Abstract
In plants, many small interfering RNAs (siRNAs) direct de novo methylation by DNA methyltransferase. DNA methylation typically occurs by RNA-directed DNA methylation (RdDM), which directs transcriptional gene silencing of transposons and endogenous transgenes. RdDM is driven by non-coding RNAs (ncRNAs) produced by DNA-dependent RNA polymerases IV and V (PolIV and PolV). The production of siRNAs is initiated by PolIV and ncRNAs produced by PolIV are precursors of 24-nucleotide siRNAs. In contrast, ncRNAs produced by PolV are involved in scaffolding RNAs. In this review, we summarize recent studies of RdDM. In particular, we focus on the mechanisms involved in chromatin remodeling by PolIV and PolV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Overview of RdDM and its function
Zemach et al. (2013) reported that DNA methylation is a conserved epigenetic process in plants and animals. In plants, many 80-nucleotide, long non-coding RNAs (lncRNAs) and 24-nucleotide, small interfering RNAs (siRNAs) direct de novo DNA methylation, which is involved in post-transcriptional gene silencing (PTGS) or transcriptional gene silencing (TGS) stimulated by biotic or abiotic stresses. This process is described as RNA-directed DNA methylation (RdDM) (Onodera et al. 2005) and is a conserved phenomenon mediated by TGS in plants, animals, and fungi (Zhang et al. 2013; Castel and Martienssen 2013). Indeed, RdDM is an essential process for suppressing transposons, DNA damage caused by stress, and DNA nucleases. The process of DNA methylation is an important mechanism for the suppression of repetitive elements (transposons) and stabilization of the genome by silencing endogenous genes (Zhang and Zhu 2011). Small RNAs (sRNAs) 20–40 nucleotides in length that are associated with PIWI-interacting RNAs (piRNAs) catalyze heterochromatic histone modification or DNA methylation recruited by lncRNAs, leading to chromatin remodeling (Zhang et al. 2013).
In plants, the RdDM pathway causes the formation of siRNAs involved in TGS. Some small binding proteins with conserved biochemical activities, such as ARGONAUTE (AGO), play similar roles in various organisms (Vaucheret 2008). In animals, non-coding RNAs (ncRNAs) are generated only by DNA-dependent RNA polymerase II (PolII), but are generated by PolII, PolIV, and PolV in plants. RNA-dependent RNA polymerase 2 (RDR2) is a small protein that receives transcribed messenger RNAs (mRNAs) from PolIV to form stem–loop structures and double-stranded RNAs (dsRNAs) (Haag et al. 2012). Some minor protein factors, known as ATP-dependent chromatin-remodeling (CLSY1) proteins, help RDR2 form dsRNAs using nuclear nucleotides (Smith et al. 2007). After forming dsRNAs 70–80 nucleotides in length, Dicer-like 3 (DCL3) protein, which is a member of the ribonuclease III (RNase III) family of enzymes, cleaves stem–loop mRNAs to form 20–24 nucleotide siRNAs with a 2-nucleotide 3′ overhang. HUA ENHANCER (HEN1) protein, which is a methyltransferase enzyme, methylates siRNAs at the 3′ end to prevent cellular nuclease activities. The presence of the RNA-induced silencing complex (RISC) transfers the 3′ overhang duplex siRNAs to the target mRNA resulting in direct silencing of expression. The RISC binds to duplex siRNAs and unwinds them with its helicase activity (Yang et al. 2006). This process is associated with the PAZ domain, which is found in the AGO, P-element-induced wimpy testis (PIWI), and ZWILLE proteins (Baumberger and Baulcombe 2005). PAZ endonuclease activity separates siRNA duplexes, resulting in the formation of appropriate antisense single-stranded siRNAs that are complementary to target mRNAs. Finally, these siRNAs are loaded onto AGO4/6/9 which leads to RdDM. RdDM targets specific sites to recruit domain rearranged methyltransferase 2 (DRM2), which is an important plant ortholog of the mammalian de novo DNA methyltransferase DNMT3, to initiate de novo DNA methylation (Cao 2002). De novo methylation initiated by DRM2 is associated with DNA methyltransferase 1 (MET 1). In plants, MET1 assists chromomethylase 3 (CMT3), a plant-specific methyltransferase enzyme, to methylate specific CG and CHG sites to cause de novo methylation (Zhu 2008; Aufsatz et al. 2004; Martinez de Alba 2013; Zhu et al. 2013).
