Abstract
Key message
A GORK homologue K + channel from the ancient desert shrub Ammopiptanthus mongolicus (Maxim.) Cheng f. shows the functional conservation of the GORK channels among plant species.
Abstract
Guard cell K+ release through the outward potassium channels eventually enables the closure of stomata which consequently prevents plant water loss from severe transpiration. Early patch-clamp studies with the guard cells have revealed many details of such outward potassium currents. However, genes coding for these potassium-release channels have not been sufficiently characterized from species other than the model plant Arabidopsis thaliana. We report here the functional identification of a GORK (for Gated or Guard cell Outward Rectifying K+ channels) homologue from the ancient desert shrub Ammopiptanthus mongolicus (Maxim.) Cheng f. AmGORK was primary expressed in shoots, where the transcripts were regulated by stress factors simulated by PEG, NaCl or ABA treatments. Patch-clamp measurements on isolated guard cell protoplasts revealed typical depolarization voltage gated outward K+ currents sensitive to the extracelluar K+ concentration and pH, resembling the fundamental properties previously described in other species. Two-electrode voltage-clamp analysis in Xenopus lavies oocytes with AmGORK reconstituted highly similar characteristics as assessed in the guard cells, supporting that the function of AmGORK is consistent with a crucial role in mediating stomatal closure in Ammopiptanthus mongolicus. Furthermore, a single amino acid mutation D297N of AmGORK eventually abolishes both the voltage-gating and its outward rectification and converts the channel into a leak-like channel, indicating strong involvement of this residue in the gating and voltage dependence of AmGORK. Our results obtained from this anciently originated plant support a strong functional conservation of the GORK channels among plant species and maybe also along the progress of revolution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Drought is one of the major environmental problems leading to severe losses in agriculture. Drought tolerance of plants is regulated by several mechanisms that work together, consisting of protecting the plant from damage and controlling plants water loss via the stomatal pores in case of water stress. A rapid plant response is stomatal closure to reduce water loss from plants in response to drought conditions (Luan 2002). In addition to its counter anions, K+ release from the surrounding guard cells is a critical process that enables the prompt closure of the stomata.
The voltage-gated outward rectifying K+ efflux (Kout) has been repeatedly evidenced in the guard cells of a variety of plant species (Ache et al. 2000; Blatt 1988; Blatt and Gradmann 1997; Hosy et al. 2003; Ilan et al. 1994; Schroeder et al. 1987). These outward currents bear certain basic functional characteristics: activated upon depolarization of the membrane potentials, voltage and external K+ dependent gating, and sensitive to external pH.
The guard cells of Vicia faba have attracted extensive interests of patch-clamp studies for the Kout currents. A bundle of earlier reports indicated that the gating of the Kout currents was sensitive to external K+. The half activation voltage shifted for approximately 50 mV to the positive direction upon tenfold increase in the extracellular K+ concentration (Blatt and Gradmann 1997). The half-time for the rising currents increased in higher concentration of external K+ (Blatt 1988). This outward current was efficiently suppressed by K+ channel blockers TEA and Ba2+ (Blatt 1988; Schroeder et al. 1987). Additionally, the steady state Kout current was markedly inhibited by acidic pH with both the maximum conductance and the half-activation voltage altered (Ilan et al. 1994).
Apart from the in vivo assessments in isolated guard cells, however, the genetic identification of genes coding for such outward rectifiers remains insufficiently achieved. The Arabidopsis GORK represents one of the best characterized Kout channel in the guard cells (Ache et al. 2000). The AtGORK was characterized as a K+-selective and K+-sensing ion channel. It was suggested that a re-organization of GORK proteins in clusters within the plane of the plasma membrane parallels its gating with external K+ concentration (Eisenach et al. 2014). AtGORK mediated depolarization-activated K+ currents which were largely inhibited by K+ channel blockers (TEA and Ba2+) and were regulated by pH (Ache et al. 2000).
Knockout mutation of the GORK gene led to loss of the outwardly rectifying K+ channel activity in the guard cell protoplast membrane (Hosy et al. 2003). Additionally, disruption of GORK activity lead to impaired stomatal closure upon darkness, and increased water loss from stomata via transpiration measurements on excised rosettes and intact plants (Hosy et al. 2003; Schroeder 2003). Therefore, in the guard cells of Arabidopsis, AtGORK essentially plays a dominant role in mediating K+ efflux that drives the stomatal closure (Hosy et al. 2003). However, AtGORK was not exclusively expressed in the guard cells, transcripts were also found in root hairs and vascular cells of the root and shoot (Becker et al. 2003; Ivashikina et al. 2001). Expression of AtGORK was enhanced in response to drought, salt stress and cold in the transcriptional level, but AtGORK expression in guard cells was ABA insensitive (Becker et al. 2003).
More recently, GORK channels have been described in Samanea saman (Moshelion et al. 2002), maize (Buchsenschutz et al. 2005) and tobacco (Dai et al. 2009), but with less details concerning the channels’ functional properties. Since our current recognition on the functionality of cloned outward rectifying K+ channels has been mainly established in the model plant Arabidopsis thaliana, it remains necessary to address whether the function and/or regulatory mechanisms of GORK homologues are highly conserved or divergent among plant species.
