attacked solely by the nonhost isolate (Fig. 1g, CD180 co-inoculation, middle). However, when the nonhost and the host isolate attacked the same epidermal cell, both isolates penetrated successfully and invaded subsequently the surrounding tissue. This was evidenced by red- and green-fluorescent infection hyphae which had branched and spread from cell to cell (Fig. 1g, TH6772 + CD180 co-inoculation, right). Remarkably, the nonhost isolate was also able to colonize the mesophyll tissue, confirming that the host isolate suppressed not only penetration but also post-penetration resistance mechanisms.
Accessibility of a Magnaporthe nonhost isolate to barley upon co-inoculation with a host isolate. a The Pyricularia penniseticola isolate CD180 is not able to cause visible disease symptoms on barley cv. Ingrid whereas b the M. oryzae isolate TH6772 causes blast on the same host. c Confocal imaging of the infection process revealed a papilla (pap)-based resistance of barley against the DsRed-tagged nonhost isolate CD180 at 48 h after inoculation. d In contrast to that, the GFP-tagged host isolate TH6772 establishes invasive hyphae (ih) at the same time point. e, f When both isolates were co-inoculated on barley primary leaves, the host isolate was applied 6 h prior to the nonhost isolate. e The attack of the same host cell by GFP-tagged TH6772 and DsRed-tagged CD180 strain enabled the nonhost strain to form invasive hyphae. f 6 days after inoculation, spindle-shaped blast symptoms were observed on co-inoculated plants filled with fungal hyphae from both isolates. g Quantitative microscopic analysis was performed on plants that were inoculated with CD180 only (single inoculation) or with CD180 and TH6772 (co-inoculation), respectively. On co-inoculated plants, interaction sites were evaluated for cells attacked by the nonhost isolate only or by both isolates 48 h after inoculation. A minimum of 100 interaction sites from at least three different primary leaves were categorized into five groups as indicated, error bars indicate standard error. The experiment was repeated once with similar result. co conidium, ap appressorium
Comparative transcriptome analysis identified 157 M. oryzae Hypothetical Effector Genes (MoHEGs)
Results of the co-inoculation experiment suggested that Magnaporthe host isolates produce potent suppressors of resistance, i.e. effectors that immune-suppress barley cells and thereby enable colonization by a nonhost isolate. Hypothesizing that the nonhost isolate is lacking these effectors, or is not expressing them due to the early abortion of infection, we performed comparative transcriptomics using epidermal peels of barley leaves inoculated either with the host or the nonhost Magnaporthe isolate. The use of epidermal peels allowed to restrict the analysis to the initial contact zone between the pathogen and the host and to highly enrich fungal transcripts compared to entire leaves. RNA of both interactions was used for a two-color microarray using the Agilent Magnaporthe (V2) Gene Expression Microarray and transcripts that accumulated to significantly higher levels (at least two-fold, P < 0.05) in the host compared to the nonhost interaction were identified. Filtering for genes that code for secreted proteins smaller than 250 amino acids identified 157 candidate effectors. 29 of these 157 genes have been identified previously as up-regulated in biotrophic, invasive hyphae of M. oryzae, including Biotrophy-Associated Secreted proteins BAS3, BAS4, PWL2 and MPG1 (Mosquera et al. 2009; Sweigard et al. 1995; Talbot et al. 1993). Based on transcript abundance, 18 candidates referred to as M. oryzae Hypothetical Effector Genes (MoHEGs) were initially selected and expression data could be validated by qRT-PCR for 16 of them (Table 1).
Transcript profiling distinguishes MoHEGs with different expression patterns in planta
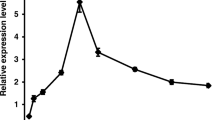
To characterize the expression profile of the 18 selected MoHEGs during infection, their transcript levels were quantified by real-time reverse transcription-PCR (qRT-PCR) in epidermal peels from primary barley leaves infected with the M. oryzae host isolate TH6772, harvested between 6 and 48 h post inoculation (h p.i.). This time course covers the biotrophic stage of infection and early mesophyll colonization (Wilson and Talbot 2009). According to their expression maximum, MoHEGs could be grouped into two categories referred to as EarlyMoHEGs and LateMoHEGs (Fig. 2; Table 1, Online Resource 3). The EarlyMoHEGs MoHEG7, MoHEG16 and MoHEG18 showed highest expression between 6 and 12 h p.i. when conidia had germinated, differentiated appressoria and started to penetrate (Fig. 2a, white bars). Expression of EarlyMoHEGs was also detected during in vitro germination (5 h) and appressoria formation (14 h) on glass slides (Fig. 2b). While transcript abundance was similar for MoHEG7 in the two in vitro samples, expression of MoHEG16 and MoHEG18 was much higher in the appressoria sample. The LateMoHEGs had an expression maximum at 24 h p.i. (Fig. 2a, grey bars), or showed a steady increase from 6 to 48 h p.i. (Fig. 2a, black bars) and were not detected during in vitro development (Fig. 2b). Expression of LateMoHEGs is therefore correlated with invasive growth in first attacked epidermal cells (24 h p.i.) and accelerated fungal colonization (48 h p.i.) and plant derived signals or stage-specific expression might be involved in triggering their expression.