The RdDM pathway
PolII depends on PolIV and PolV and has a unique function in the RdDM pathway. PolIV generates single-stranded RNAs (ssRNAs), which are presumed to be precursors of siRNAs. Most 20–24 nucleotide siRNAs are derived from repetitive sequences or transposons. The biogenesis of these siRNAs depends on the plant-specific RdDM pathway in which methylated single-stranded siRNAs are associated with AGO4/6/9 proteins (Zhang and Zhu 2011; Martinez de Alba 2013; Zhu et al. 2013; Havecker et al. 2010). Altered functions among AGO4/6/9 proteins are related to the location of expression in the plant. According to Havecker et al. (2010) AGO4 is typically expressed in plants from buds to roots, but AGO6 is expressed in the apical meristems of shoots or roots and in regenerating tissues. Association of AGO4/6/9 with methylated single-stranded siRNAs triggers de novo methylation leading to transcriptional silencing of repetitive sequences and transposons (Henderson and Jacobsen 2007; Zaratiegui et al. 2007).
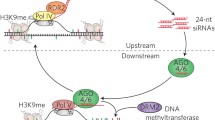
As shown in Fig. 1, PolIV associates with both SNF2-like chromatin-remodeling factor, called CLSY1 (Pontier et al. 2005; Kanno et al. 2005; Herr et al. 2005; Onodera et al. 2005), and a small protein known as SAWADEE HOMEODOMAIN HOMOLOG 1 (SHH1), which detects histone H3K9me2 (Law et al. 2011; He et al. 2009). DNA transcription factor 1 (DTF1) associates with SHH1 and contributes to the detection of the exact target by PolIV. This protein may assist DNA-directed RNA polymerase IV subunit 1 (NRPD1) which is the largest subunit of PolIV (Law et al. 2011). The DTF1 gene is plant specific and encodes a DTF1 protein 167 amino acids in length, and contains a cryptic homeodomain in the N-terminal region and a C-terminal domain called SAWADEE that is conserved in plants (Mukherjee et al. 2009). The cryptic homeodomain is a conserved DNA-binding domain with a three-helix structure (Billeter 1996). In contrast, the SAWADEE domain adopts a specific chromo barrel structure involved in detecting and binding to modified histones (Zhang et al. 2013; Law et al. 2013). Mutations in genes encoding DTF1 and PolIV subunit NRPD1 have significant effects on siRNA levels. The nrpd1 mutant exhibited a reduction in the level of siRNAs of less than 10 %, whereas the dtf1 mutant exhibited a decrease of approximately 28 % in the level of siRNAs. Studies on mutations also showed that the mutation in nrpe1 leads to a decrease in the level of siRNAs of ~50 % (Zhang et al. 2013). Based on these results, mutations in downstream genes involved in RdDM cause greater decreases in siRNA levels than mutations in upstream genes and lead to decreased DNA methylation across the entire genome. These studies also showed that association of DTF1 protein with PolIV is necessary for the initiation of RdDM. The specific function of the SAWADEE domain is the detection of histone H3 peptides methylated at lysines 2, 3, and 9 (Zhang et al. 2013). Thus, DTF1 recruits PolIV to methylate histone H3K9 (Law et al. 2013).