Ammopiptanthus mongolicus (Maxim) Cheng f. is an endangered fabaceous shrub from the Tertiary period (from 65 to 2 million years ago, the age that mammals begun). The plant inhabits primarily in the northwest desert of China where the annual precipitation is often less than 50–100 mm, but the mean annual evaporation can be up to 3000 mm (Liu et al. 2013; Wu et al. 2014). The ancient origination and the survival over persistent arid environment make this species useful for resolving mechanisms involved in drought adaptation/resistance. Therefore, in this report, we cloned and functionally characterized the GORK homologue from this particular plant in order to provide a clue to the functional conservation between the known knowledge achieved in Arabidopsis. We applied both patch-clamp measurements in the guard cell protoplasts of Ammopiptanthus mongolicus and heterologous characterization in Xenopus oocytes to integrate the properties of the cloned channel to the outward currents recorded from the guard cells.
Materials and methods
Plant growth
Seeds of A. mongolicus were surface sterilized, washed and soaked in water for 2 days at 26 °C and placed in sterile Petri dishes before germinated on moist filter paper. Seedlings were transferred to half strength Hoagland solution in a greenhouse at approximately 26 °C with a photoperiod of 16 h light and 8 h dark for 4 weeks. Uniform seedlings were subjected to treatments with 18 % PEG-6000 (water potential of ca. −0.4 MPa), 100 mM NaCl or 100 μM ABA (Sigma) supplemented into half strength Hoagland solution. Shoots and roots were harvested separately at different time points (1, 3, 6, 12, 24 or 48 h) and immediately frozen in liquid nitrogen for RNA extraction. Controls were without any treatments.
Cloning of AmGORK
According to the coding sequence of the Arabidopsis GORK (At5g37500), we searched over our A. mongolicus RNA-Seq contigs (data not published) for homologous fragments. A complete coding sequence (CDS) was then successfully assembled and primers (Forward: 5′-CTCGAG ATGCATGGCGGTGACGGAG-3′ and reverse: 5′-GCGGCCGCTTATGCTCCTCTCTGTGCTTCACTC-3′) were retrieved for high-fidelity PCR (PrimerStar DNA polymerase, TAKARA) amplification of the AmGORK CDS. cDNAs from Ammopiptanthus mongolicus shoots were used as templates. After an initial denaturation of the template cDNA for 3 min at 95 °C, the amplification was performed for 30 cycles each consisting of 30 s at 95 °C, 30 s at 60 °C and 150 s at 72 °C, with a final extension for 10 min at 72 °C. Amplified products were purified, cloned into PMD 19-T vector (TAKARA) and sequenced. Verified sequence was submitted to the GeneBank (KP979749). For expression in Xenopus oocytes, the CDS of AmGORK was subcloned into the pCI vector (Promega, Beijing) between the XhoI and NotI sites.
Single mutation AmGORK-D297N was generated by overlaping PCR as described by Poree (Poree et al. 2005). Primers (M1: 5′-GGTAAGCACCAAGAATCATGTTAAAGGAG-3′; M2: 5′-CTCCTTTAACATGATTCTTGGTGCTTACC-3′) were used to introduce the site-directed mutation in AmGORK, and then subcloned into pCI vector for electrophysiological experiments after sequencing.
RNA extraction and real-time PCR
Total RNA was isolated from roots or shoots of 4-week-old seedlings with the TRIZOL reagent (Life technologies, Shanghai) following the manufacturer’s instruction. Total RNA (1.5 μg) was reversely transcribed into single strand cDNAs using Oligo-dT18 primer and the reverse transcriptase (TAKARA) according to the manufacturer’s instruction. For Real-Time Quantitative PCR analyses, PCR amplification was performed in a 20 μL reaction system containing 2.5 μL of 5× diluted cDNA templates, 0.2 μM of each forward and reverse primers and 10 μL SYBR Green PCR Master Mix (TOYOBO). The reaction was performed with CFX96 Real-Time PCR cycler programmed with: 45 cycles of 95 °C for 15 s, 60 °C for 15 s and 72 °C for 15 s. Specific primers (Forward: 5′-AAGAGGATAGAAGGGAGG-3′; Revese: 5′- ATAAAATACAGGAACCGT-3′) of AmGORK were used. AmEIF1 (Forward: 5′-CTGACATGCGCCGTAGGAACG-3′; Reverse: 5′-CCCTGCTTATGCCAGTCTTTT-3′) was used as an endogenous control (Shi et al. 2012).
Patch-clamp measurements on guard cell protoplasts
Isolation of A. mongolicus guard cell protoplasts for patch clamp was improved from method as described in Pei et al. (1997). Four-week-old A. mongolicus seedlings were used in this work. The guard cell protoplasts were isolated by enzymatic digestion from the young leaves. Epidermal tissues were collected by a fresh (sharp) razor blade. These epidermal tissues were incubated in 5 mL of medium containing 1 % Cellulase RS, 0.5 % Macerozyme R-10, 0.5 % BSA, 0.1 mM KCl, 0.1 mM CaCl2, 10 mM ascorbic acid and 500 mM D-sorbitol for 4 h at 23 °C on a circular shaker at 80 rpm. The whole cell mode was used to record outward rectifier potassium channel currents. The bath solution contained 30 mM KCl or 1 mM KCl, 1 mM CaCl2, 2 mM MgCl2 and 10 mM MES or 10 mM HEPES (Tris, pH 5.6 or 7.4). The pipette solution contained 30 mM KCl, 70 mM K-Glu, 2 mM MgCl2, 2 mM CaCl2,6.7 mM EGTA and 10 mM HEPES (Tris, pH 7.1). Fresh 5 mM ATP was added into pipette solution. Osmolarity was adjusted by d-sorbitol, 485 and 500 mmol kg−1 separately for bath solution and pipette solution. The voltage pulses were from −40 mV to +80 mV with a +20 mV increment.