Transcripts of Magnaporthe oryzae Hypothetical Effector Genes (MoHEGs) accumulate with distinct profiles. a Relative transcript abundance of selected MoHEGs was quantified in barley epidermal peels harvested at 6, 12, 24 and 48 h after inoculation with M. oryzae host isolate TH6772 via real-time reverse transcription-PCR. MoHEGs with an early expression maximum are displayed in white (EarlyMoHEGs), MoHEGs with a late expression maximum in grey or black, respectively (LateMoHEGs). b Relative transcript abundances of selected MoHEGs were examined in conidia germinated on glass slides after 5 or 14 h, discriminated by the absence or presence of appressoria, respectively. Only transcripts of EarlyMoHEGs could be detected in these samples, whereas abundances of LateMoHEG transcripts were extremely low or below the detection limit (for MoHEG9, MoHEG11, MoHEG13 or MoHEG14), respectively. Expression levels are shown relative to the mean expression of α-Tubulin. Graphs show mean values and standard deviations calculated from three technical replicate measurements of one experiment. Results were confirmed in three independent biological replicates and in an extended time-course study (Online Resource 3). BAS Biotrophy-Associated Secreted protein
HEG orthologs from M. grisea show similar expression profiles as MoHEGs
To investigate whether MoHEGs are conserved in other closely related fungi of the genus Pyricularia, orthologs were searched by BLAST in the genome of the crabgrass (Digitaria sanguinalis) pathogen M. grisea (Couch and Kohn 2002) (Fig. 3a). This identified 14 orthologs of MoHEGs that were named M. grisea Hypothetical Effector Genes (MgHEGs) and numbered as their MoHEG orthologs. Mean amino acid identity of mature MoHEGs and MgHEGs was 73 % (Table 2). Sequence information for MgHEG5 and MgHEG18 were not complete at the 5′-terminus, preventing analysis of the signal peptides.
Transcript profiles of selected Magnaporthe grisea Hypothetical Effector Genes (MgHEGs) are similar to those of MoHEGs. a The M. grisea isolate BR29 causes blast symptoms on its host plant Digitaria sanguinalis, whereas it is not able to cause disease on barley cv. Ingrid. However, BR29 is able to infect the immune-compromised barley cultivar Nigrate. b Relative transcript abundances of selected MgHEGs were quantified by real-time reverse transcription-PCR in epidermal peels of Nigrate leaves inoculated with BR29 and harvested 6, 12, 24 and 48 h after inoculation. MgHEGs with early expression maxima are displayed in white (EarlyMgHEGs), MgHEGs with expression maxima at 24 or 48 h after inoculation in grey or black, respectively (LateMgHEGs). c Relative transcript abundances of selected MgHEGs were also studied in germinated conidia harvested from glass slides 5 h after application. Expression levels are calculated relative to the mean expression of α-Tubulin. Error bars show standard deviation of three technical replicate measurements of one experiment. Experiment was repeated with similar results
Using whole leaf material or epidermal peels from M. grisea isolate BR29-infected D. sanguinalis plants did not allow the detection of fungal transcripts in qRT-PCR experiments. Therefore, epidermal peels from leaves of the barley cultivar Nigrate, which is a universal susceptible host for M. grisea, were used to study expression of MgHEGs during infection (Fig. 3a) (Hyon et al. 2012; Nga et al. 2012). Barley cultivars other than Nigrate show nonhost resistance to BR29. Data from initial experiments suggested that the EarlyMoHEG orthologs MgHEG7 and MgHEG16 behaved similar to MoHEGs since they were expressed during in vitro germination and appressorium formation and had their expression maximum early during infection (Fig. 3b, c). On the contrary, MgHEG18 behaved different from MoHEG18 as it was not expressed in vitro and had its expression maximum at 24 h p.i. The LateMoHEG orthologs showed LateHEG expression profiles with no in vitro expression and later in planta expression maxima. According to these pilot but mutually supporting experiments, orthologous MgHEGs and MoHEGs in most instances have similar expression profiles with differences merely related to the timing of the expression maxima.
Functional analysis revealed that MoHEG16 but not MoHEG18 is crucial for plant infection
It has been reported that deletion of the LateMoHEGs BAS3 and BAS4 did not affect virulence of the blast fungus (Mosquera et al. 2009). We therefore concentrated for functional analysis on EarlyMoHEGs and created knockout mutants for MoHEG16 and MoHEG18 in the M. oryzae isolate 70–15. Δmoheg18 mutants were not altered in virulence on barley (Online Resource 4a), while Δmoheg16 mutants showed less disease symptoms than the wild type (Fig. 4a). The latter phenotype was correlated at the cellular level with an increased number of hyphae arrested at the appressorium stage (with or without papilla formation) and less successful tissue colonization reflected by a reduced proportion of interaction sites showing collapsed mesophyll cells 72 h p.i. (Fig. 4b, Online Resource 5). Therefore, early induction of MoHEG16 seems to be required for successful invasion of epidermal cells and to prepare the host tissue for transition from epidermis to mesophyll colonization.
Mutant analysis reveals that Magnaporthe oryzae Hypothetical Effector Gene16 (MoHEG16) is required for full virulence of M. oryzae. Barley plants cv. Vada were inoculated with conidia (250,000 spores mL−1) of the M. oryzae isolate 70–15 (wild type, wt) or three independent Δmoheg16-mutants. a Infection phenotypes were evaluated macroscopically after 4 days. b For a quantitative microscopic analysis by bright field and fluorescence microscopy, at least three primary leaves were harvested 72 h after inoculation and a minimum of 100 interaction sites was analyzed per leaf and classified as indicated (app melanized appressorium with no visible fluorescence of plant tissue, pap melanized appressorium with local fluorescence underneath, fluo accumulation of fluorescent material in attacked epidermal cell, ih fluorescent epidermal cell infected by bulbous invasive hyphae, col fluorescent epidermal cell with invasive hyphae and collapsed mesophyll tissue). This approach was independently repeated three times and results were combined. Frequencies are given as mean values with standard errors. Asterisks (paired t test) and hash keys (Mann–Whitney-Rank-Sum-test) indicate statistically significant differences to the wild type isolate (P ≤ 0.05)
MoHEG13 acts as a cell death suppressor in N. benthamiana and is required for virulence of M. oryzae
LateMoHEGs show highest expression around the biotrophy-necrotrophy-switch in the barley-M. oryzae interaction (Fig. 2a), suggesting that they could act either as inducers or suppressors of cell death. Therefore, their ability to induce or antagonize cell death was analyzed in an Agrobacterium tumefaciens-mediated transient expression assay in Nicotiana benthamiana. Previous studies have demonstrated that NLPs are appropriate cell death inducers in such assays (Kleemann et al. 2012). The genome of M. oryzae contains four NLPs (Gijzen and Nürnberger 2006), two type-1 NLPs, MoNLP1 and MoNLP4 (MGG_08454, MGG_10532), and two type-2 NLPs, MoNLP2 and MoNLP3 (MGG_00401, MGG_02332), that all contain the characteristic hepta-peptide motif GHRHDWE (Fig. 5a) (Gijzen and Nürnberger 2006).