RNA-directed DNA methylation in plants. The SHH1/DTF1 complex recruits PolIV to chromatin and CLSY1 causes it to initiate RdDM. PolIV transcribes methylated DNA, transposable elements, or repetitive sequences into ssRNA. RDR2 converts ssRNA into dsRNA by interacting with IDN2/RDM12. The SR45 splicing factor acts on PolIV-RDR2 upstream of RdDM to drive siRNA biogenesis. The dsRNA is cleaved by DCL3 into 24-nucleotide siRNA duplexes with 3′ overhangs, which are methylated by HEN1. SiRNA duplexes are converted to one strand and loaded into the RITS complex containing AGO. In contrast, PolV transcribes scaffold RNA from the DNA template. The DDR complex containing RDM1, DRD1, DMS3, and DMS11 associates with PolV to mediate scaffold RNA transcription to recruit DRM2, leading to DNA methylation at specific target loci. KTF1 interacts with the AGO–siRNA complex factor associated with RDM16 leading to the recruitment of STA1, POZ1 splicing factors. RDM12/IDN2 associates with SWI3B to stabilize the interactions between siRNAs and the scaffold RNA to regulate nucleosome positioning
As shown in Fig. 1, methylated DNA is transcribed into ssRNAs by PolIV, which recruits RDR2 leading to the formation of dsRNAs from ssRNAs using RNA-directed DNA methylation 4 (RDM4) protein. RDR2 recruits the INVOLVED IN DE NOVO 2 (IDN2) protein, which is associated with the RNA-directed DNA methylation 12 (RDM12) protein (Xie et al. 2004). Subsequently, DCL3 cleaves the dsRNAs into 24-nucleotide siRNA duplexes to recruit HEN1 to methylate 3′ overhang siRNA duplexes resulting in the avoidance of endonuclease enzyme activities (Xie et al. 2004). Then, methylated siRNA duplexes are loaded onto a RISC-like complex known as the RNA-induced transcriptional silencing (RITS) complex (Verdel et al. 2004). The RITS complex, which includes AGO4/6/9 protein, directs both CG and non-CG DNA methylation leading to the detection of the target loci on the RNA scaffold. This function is related to transcription of the RNA scaffold by PolV interacting with RITS resulting in the recruitment of the DDR complex (Law et al. 2010). The DDR complex is an association of proteins that act widely in silencing but are defective in distinct areas. PolV recruits chromatin-remodeling proteins including defective RNA-directed DNA methylation 1 (DRD1), defective meristem silencing 3 (DMS3), and RDM1 (Law et al. 2010; Kanno et al. 2004).
A chromatin-remodeling factor called SNF2 binds to RDM1 leading to the formation of DMS3, DMS11/GHKL ATPase complex (Lorkovic et al. 2012). In contrast, PolV recruits de novo methyltransferase proteins such as domain rearranged methyltransferase 2 (DRM2) during transcription of the RNA scaffold and interacts with AGO4/6/9–siRNA complex, resulting in linkage of RDM3 and KOW domain-containing transcription factor 1 (KTF1), which is a conserved PolII transcriptional elongation factor (He et al. 2009). The RDM12/IDN2 complex acts as a stabilizer to regulate interactions between AGO4/6/9–siRNAs and PolV (Ausin et al. 2009).