Heterologous expression in Xenopus oocytes
Oocytes were injected with 59.8 nL (Nanoliter 2000, WPI) of AmGORK-pCI (1.5 μg/μL) or same volume of sterilized water for control, and then kept at 20 °C in ND96 solution (96 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 5 mM Hepes/NaOH, pH 7.4) supplemented with 50 mg/L of gentamicin until electrophysiological recordings. Two days after injection, two-electrode voltage-clamp (TEVC) tests on oocytes and data analysis were performed as described by Su et al. (2005). Electrode was filled with 3 M KCl. Recording solutions was comprised of 1 mM MgCl2, 1.8 mM CaCl2, 5 mM Hepes/NaOH, pH 7.4, or 5 mM MES/NaOH, pH 5.4, and KCl as indicated. NaCl was used for adjusting the constant ionic strength in all solution. Further used solutions were described in the figure legends. The voltage pulses ranged from −60 mV to +40 mV with a +10 mV increment.
Results
Cloning and sequence analysis of AmGORK
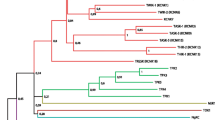
A homologous screening of A. mongolicus RNA-Seq (our unpublished data) for the Arabidopsis GORK revealed several partial fragments and a raw complete coding region were obtained by assembling the hit contigs. Then a full-length open reading frame of 2475 bp was amplified with high-fidelity PCR. The coding sequence was verified by sequencing and submitted to the GenBank (accession number: KP979749). This cDNA was designated as AmGORK (for a putative A. mongolicus Gated Outward Rectifying K+ channel). AmGORK coded for a deduced polypeptide of 824 amino acids showing high degrees of homologies to known outward rectifying channels from Arabidopsis, Samanea saman, poplar, maize and tobacco (Ache et al. 2000; Buchsenschutz et al. 2005; Dai et al. 2009; Gaymard et al. 1998; Langer et al. 2002; Moshelion et al. 2002). AmGORK displayed typical structure features of plant Shaker family K+ channels, such as six transmembrane domains, the P-loop between S5 and S6, the conserved GYGD motif in the P-loop (Fig. 1a), a putative cyclic nucleotide binding domain and the ankyrin repeat sequences (supplemental data1). According to the phylogenetic analysis shown in Fig. 1b, AmGORK was closest related to the outward potassium channels with 64.4, 58.4 and 57.3 % overall identities to the poplar PTORK (accession no. CAC05488.1) (Langer, et al. 2002), SKOR (At3g02850.1) and GORK (At5g37500.1), respectively. And to lesser extents AmGORK also showed certain homologies to the inward rectifiers (Fig. 1b).
Phylogenetic and expression analysis of AmGORK. a Alignment of the AmGORK pore domains amino acid sequence with outward potassium channels from other plant species. b Phylogenetic analysis of polypeptides plant Shaker-type potassium channel sequences. Accession numbers: PtORK, CAC05488.1; AtGORK, At5g37500.1; AtSKOR, At3g02850.1; SPORK, CAC10514.1; ZMK2, NP_001105120.1; SPICK2, AAD39492.1; SPICK1, AAD16278.1; PTK2, CAC05489.1; AtAKT2/3, At4g22200.1; KST1, NP_001275475.1; AtKAT1, At5g46240.1; AtKAT2, At4g18290.1; KPT1, CAC87141.1; SIRK, AF359521.1; SKT1, AF237951; LKT1, X96390; ZMK1, Y07632.1; TaAKT1, AF207745; AtSPIK, At2g25600.1; AtAKT5, At4g32500.1; AtKC1, At4g32650; KDC1, AJ249962; MIRK, NP_001284388.1; OsAKT1, LOC_Os01g45990.1; OsKAT1, LOC_Os01g55200.1; OsKAT2, LOC_Os01g11250.1; ZmK2.1, AAR21353.1; KZM1, CAD18901.1; NTORK1, AB196792.1;ZORK, AAW82753. The phylogenetic tree was constructed using MEGA 6. c Transcript levels of AmGORK were evaluated in shoots and roots by real-time quantitative RT-PCR. Four-week-old seedlings were transferred to hydroponic medium supplemented with 18 % PEG (m/v) (D), 200 mM NaCl (E) or 100 µM ABA (F) followed by transcript measurements in shoots. Transcripts of AmGORK were measured by quantitative RT-PCR and normalized to control seedlings grown under stress-free conditions (0 h), and expression was reported as mean ± SEM from three independent experiments
Expression analysis of AmGORK
In general consistence with the guard cell expressed GORK gene of Arabidopsis (Ache et al. 2000; Hosy et al. 2003), the transcripts of AmGORK were also primarily accumulated in the green parts of A. mongolicus seedlings, though also detectable in roots (Fig. 1c); therefore we further focused on the expression analysis in the shoots. Upon time coursed quantification of the AmGORK transcripts in shoots by qRT-PCR analysis, the expression was slightly suppressed during the first 12 h of abiotic stresses subjected to the root including PEG (18 % PEG-6000, w/v), NaCl (200 mM) or ABA (100 μM) treatments (Fig. 1d–f). Persistent stresses to the root for 24 h resulted in initiation of the induction of AmGORK transcripts in shoots (PEG and ABA treatments) followed by relatively stronger induction (onefold increase as compared to the initial abundances) at 48 h of treatments (Fig. 1d, f). The induction of AmGORK expression, to certain extent, coincided with an abiotic stress responsive guard cell closure enabled by strengthening K+ release through the GORK channel and showed a delayed response to the stimuli initially subjected to the roots.