Expression of MoNLPs in Nicotiana benthamiana induces necrosis. a All MoNLPs possess the heptapeptide motif characteristic for the NLP protein family and also present in Colletotrichum higginsianum (Ch) NLP1. b Relative transcript abundance of MoNLPs in barley epidermal peels harvested after inoculation with the M. oryzae host isolate TH6772 at time points indicated. Expression was only detected for MoNLP1 and MoNLP4 and both genes had maximum transcript abundance at 24 h after inoculation. Expression levels are calculated relative to the mean expression of α-Tubulin. Error bars show the standard deviation of three technical replicates of one experiment. The experiment was repeated with similar results. c N. benthamiana leaves were transiently transformed by infiltration of A. tumefaciens clones carrying constructs for MoNLPs, YFP (negative control) or ChNLP1, a known cell death inducer from C. higginsianum (positive control). MoNLP1, MoNLP2, MoNLP4 and ChNLP1 (white script) induced necroses in the infiltrated zone. MoNLP3 and YFP (black script) did not induce necrosis. Photos were taken 6 days after infiltration. d Necrosis-inducing MoNLPs or ChNLP1 were co-expressed with ChEC3, a suppressor of ChNLP1-triggered necrosis. This revealed that ChEC3 suppresses also necroses induced by MoNLP1, MoNLP2 or MoNLP4. Photos were taken 6 days after infiltration. ChEC3 Colletotrichum higginsianum Effector Candidate 3, NLP Necrosis-and ethylene-inducing protein-1-like protein
MoNLP1 was among the 157 M. oryzae candidate effectors identified in our microarray analyses (see above). Transcript profiling by qRT-PCR revealed expression maxima for MoNLP1 and MoNLP4 at 24 h p.i. similar to LateHEGs (Fig. 5b), while expression of MoNLP2 and MoNLP3 was generally low and did not vary during the infection process. Since NLPs were reported to induce cell death only in dicots (Bailey 1995), which are no hosts for M. oryzae, the exact role of MoNLPs during infection remains to be established. Cell death induction by MoNLPs was tested in N. benthamiana by A. tumefaciens-mediated transient expression assays using MoNLP full length cDNA constructs comprising the secretion signal and Colletotrichum higginsianum (Ch) NLP1 as a positive control (Kleemann et al. 2012). All MoNLPs, with the exception of MoNLP3, caused necroses comparable to ChNLP1 (Fig. 5c). We further established that the C. higginsianum effector candidate ChEC3 that suppresses ChNLP1-mediated cell death (Kleemann et al. 2012), exhibited the same activity against MoNLPs (Fig. 5d). This suggests a general mode of interaction between ChEC3 and NLPs from different species. None of the LateMoHEGs showed a cell death-inducing activity when expressed in N. benthamiana leaves (Fig. 6a for MoHEG1, MoHEG2, MoHEG11 and MoHEG13, not shown for the others). When co-expressed with MoNLPs, the majority of MoHEGs also failed to suppress MoNLP-mediated cell death (Fig. 6b for MoHEG2 and MoHEG14, not shown for the others). The sole exception was MoHEG13 which suppressed cell death induced by MoNLP1, MoNLP2 and MoNLP4 to a similar extent as ChEC3 (Fig. 6c). MoHEG13-mediated cell death suppression was very robust and observed in 90 % of incidences from independent replicate experiments indicating that it is a potent inhibitor of NLP-triggered cell death. To study the role of MoHEG13 in plant infection, we used a Δmoheg13 mutant kindly provided by Hiromasa Saitoh (Iwate Biotechnology Research Center, Kitakami, Japan) (Saitoh et al. 2012). Using two different spore concentrations for inoculation of barley leaves, we observed reduced numbers of disease symptoms after inoculation with the mutant isolate compared to the wild type isolate (Fig. 7a). Microscopic analysis revealed no differences in pre-penetration development of both isolates at 12 h p.i., neither in spore germination nor in appressorium formation (data not shown). However, the lower penetration success of the Δmoheg13 mutant observed at 24 h p.i. suggests that MoHEG13 plays an important role in the early invasion of epidermal host cells (Fig. 7b).