In addition, IDN2 stimulates the switch subunit 3B (SWI3B) protein, which is a subunit of the SWI/SNF DNA remodeling complex, to interact with PolIV, resulting in regulation of the position of DNA methylation inside the nucleus (Zhu et al. 2013). Recently, PolII has been also suggested to play a key role in the RdDM pathway. PolII recruits PolIV and PolV dependent on siRNAs in the exact DNA target (Zheng et al. 2009). Many coordinators regulate the activity of effector proteins in RdDM. In yeast, for example, a transcription factor complex containing RNA PolII (IWR1)/RDM4/DMS4 regulates interactions between PolII, PolIV, and PolV (He et al. 2009; Kanno et al. 2010). In addition, an ATPase domain termed Morpheus’ molecule 1 (MOM1), which is a protein homologous to the SWI2/SNF2 chromatin remolding complex, interacts with PolIV and PolV, resulting in regulation of TGS (Yokthongwattana et al. 2010). According to Zhang et al. (2013), the complex of the zinc finger (ZnF) and OCRE domain-containing Protein 1 (ZOP1) is another splicing factor that affects RdDM directly. Zhang et al. (2013) reported that ZOP1 is expressed downstream of RdDM and is necessary for the pathway (Fig. 1). Moreover, immunofluorescence assays showed that mutation of the ZOP1 gene in the zop1 mutant led to decreased PolIV levels and an eventual reduction in RdDM function (Zhang et al. 2013). ZOP1 has been reported to regulate the RdDM pathway directly in association with NPRE1 and DRM2 (Huang and Zhu 2014). In contrast, ZOP1 appears in condensed nucleolus foci including Cajal bodies, which function as the small ribonucleoprotein particle (snRNP) assembly center containing the U1, U2, U5, and U4/U6 snRNAs (Sanford et al. 2005; Morris 2008) and is required for AGO4/6/9 complex formation with 3′ overhang siRNAs (Li et al. 2008). Although, ZOP1 affects PolII and regulates its role in pre-mRNA splicing separate from RdDM (Zhang et al. 2013), recent studies showed that PolII also participates in the RdDM pathway through the generation of non-coding transcripts, leading to the recruitment of AGO4/6/9 (Zheng et al. 2009).
TGS and DNA methylation in the RdDM pathway require specific DNA methyltransferases such as MET1, CMT3, and DRM2 (Kankel et al. 2003). The balance between DNA methylation and DNA demethylation is a requirement for cell survival and is the reason that the action of DNA methyltransferase enzymes is balanced with the action of DNA demethylating glycosylase enzymes, such as Demeter (DME), Demeter-like 2 (DML2), and Demeter-like 3 (DML3) or repressor of silencing 1 (ROS1) and ROS3 (Zheng et al. 2008).
In histone modification, lysines 9 and 27 of histone H3 can be either methylated or acetylated through chromatin remodeling. Some components implicated as being involved in histone modifications are histone deacetylase 6 (HDA6), decreased in DNA methylation 1 (DDM1), ubiquitin protease 26 (UBP26, which deubiquitinates histone H2B and is required for DNA methylation, especially heterochromatic histone H3 methylation), DRD1, and histone methyltransferase SU-(Var)-2-9 homolog (SUVH2-9) (Ebbs and Bender 2006; Ebbs et al. 2005; Aufsatz et al. 2002; Sridhar et al. 2007).
Histone methyltransferase SUHV4 is necessary for the methylation of CHG sites through binding of the SRA methyl-cytosine-binding domain and CMT3, which binds to histone H3 methylated via the chromo domain tail of SUVH4 (Johnson et al. 2007). Thus, histone modification plays an important role in DNA methylation through both methylation and acetylation.
Studies of the RdDM pathway have shown that the RDM family proteins are important regulatory factors with various functions that regulate this pathway. For instance, RDM4 encodes a conserved transcription factor that affects both PolV by transcription of DNA intergenic sequences and PolII by transcription with a pleiotropic effect so, regulates PolII and PolV (He et al. 2009). Nevertheless, the RDM12 protein, which is identified as IDN2 and includes XS domain-containing SGS3 protein, regulates PolIV leading to transcription of 5′ overhang dsRNAs via a facilitator for RDR2, but it functions as a stabilizer of the interaction between the complex of AGO-3′ methylated overhang siRNAs and PolV results in transcription of scaffold RNAs (Ausin et al. 2009; Zhu et al. 2013). Additional studies revealed that RDM16 participates in pre-mRNA splicing and affects the RdDM pathway directly (Huang et al. 2013). A mutation in RDM16 caused PolV transcripts to decrease but chromatin immunoprecipitation (ChIP) assays showed that the content of RDM16 in PolV target loci was rich (Huang et al. 2013). This observation emphasizes that PolV recruits RDM16 resulting in the regulation of PolV transcription (Huang et al. 2013) (Fig. 1).