Properties of the outward K+ currents in A. mongolicus guard cell protoplasts
Outward rectifying K+ channels expressed in the guard cells have known to mediate K+ efflux that drives the stomatal closure (Hosy et al. 2003). To explore characterizations of outward K+ currents in A. mongolicus guard cell protoplasts, whole-cell patch-clamp experiments were performed as shown in Fig. 2. In response to depolarizing membrane potentials, typical and large K +out currents were present in both voltage- and time-dependent manners (Fig. 2a). In addition, the outward rectifying currents declined when external K+ concentration increased from 1 to 30 mM (reduced by 47 % at membrane potential of +40 mV) as well as external acidification from pH 7.4 to 5.6 (reduced by 63 % at +40 mV) (Fig. 2b, c), indicating Kout currents in guard cells were voltage and K+ concentration dependent, suppressed by extracelluar acidification, which were reminiscent of typical outward currents described in Vicia faba (Blatt 1988; Blatt and Gradmann 1997; Ilan et al. 1994; Schroeder et al. 1987) and Arabidopsis (Ache et al. 2000; Hosy et al. 2003). The results imply that a GORK-related channel may act in the guard cells of A. mongolicus.
Patch-clamp measurements of guard cell protoplasts of A. mongolicus. a Whole-cell recording of outward K+ currents in the presence of 1 mM KCl in the bath solution were made in guard cell protoplasts of A. mongolicus. K +out currents were activated by voltage pulses with 20 mV increment from −40 mV to +80 mV. Average current–voltage relationships in response to K+ concentration increment, b from 1 (solid square) to 30 mM (solid circle) or external pH drop, c from pH 7.4 (solid square) to 5.6 (solid circle). Results were shown as mean ± SE (n = 5 or 6)
Functional expression of AmGORK in oocytes
In order to obtain functional details of AmGORK, two-electrode voltage-clamp experiments were performed on AmGORK-expressing oocytes. Upon the presence of extracelluar K+, outward currents were elicited in response to depolarization voltage pulses imposed to oocyte membrane (from −60 to +40 mV) in oocytes injected with AmGORK (Fig. 3a). However, as demonstrated for the Arabidopsis outward channels AtGORK (Ache et al. 2000) and AtSKOR (Gaymard et al. 1998), the current amplitudes gradually diminished along increased K+ concentrations (10 to 100 mM) presented outside of the oocytes (Fig. 3a, b), indicating a strong dependence of AmGORK mediated K+ release on the external K+ concentration. Visualized from the current–voltage curves presented in Fig. 3b, the AmGORK currents displayed a clear outward-rectification and voltage-gating characteristic. The reversal potential (Vrev) of AmGORK was analyzed at different external K+ concentrations (Fig. 3c). Vrev shifted by 52.4 ± 2.3 mV for a tenfold increase in external K+ concentration, which indicated that AmGORK mainly carried K+. The inhibition of the AmGORK currents showed a reversed saturation kinetic in response to K+ concentrations, with a predicted inhibition coefficient of 59.5 ± 5.5 mM at +40 mV and 26.3 ± 3.2 mM at +20 mV (Fig. 3d). AmGORK was further demonstrated by analysis of the channel’s gating properties with respect to the membrane voltages and concentrations of external K+ (Fig. 3e). AmGORK channel opened only upon the depolarization progress of the membrane potentials with increased open probabilities at more depolarized voltages. External K+ modulated the current amplitudes by shifting the half maximal activation potentials (E 50): gradually increasing the external K+ concentrations from 10 to 50 or 100 mM leading to progressively positive shifts of the E 50 values from −18.5 ± 2.9 mV (10 mM K+) to 23.9 ± 2.5 mV (50 mM K+) or 32.9 ± 4 mV (100 mM K+) (Fig. 3e). Furthermore, the activation half-time of the AmGORK was 526.7 ± 16.6, 806 ± 20 and 947 ± 21.5 ms in the presence of 10, 50 and 100 mM K+, respectively (Fig. 3f, compare the activation half-time under 10 mM K+ to that under 50 or 100 mM K+). To this end, AmGORK mediated both voltage- and external K+-dependent (or gated) K+ release similar to that demonstrated for the Arabidopsis GORK (Ache et al. 2000) and SKOR (Gaymard et al. 1998).