MoHEG13 is able to antagonize MoNLP-derived cell death. a MoHEGs were tested for their cell death-inducing activity by expressing them transiently in Nicotiana benthamiana leaves. YFP and ChNLP1 were used as negative and positive controls. No cell death inducing MoHEGs were identified and representative results are shown for MoHEG1, MoHEG2, MoHEG11 and MoHEG13. Photos were taken 6 days after infiltration. b MoHEGs were evaluated for their ability of antagonizing MoNLP1-triggered cell death by co-infiltration. Co-infiltration of MoNLP1 and ChEC3 served as positive control (cell death is suppressed) and co-expression of MoNLP1 and YFP as negative control (cell death is not suppressed). Only leaves which showed the expected result for both controls were further evaluated for the cell death suppression activity of MoHEGs. MoHEG13 was identified as the sole candidate suppressing MoNLP1-induced cell death and this phenotype was observed in 90 % of incidences in several biological replicates (51 leaves were analyzed in total). Negative results are shown for MoHEG2 and MoHEG14 as representatives for the MoHEGs lacking cell death suppressing activity. Photos were taken 6 days after infiltration. c Suppression of MoNLP2- or MoNLP4-triggered cell death by MoHEG13 was analyzed as in b and showed cell death suppressing activity for MoHEG13. ChEC3 Colletotrichum higginsianum Effector Candidate 3, HEG Hypothetical Effector Gene, NLP Necrosis-and ethylene-inducing protein-1-like protein
Mutant analysis revealed that MoHEG13 is required for full virulence of Magnaporthe oryzae. a Seven-days-old barley plants of cv. Vada were spray inoculated with two different spore concentrations of M. oryzae mutant Δmoheg13 or the respective wild type isolate INA72. Macroscopic phenotypes were evaluated 8 days after inoculation. b For quantitative microscopy, three barley leaves inoculated with INA72 or Δmoheg13 (200,000 spores mL−1), respectively, were harvested 24 h post inoculation. For each leaf, approximately 100 interaction sites with appressorium were evaluated for presence or absence of a penetration hypha in the attacked epidermal cell. The percentage of penetrated cells is given as mean with standard error of three leaves. Asterisk indicates the statistically significant difference between INA72 and Δmoheg13 (t test, P ≤ 0.05). One representative experiment of three independent replicates with similar results is shown
Discussion
Effective penetration and post-penetration resistance mechanisms prevent colonization of plants by nonhost pathogens. Host pathogens, by contrast, are successful due to effector-mediated suppression of plant immune responses. While such effectors are well-studied in bacteria and oomycetes, less is known for fungal plant pathogens. One cause for this lack of knowledge is the absence of conserved sequence motifs such as the RXLR-pattern known from oomycetes which complicates in silico identification of fungal effectors (Whisson et al. 2007). Thus, at present, plant pathologists rely on a gene by gene investigation to identify and functionally characterize effectors from fungi. In M. oryzae, at least 851 genes were described to encode small secreted proteins that could potentially act as effectors (Chen et al. 2013; Dean et al. 2005; Mosquera et al. 2009), but only for a very limited number a virulence function was confirmed. In the present study we added further pieces to that puzzle by describing biological functions for two so far unexplored M. oryzae effector candidates.
Our study was driven by the initial observation that a P. penniseticola isolate that establishes a nonhost interaction with barley (Fig. 1a, c) could penetrate and proliferate in barley cells that had been invaded by a closely related M. oryzae host isolate (Fig. 1e, f). This phenomenon of induced susceptibility is supposed to rely on the suppression of nonhost resistance in co-infected cells and is not restricted to blast disease. Kunoh et al. (1986) showed e.g. that at the single cell level conidia from a nonhost pea powdery mildew were able to penetrate barley when attacking cells that were simultaneously invaded by the host powdery mildew pathogen Blumeria graminis f.sp. hordei (Bgh). Carver and his co-workers (Lyngkjær and Carver 1999; Lyngkjær et al. 2001) followed this in more detail and revealed that also race-specific resistance was overcome in cells that had been penetrated by a virulent Bgh isolate. Projecting these observations onto the molecular level, we hypothesize that the M. oryzae host isolate secretes potent effector molecules during barley colonization that render cells susceptible even for a nonhost pathogen. If these effectors are missing, as in the case of a nonhost isolate, the plant can successfully establish defense reactions such as papilla formation (Online Resource 5c, d). We investigated whether differences in the effector repertoires of host and nonhost isolates are reflected in their stage-specific transcriptomes using a microarray approach. Thereby, we obtained a list of M. oryzae effector candidates and transcript profiling allowed grouping of these genes into two categories according to their expression profile: EarlyMoHEGs with maximal transcript abundance at 12 h p.i. and expression during in vitro germination and appressorium formation and LateMoHEGs, with maximal expression between 24 and 48 h p.i. (Fig. 2). While some LateMoHEGs already have been identified in other studies (for references see Table 1), to our best knowledge, no information about a role in pathogenicity is available for genes from the group of EarlyMoHEGs. This might be due to the fact that these genes are expressed at very early time points during infection when fungal biomass is quite low and consequently transcript detection is difficult. The use of epidermal peels, which is not possible for rice, therefore might have been a crucial experimental advantage of our strategy. EarlyMoHEGs such as MoHEG7 and MoHEG16 were only reported in a transcriptome study using batches of in vitro grown M. oryzae material (Gowda et al. 2006).
Only few M. oryzae effectors have been characterized functionally and shown to be important for virulence of the blast fungus. This is largely due to the fact that mutant analysis is hampered by the high degree of functional redundancy among effectors and single knockout mutants were therefore not attenuated in their virulence (Mosquera et al. 2009; Saitoh et al. 2012). Thus, we concentrated on functional analysis of the class of EarlyMoHEGs that had not been identified previously and screened LateMoHEGs for cell death-related phenotypes in a heterologous plant system prior to mutant analysis. Both approaches were successful and led to the identification of new M. oryzae effectors that are required for full virulence of the blast fungus.