Splicing factors play critical roles in the RdDM pathway. The SR45 gene encodes the SR45 splicing factor, which contains a domain that is rich in serine and arginine and is essential for pre-mRNA splicing associated with PolIV and directs RDR2 leading to the production of dsRNAs (Huang and Zhu 2014). This factor is also suggested to be recruited by PolIV and RDR2 downstream of siRNA biogenesis (Huang and Zhu 2014). Ausin et al. (2012) stated that the mutation in this gene in the sr45 mutant caused DNA methylation to decrease and caused a decreased flowering phenotype. The sr45 mutation also caused a decrease in the AGO4 content and removed it from the RdDM pathway upstream of siRNA biogenesis (Li et al. 2006). Based on this result, SR45 should be expressed upstream of the RdDM pathway, leading to regulation of the pathway (Ausin et al. 2012).
As shown in Fig. 1, SRA1 (U5 snRNP) acts as an additional splicing factor involved in RdDM (Dou et al. 2013). Recent studies showed that SRA1 regulates the content of siRNAs by affecting PolV. Also, these studies indicated that SRA1 appears in Cajal bodies similar to POZ1 and associates with NRPE1 (Dou et al. 2013). Thus, SRA1 functions downstream of the RdDM pathway, leading to effects on PolV transcription and the regulation of siRNA biogenesis.
Conclusion
Many questions remain to be answered regarding RdDM and gene silencing by TGS and epigenetics in cells. For instance, which additional specific proteins are involved in the interaction with DRM2? Which additional specific enzymes such as splicing factors are involved in this pathway? Furthermore, many components of the RdDM pathway remain to be identified using biochemical and biological approaches and genetic screening.
Author contribution statement
In this review, all the authors have been directed by Dr. Ali Movahedi as the first author. Also, Dr. Weibu Sun and Dr. Jiaxin Zhang contributed equally as the second author and Prof. Qiang Zhuge is the correspondent in this review. The authors have no conflict of interest to claim.
Abbreviations
- lncRNAs:
-
Long non-coding RNAs
- siRNAs:
-
Small interfering RNAs
- PTGS:
-
Post-transcriptional gene silencing
- TGS:
-
Transcriptional gene silencing
- RdDM:
-
RNA-directed DNA methylation
- AGO:
-
ARGONAUTE
- PolII:
-
RNA polymerase II
- RDR2:
-
RNA-dependent RNA polymerase 2
- dsRNAs:
-
Double-stranded RNAs
- DRM2:
-
Domain rearranged methyltransferase 2
- RDM:
-
RNA-directed DNA methylation
- RITS:
-
RNA-induced transcriptional silencing complex
References
Aufsatz W, Mette MF, van der Winden J, Matzke M, Matzke AJ (2002) HDA6, a putative histone deacetylase needed to enhance DNA methylation induced by double-stranded RNA. EMBO J 21:6832–6841
Aufsatz W, Mette MF, Matzke AJ, Matzke M (2004) The role of MET1 in RNA-directed de novo and maintenance methylation of CG dinucleotides. Plant Mol Biol 54:793–804
Ausin I, Mockler TC, Chory J, Jacobsen SE (2009) IDN1 and IDN2 are required for de novo DNA methylation in Arabidopsis thaliana. Nat Struct Mol Biol 16:1325–1327
Ausin I, Greenberg MVC, Li CF, Jacobsen SE (2012) The splicing factor SR45 affects the RNA-directed DNA methylation pathway in Arabidopsis. Epigenetics 7:29–33