Heterologous expression of AmGORK in Xenopus oocytes. a Outwardly rectifying currents were recorded in an oocyte expressing AmGORK. Voltages applied from a holding potential of −40 mV ranged from −60 to +40 mV with an increment of 10 mV. K+ concentration in the bath solution was 10, 50 or 100 mM, pH 7.4. b Current–voltage relationships at steady state in 10 mM K+ (solid circle), 50 mM K+ (solid square) or 100 mM K+ (solid triangle up). Currents were normalized to values measured in 10 mM KCl at +40 mV. Results were shown as mean ± SE (n = 7 or 8). c Reversal potential (Vrev) was obtained from analysis of deactivating currents. Holding potential was −40 mV. The activating prepulse at +40 mV lasted 3 s. Tail currents were recorded during a 2 s pulse range from −80 to +40 mV in 10 mV increments. Reversal potential were plotted against K+ concentrations (10, 50 and 100 mM, pH 7.4, in log([K+]) scales). The straight line was obtained from a linear regression: Vrev (mV) = 52.4 ± 2.3 × log([K+]) − 109. Results were shown as mean ± SE (n = 5). d Currents recorded at +20 and +40 mV plotted versus the bath K+ concentration (10, 25, 50, 75 and 100 mM, pH 7.4). A predicted inhibition coefficient was fitted with a hyperbolic decay equation. Currents were normalized to values measured in 10 mM KCl at +40 mV. Results were shown as mean ± SE (n = 7 or 8). e Analysis of AmGORK gating property. The line is a fit of voltage-dependent gating at different external K+ concentration (10, 50 or 100 mM, pH7.4) with a sigmoidal equation y = a/{1 + exp[−(x − x 0)/b]}. Results were shown as mean ± SE (n = 6). f The time constants of activating current. The half times were obtained by a single exponential equation fitted to activating current from at +40 mV. Results were shown as mean ± SE (n = 6). Unpaired Student’s t-tests were applied to assess significance. **P < 0.01
In good accordance to the voltage-gated plant K+ channels reported so far, AmGORK was similarly inhibited by external application of classical K+ channel blockers Ba2+ and tetraethylammonium (TEA) (Fig. 4a, b, d). The currents blockage by Ba2+ exhibited voltage-dependent manner. The outward steady-state currents were blocked by 59 ± 1.3 % at potentials of +10 mV and 27 ± 2.3 % at potentials of +40 mV with 5 mM Ba2+ (Fig. 4c). Whereas, the TEA (25 mM) blockade by 77 ± 3 % showed independent of the membrane potentials (Fig. 4e). These results provided further indications that AmGORK acts as a K+-selective channel.
Blockage of outward currents by the external application of K+ channel blockers Ba2+ and TEA. a The outward currents of the AmGORK were blocked by external Ba2+ and TEA. K+ concentration was 10 mM. 5 mM Ba2+ or 25 mM TEA was added into the bath solution. b AmGORK blockage by 5 mM Ba2+ (solid square) at steady state. K+ concentration was 10 mM (pH 7.4). Currents were normalized to values measured in 0 mM Ba2+ (solid circle) at +40 mV. Results were shown as mean ± SE (n = 7). c Inhibition percentage of the AmGORK current blocked by external Ba2+ (5 mM) at different membrane voltages. Data are shown as the percentage of the control (0 mM Ba2+). Results were shown as mean ± SE (n = 7). d AmGORK blockage by 25 mM TEA (solid square) at steady state. K+ concentration was 10 mM (pH 7.4). Currents were normalized to values measured in 0 mM TEA (solid circle) at +40 mV. Results were shown as mean ± SE (n = 6). e Inhibition percentage of the AmGORK currents blocked by external TEA at different membrane voltages. Data are shown as the percentage of the control (0 mM TEA). Results were shown as mean ± SE (n = 6)
In addition to the major voltage-control, extracellular acidification and external Ca2+ were also reported to regulate many plant K+ channels (Blatt 1992; Blatt and Gradmann 1997; Ilan et al. 1994). Therefore, we evaluated the effects of pH and external Ca2+ on the currents of AmGORK. As depicted in Fig. 5, a pH drop from 7.4 to 5.4 resulted in 77 ± 2.5 % reduction of AmGORK currents at +40 mV (Fig. 5a–c). The half activation potential was altered by external pH (ΔE50 = 16.3 ± 2.3 mV, pH 7.4 versus 5.4). External application of ~ tenfold higher concentration of Ca2+ (20 mM), as shown in Fig. 5d, e, resulted in little influence on the macroscopic currents of AmGORK.