The EarlyMoHEG16 proved to be important for full virulence since Δmoheg16 knockout mutant isolates are attenuated in virulence on barley. At the cellular level, this phenotype was associated with reduced success in epidermal penetration and a retarded transition of invasive hyphae into the mesophyll suggesting important activities of MoHEG16 at different infection stages (Fig. 4). The mode of action and molecular function of MoHEG16 remains to be established but sequence analysis revealed that MoHEG16 possesses a YFWxC motif at its N terminus and contains a cysteine-rich EGF-like domain (CFEM domain, Pfam PF05730). The YFWxC motif which is also present in MoHEG17 and MoHEG18 is frequently found in the N terminus of effectors from haustorium-forming fungi such as powdery mildews or rusts (Godfrey et al. 2010). However, its function is not known. The CFEM domain is an extracellular, fungal-specific domain containing eight cysteine residues and was found in proteins with significant similarity to G-protein-coupled receptors (Kulkarni et al. 2003, 2005). In addition, CFEM-containing proteins were reported to be induced in M. oryzae in response to treatment with the bacterial antagonist Lysobacter enzymogenes, suggesting a role in defense against bacteria (Mathioni et al. 2013).
Investigation of cell death-related functions of LateMoHEGs showed that none of them induced cell death when heterologously expressed in N. benthamiana. A similar screening of in planta expressed effector candidates identified four effectors from M. oryzae (Cell Death Inducing Protein, MoCDIP) that induced cell death in rice and N. benthamiana and one that was only active on rice protoplasts (Chen et al. 2013). These MoCIDPs showed no overlap with MoHEGs or those candidates from the larger list of 157 effectors identified by our microarray approach. Lack of cell death-inducing effectors in our candidate list might be due to different experimental set-ups (whole leaf rice material vs. barley epidermal peels) and a focus in our study on effectors expressed during early biotrophic infection.
Screening of LateMoHEGs for cell death-suppressing activity identified MoHEG13 as a potential cell death suppressor that counteract NLP-induced necrosis similar to ChEC3, a cell death suppressor from C. higgensianum (Kleemann et al. 2012). In the future, it will be interesting to test if MoHEG13 acts on other types of cell death such as MoCIDP- or R protein-induced cell death. Mutant analysis revealed that MoHEG13 is required for full virulence of M. oryzae and that the mutant provokes lesser symptoms than the wild type and shows reduced penetration 24 h p.i., the time point when the corresponding gene shows maximal expression. Although only one Δmoheg13 mutant could be examined results from cell death suppression and inoculation experiments convergently suggests that MoHEG13 has an important role during early infection. It will be particularly interesting to determine if its virulence function is related to cell death suppression during barley infection and by which mechanism it operates. In addition, it will be important to verify if MoHEG13 has similar roles in rice infection where no phenotype has been detected for the Δmoheg13 mutant in a previous large scale study (Saitoh et al. 2012).
To establish cell death suppression assays for M. oryzae candidate effectors, we cloned NLPs present in the M. oryzae genome and performed an initial characterization of this ubiquitous family of virulence factors in the blast fungus. M. oryzae has two type-1 and two type-2 NLPs. Both types of NLPs are known to act as inducers of necrosis in dicotyledonous plants, but our data showed that only MoNLP1 and 4 (both type-1 NLPs) and the MoNLP3 (type-2 NLP) induced necrosis in N. benthamiana while MoNLP2 was inactive. Interestingly, only the two type-1 NLPs are expressed during barley infection suggesting that type-2 NLPs do not contribute to virulence of the blast fungus in barley. The mechanism of cell death induction can be supposed to be similar for type-1 and type-2 NLPs since cell death is suppressed in all cases by MoHEG13 and ChEC3. Since NLPs are known to induce no cell death in monocotyledonous plants (Bailey 1995) and since the expression maxima of MoNLP1 and 4 are at 24 h p.i. when the M. oryzae-barley interaction is fully biotrophic, their role during rice or barley infection remains to be established.
Taken together, our study identified novel effector candidates of the rice blast fungus by a combination of comparative transcriptomics, effector expression profiling and heterologous functional screening. Two of these candidates, MoHEG13 and MoHEG16, were demonstrated by mutant analysis to be crucial for plant infection, illustrating the power of our approach.
Author contribution statement
VM performed most experiments. SL and PMB helped in BR29-related experiments and N. benthamiana infiltration assays, respectively. RD started the initial experiments by performing and interpreting microarray analyses supervised by KA and ET. DW performed co-inoculation experiments and RD analyzed the MoHEG13 mutant. US conceived and designed experiments. TK provided sequence information for isolate BR29 and helped in designing experiments. VM drafted and ET, TK and US finalized the manuscript. DW finalized figures and edited the manuscript. All authors read and approved the manuscript.