Baumberger N, Baulcombe DC (2005) Arabidopsis ARGONAUTE1 is an RNA slicer that 294 selectively recruits microRNAs and short interfering RNAs. Proc Natl Acad Sci USA 295(102):11928–11933
Billeter M (1996) Homeodomain-type DNA recognition. Prog Biophys Mol Biol 66:211–225
Cao X, Jacobsen SE (2002) Role of the Arabidopsis DRM methyltransferases in de novo DNA methylation and gene silencing. Curr Biol 12:1138–1144
Castel SE, Martienssen RA (2013) RNA interference in the nucleus: roles for small RNAs in transcription, epigenetics and beyond. Nat Rev Genet 14:100–112
Dou K, Huang CF, Ma ZY, Zhang CJ, Zhou JX, Huang HW, Cai T, Tang K, Zhu JK, He XJ (2013) The PRP6-like splicing factor STA1 is involved in RNA-directed DNA methylation by facilitating the production of PolV-dependent scaffold RNAs. Nucl Acids Res 41:8489–8502
Ebbs ML, Bender J (2006) Locus-specific control of DNA methylation by the Arabidopsis SUVH5 histone methyltransferase. Plant Cell 18:1166–1176
Ebbs ML, Bartee L, Bender J (2005) H3 lysine 9 methylation is maintained on a transcribed inverted repeat by combined action of SUVH6 and SUVH4 methyltransferases. Mol Cell Biol 25:10507–10515
Haag JR, Ream TS, Marasco M, Nicora CD, Norbeck AD, Pasa-Tolic L, Pikaard CS (2012) In vitro transcription activities of PolIV, PolV, and RDR2 reveal coupling of PolIV and RDR2 for dsRNA synthesis in plant RNA silencing. Mol Cell 48:811–818
Havecker ER, Wallbridge LM, Hardcastle TJ, Bush MS, Kelly KA, Dunn RM, Schwach F, Doonan JH, Baulcombe DC (2010) The Arabidopsis RNA-directed DNA methylation argonautes functionally diverge based on their expression and interaction with target loci. Plant Cell 22:321–334
He XJ, Hsu YF, Pontes O, Zhu J, Lu J, Bressan RA, Pikaard C, Wang CS, Zhu JK (2009a) NRPD4, a protein related to the RPB4 subunit of RNA polymerase II, is a component of RNA polymerases IV and V and is required for RNA-directed DNA methylation. Genes Dev 23:318–330
He XJ, Hsu YF, Zhu S, Liu HL, Pontes O, Zhu J, Cui X, Wang CS, Zhu JK (2009b) A conserved transcriptional regulator is required for RNA directed DNA methylation and plant development. Genes Dev 23:2717–2722
Henderson IR, Jacobsen SE (2007) Epigenetic inheritance in plants. Nature 447:418–424
Herr AJ, Jensen MB, Dalmay T, Baulcombe DC (2005) RNA polymerase IV directs silencing of endogenous DNA. Science 308:118–120
Huang CF, Zhu JK (2014) RNA splicing factors and RNA-directed DNA methylation. Biology 3:243–254
Huang CF, Miki D, Tang K, Zhou HR, Zheng Z, Chen W, Ma ZY, Yang L, Zhang H, Liu R (2013) A Pre-mRNA-splicing factor is required for RNA-directed DNA methylation in Arabidopsis. PLoS Genet 9:e1003779
Johnson LM, Bostick M, Zhang X, Kraft E, Henderson I, Callis J, Jacobsen SE (2007) The SRA methyl-cytosine-binding domain links DNA and histone methylation. Curr Biol 17:379–384
Kankel MW, Ramsey DE, Stokes TL, Flowers SK, Haag JR, Jeddeloh JA, Riddle NC, Verbsky ML, Richards EJ (2003) Arabidopsis MET1 cytosine methyltransferase mutants. Genetics 163:1109–1122
Kanno T, Mette MF, Kreil DP, Aufsatz W, Matzke M, Matzke AJ (2004) Involvement of putative SNF2 chromatin remodeling protein DRD1 in RNA-directed DNA methylation. Curr Biol 14:801–805