Effect of external pH and Ca2+ on steady-state AmGORK current. a Expression of AmGORK in oocytes appeared outward currents with voltage application from a holding potential of −40 mV ranged from −60 to +40 mV with an increment of 10 mV. Outward currents were reduced in the presence of 10 mM K+ in the bath solution, by decreasing external pH from 7.4 to 5.4. b Steady-state currents in response to pH 7.4 (solid circle) and pH 5.4 (solid square) were measured at varied membrane potentials. Currents were normalized to values measured in pH 7.4 at +40 mV. Results were shown as mean ± SE (n = 9). c Inhibition percentage of the AmGORK currents blocked by external protons at different membrane voltages. Results were shown as mean ± SE (n = 9). d Outward currents were recorded in the presence of 10 mM K+ in the bath solution, by increasing external Ca2+ from 1.8 to 20 mM. e Relationship of steady-state current to voltage at 1.8 mM Ca2+ (solid circle) and 20 mM Ca2+ (solid square). K+ concentration in the bath solution was 10 mM, pH 7.4. AmGORK was insensitive to external Ca2+. Currents were normalized to values measured in 1.8 mM Ca2+ at 40 mV. Results were shown as mean ± SE (n = 4)
A single amino acid mutation (D297N) abolishes the voltage sensitivity as well as the rectification of AmGORK
The rectification and the voltage sensitivity are the two major signatures of a K+ channel. Although the Shaker-type K+ channels of plants exhibit divergent voltage-sensitivity and/or distinct rectification, they share high degrees of amino acid sequence and structural similarities. It is reported that amino acids in the S6 transmembrane domain of SKOR, another outward K+ channel of Arabidopsis, are strongly involved in the voltage control, sensitivity to external K+ as well as the rectification of the channel (Johansson et al. 2006; Li et al. 2008). To obtain further molecular insights into the voltage/rectification determinants of AmGORK, with a close alignment of the S6 sequence among AmGORK and known Kout and Kin channels, we generated a D297N single site mutation (Fig. 6a). As shown in Fig. 6b, the neutralization of the negatively charged aspartic acid by asparagine (AmGORK-D297N) resulted in loss of both the strictly voltage-gating and outwardly rectifying properties, and upon membrane depolarization, leak-like inward currents could develop. When 25 mM TEA was added into the external medium, the currents were blocked by 67 ± 8 % (Fig. 6b, c), suggesting that the leak-like current is likely carried by K+. Therefore, the D297N mutation of AmGORK eventually resembled a leak-like weak rectifier such as AKT2/3 (Lacombe et al. 2000).
Single amino acid mutation (D297N) converts AmGORK into an inward rectifier. a Comparison of the amino acid sequences of the S6 regions of inward and outward K+ channels in plants. b Currents recorded from oocytes expression single mutant D297N by the TEVC measurement, with voltage application from a holding potential of −40 mV ranged from −60 to +40 mV with an increment of 10 mV. The bath solution contained 10 mM K+, pH 7.4 (left). Currents of mutant D297N were recorded in bath solution including 10 mM K+ and 25 mM TEA, pH 7.4 (right). c Mutant D297N blockage by 25 mM TEA (solid square) at steady state. K+ concentration was 10 mM (pH 7.4). Results were shown as mean ± SE (n = 5)
Discussion
Since the late 1980’s depolarization-activated outward K+ currents have been repeatedly reported by patch-clamp studies with plant guard cells (Blatt 1988; Blatt and Gradmann 1997; Schroeder et al. 1987). Among several plant species engaged, Vicia faba has attributed the most interests. The guard cell outwardly rectifying currents recognized so far share similar fundamental properties featured as both voltage- and external K+-dependencies of the channel’s gating, and the inhibitory regulation upon extracellular acidification (Ache et al. 2000; Blatt 1988; Blatt and Gradmann 1997; Hosy et al. 2003; Ilan et al. 1994; Schroeder et al. 1987). The currents recorded in guard cells of A. mongolicus (Fig. 2), largely resemble those previously obtained in Vicia faba (Blatt 1988; Blatt and Gradmann 1997; Schroeder et al. 1987) and Arabidopsis (Ache et al. 2000; Eisenach et al. 2014; Hosy et al. 2003), suggesting that this type of K+ efflux is highly conserved in guard cells of various plant species, and rationally is mediated by a similar channel protein.
The molecular identification of such current applauses mostly, the discovery of the GORK channel in Arabidopsis (Ache et al. 2000; Eisenach et al. 2014; Hosy et al. 2003). In Arabidopsis nine Shaker type K+ channels have been identified, with only SKOR and GORK exhibited clear outward rectification. SKOR is a root stelar located K+ release channel primarily responsible for the uploading of K+ to the green tissues (Gaymard et al. 1998). Although GORK is designated as a guard cell outward rectifier, and indeed plays a dominant role in mediating the Kout currents in guard cells (Hosy et al. 2003), its localization, however, is not exclusively constrained in the guard cells. The transcripts of AtGORK are detectable in vascular tissues of the root and shoot and also weakly in the root hairs (Ache et al. 2000; Becker et al. 2003; Hosy et al. 2003; Ivashikina et al. 2001). Importantly, despite the different localization and accordingly distinct physiological relevance they coordinate in Arabidopsis, GORK and SKOR share major functional similarities characterized as voltage-gated Kout channels, and they both are dependent on external K+ and regulated by pH (Ache et al. 2000; Gaymard et al. 1998; Lacombe et al. 2000). This provides an implication that the functional features of outwardly rectifying K+ channels are possibly conserved regardless their different competition in the plant.
The presence of GORK genes has been described in Samanea saman (Moshelion et al. 2002), maize (Buchsenschutz et al. 2005) and tobacco (Dai et al. 2009), and their expression profiles coincide with a role in regulating the movement of stomata. Though to the functional respects, it remains necessary to clarify whether the GORK homologues of various plant species act similarly or engage distinctive regulatory mechanisms that satisfy particular needs of the plants of origin.