References
Bailey BA (1995) Purification of a protein from culture filtrates of Fusarium oxysporum that induces ethylene and necrosis in leaves of Erythroxylum coca. Phytopathology 85:1250–1255
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B (Methodol) 57:289–300
Catlett NL, Lee DN, Yoder OC, Turgeon BG (2003) Split-marker recombination for efficient targeted deletion of fungal genes. Fungal Genet Newsl 50:9–11
Cesari S, Thilliez G, Ribot C, Chalvon V, Michel C, Jauneau A, Rivas S, Alaux L, Kanzaki H, Okuyama Y, Morel J-B, Fournier E, Tharreau D, Terauchi R, Kroj T (2013) The rice resistance protein pair RGA4/RGA5 recognizes the Magnaporthe oryzae effectors AVR-Pia and AVR1-CO39 by direct binding. Plant Cell 25:1463–1481
Chen S, Songkumarn P, Venu RC, Gowda M, Bellizzi M, Hu J, Liu W, Ebbole D, Meyers B, Mitchell T, Wang G-L (2013) Identification and characterization of in planta-expressed secreted effector proteins from Magnaporthe oryzae that induce cell death in rice. Mol Plant Microbe Interact 26:191–202
Couch BC, Kohn LM (2002) A multilocus gene genealogy concordant with host preference indicates segregation of a new species, Magnaporthe oryzae, from M. grisea. Mycologia 94:683–693
Dean RA, Talbot NJ, Ebbole DJ, Farman ML, Mitchell TK, Orbach MJ, Thon M, Kulkarni R, Xu J-R, Pan H, Read ND, Lee Y-H, Carbone I, Brown D, Oh YY, Donofrio N, Jeong JS, Soanes DM, Djonovic S, Kolomiets E, Rehmeyer C, Li W, Harding M, Kim S, Lebrun M-H, Bohnert H, Coughlan S, Butler J, Calvo S, Ma L-J, Nicol R, Purcell S, Nusbaum C, Galagan JE, Birren BW (2005) The genome sequence of the rice blast fungus Magnaporthe grisea. Nature 434:980–986
Delventhal R, Falter C, Strugala R, Zellerhoff N, Schaffrath U (2014) Ectoparasitic growth of Magnaporthe on barley triggers expression of the putative barley wax biosynthesis gene CYP96B22 which is involved in penetration resistance. BMC Plant Biol 14:26
Dudler R, Hertig C (1992) Structure of an mdr-like gene from Arabidopsis thaliana. Evolutionary implications. J Biol Chem 267:5882–5888
Eckert M, Maguire K, Urban M, Foster S, Fitt B, Lucas J, Hammond-Kosack K (2005) Agrobacterium tumefaciens-mediated transformation of Leptosphaeria spp. and Oculimacula spp. with the reef coral gene DsRed and the jellyfish gene gfp. FEMS Microbiol Lett 253:67–74
Faivre-Rampant O, Thomas J, Allegre M, Morel JB, Tharreau D, Notteghem JL, Lebrun MH, Schaffrath U, Piffanelli P (2008) Characterization of the model system rice-Magnaporthe for the study of nonhost resistance in cereals. New Phytol 180:899–910
Fisher MC, Henk DA, Briggs CJ, Brownstein JS, Madoff LC, McCraw SL, Gurr SJ (2012) Emerging fungal threats to animal, plant and ecosystem health. Nature 484:186–194
Gentleman R, Carey V, Bates D, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, Hornik K, Hothorn T, Huber W, Iacus S, Irizarry R, Leisch F, Li C, Maechler M, Rossini A, Sawitzki G, Smith C, Smyth G, Tierney L, Yang J, Zhang J (2004) Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 5:R80
Gijzen M, Nürnberger T (2006) Nep1-like proteins from plant pathogens: recruitment and diversification of the NPP1 domain across taxa. Phytochemistry 67:1800–1807
Giraldo MC, Dagdas YF, Gupta YK, Mentlak TA, Yi M, Martinez-Rocha AL, Saitoh H, Terauchi R, Talbot NJ, Valent B (2013) Two distinct secretion systems facilitate tissue invasion by the rice blast fungus Magnaporthe oryzae. Nat Commun 4:1996
Godfrey D, Bohlenius H, Pedersen C, Zhang Z, Emmersen J, Thordal-Christensen H (2010) Powdery mildew and rust fungal effector candidates share N-terminal Y/F/WxC-motif. BMC Genom 11:317
Gowda M, Venu R, Raghupathy M, Nobuta K, Li H, Wing R, Stahlberg E, Couglan S, Haudenschild C, Dean R, Nahm B-H, Meyers B, Wang G-L (2006) Deep and comparative analysis of the mycelium and appressorium transcriptomes of Magnaporthe grisea using MPSS, RL-SAGE, and oligoarray methods. BMC Genom 7:310
Heath MC (2000) Nonhost resistance and nonspecific plant defenses. Curr Opin Plant Biol 3:315–319
Horton P, Park KJ, Obayashi T, Nakai K (2006) Protein subcellular localization prediction with WOLF PSORT. Ser Adv Bioinform 3:39–48
Horton P, Park KJ, Obayashi T, Fujita N, Harada H, Adams-Collier CJ, Nakai K (2007) WoLF PSORT: protein localization predictor. Nucleic Acids Res 35:W585–W587
Hyon G-S, Nga N, Chuma I, Inoue Y, Asano H, Murata N, Kusaba M, Tosa Y (2012) Characterization of interactions between barley and various host-specific subgroups of Magnaporthe oryzae and M. grisea. J Gen Plant Pathol 78:237–246
Jarosch B, Kogel KH, Schaffrath U (1999) The ambivalence of the barley Mlo locus: mutations conferring resistance against powdery mildew (Blumeria graminis f. sp. hordei) enhance susceptibility to the rice blast fungus Magnaporthe grisea. Mol Plant Microbe Interact 12:508–514
Jarosch B, Collins NC, Zellerhoff N, Schaffrath U (2005) RAR1, ROR1, and the actin cytoskeleton contribute to basal resistance to Magnaporthe grisea in barley. Mol Plant Microbe Interact 18:397–404
Jia Y, McAdams SA, Bryan GT, Hershey HP, Valent B (2000) Direct interaction of resistance gene and avirulence gene products confers rice blast resistance. EMBO J 19:4004–4014
Jones JDG, Dangl JL (2006) The plant immune system. Nature 444:323–329