Kanno T, Huettel B, Mette MF, Aufsatz W, Jaligot E, Daxinger L, Kreil DP, Matzke M, Matzke AJ (2005) A typical RNA polymerase subunit required for RNA-directed DNA methylation. Nat Genet 37:761–765
Kanno T, Bucher E, Daxinger L, Huettel B, Kreil DP, Breinig F, Lind M, Schmitt MJ, Simon SA, Gurazada SG, Meyers BC, Lorkovic ZJ, Matzke AJ, Matzke M (2010) RNA-directed DNA methylation and plant development require an IWR1-type transcription factor. EMBO Rep 11:65–71
Law JA, Ausin I, Johnson LM, Vashisht AA, Zhu JK, Wohlschlegel JA, Jacobsen SE (2010) A protein complex required for polymerase V transcripts and RNA-directed DNA methylation in Arabidopsis. Curr Biol 20:951–956
Law JA, Vashisht AA, Wohlschlegel JA, Jacobsen SE (2011) SHH1, a homeodomain protein required for DNA methylation, as well as RDR2, RDM4, and chromatin remodeling factors, associate with RNA polymerase IV. PLoS Genet 7:e1002195
Law JA, Du J, Hale CJ, Feng S, Krajewski K, Palanca AM, Strahl BD, Patel DJ, Jacobsen SE (2013) Polymerase IV occupancy at RNA-directed DNA methylation sites requires SHH1. Nature 498:385–389
Li CF, Pontes O, El-Shami M, Henderson IR, Bernatavichute YV, Chan SWL, Lagrange T, Pikaard CS, Jacobsen SE (2006) An ARGONAUTE4-containing nuclear processing center co-localized with Cajal bodies in Arabidopsis thaliana. Cell 126:93–106
Li CF, Henderson IR, Song L, Fedoroff N, Lagrange T, Jacobsen SE (2008) Dynamic regulation of ARGONAUTE4 within multiple nuclear bodies in Arabidopsis thaliana. PLoS Genet 4:e27
Lorkovic ZJ, Naumann U, Matzke AJ, Matzke M (2012) Involvement of a GHKL ATPase in RNA-directed DNA methylation in Arabidopsis thaliana. Curr Biol 22:933–938
Martinez de Alba AE, Elvira-Matelot E, Vaucheret H (2013) Gene silencing in plants: a diversity of pathways. Biochim Biophys Acta. doi:10.1016/j.bbagrm.2013.10.005
Morris GE (2008) The Cajal body. Biochim Biophys Acta 1783:2108–2115
Mukherjee K, Brocchieri L, Bürglin TR (2009) A comprehensive classification and evolutionary analysis of plant homeobox genes. Mol Biol Evol 26:2775–2794
Onodera Y, Haag JR, Ream T, Costa Nunes P, Pontes O, Pikaard CS (2005) Plant nuclear RNA polymerase IV mediates siRNA and DNA methylation-dependent heterochromatin formation. Cell 120:613–622
Pontier D, Yahubyan G, Vega D, Bulski A, Saez-Vasquez J, Hakimi MA, Lerbs-Mache S, Colot V, Lagrange T (2005) Reinforcement of silencing at transposons and highly repeated sequences requires the concerted action of two distinct RNA polymerases IV in Arabidopsis. Genes Dev 19:2030–2040
Sanford JR, Ellis J, Caceres JF (2005) Multiple roles of arginine/serine-rich splicing factors in RNA processing. Biochem Soc Trans 33:443–446
Smith LM, Pontes O, Searle I, Yelina N, Yousafzai FK, Herr AJ, Pikaard CS, Baulcombe DC (2007) An SNF2 protein associated with nuclear RNA silencing and the spread of a silencing signal between cells in Arabidopsis. Plant Cell 19:1507–1521
Sridhar VV, Kapoor A, Zhang K, Zhu J, Zhou T, Hasegawa PM, Bressan RA, Zhu JK (2007) Control of DNA methylation and heterochromatic silencing by histone H2B deubiquitination. Nature 447:735–738
Vaucheret H (2008) Plant ARGONAUTES. Trends Plant Sci 2008(13):350–358