Ammopiptanthus mongolicus (Maxim.) Cheng f., is a leguminous shrub with ancient origin tracing back to the age that mammals begun. The plant has survived over extremely arid environment along a long historical era. This plant develops morphological as well as biochemical/mechanism based adaptations to the consistent drought stresses (Liu et al. 2013; Wu et al. 2014). Therefore, we are particularly interested in the assessment of mechanisms related to persistent drought stress resistance using this plant as a model. Our previous RNA-Seq analysis aiming at identifying sensitive genes to severe water stresses (Man Jin et al., submitted) provides ample resources for cloning of homologous new genes including GORK. Besides the high degrees (especially within the transmembrane domains and the pore motifs) of amino acid sequence similarities to the well-defined Arabidopsis GORK (Fig. 1a, b and supplemental data 1), the predominant expression in the green parts and the regulation by typical stress conditions bring AmGORK closer to a guard cell GORK channel (Fig. 1c). The consistence in principally functional properties between the outward K+ currents in the guard cells (Fig. 2) and the cloned AmGORK assessed in the oocytes (Fig. 3, for instance) provides further evidence that AmGORK is very likely a key K+ release channel in the guard cells of A. mongolicus.
To the functional respects, AmGORK, as its Arabidopsis counterpart (Ache et al. 2000), or even similar to the SKOR channel (Gaymard et al. 1998), is activated upon depolarization of membrane potentials, showing both voltage- and external K+-dependent gating (Fig. 3). The macroscopic amplitudes show a saturate inhibitory relationship with the concentrations of external K+ with a coefficient of 59.5 ± 5.5 mM at +40 mV or 26.3 ± 3.2 mM at +20 mV (Fig. 3d). Similar as described in Vicia faba guard cells (Blatt and Gradmann 1997), increased external K+ results in positive shifts of the half-activation voltages also by approximately 50 mV per decade of K+ concentration [compare data in Fig. 3e with (Blatt and Gradmann 1997)]. Meanwhile, the half-times of AmGORK current rising are altered in response to Kext with faster activation under lower concentration of Kext (Fig. 3f), also consisting with earlier findings in the guard cells of Vicia faba (Blatt 1988). As a common recognition to the voltage-gated K+ channels, AmGORK is inhibited by classic channel blockers Ba2+ and TEA (Fig. 4).
The guard cell outward rectifying current in Vicia faba (Ilan et al. 1994) and the cloned Arabidopsis GORK (Ache et al. 2000) and SKOR (Gaymard et al. 1998) are sensitive to the extracellular pH. Similarly, the Kout current in A. mongolicus guard cells is significantly suppressed upon acidification external to the cells (Fig. 2). In the heterologous expression system, cloned AmGORK current is reduced by over 70 % following a 2 pH-unit acidification and the half activation voltage shifts to the positive directions (Fig. 5a, b).
To this end, this bundle of functional analyses indicate that the function of AmGORK, to a great extent, resembles its Arabidopsis counterpart, and may also similar to the potential GORK (if being identified and cloned in future) of Vicia faba.
Extensive site-directed mutation analyses with voltage-gated K+ channels have provided convincing supports that many key functional properties are determined by particular structure elements. Our results have shown that in AmGORK, a single amino acid mutation D297N of the S6 segment eventually abolishes both the voltage-gating and its outward rectification, thus converts AmGORK into a leak-like channel resembling the characteristic of AKT2/3 (Lacombe et al. 2000). This provides additional supports to the findings that amino acids of the S6 domain, are strongly involved in the gating and voltage dependence of plant Kout channels (Johansson et al. 2006; Li et al. 2008).
In summary, by functional identification of a GORK homologue from the ancient plant species A. mongolicus, we provide evidence that the function and/or major regulatory mechanisms of the guard cell K+ release channel of this type, is strongly conserved among plant species and may also along the long historic progress of evolution.
Author contribution statement
YHS, JLL and HCZ designed the work, JLL performed all electrophysiology measurements, LH carried out all molecular work, MJ and YGZ prepared plant materials, JLL and YHS wrote the paper. YHS and HCZ supervised the study. All authors read and approved the manuscript.