Kankanala P, Czymmek K, Valent B (2007) Roles for rice membrane dynamics and plasmodesmata during biotrophic invasion by the blast fungus. Plant Cell 19:706–724
Kanzaki H, Yoshida K, Saitoh H, Fujisaki K, Hirabuchi A, Alaux L, Fournier E, Tharreau D, Terauchi R (2012) Arms race co-evolution of Magnaporthe oryzae AVR-Pik and rice Pik genes driven by their physical interactions. Plant J 72:894–907
Kershaw MJ, Talbot NJ (2009) Genome-wide functional analysis reveals that infection-associated fungal autophagy is necessary for rice blast disease. Proc Natl Acad Sci 106:15967–15972
Khang CH, Berruyer R, Giraldo MC, Kankanala P, Park S-Y, Czymmek K, Kang S, Valent B (2010) Translocation of Magnaporthe oryzae effectors into rice cells and their subsequent cell-to-cell movement. Plant Cell 22:1388–1403
Kleemann J, Rincon-Rivera LJ, Takahara H, Neumann U, van Themaat EVL, van der Does HC, Hacquard S, Stüber K, Will I, Schmalenbach W, Schmelzer E, O’Connell RJ (2012) Sequential delivery of host-induced virulence effectors by appressoria and intracellular hyphae of the phytopathogen Colletotrichum higginsianum. PLoS Pathog 8:e1002643
Kulkarni RD, Kelkar HS, Dean RA (2003) An eight-cysteine-containing CFEM domain unique to a group of fungal membrane proteins. Trends Biochem Sci 28:118–121
Kulkarni R, Thon M, Pan H, Dean R (2005) Novel G-protein-coupled receptor-like proteins in the plant pathogenic fungus Magnaporthe grisea. Genome Biol 6:R24
Kunoh H, Kuroda K, Hayashimoto A, Ishizaki H (1986) Induced susceptibility and enhanced resistance at the cellular level in barley coleoptiles. II. Timing and localization of induced susceptibility in a single coleoptile cell and its transfer to an adjacent cell. Can J Bot 64:889–895
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(−Delta Delta C) method. Methods 25:402–408
Loehrer M, Botterweck J, Jahnke J, Mahlmann DM, Gaetgens J, Oldiges M, Horbach R, Deising H, Schaffrath U (2014) In vivo assessment by Mach-Zehnder double-beam interferometry of the invasive force exerted by the Asian soybean rust fungus (Phakopsora pachyrhizi). New Phytol 203:620–631
Lyngkjær MF, Carver TLW (1999) Induced accessibility and inaccessibility to Blumeria graminis f. sp hordei in barley epidermal cells attacked by a compatible isolate. Physiol Mol Plant Pathol 55:151–162
Lyngkjær MF, Carver TLW, Zeyen RJ (2001) Virulent Blumeria graminis infection induces penetration susceptibility and suppresses race-specific hypersensitive resistance against avirulent attack in Mla1-barley. Physiol Mol Plant Pathol 59:243–256
Mathioni S, Beló A, Rizzo C, Dean R, Donofrio N (2011) Transcriptome profiling of the rice blast fungus during invasive plant infection and in vitro stresses. BMC Genomics 12:1–20
Mathioni SM, Patel N, Riddick B, Sweigard JA, Czymmek KJ, Caplan JL, Kunjeti SG, Kunjeti S, Raman V, Hillman BI, Kobayashi DY, Donofrio NM (2013) Transcriptomics of the rice blast fungus Magnaporthe oryzae in response to the bacterial antagonist Lysobacter enzymogenes reveals candidate fungal defense response genes. PLoS One 8:e76487
Mentlak TA, Kombrink A, Shinya T, Ryder LS, Otomo I, Saitoh H, Terauchi R, Nishizawa Y, Shibuya N, Thomma BPHJ, Talbot NJ (2012) Effector-mediated suppression of chitin-triggered immunity by Magnaporthe oryzae is necessary for rice blast disease. Plant Cell Online 24:322–335
Mosquera G, Giraldo MC, Khang CH, Coughlan S, Valent B (2009) Interaction transcriptome analysis identifies Magnaporthe oryzae BAS1-4 as biotrophy-associated secreted proteins in rice blast disease. Plant Cell 21:1273–1290
Motteram J, Küfner I, Deller S, Brunner F, Hammond-Kosack KE, Nürnberger T, Rudd JJ (2009) Molecular characterization and functional analysis of MgNLP, the sole NPP1 domain-containing protein, from the fungal wheat leaf pathogen Mycosphaerella graminicola. Mol Plant Microbe Interact 22:790–799
Mysore KS, Ryu CM (2004) Nonhost resistance: How much do we know? Trends Plant Sci 9:97–104
Nga NTT, Hau VTB, Tosa Y (2009) Identification of genes for resistance to a Digitaria isolate of Magnaporthe grisea in common wheat cultivars. Genome 52:801–809
Nga NTT, Inoue Y, Chuma I, Hyon G-S, Okada K, Vy TTP, Kusaba M, Tosa Y (2012) Identification of a novel locus Rmo2 conditioning resistance in barley to host-specific subgroups of Magnaporthe oryzae. Phytopathology 102:674–682
Odenbach D, Breth B, Thines E, Weber RWS, Anke H, Foster AJ (2007) The transcription factor Con7p is a central regulator of infection-related morphogenesis in the rice blast fungus Magnaporthe grisea. Mol Microbiol 64:293–307
Petersen TN, Brunak S, von Heijne G, Nielsen H (2011) SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods 8:785–786
Pritchard L, Birch PRJ (2014) The zigzag model of plant–microbe interactions: is it time to move on? Mol Plant Pathol 15:865–870
Qutob D, Kemmerling B, Brunner F, Küfner I, Engelhardt S, Gust AA, Luberacki B, Seitz HU, Stahl D, Rauhut T, Glawischnig E, Schween G, Lacombe B, Watanabe N, Lam E, Schlichting R, Scheel D, Nau K, Dodt G, Hubert D, Gijzen M, Nürnberger T (2006) Phytotoxicity and innate immune responses induced by Nep1-like proteins. Plant Cell 18:3721–3744
R Development Core Team (2009). http://www.R-project.org. Accessed 15 July 2009