Verdel A, Jia S, Gerber S, Sugiyama T, Gygi S, Grewal SI, Moazed D (2004) RNAi-mediated targeting of heterochromatin by the RITS complex. Science 303:672–676
Xie Z, Johansen LK, Gustafson AM, Kasschau KD, Lellis AD, Zilberman D, Jacobsen SE, Carrington JC (2004) Genetic and functional diversification of small RNA pathways in plants. PLoS Biol 2:E104
Yang Z, Ebright YW, Yu B, Chen X (2006) HEN1 recognizes 21–24 nt small RNA duplexes and deposits a methyl group onto the 2′ OH of the 3′ terminal nucleotide. Nucl Acids Res 34:667–675
Yokthongwattana C, Bucher E, Caikovski M, Vaillant I, Nicolet J, Mittelsten Scheid O, Paszkowski J (2010) MOM1 and Pol-IV/V interactions regulate the intensity and specificity of transcriptional gene silencing. EMBO J 29:340–351
Zaratiegui M, Irvine DV, Martienssen RA (2007) Noncoding RNAs and gene silencing. Cell 128:763–776
Zemach A, Kim MY, Hsieh PH, Coleman-Derr D, EshedWilliams L, Thao K, Harmer SL, Zilberman D (2013) The Arabidopsis nucleosome remodeler DDM1 allows DNA methyltransferases to access H1-containing heterochromatin. Cell 153:193–205
Zhang H, Zhu JK (2011) RNA-directed DNA methylation. Curr Opin Plant Biol 14:142–147
Zhang H, He X, Zhu J (2013) RNA-directed DNA methylation in plants: where to start. RNA Biol 10 (In press)
Zhang H, Ma ZY, Zeng L, Tanaka K, Zhang CJ, Ma J, Bai G, Wang P, Zhang SW, Liu ZW (2013b) DTF1 is a core component of RNA-directed DNA methylation and may assist in the recruitment of PolIV. Proc Natl Acad Sci USA 110:8290–8295
Zhang CJ, Zhou JX, Liu J, Ma ZY, Zhang SW, Dou K, Huang HW, Cai T, Liu R, Zhu JK, He XJ (2013c) The splicing machinery promotes RNA-directed DNA methylation and transcriptional silencing in Arabidopsis. EMBO J 32:1128–1140
Zheng X, Pontes O, Zhu J, Miki D, Zhang F, Li WX, Iida K, Kapoor A, Pikaard CS, Zhu JK (2008) ROS3 is an RNA-binding protein required for DNA demethylation in Arabidopsis. Nature 455:1259–1262
Zheng B, Wang Z, Li S, Yu B, Liu JY, Chen X (2009) Intergenic transcription by RNA polymerase II coordinates PolIV and PolV in siRNA-directed transcriptional gene silencing in Arabidopsis. Genes Dev 23:2850–2860
Zhu JK (2008) Epigenome sequencing comes of age. Cell 133:395–397
Zhu Y, Rowley MJ, Bohmdorfer G, Wierzbicki AT (2013) A SWI/SNF chromatin remodeling complex acts in noncoding RNA-mediated transcriptional silencing. Mol Cell 49:298–309
Acknowledgments
This work was supported by the National Science Foundation of China (No. 31170561), the International Science and Technology Cooperation Program of China (2014DFG32440), the Priority Academic Program Development of Jiangsu Higher Education Institutions, the Program for Innovative Research Team in University of Educational Department and Jiangsu Province, China.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by N. Stewart.
W. Sun and J. Zhang contributed equally to this review.
Rights and permissions
About this article
Cite this article
Movahedi, A., Sun, W., Zhang, J. et al. RNA-directed DNA methylation in plants. Plant Cell Rep 34, 1857–1862 (2015). https://doi.org/10.1007/s00299-015-1839-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-015-1839-0