References
Ache P, Becker D, Ivashikina N, Dietrich P, Roelfsema MR, Hedrich R (2000) GORK, a delayed outward rectifier expressed in guard cells of Arabidopsis thaliana, is a K+-selective, K+-sensing ion channel. FEBS Lett 486:93–98
Becker D, Hoth S, Ache P, Wenkel S, Roelfsema MRG, Meyerhoff O, Hartung W, Hedrich R (2003) Regulation of the ABA-sensitive Arabidopsis potassium channel gene GORK in response to water stress. FEBS Lett 554:119–126
Blatt MR (1988) Potassium-Dependent, Bipolar Gating of K+ Channels in Guard-Cells. J Membr Biol 102:235–246
Blatt MR (1992) K+ channels of stomatal guard-cells—characteristics of the inward rectifier and its control by pH. J Gen Physiol 99:615–644
Blatt MR, Gradmann D (1997) K+-sensitive gating of the K+ outward rectifier in Vicia guard cells. J Membr Biol 158:241–256
Buchsenschutz K, Marten I, Becker D, Philippar K, Ache P, Hedrich R (2005) Differential expression of K+ channels between guard cells and subsidiary cells within the maize stomatal complex. Planta 222:968–976
Dai XY, Su YR, Wei WX, Wu JS, Fan YK (2009) Effects of top excision on the potassium accumulation and expression of potassium channel genes in tobacco. J Exp Bot 60:279–289
Eisenach C, Papanatsiou M, Hillert EK, Blatt MR (2014) Clustering of the K+ channel GORK of Arabidopsis parallels its gating by extracellular K+. Plant J 78:203–214
Gaymard F, Pilot G, Lacombe B, Bouchez D, Bruneau D, Boucherez J, Michaux-Ferriere N, Thibaud JB, Sentenac H (1998) Identification and disruption of a plant shaker-like outward channel involved in K+ release into the xylem sap. Cell 94:647–655
Hosy E, Vavasseur A, Mouline K, Dreyer I, Gaymard F, Poree F, Boucherez J, Lebaudy A, Bouchez D, Very AA, Simonneau T, Thibaud JB, Sentenac H (2003) The Arabidopsis outward K+ channel GORK is involved in regulation of stomatal movements and plant transpiration. Proc Natl Acad Sci USA 100:5549–5554
Ilan N, Schwartz A, Moran N (1994) External pH effects on the depolarization-activated K-channels in guard-cell protoplasts of Vicia faba. J Gen Physiol 103:807–831
Ivashikina N, Becker D, Ache P, Meyerhoff O, Felle HH, Hedrich R (2001) K+ channel profile and electrical properties of Arabidopsis root hairs. FEBS Lett 508:463–469
Johansson I, Wulfetange K, Poree F, Michard E, Gajdanowicz P, Lacombe B, Sentenac H, Thibaud JB, Mueller-Roeber B, Blatt MR, Dreyer I (2006) External K+ modulates the activity of the Arabidopsis potassium channel SKOR via an unusual mechanism. Plant J 46:269–281
Lacombe B, Pilot G, Gaymard F, Sentenac H, Thibaud JB (2000a) pH control of the plant outwardly-rectifying potassium channel SKOR. FEBS Lett 466:351–354
Lacombe B, Pilot G, Michard E, Gaymard F, Sentenac H, Thibaud JB (2000b) A shaker-like K+ channel with weak rectification is expressed in both source and sink phloem tissues of Arabidopsis. Plant Cell 12:837–851
Langer K, Ache P, Geiger D, Stinzing A, Arend M, Wind C, Regan S, Fromm J, Hedrich R (2002) Poplar potassium transporters capable of controlling K+ homeostasis and K+-dependent xylogenesis. Plant Journal 32:997–1009
Li LG, Liu K, Hu Y, Li DP, Luan S (2008) Single mutations convert an outward K+ channel into an inward K+ channel. Proc Natl Acad Sci USA 105:2871–2876
Liu M, Shi J, Lu C (2013) Identification of stress-responsive genes in Ammopiptanthus mongolicus using ESTs generated from cold- and drought-stressed seedlings. BMC Plant Biol 13:88
Luan S (2002) Signalling drought in guard cells. Plant, Cell Environ 25:229–237
Moshelion M, Becker D, Czempinski K, Mueller-Roeber B, Attali B, Hedrich R, Moran N (2002) Diurnal and circadian regulation of putative potassium channels in a leaf moving organ. Plant Physiol 128:634–642
Pei ZM, Kuchitsu K, Ward JM, Schwarz M, Schroeder JI (1997) Differential abscisic acid regulation of guard cell slow anion channels in Arabidopsis wild-type and abi1 and abi2 mutants. Plant Cell 9:409–423
Poree F, Wulfetange K, Naso A, Carpaneto A, Roller A, Natura G, Bertl A, Sentenac H, Thibaud JB, Dreyer I (2005) Plant K in and K out channels: approaching the trait of opposite rectification by analyzing more than 250 KAT1-SKOR chimeras. Biochemical And Biophysical Research Communications 332:465–473
Schroeder JI (2003) Knockout of the guard cell K+ out channel and stomatal movements. Proc Natl Acad Sci USA 100:4976–4977
Schroeder JI, Raschke K, Neher E (1987) Voltage dependence of K+ channels in guard-cell protoplasts. Proc Natl Acad Sci USA 84:4108–4112
Shi J, Liu M, Shi J, Zheng G, Wang Y, Wang J, Chen Y, Lu C, Yin W (2012) Reference gene selection for qPCR in Ammopiptanthus mongolicus under abiotic stresses and expression analysis of seven ROS-scavenging enzyme genes. Plant Cell Rep 31:1245–1254
Su YH, North H, Grignon C, Thibaud JB, Sentenac H, Very AA (2005) Regulation by external K+ in a maize inward Shaker channel targets transport activity in the high concentration range. Plant Cell 17:1532–1548
Wu YQ, Wei W, Pang XY, Wang XF, Zhang HL, Dong B, Xing YP, Li XU, Wang MY (2014) Comparative transcriptome profiling of a desert evergreen shrub, Ammopiptanthus mongolicus, in response to drought and cold stresses. BMC Genom 15:671
Acknowledgments
This work was supported by the National Science Foundation of China (91125028) and the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB15030202) and a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Y.-I. Park.
J. Li and H. Zhang contribute equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, J., Zhang, H., Lei, H. et al. Functional identification of a GORK potassium channel from the ancient desert shrub Ammopiptanthus mongolicus (Maxim.) Cheng f.. Plant Cell Rep 35, 803–815 (2016). https://doi.org/10.1007/s00299-015-1922-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-015-1922-6