Rho HS, Kang S, Lee YH (2001) Agrobacterium tumefaciens-mediated transformation of the plant pathogenic fungus, Magnaporthe grisea. Mol Cells 12:407–411
Saitoh H, Fujisawa S, Mitsuoka C, Ito A, Hirabuchi A, Ikeda K, Irieda H, Yoshino K, Yoshida K, Matsumura H, Tosa Y, Win J, Kamoun S, Takano Y, Terauchi R (2012) Large-scale gene disruption in Magnaporthe oryzae identifies MC69, a secreted protein required for infection by monocot and dicot fungal pathogens. PLoS Pathog 8:e1002711
Schulze-Lefert P, Panstruga R (2011) A molecular evolutionary concept connecting nonhost resistance, pathogen host range, and pathogen speciation. Trends Plant Sci 16:117–125
Smyth GK (2004) Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 3:1–25
Sweigard JA, Carroll AM, Kang S, Farrall L, Chumley FG, Valent B (1995) Identification, cloning, and characterization of PWL2, a gene for host species specificity in the rice blast fungus. Plant Cell 7:1221–1233
Talbot NJ (2003) On the trail of a cereal killer: investigating the biology of Magnaporthe grisea. Annu Rev Microbiol 57:177–202
Talbot NJ, Ebbole DJ, Hamer JE (1993) Identification and characterization of MPG1, a gene involved in pathogenicity from the rice blast fungus Magnaporthe grisea. Plant Cell 5:1575–1590
Thordal-Christensen H (2003) Fresh insights into processes of nonhost resistance. Curr Opin Plant Biol 6:351–357
Tucker S, Orbach M (2007) Agrobacterium-mediated transformation to create an insertion library in Magnaporthe grisea. In: Ronald P (ed) Plant-pathogen interactions. Humana Press, New York, pp 57–68
Untergasser A, Nijveen H, Rao X, Bisseling T, Geurts R, Leunissen JAM (2007) Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res 35:W71–W74
Voegele RT, Schmid A (2011) RT real-time PCR-based quantification of Uromyces fabae in planta. FEMS Microbiol Lett 322:131–137
Whisson SC, Boevink PC, Moleleki L, Avrova AO, Morales JG, Gilroy EM, Armstrong MR, Grouffaud S, van West P, Chapman S, Hein I, Toth IK, Pritchard L, Birch PRJ (2007) A translocation signal for delivery of oomycete effector proteins into host plant cells. Nature 450:115–118
Wilson RA, Talbot NJ (2009) Under pressure: investigating the biology of plant infection by Magnaporthe oryzae. Nat Rev Microbiol 7:185–195
Wu J, Kou Y, Bao J, Li Y, Tang M, Zhu X, Ponaya A, Xiao G, Li J, Li C, Song M-Y, Cumagun CJR, Deng Q, Lu G, Jeon JS, Naqvi NI, Zhou B (2015) Comparative genomics identifies the Magnaporthe oryzae avirulence effector AvrPi9 that triggers Pi9-mediated blast resistance in rice. New Phytologist 206:1463–1475
Yu J-H, Hamari Z, Han K-H, Seo J-A, Reyes-Domínguez Y, Scazzocchio C (2004) Double-joint PCR: a PCR-based molecular tool for gene manipulations in filamentous fungi. Fungal Genet Biol 41:973–981
Zellerhoff N, Jarosch B, Groenewald JZ, Crous PW, Schaffrath U (2006) Nonhost resistance of barley is successfully manifested against Magnaporthe grisea and a closely related Pennisetum-infecting lineage but is overcome by Magnaporthe oryzae. Mol Plant Microbe Interact 19:1014–1022
Zellerhoff N, Himmelbach A, Dong W, Bieri S, Schaffrath U, Schweizer P (2010) Nonhost resistance of barley to different fungal pathogens is associated with largely distinct, quantitative transcriptional responses. Plant Physiol 152:2053–2066
Zhang S, Xu J-R (2014) Effectors and effector delivery in Magnaporthe oryzae. PLoS Pathog 10:e1003826
Zhang H, Li D, Wang M, Liu J, Teng W, Cheng B, Huang Q, Wang M, Song W, Dong S, Zheng X, Zhang Z (2012) The Nicotiana benthamiana mitogen-activated protein kinase cascade and WRKY transcription factor participate in Nep1Mo-triggered plant responses. Mol Plant Microbe Interact 25:1639–1653
Zipfel C (2014) Plant pattern-recognition receptors. Trends Immunol 35:345–351
Acknowledgments
The authors are grateful to Nick Talbot, Lauren Ryder (both University of Exeter, UK) and Holger Deising, Ralf Horbach and Jan-Jorrit Krijger (all Martin-Luther-University Halle-Wittenberg, Germany) for introducing Valerie Mogga into Magnaporthe transformation and for sharing material. Didier Tharreau (CIRAD, Montpellier, France) is kindly acknowledged for providing different Magnaporthe isolates. Jochen Kleemann und Richard O’Connell (both MPIPZ, Cologne, Germany) helped with the N. benthamiana infiltration assay and shared material. We are also grateful to Andrew J. Foster (IBWF, Kaiserslautern, Germany) for providing the pCAMB-HPT vector and to Hiromasa Saitoh (Iwate Biotechnology Research Center, Kitakami, Japan) for sharing Δmoheg13 and wild type isolate INA72. Denise Weidenbach was funded in the framework of the Federal Ministry of Education and Research funding activity ‘Plant Biotechnology for the future, PLANT 2030’ within the project ‘BarleyFortress’.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by E. Guiderdoni.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mogga, V., Delventhal, R., Weidenbach, D. et al. Magnaporthe oryzae effectors MoHEG13 and MoHEG16 interfere with host infection and MoHEG13 counteracts cell death caused by Magnaporthe-NLPs in tobacco. Plant Cell Rep 35, 1169–1185 (2016). https://doi.org/10.1007/s00299-016-1943-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-016-1943-9